Loss of Cdc42 in Exocrine Acini Decreases Saliva Secretion but Increases Tear Secretion—A Potential Model of Exocrine Gland Senescence
Abstract
:1. Introduction
2. Results
2.1. Acinar Cell-Specific Cdc42 Conditional Knockout Mice Had Altered Luminal Structures in SGs and LGs
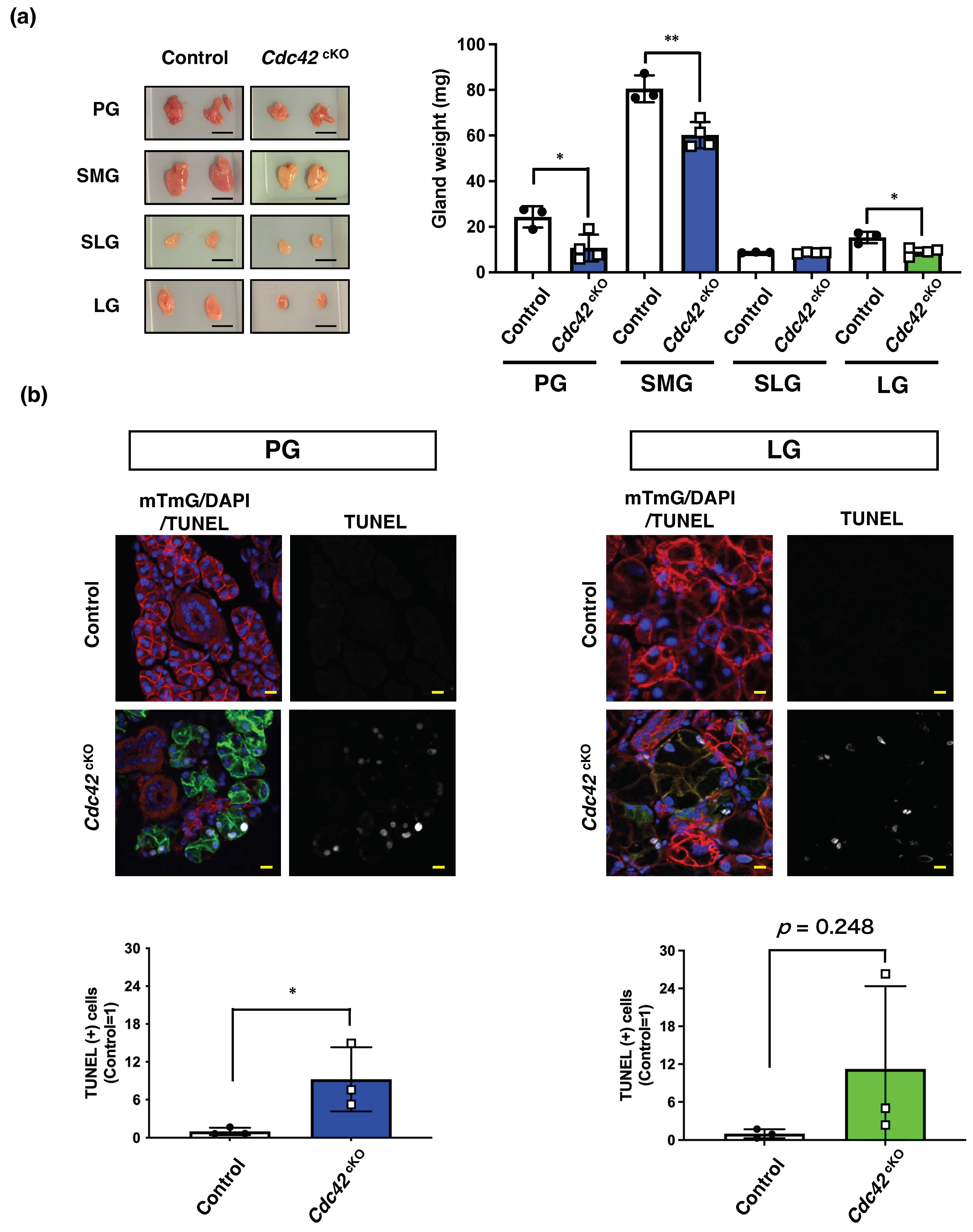
2.2. Cdc42cKO Reduces the Number of Acinar Cells in Exocrine Glands by Inducing Apoptotic Cell Death
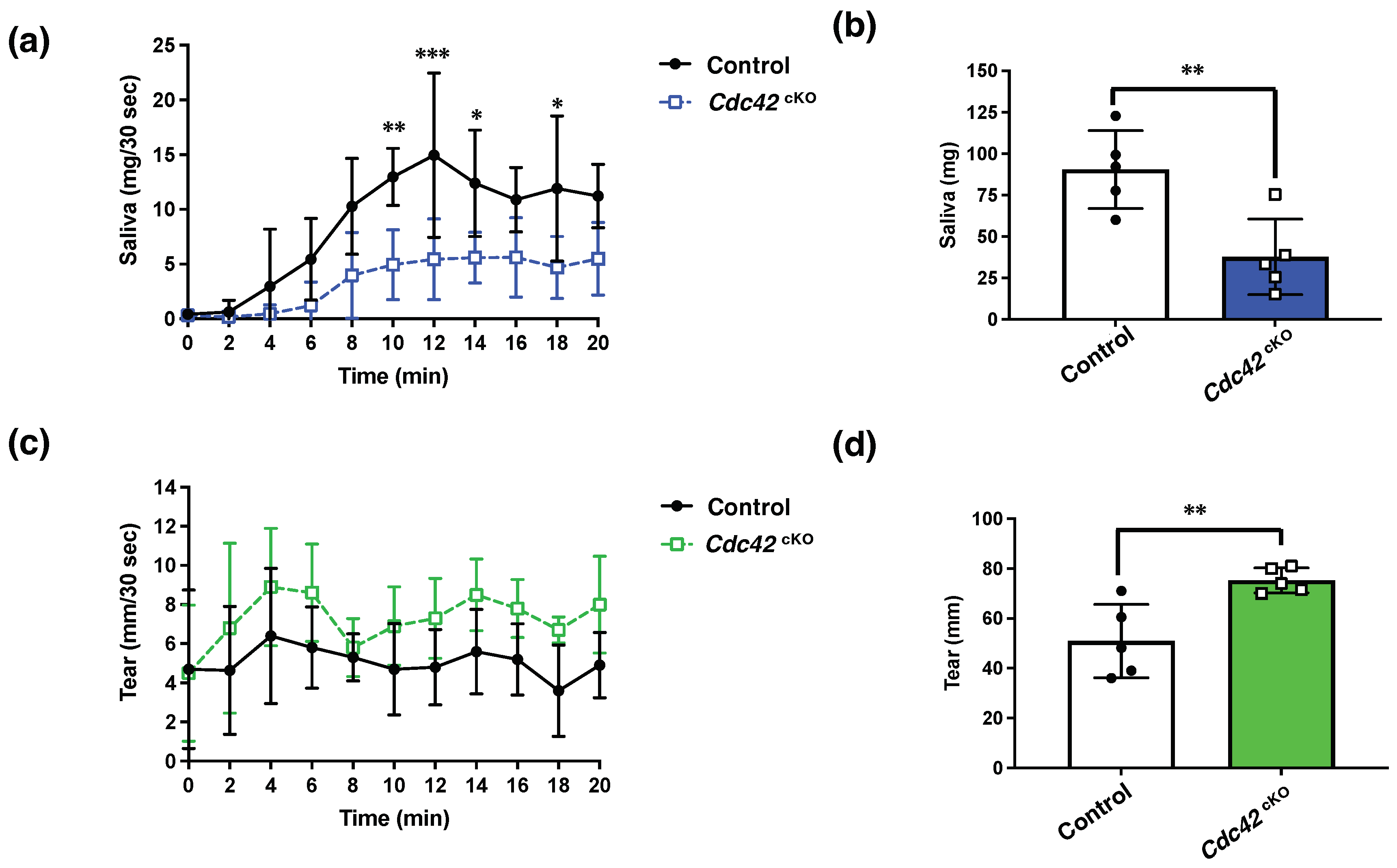
2.3. Cdc42 Depletion Decreased Pilocarpine-Stimulated Saliva Secretion, but Increased Tear Secretion
2.4. AQP5 Protein in Acinar Cells Is Downregulated in PGs but Upregulated in LGs
3. Discussion
3.1. Differences in Water Secretion Function and AQP5 Expression between SGs and LGs
3.2. Mechanism of Cdc42’s Regulation of AQP5 Expression
3.3. Acinar Cdc42 Deficiency Induced Apoptosis
3.4. Cdc42cKO Mice as a Potential Mouse Model for Aging Exocrine Glands
4. Materials and Methods
4.1. Materials
4.2. Mouse Strains
4.3. Tamoxifen Injection
4.4. Anesthesia Administration
4.5. RNA Extraction and Real-Time RT PCR (qPCR) Analysis
4.6. Immunofluorescent Staining and Imaging
4.7. Measurement of Gland Weight
4.8. In Situ Apoptosis Analysis
4.9. Measurement of Pilocarpine-Induced Saliva and Tear Volumes
4.10. Western Blot Analysis
4.11. Immunohistochemistry
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stephens, L.C.; Schultheiss, T.E.; Price, R.E.; Ang, K.K.; Peters, L.J. Radiation apoptosis of serous acinar cells of salivary and lacrimal glands. Cancer 1991, 67, 1539–1543. [Google Scholar] [CrossRef] [PubMed]
- Aqrawi, L.A.; Galtung, H.K.; Vestad, B.; Ovstebo, R.; Thiede, B.; Rusthen, S.; Young, A.; Guerreiro, E.M.; Utheim, T.P.; Chen, X.; et al. Identification of potential saliva and tear biomarkers in primary Sjogren’s syndrome, utilising the extraction of extracellular vesicles and proteomics analysis. Arthritis Res. Ther. 2017, 19, 14. [Google Scholar] [CrossRef]
- Aqrawi, L.A.; Chen, X.; Hynne, H.; Amdal, C.; Reppe, S.; Aass, H.C.D.; Rykke, M.; Hove, L.H.; Young, A.; Herlofson, B.B.; et al. Cytokines Explored in Saliva and Tears from Radiated Cancer Patients Correlate with Clinical Manifestations, Influencing Important Immunoregulatory Cellular Pathways. Cells 2020, 9, 2050. [Google Scholar] [CrossRef]
- Das, N.; Menon, N.G.; de Almeida, L.G.N.; Woods, P.S.; Heynen, M.L.; Jay, G.D.; Caffery, B.; Jones, L.; Krawetz, R.; Schmidt, T.A.; et al. Proteomics Analysis of Tears and Saliva from Sjogren’s Syndrome Patients. Front. Pharmacol. 2021, 12, 787193. [Google Scholar] [CrossRef] [PubMed]
- Scully, C.; Felix, D.H. Oral Medicine—Update for the dental practitioner. Dry mouth and disorders of salivation. Br. Dent. J. 2005, 199, 423–427. [Google Scholar] [CrossRef]
- Humphrey, S.P.; Williamson, R.T. A review of saliva: Normal composition, flow, and function. J. Prosthet. Dent. 2001, 85, 162–169. [Google Scholar] [CrossRef]
- Pflugfelder, S.C.; Stern, M.E. Biological functions of tear film. Exp. Eye Res. 2020, 197, 108115. [Google Scholar] [CrossRef]
- Barrows, C.M.L.; Wu, D.; Farach-Carson, M.C.; Young, S. Building a Functional Salivary Gland for Cell-Based Therapy: More than Secretory Epithelial Acini. Tissue Eng. Part A 2020, 26, 1332–1348. [Google Scholar] [CrossRef]
- Etienne-Manneville, S. Cdc42—The centre of polarity. J. Cell Sci. 2004, 117 Pt 8, 1291–3000. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Ma, L.; Parrini, M.C.; Mao, X.; Lopez, M.; Wu, C.; Marks, P.W.; Davidson, L.; Kwiatkowski, D.J.; Kirchhausen, T.; et al. Cdc42 is required for PIP2-induced actin polymerization and early development but not for cell viability. Curr. Biol. 2000, 10, 758–765. [Google Scholar] [CrossRef] [PubMed]
- Shitara, A.; Malec, L.; Ebrahim, S.; Chen, D.; Bleck, C.; Hoffman, M.P.; Weigert, R. Cdc42 negatively regulates endocytosis during apical membrane maintenance in live animals. Mol. Biol. Cell 2019, 30, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Lemercier, C.; To, R.Q.; Swanson, B.J.; Lyons, G.E.; Konieczny, S.F. Mist1: A novel basic helix-loop-helix transcription factor exhibits a developmentally regulated expression pattern. Dev. Biol. 1997, 182, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Pin, C.L.; Bonvissuto, A.C.; Konieczny, S.F. Mist1 expression is a common link among serous exocrine cells exhibiting regulated exocytosis. Anat. Rec. 2000, 259, 157–167. [Google Scholar] [CrossRef]
- Farmer, D.T.; Nathan, S.; Finley, J.K.; Shengyang Yu, K.; Emmerson, E.; Byrnes, L.E.; Sneddon, J.B.; McManus, M.T.; Tward, A.D.; Knox, S.M. Defining epithelial cell dynamics and lineage relationships in the developing lacrimal gland. Development 2017, 144, 2517–2528. [Google Scholar] [CrossRef] [PubMed]
- Winston, D.C.; Hennigar, R.A.; Spicer, S.S.; Garrett, J.R.; Schulte, B.A. Immunohistochemical localization of Na+, K+-ATPase in rodent and human salivary and lacrimal glands. J. Histochem. Cytochem. 1988, 36, 1139–1145. [Google Scholar] [CrossRef]
- Tsubota, K.; Hirai, S.; King, L.S.; Agre, P.; Ishida, N. Defective cellular trafficking of lacrimal gland aquaporin-5 in Sjogren’s syndrome. Lancet 2001, 357, 688–689. [Google Scholar] [CrossRef]
- Ohashi, Y.; Tsuzaka, K.; Takeuchi, T.; Sasaki, Y.; Tsubota, K. Altered distribution of aquaporin 5 and its C-terminal binding protein in the lacrimal glands of a mouse model for Sjögren’s syndrome. Curr. Eye Res. 2008, 33, 621–629. [Google Scholar] [CrossRef]
- Liu, Y.; Di, G.; Hu, S.; Zhao, T.; Xu, X.; Wang, X.; Chen, P. Expression Profiles of CircRNA and mRNA in Lacrimal Glands of AQP5−/− Mice With Primary Dry Eye. Front. Physiol. 2020, 11, 1010. [Google Scholar] [CrossRef]
- Matsuzaki, T.; Susa, T.; Shimizu, K.; Sawai, N.; Suzuki, T.; Aoki, T.; Yokoo, S.; Takata, K. Function of the membrane water channel aquaporin-5 in the salivary gland. Acta Histochem. Cytochem. 2012, 45, 251–259. [Google Scholar] [CrossRef]
- Sasaki, Y.; Tsubota, K.; Kawedia, J.D.; Menon, A.G.; Yasui, M. The difference of aquaporin 5 distribution in acinar and ductal cells in lacrimal and parotid glands. Curr. Eye Res. 2007, 32, 923–929. [Google Scholar] [CrossRef]
- D’Agostino, C.; Parisis, D.; Chivasso, C.; Hajiabbas, M.; Soyfoo, M.S.; Delporte, C. Aquaporin-5 Dynamic Regulation. Int. J. Mol. Sci. 2023, 24, 1889. [Google Scholar] [CrossRef] [PubMed]
- Satoh, K.; Narita, T.; Matsuki-Fukushima, M.; Okabayashi, K.; Ito, T.; Senpuku, H.; Sugiya, H. E2f1-deficient NOD/SCID mice have dry mouth due to a change of acinar/duct structure and the down-regulation of AQP5 in the salivary gland. Pflug. Arch. 2013, 465, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, Y.; Bouley, R.; Chen, Y.; Matsuzaki, T.; Nunes, P.; Hasler, U.; Brown, D.; Lu, H.A. Simvastatin enhances aquaporin-2 surface expression and urinary concentration in vasopressin-deficient Brattleboro rats through modulation of Rho GTPase. Am. J. Physiol. Ren. Physiol. 2011, 301, F309–F318. [Google Scholar] [CrossRef] [PubMed]
- Haanen, C.; Vermes, I. Apoptosis and inflammation. Mediat. Inflamm. 1995, 4, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Wallach, D.; Kovalenko, A. Keeping inflammation at bay. eLife 2014, 25, e02583. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Ohbo, K.; Takakura, A.; Takebayashi, H.; Okada, T.; Abe, K.; Nabeshima, Y. Sgn1, a basic helix-loop-helix transcription factor delineates the salivary gland duct cell lineage in mice. Dev. Biol. 2001, 240, 517–530. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Zhang, L.; Chen, Y.; Zhang, H.; Zhang, Q.; Li, R.; Ma, J.; Li, Z.; Yu, C.; Lai, Y.; et al. Cdc42 deficiency induces podocyte apoptosis by inhibiting the Nwasp/stress fibers/YAP pathway. Cell Death Dis. 2016, 7, e2142. [Google Scholar] [CrossRef] [PubMed]
- Smits, K.; Iannucci, V.; Stove, V.; Van Hauwe, P.; Naessens, E.; Meuwissen, P.J.; Arien, K.K.; Bentahir, M.; Plum, J.; Verhasselt, B. Rho GTPase Cdc42 is essential for human T-cell development. Haematologica 2010, 95, 367–375. [Google Scholar] [CrossRef]
- Li, G.; Wang, Y.; Guo, X.B.; Zhao, B. CDC42 Regulates Cell Proliferation and Apoptosis in Bladder Cancer via the IQGAP3-Mediated Ras/ERK Pathway. Biochem. Genet. 2022, 60, 2383–2398. [Google Scholar] [CrossRef]
- Shikama, Y.; Kurosawa, M.; Furukawa, M.; Ishimaru, N.; Matsushita, K. Involvement of adiponectin in age-related increases in tear production in mice. Aging 2019, 11, 8329–8346. [Google Scholar] [CrossRef]
- McClellan, A.J.; Volpe, E.A.; Zhang, X.; Darlington, G.J.; Li, D.Q.; Pflugfelder, S.C.; de Paiva, C.S. Ocular surface disease and dacryoadenitis in aging C57BL/6 mice. Am. J. Pathol. 2014, 184, 631–643. [Google Scholar] [CrossRef] [PubMed]
- Yue, B. Structural features of submandibular glands in the elderly. Kokubyo Gakkai Zasshi 2007, 74, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Umbayev, B.; Safarova Yantsen, Y.; Yermekova, A.; Nessipbekova, A.; Syzdykova, A.; Askarova, S. Role of a small GTPase Cdc42 in aging and age-related diseases. Biogerontology 2023, 24, 27–46. [Google Scholar] [CrossRef]
- Wang, L.; Yang, L.; Debidda, M.; Witte, D.; Zheng, Y. Cdc42 GTPase-activating protein deficiency promotes genomic instability and premature aging-like phenotypes. Proc. Natl. Acad. Sci. USA 2007, 104, 1248–1253. [Google Scholar] [CrossRef] [PubMed]
- Muzumdar, M.D.; Tasic, B.; Miyamichi, K.; Li, L.; Luo, L. A global double-fluorescent Cre reporter mouse. Genesis 2007, 45, 593–605. [Google Scholar] [CrossRef]
- Ohno, Y.; Satoh, K.; Shitara, A.; Into, T.; Kashimata, M. Arginase 1 is involved in lacrimal hyposecretion in male NOD mice, a model of Sjogren’s syndrome, regardless of dacryoadenitis status. J. Physiol. 2020, 598, 4907–4925. [Google Scholar] [CrossRef]
- Nagase, H.; Higashi, S.L.; Iweka, C.A.; Pearson, C.S.; Hirata, Y.; Geller, H.M.; Katagiri, Y. Reliable and sensitive detection of glycosaminoglycan chains with immunoblots. Glycobiology 2021, 31, 116–125. [Google Scholar] [CrossRef]
- Higashi, S.L.; Yagyu, K.; Nagase, H.; Pearson, C.S.; Geller, H.M.; Katagiri, Y. Old but not obsolete: An enhanced high-speed immunoblot. J. Biochem. 2020, 168, 15–22. [Google Scholar] [CrossRef]



| Gene Name | Direction | Primer Sequence (5′−3′) | Annealing Temperature (°C) | Reference |
|---|---|---|---|---|
| Cdc42 | Forward | GTG TGT TGT TGT TGG TGA TGG T | 60 | This study |
| Reverse | AGT CCA AGA GTG TAT GGC TCT | |||
| Aqp5 | Forward | CAT CTT CTA CGT GGC AGC CC | 60 | This study |
| Reverse | ATT AAC TCC ACC ACC ACG GC | |||
| Gapdh | Forward | AGG CCG GTG CTG AGT ATG TC | 60 | |
| Reverse | TGC CTG CTT CAC CAC CTT CT |
| Antibody | Species | Dilution for WB | Dilution for IF, IHC | Campany (Catalog#) |
|---|---|---|---|---|
| AQP5 | pAb, Rb | 1:6K | 1:200, 1:300 | Millipore (AB15858) |
| GAPDH | pAb, Rb | 1:20K | − | Gene Tex (GTX100118) |
| Rb IgG-F(ab’)2-HRP | pAb, Gt | 1:20K | 1:500 | Millipore (AQ132P) |
| Rb IgG H&L-647 | pAb, Donkey | − | 1:300 | abcam (ab150075) |
| Streptavidin-HRP | − | − | 1:1K | abcam (ab7403) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagase, H.; Shitara, A.; Ohno, Y.; Satoh, K.; Kashimata, M. Loss of Cdc42 in Exocrine Acini Decreases Saliva Secretion but Increases Tear Secretion—A Potential Model of Exocrine Gland Senescence. Int. J. Mol. Sci. 2023, 24, 17220. https://doi.org/10.3390/ijms242417220
Nagase H, Shitara A, Ohno Y, Satoh K, Kashimata M. Loss of Cdc42 in Exocrine Acini Decreases Saliva Secretion but Increases Tear Secretion—A Potential Model of Exocrine Gland Senescence. International Journal of Molecular Sciences. 2023; 24(24):17220. https://doi.org/10.3390/ijms242417220
Chicago/Turabian StyleNagase, Haruna, Akiko Shitara, Yuta Ohno, Keitaro Satoh, and Masanori Kashimata. 2023. "Loss of Cdc42 in Exocrine Acini Decreases Saliva Secretion but Increases Tear Secretion—A Potential Model of Exocrine Gland Senescence" International Journal of Molecular Sciences 24, no. 24: 17220. https://doi.org/10.3390/ijms242417220
APA StyleNagase, H., Shitara, A., Ohno, Y., Satoh, K., & Kashimata, M. (2023). Loss of Cdc42 in Exocrine Acini Decreases Saliva Secretion but Increases Tear Secretion—A Potential Model of Exocrine Gland Senescence. International Journal of Molecular Sciences, 24(24), 17220. https://doi.org/10.3390/ijms242417220





