Seawater Accelerated the Aging of Polystyrene and Enhanced Its Toxic Effects on Caenorhabditis elegans
Abstract
:1. Introduction
2. Results
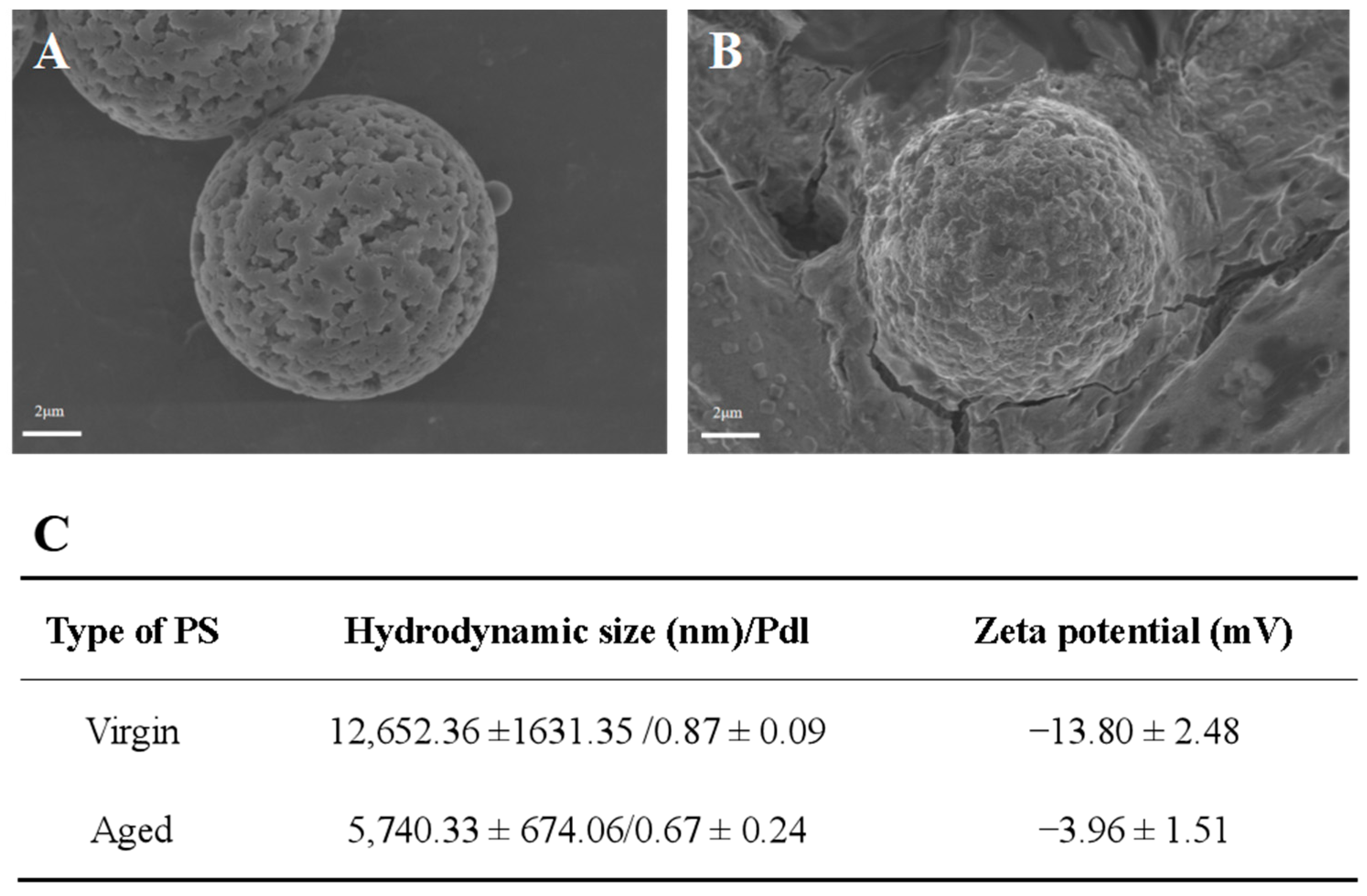
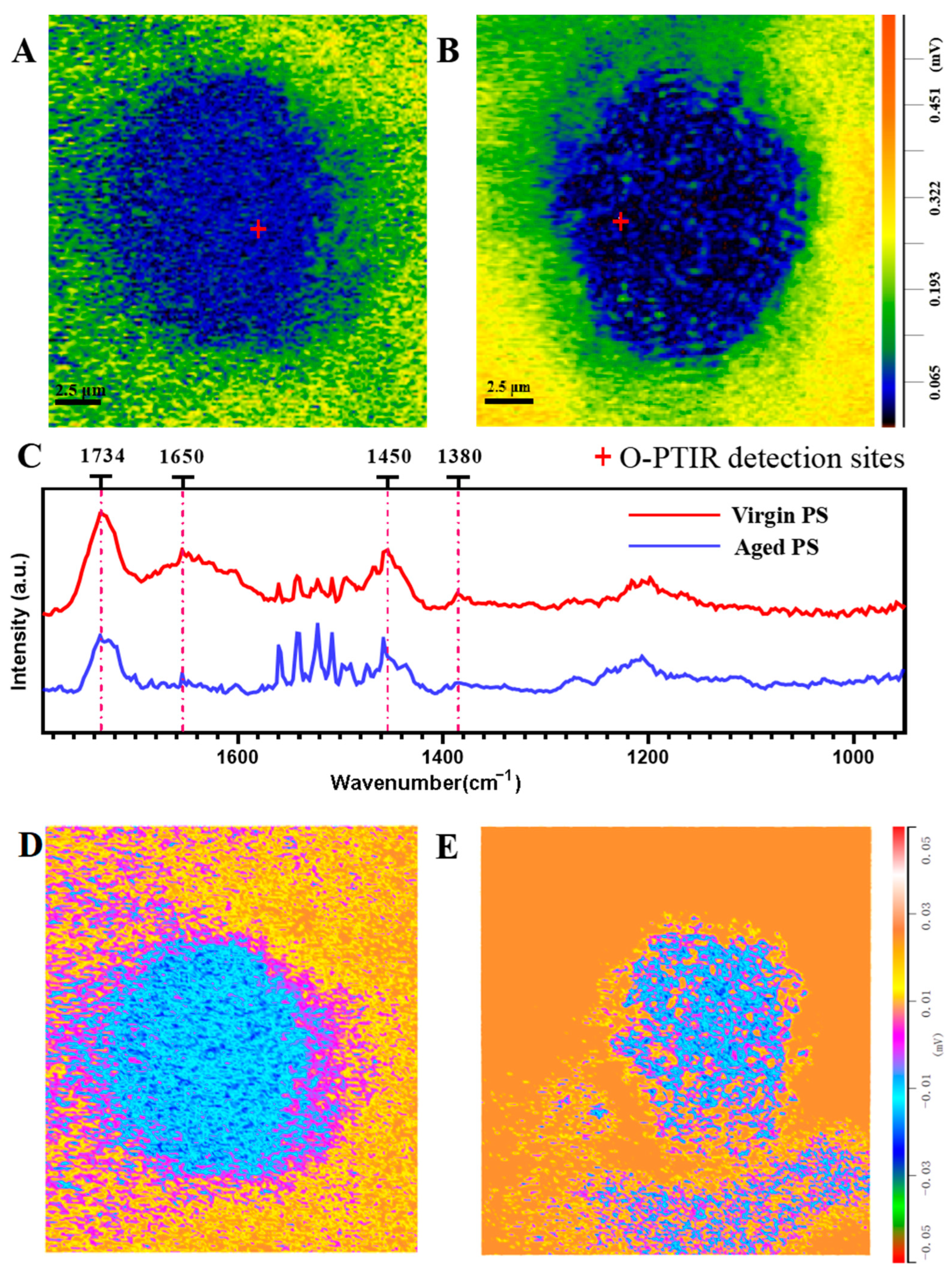
2.1. Characterization of Virgin and Aged Polystyrene (PS)
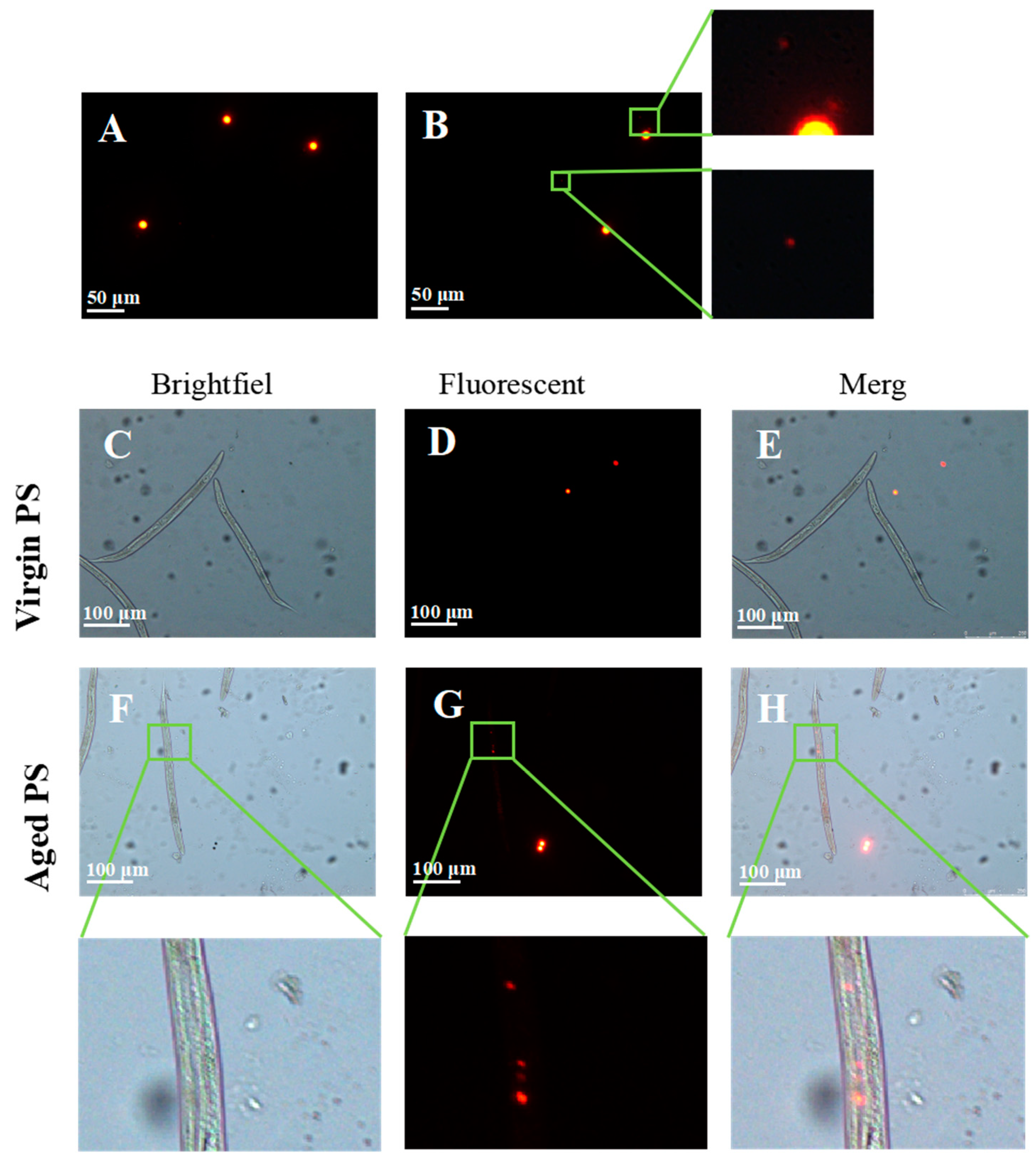
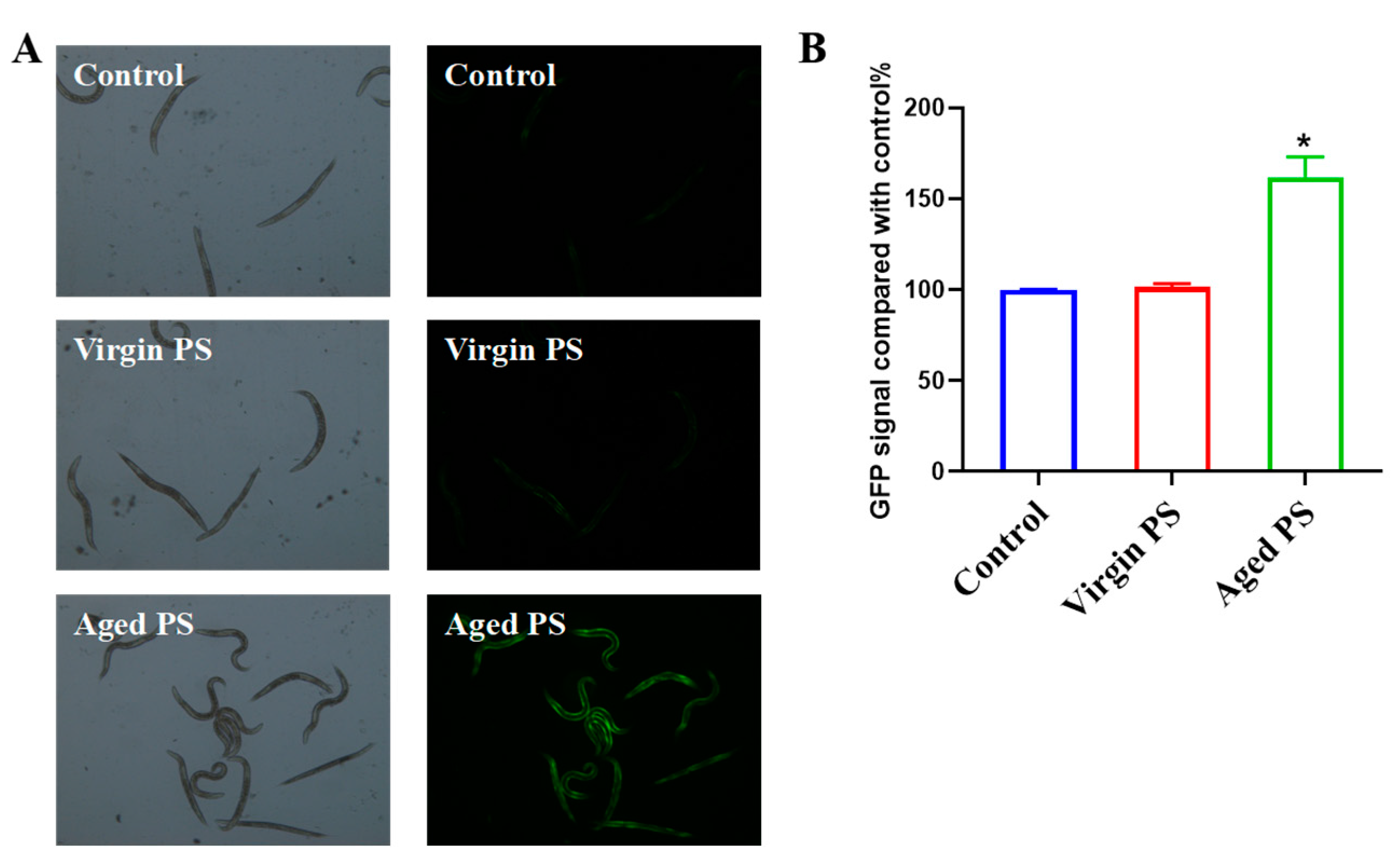
2.2. Secondary Microplastics (MPs) Ingested by C. elegans
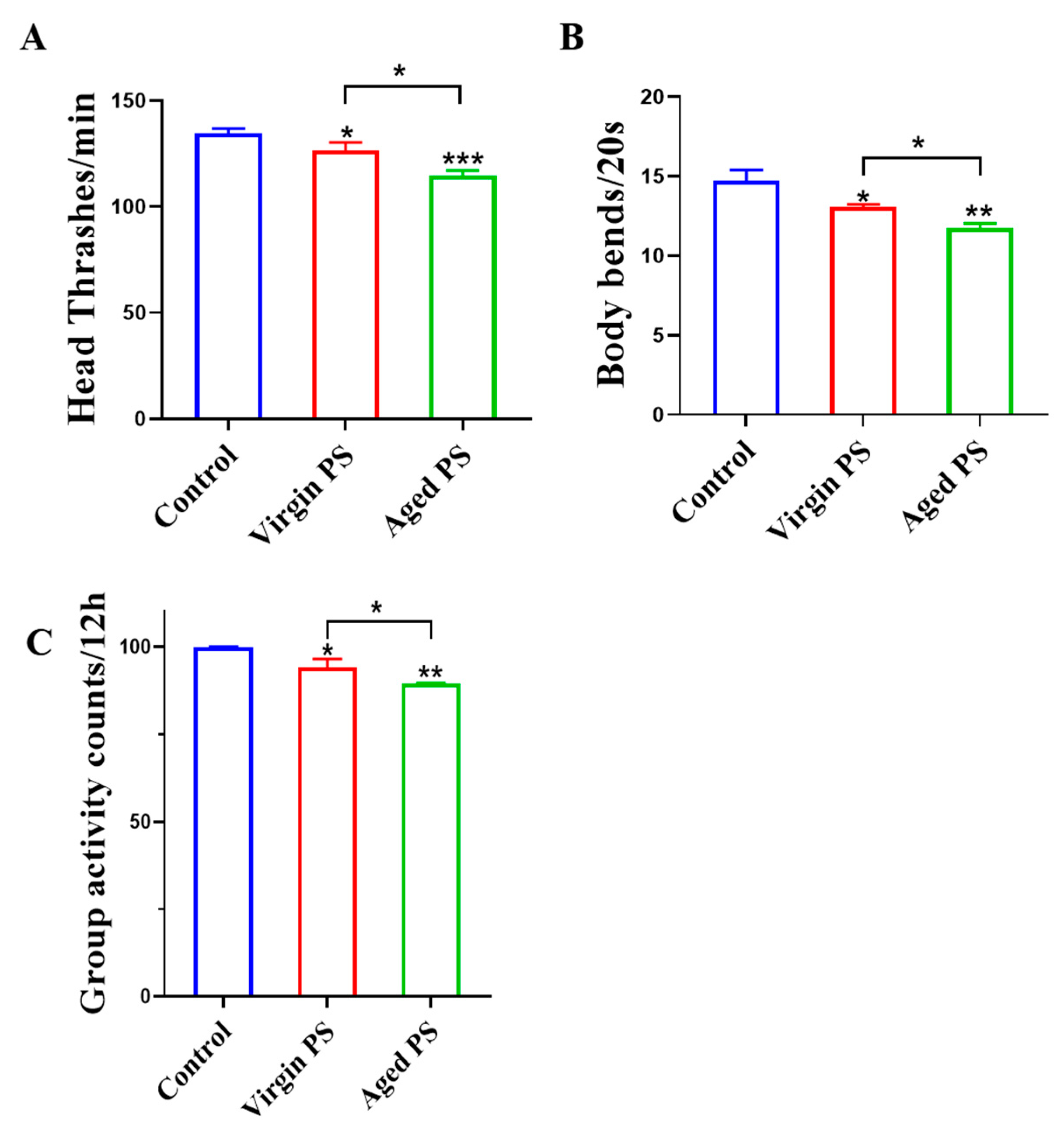
2.3. Effects of Virgin and Aged PS on Locomotion Behavior in C. elegans
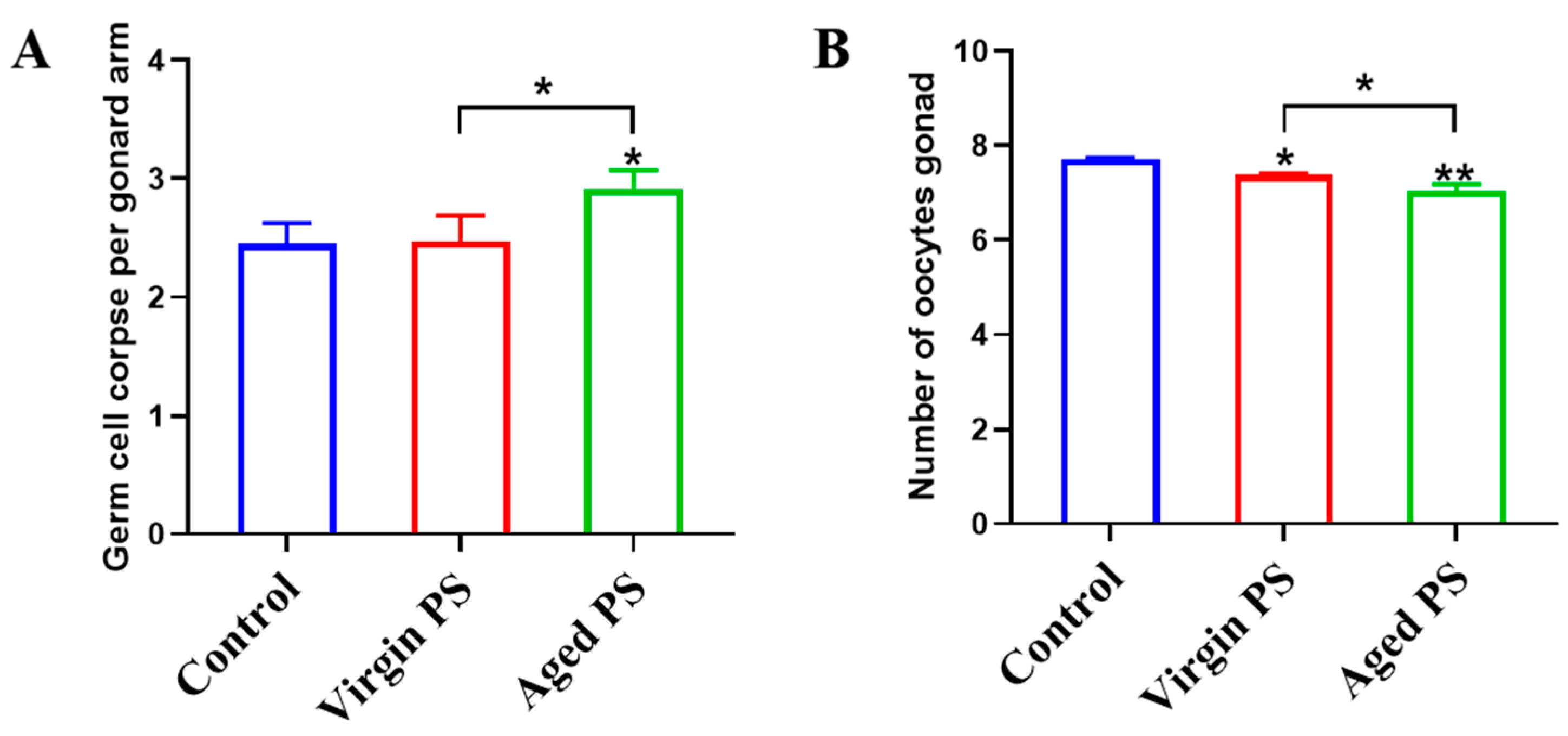
2.4. Effects of Virgin and Aged PS on Germ Cell Apoptosis and Oocytes Development in C. elegans
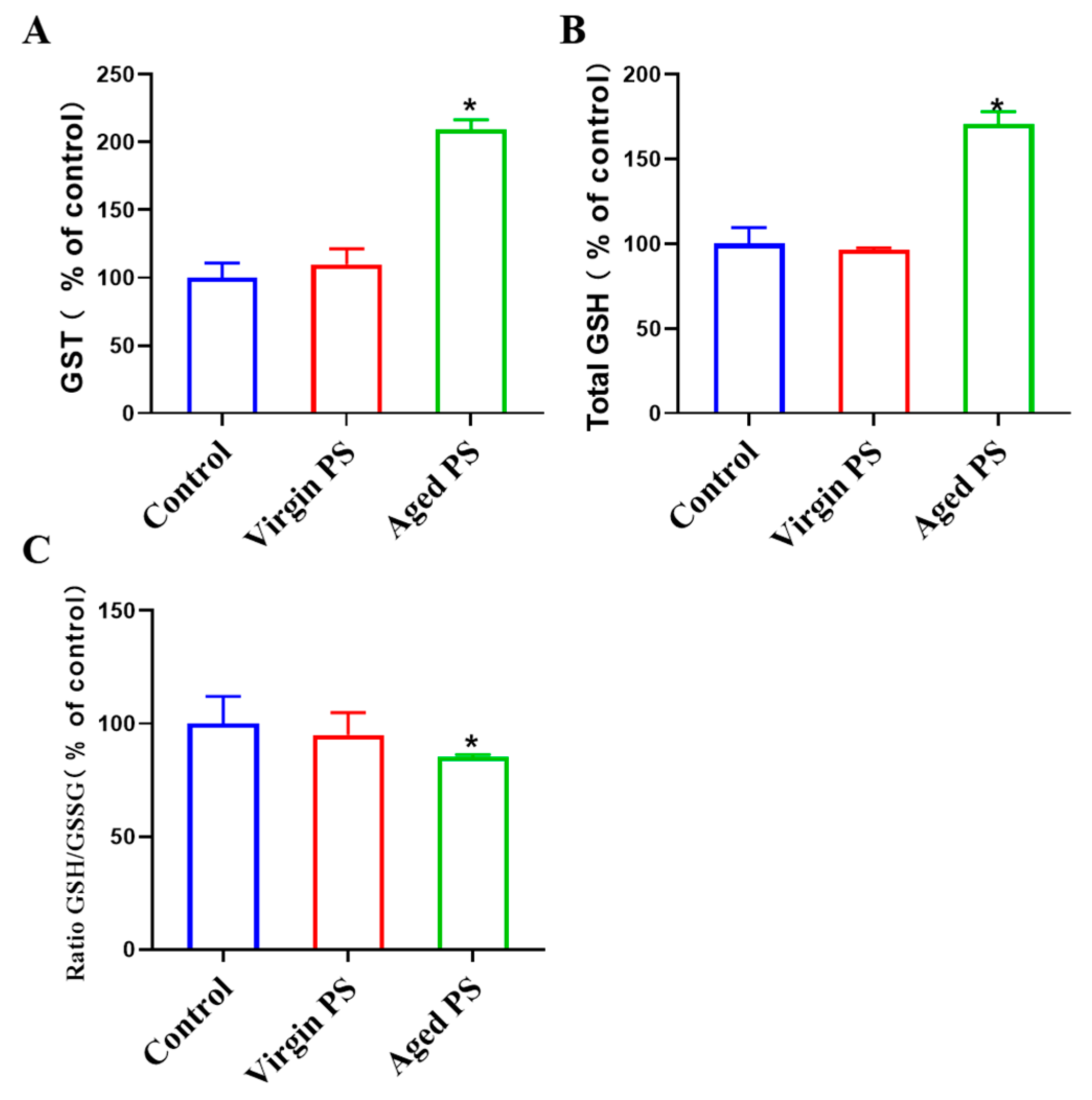
2.5. Aged PS Causes Oxidative Stress in C. elegans
3. Discussion
4. Materials and Methods
4.1. Modeling the Aging Process of Microplastics (PS) in Standard Seawater
4.2. Characterization of Physicochemical Properties of Virgin and Aged PS
4.3. C. elegans Strains and Exposure Concentration
4.4. In Vivo Distributions of Virgin and Aged Orange Fluorescent PS in C. elegans
4.5. Locomotor Behavior Assay
4.6. Viability Assay
4.7. Fertility Assay
4.8. Activation of the Antioxidative Response
4.9. Measurements of Antioxidant Enzyme Activities
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thompson, R.C.; Olsen, Y.; Mitchell, R.P.; Davis, A.; Rowland, S.J.; John, A.W.G.; McGonigle, D.; Russell, A.E. Lost at Sea: Where Is All the Plastic? Science 2004, 304, 838. [Google Scholar] [CrossRef]
- Li, C.; Wang, X.; Liu, K.; Zhu, L.; Wei, N.; Zong, C.; Li, D. Pelagic Microplastics in Surface Water of the Eastern Indian Ocean during Monsoon Transition Period: Abundance, Distribution, and Characteristics. Sci. Total Environ. 2021, 755, 142629. [Google Scholar] [CrossRef]
- Wang, S.; Chen, H.; Zhou, X.; Tian, Y.; Lin, C.; Wang, W.; Zhou, K.; Zhang, Y.; Lin, H. Microplastic Abundance, Distribution and Composition in the Mid-West Pacific Ocean. Environ. Pollut. 2020, 264, 114125. [Google Scholar] [CrossRef]
- Patti, T.B.; Fobert, E.K.; Reeves, S.E.; Burke Da Silva, K. Spatial Distribution of Microplastics around an Inhabited Coral Island in the Maldives, Indian Ocean. Sci. Total Environ. 2020, 748, 141263. [Google Scholar] [CrossRef]
- Suaria, G.; Cappa, P.; Perold, V.; Aliani, S.; Ryan, P.G. Abundance and Composition of Small Floating Plastics in the Eastern and Southern Sectors of the Atlantic Ocean. Mar. Pollut. Bull. 2023, 193, 115109. [Google Scholar] [CrossRef]
- Ikenoue, T.; Nakajima, R.; Fujiwara, A.; Onodera, J.; Itoh, M.; Toyoshima, J.; Watanabe, E.; Murata, A.; Nishino, S.; Kikuchi, T. Horizontal Distribution of Surface Microplastic Concentrations and Water-Column Microplastic Inventories in the Chukchi Sea, Western Arctic Ocean. Sci. Total Environ. 2023, 855, 159564. [Google Scholar] [CrossRef] [PubMed]
- Aves, A.R.; Revell, L.E.; Gaw, S.; Ruffell, H.; Schuddeboom, A.; Wotherspoon, N.E.; LaRue, M.; McDonald, A.J. First Evidence of Microplastics in Antarctic Snow. Cryosphere 2022, 16, 2127–2145. [Google Scholar] [CrossRef]
- Laskar, N.; Kumar, U. Plastics and Microplastics: A Threat to Environment. Environ. Technol. Innov. 2019, 14, 100352. [Google Scholar] [CrossRef]
- Kentin, E.; Kaarto, H. An EU Ban on Microplastics in Cosmetic Products and the Right to Regulate. RECIEL 2018, 27, 254–266. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, J.; Huang, W.; He, Y. Laboratory Simulated Aging Methods, Mechanisms and Characteristic Changes of Microplastics: A Review. Chemosphere 2023, 315, 137744. [Google Scholar] [CrossRef] [PubMed]
- Guerranti, C.; Martellini, T.; Perra, G.; Scopetani, C.; Cincinelli, A. Microplastics in Cosmetics: Environmental Issues and Needs for Global Bans. Environ. Toxicol. Pharmacol. 2019, 68, 75–79. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, Q.; Zhang, C.; Zhang, J.; Dong, Z.; Xu, Q. Aging Simulation of Thin-Film Plastics in Different Environments to Examine the Formation of Microplastic. Water Res. 2021, 202, 117462. [Google Scholar] [CrossRef]
- Zhu, K.; Jia, H.; Sun, Y.; Dai, Y.; Zhang, C.; Guo, X.; Wang, T.; Zhu, L. Long-Term Phototransformation of Microplastics under Simulated Sunlight Irradiation in Aquatic Environments: Roles of Reactive Oxygen Species. Water Res. 2020, 173, 115564. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Jia, H.; Zhao, S.; Xia, T.; Guo, X.; Wang, T.; Zhu, L. Formation of Environmentally Persistent Free Radicals on Microplastics under Light Irradiation. Environ. Sci. Technol. 2019, 53, 8177–8186. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Xian, Z.; Jin, X.; Liang, S.; Chen, Z.; Pan, B.; Wu, B.; Ok, Y.S.; Gu, C. Photo-Aging of Polyvinyl Chloride Microplastic in the Presence of Natural Organic Acids. Water Res. 2020, 183, 116082. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.K.; Hong, S.H.; Jang, M.; Han, G.M.; Jung, S.W.; Shim, W.J. Combined Effects of UV Exposure Duration and Mechanical Abrasion on Microplastic Fragmentation by Polymer Type. Environ. Sci. Technol. 2017, 51, 4368–4376. [Google Scholar] [CrossRef] [PubMed]
- Guan, Q.; Yang, H.; Zhao, Y.; Han, Z.; Ling, Z.; Yang, K.; Yin, C.; Yu, S. Microplastics Release from Victuals Packaging Materials during Daily Usage. EcoMat 2021, 3, e12107. [Google Scholar] [CrossRef]
- Zhou, G.; Wu, Q.; Tang, P.; Chen, C.; Cheng, X.; Wei, X.-F.; Ma, J.; Liu, B. How Many Microplastics Do We Ingest When Using Disposable Drink Cups? J. Hazard. Mater. 2023, 441, 129982. [Google Scholar] [CrossRef]
- Su, Y.; Hu, X.; Tang, H.; Lu, K.; Li, H.; Liu, S.; Xing, B.; Ji, R. Steam Disinfection Releases Micro(Nano)Plastics from Silicone-Rubber Baby Teats as Examined by Optical Photothermal Infrared Microspectroscopy. Nat. Nanotechnol. 2022, 17, 76–85. [Google Scholar] [CrossRef]
- Khoshnamvand, M.; Hanachi, P.; Ashtiani, S.; Walker, T.R. Toxic Effects of Polystyrene Nanoplastics on Microalgae Chlorella vulgaris: Changes in Biomass, Photosynthetic Pigments and Morphology. Chemosphere 2021, 280, 130725. [Google Scholar] [CrossRef]
- Anbumani, S.; Kakkar, P. Ecotoxicological Effects of Microplastics on Biota: A Review. Environ. Sci. Pollut. Res. 2018, 25, 14373–14396. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, X.; Wang, J.; Tan, L. Toxic Effects of Microplastic on Marine Microalgae Skeletonema costatum: Interactions between Microplastic and Algae. Environ. Pollut. 2017, 220, 1282–1288. [Google Scholar] [CrossRef]
- Besseling, E.; Quik, J.T.K.; Sun, M.; Koelmans, A.A. Fate of Nano- and Microplastic in Freshwater Systems: A Modeling Study. Environ. Pollut. 2017, 220, 540–548. [Google Scholar] [CrossRef]
- Bosker, T.; Olthof, G.; Vijver, M.G.; Baas, J.; Barmentlo, S.H. Significant Decline of Daphnia magna Population Biomass Due to Microplastic Exposure. Environ. Pollut. 2019, 250, 669–675. [Google Scholar] [CrossRef]
- Wang, X.; Liu, L.; Zheng, H.; Wang, M.; Fu, Y.; Luo, X.; Li, F.; Wang, Z. Polystyrene Microplastics Impaired the Feeding and Swimming Behavior of Mysid Shrimp Neomysis japonica. Mar. Pollut. Bull. 2020, 150, 110660. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Yang, G.; Lu, L.; Zheng, Y.; Zhang, Q.; Zhang, X.; Tian, H.; Wang, W.; Ru, S. Low Level of Polystyrene Microplastics Decreases Early Developmental Toxicity of Phenanthrene on Marine Medaka (Oryzias melastigma). J. Hazard. Mater. 2020, 385, 121586. [Google Scholar] [CrossRef]
- Li, Y.; Yang, G.; Wang, J.; Lu, L.; Li, X.; Zheng, Y.; Zhang, Z.; Ru, S. Microplastics Increase the Accumulation of Phenanthrene in the Ovaries of Marine Medaka (Oryzias melastigma) and Its Transgenerational Toxicity. J. Hazard. Mater. 2022, 424, 127754. [Google Scholar] [CrossRef] [PubMed]
- Waring, R.H.; Harris, R.M.; Mitchell, S.C. Plastic Contamination of the Food Chain: A Threat to Human Health? Maturitas 2018, 115, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Mercogliano, R.; Avio, C.G.; Regoli, F.; Anastasio, A.; Colavita, G.; Santonicola, S. Occurrence of Microplastics in Commercial Seafood under the Perspective of the Human Food Chain. A Review. J. Agric. Food Chem. 2020, 68, 5296–5301. [Google Scholar] [CrossRef]
- Cao, Z.; Wang, M.; Zhou, T.; Xu, A.; Du, H. Whole-Genome Sequencing Reveals Germ Cell Mutagenicity of α-Endosulfan in Caenorhabditis elegans. Environ. Sci. Technol. 2022, 56, 16024–16032. [Google Scholar] [CrossRef]
- Chen, H.; Hua, X.; Li, H.; Wang, C.; Dang, Y.; Ding, P.; Yu, Y. Transgenerational Neurotoxicity of Polystyrene Microplastics Induced by Oxidative Stress in Caenorhabditis elegans. Chemosphere 2021, 272, 129642. [Google Scholar] [CrossRef] [PubMed]
- Schöpfer, L.; Menzel, R.; Schnepf, U.; Ruess, L.; Marhan, S.; Brümmer, F.; Pagel, H.; Kandeler, E. Microplastics Effects on Reproduction and Body Length of the Soil-Dwelling Nematode Caenorhabditis elegans. Front. Environ. Sci. 2020, 8, 41. [Google Scholar] [CrossRef]
- Chen, H.; Yang, Y.; Wang, C.; Hua, X.; Li, H.; Xie, D.; Xiang, M.; Yu, Y. Reproductive Toxicity of UV-Photodegraded Polystyrene Microplastics Induced by DNA Damage-Dependent Cell Apoptosis in Caenorhabditis elegans. Sci. Total Environ. 2022, 811, 152350. [Google Scholar] [CrossRef]
- Rubin, A.E.; Sarkar, A.K.; Zucker, I. Questioning the Suitability of Available Microplastics Models for Risk Assessment—A Critical Review. Sci. Total Environ. 2021, 788, 147670. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.L.-H.; Chiang, S.; Kalinowski, D.S.; Bae, D.-H.; Sahni, S.; Richardson, D.R. The Role of the Antioxidant Response in Mitochondrial Dysfunction in Degenerative Diseases: Cross-Talk between Antioxidant Defense, Autophagy, and Apoptosis. Oxid. Med. Cell. Longev. 2019, 2019, 6392763. [Google Scholar] [CrossRef]
- Zheng, X.; Liu, X.; Zhang, L.; Wang, Z.; Yuan, Y.; Li, J.; Li, Y.; Huang, H.; Cao, X.; Fan, Z. Toxicity Mechanism of Nylon Microplastics on Microcystis aeruginosa through Three Pathways: Photosynthesis, Oxidative Stress and Energy Metabolism. J. Hazard. Mater. 2022, 426, 128094. [Google Scholar] [CrossRef]
- Palaniappan, S.; Sadacharan, C.M.; Rostama, B. Polystyrene and Polyethylene Microplastics Decrease Cell Viability and Dysregulate Inflammatory and Oxidative Stress Markers of MDCK and L929 Cells In Vitro. Expo. Health 2022, 14, 75–85. [Google Scholar] [CrossRef] [PubMed]
- An, R.; Wang, X.; Yang, L.; Zhang, J.; Wang, N.; Xu, F.; Hou, Y.; Zhang, H.; Zhang, L. Polystyrene Microplastics Cause Granulosa Cells Apoptosis and Fibrosis in Ovary through Oxidative Stress in Rats. Toxicology 2021, 449, 152665. [Google Scholar] [CrossRef]
- Jeon, S.; Lee, D.-K.; Jeong, J.; Yang, S.I.; Kim, J.-S.; Kim, J.; Cho, W.-S. The Reactive Oxygen Species as Pathogenic Factors of Fragmented Microplastics to Macrophages. Environ. Pollut. 2021, 281, 117006. [Google Scholar] [CrossRef]
- Das, A. The Emerging Role of Microplastics in Systemic Toxicity: Involvement of Reactive Oxygen Species (ROS). Sci. Total Environ. 2023, 895, 165076. [Google Scholar] [CrossRef]
- Yang, C.; Gao, X. Impact of Microplastics from Polyethylene and Biodegradable Mulch Films on Rice (Oryza sativa L.). Sci. Total Environ. 2022, 828, 154579. [Google Scholar] [CrossRef]
- Silva, C.J.M.; Patrício Silva, A.L.; Campos, D.; Machado, A.L.; Pestana, J.L.T.; Gravato, C. Oxidative Damage and Decreased Aerobic Energy Production Due to Ingestion of Polyethylene Microplastics by Chironomus riparius (Diptera) Larvae. J. Hazard. Mater. 2021, 402, 123775. [Google Scholar] [CrossRef] [PubMed]
- Jeong, C.-B.; Won, E.-J.; Kang, H.-M.; Lee, M.-C.; Hwang, D.-S.; Hwang, U.-K.; Zhou, B.; Souissi, S.; Lee, S.-J.; Lee, J.-S. Microplastic Size-Dependent Toxicity, Oxidative Stress Induction, and p-JNK and p-P38 Activation in the Monogonont Rotifer (Brachionus koreanus). Environ. Sci. Technol. 2016, 50, 8849–8857. [Google Scholar] [CrossRef]
- Kim, J.-H.; Yu, Y.-B.; Choi, J.-H. Toxic Effects on Bioaccumulation, Hematological Parameters, Oxidative Stress, Immune Responses and Neurotoxicity in Fish Exposed to Microplastics: A Review. J. Hazard. Mater. 2021, 413, 125423. [Google Scholar] [CrossRef] [PubMed]
- Solomando, A.; Capó, X.; Alomar, C.; Álvarez, E.; Compa, M.; Valencia, J.M.; Pinya, S.; Deudero, S.; Sureda, A. Long-Term Exposure to Microplastics Induces Oxidative Stress and a pro-Inflammatory Response in the Gut of Sparus aurata Linnaeus, 1758. Environ. Pollut. 2020, 266, 115295. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Liu, Z.; Wu, D.; Chen, M.; Lv, W.; Zhao, Y. Accumulation of Polystyrene Microplastics in Juvenile Eriocheir sinensis and Oxidative Stress Effects in the Liver. Aquat. Toxicol. 2018, 200, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Chen, H.; Hua, X.; Dang, Y.; Han, Y.; Yu, Z.; Chen, X.; Ding, P.; Li, H. Polystyrene Microplastics (PS-MPs) Toxicity Induced Oxidative Stress and Intestinal Injury in Nematode Caenorhabditis elegans. Sci. Total Environ. 2020, 726, 138679. [Google Scholar] [CrossRef]
- Sivaselvam, S.; Mohankumar, A.; Thiruppathi, G.; Sundararaj, P.; Viswanathan, C.; Ponpandian, N. Engineering the Surface of Graphene Oxide with Bovine Serum Albumin for Improved Biocompatibility in Caenorhabditis elegans. Nanoscale Adv. 2020, 2, 5219–5230. [Google Scholar] [CrossRef]
- Mohankumar, A.; Devagi, G.; Shanmugam, G.; Nivitha, S.; Sundararaj, P.; Dallemer, F.; Kalaivani, P.; Prabhakaran, R. Organoruthenium(II) Complexes Attenuate Stress in Caenorhabditis elegans through Regulating Antioxidant Machinery. Eur. J. Med. Chem. 2019, 168, 123–133. [Google Scholar] [CrossRef]
- Wu, X.; Zhao, X.; Chen, R.; Liu, P.; Liang, W.; Wang, J.; Teng, M.; Wang, X.; Gao, S. Wastewater Treatment Plants Act as Essential Sources of Microplastic Formation in Aquatic Environments: A Critical Review. Water Res. 2022, 221, 118825. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, T.; Qu, G.; Jia, H.; Zhu, L. Probing the Aging Processes and Mechanisms of Microplastic under Simulated Multiple Actions Generated by Discharge Plasma. J. Hazard. Mater. 2020, 398, 122956. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Wu, Z.; Wang, J.; Li, Y.; Chu, R.; Pei, Y.; Ma, J. Effect of Landfill Age on the Physical and Chemical Characteristics of Waste Plastics/Microplastics in a Waste Landfill Sites. Environ. Pollut. 2022, 306, 119366. [Google Scholar] [CrossRef]
- Kadac-Czapska, K.; Knez, E.; Gierszewska, M.; Olewnik-Kruszkowska, E.; Grembecka, M. Microplastics Derived from Food Packaging Waste—Their Origin and Health Risks. Materials 2023, 16, 674. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhang, X.; Liu, J.; Li, Z.; Zhang, Z.; Gong, Y.; Bai, X.; Tan, C.; Li, H.; Li, J.; et al. Ageing Characteristics and Microplastic Release Behavior from Rainwater Facilities under ROS Oxidation. Sci. Total Environ. 2023, 866, 161397. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; She, Z.; Xiong, X.; Ouyang, G.; Luo, Z. Automated Analysis of Microplastics Based on Vibrational Spectroscopy: Are We Measuring the Same Metrics? Anal. Bioanal. Chem. 2022, 414, 3359–3372. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhu, J.; He, Y.; Wang, J.; Zeng, N.; Zhan, X. Photoaging Process and Mechanism of Four Commonly Commercial Microplastics. J. Hazard. Mater. 2023, 451, 131151. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Tang, S.; Wang, P.; Lu, B.; Li, K.; Jin, W.; He, X. From Source to Sink: Review and Prospects of Microplastics in Wetland Ecosystems. Sci. Total Environ. 2021, 758, 143633. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Liu, C.; He, D.; Xu, J.; Sun, J.; Li, J.; Pan, X. Environmental Behaviors of Microplastics in Aquatic Systems: A Systematic Review on Degradation, Adsorption, Toxicity and Biofilm under Aging Conditions. J. Hazard. Mater. 2022, 423, 126915. [Google Scholar] [CrossRef] [PubMed]
- Rauchschwalbe, M.-T.; Fueser, H.; Traunspurger, W.; Höss, S. Bacterial Consumption by Nematodes Is Disturbed by the Presence of Polystyrene Beads: The Roles of Food Dilution and Pharyngeal Pumping. Environ. Pollut. 2021, 273, 116471. [Google Scholar] [CrossRef]
- Ding, J.; Zhang, S.; Razanajatovo, R.M.; Zou, H.; Zhu, W. Accumulation, Tissue Distribution, and Biochemical Effects of Polystyrene Microplastics in the Freshwater Fish Red Tilapia (Oreochromis niloticus). Environ. Pollut. 2018, 238, 1–9. [Google Scholar] [CrossRef]
- Covernton, G.A.; Cox, K.D.; Fleming, W.L.; Buirs, B.M.; Davies, H.L.; Juanes, F.; Dudas, S.E.; Dower, J.F. Large Size (>100-μm) Microplastics Are Not Biomagnifying in Coastal Marine Food Webs of British Columbia, Canada. Ecol. Appl. 2022, 32, e2654. [Google Scholar] [CrossRef] [PubMed]
- An, D.; Na, J.; Song, J.; Jung, J. Size-Dependent Chronic Toxicity of Fragmented Polyethylene Microplastics to Daphnia magna. Chemosphere 2021, 271, 129591. [Google Scholar] [CrossRef]
- Wang, Q.; Wangjin, X.; Zhang, Y.; Wang, N.; Wang, Y.; Meng, G.; Chen, Y. The Toxicity of Virgin and UV-Aged PVC Microplastics on the Growth of Freshwater Algae Chlamydomonas reinhardtii. Sci. Total Environ. 2020, 749, 141603. [Google Scholar] [CrossRef]
- Choi, J.S.; Jung, Y.-J.; Hong, N.-H.; Hong, S.H.; Park, J.-W. Toxicological Effects of Irregularly Shaped and Spherical Microplastics in a Marine Teleost, the Sheepshead Minnow (Cyprinodon variegatus). Mar. Pollut. Bull. 2018, 129, 231–240. [Google Scholar] [CrossRef]
- Yu, C.-W.; Luk, T.C.; Liao, V.H.-C. Long-Term Nanoplastics Exposure Results in Multi and Trans-Generational Reproduction Decline Associated with Germline Toxicity and Epigenetic Regulation in Caenorhabditis elegans. J. Hazard. Mater. 2021, 412, 125173. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, C.; Li, M.; Ke, J.; Huang, Y.; Bian, Y.; Guo, S.; Wu, Y.; Han, Y.; Liu, M. Neurodevelopmental Toxicity of Polystyrene Nanoplastics in Caenorhabditis elegans and the Regulating Effect of Presenilin. ACS Omega 2020, 5, 33170–33177. [Google Scholar] [CrossRef]
- Chen, W.; Chen, Z.; Shan, S.; Wu, A.; Zhao, C.; Ye, X.; Zheng, X.; Zhu, R. Cyanidin-3-O-Glucoside Promotes Stress Tolerance and Lifespan Extension of Caenorhabditis elegans Exposed to Polystyrene via DAF-16 Pathway. Mech. Ageing Dev. 2022, 207, 111723. [Google Scholar] [CrossRef] [PubMed]
- Shang, X.; Lu, J.; Feng, C.; Ying, Y.; He, Y.; Fang, S.; Lin, Y.; Dahlgren, R.; Ju, J. Microplastic (1 and 5 Μm) Exposure Disturbs Lifespan and Intestine Function in the Nematode Caenorhabditis elegans. Sci. Total Environ. 2020, 705, 135837. [Google Scholar] [CrossRef]
- Kim, H.M.; Lee, D.-K.; Long, N.P.; Kwon, S.W.; Park, J.H. Uptake of Nanopolystyrene Particles Induces Distinct Metabolic Profiles and Toxic Effects in Caenorhabditis elegans. Environ. Pollut. 2019, 246, 578–586. [Google Scholar] [CrossRef] [PubMed]
- Fu, D.; Zhang, Q.; Fan, Z.; Qi, H.; Wang, Z.; Peng, L. Aged Microplastics Polyvinyl Chloride Interact with Copper and Cause Oxidative Stress towards Microalgae Chlorella vulgaris. Aquat. Toxicol. 2019, 216, 105319. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zheng, H.; Zhao, J.; Luo, X.; Wang, Z.; Xing, B. Photodegradation Elevated the Toxicity of Polystyrene Microplastics to Grouper (Epinephelus moara) through Disrupting Hepatic Lipid Homeostasis. Environ. Sci. Technol. 2020, 54, 6202–6212. [Google Scholar] [CrossRef]
- Chen, H.; Hua, X.; Yang, Y.; Wang, C.; Jin, L.; Dong, C.; Chang, Z.; Ding, P.; Xiang, M.; Li, H.; et al. Chronic Exposure to UV-Aged Microplastics Induces Neurotoxicity by Affecting Dopamine, Glutamate, and Serotonin Neurotransmission in Caenorhabditis elegans. J. Hazard. Mater. 2021, 419, 126482. [Google Scholar] [CrossRef]
- Liu, P.; Lu, K.; Li, J.; Wu, X.; Qian, L.; Wang, M.; Gao, S. Effect of Aging on Adsorption Behavior of Polystyrene Microplastics for Pharmaceuticals: Adsorption Mechanism and Role of Aging Intermediates. J. Hazard. Mater. 2020, 384, 121193. [Google Scholar] [CrossRef]
- Cole, M.; Lindeque, P.; Fileman, E.; Halsband, C.; Goodhead, R.; Moger, J.; Galloway, T.S. Microplastic Ingestion by Zooplankton. Environ. Sci. Technol. 2013, 47, 6646–6655. [Google Scholar] [CrossRef]
- Kawamura, G.; Kasedou, T.; Tamiya, T.; Watanabe, A. Colour Preference of Five Marine Fishes: Bias for Natural and Yellow-Dyed Krill in Laboratory Tanks, Sea Cages and an Earthen Pond. Mar. Freshw. Behav. Physiol. 2010, 43, 169–182. [Google Scholar] [CrossRef]
- Fueser, H.; Mueller, M.-T.; Weiss, L.; Höss, S.; Traunspurger, W. Ingestion of Microplastics by Nematodes Depends on Feeding Strategy and Buccal Cavity Size. Environ. Pollut. 2019, 255, 113227. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Chang, Y.; Zhang, T.; Qiao, Y.; Klobučar, G.; Li, M. Toxicological Effects of Polystyrene Microplastics on Earthworm (Eisenia fetida). Environ. Pollut. 2020, 259, 113896. [Google Scholar] [CrossRef] [PubMed]
- Truong, V.; Jun, M.; Jeong, W. Role of Resveratrol in Regulation of Cellular Defense Systems against Oxidative Stress. BioFactors 2018, 44, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Anjum, N.A.; Hasanuzzaman, M.; Gill, R.; Trivedi, D.K.; Ahmad, I.; Pereira, E.; Tuteja, N. Glutathione and Glutathione Reductase: A Boon in Disguise for Plant Abiotic Stress Defense Operations. Plant Physiol. Biochem. 2013, 70, 204–212. [Google Scholar] [CrossRef]
- Hee Jo, E.; Eun Moon, J.; Han Chang, M.; Jin Lim, Y.; Hyun Park, J.; Hee Lee, S.; Rae Cho, Y.; Cho, A.E.; Pil Pack, S.; Kim, H.-W.; et al. Sensitization of GSH Synthesis by Curcumin Curtails Acrolein-Induced Alveolar Epithelial Apoptosis via Keap1 Cysteine Conjugation: A Randomized Controlled Trial and Experimental Animal Model of Pneumonitis. J. Adv. Res. 2023, 46, 17–29. [Google Scholar] [CrossRef]
- Danes, J.M.; Palma, F.R.; Bonini, M.G. Arsenic and Other Metals as Phenotype Driving Electrophiles in Carcinogenesis. Semin. Cancer Biol. 2021, 76, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Hou, J.; Zhu, Y.; Lin, D. Multigenerational Exposure to TiO2 Nanoparticles in Soil Stimulates Stress Resistance and Longevity of Survived C. elegans via Activating Insulin/IGF-like Signaling. Environ. Pollut. 2020, 263, 114376. [Google Scholar] [CrossRef] [PubMed]
- Neumann, C.; Baesler, J.; Steffen, G.; Nicolai, M.M.; Zubel, T.; Aschner, M.; Bürkle, A.; Mangerich, A.; Schwerdtle, T.; Bornhorst, J. The Role of Poly(ADP-Ribose) Polymerases in Manganese Exposed Caenorhabditis elegans. J. Trace Elem. Med. Biol. 2020, 57, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Wang, S.; Jin, Y.; Xue, W.; Zhong, Y.; Wang, H.; An, J.; Li, H. Polystyrene Nanoparticles Induced Neurodevelopmental Toxicity in Caenorhabditis elegans through Regulation of Dpy-5 and Rol-6. Ecotoxicol. Environ. Saf. 2021, 222, 112523. [Google Scholar] [CrossRef]
- Prokić, M.D.; Radovanović, T.B.; Gavrić, J.P.; Faggio, C. Ecotoxicological Effects of Microplastics: Examination of Biomarkers, Current State and Future Perspectives. TrAC Trends Anal. Chem. 2019, 111, 37–46. [Google Scholar] [CrossRef]
- Cheng, L.; Yang, B.; Du, H.; Zhou, T.; Li, Y.; Wu, J.; Cao, Z.; Xu, A. Moderate Intensity of Static Magnetic Fields Can Alter the Avoidance Behavior and Fat Storage of Caenorhabditis elegans via Serotonin. Environ. Sci. Pollut. Res. 2022, 29, 43102–43113. [Google Scholar] [CrossRef]
- Wu, T.; He, K.; Zhan, Q.; Ang, S.; Ying, J.; Zhang, S.; Zhang, T.; Xue, Y.; Tang, M. MPA-Capped CdTe Quantum Dots Exposure Causes Neurotoxic Effects in Nematode Caenorhabditis elegans by Affecting the Transporters and Receptors of Glutamate, Serotonin and Dopamine at the Genetic Level, or by Increasing ROS, or Both. Nanoscale 2015, 7, 20460–20473. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, T.; Wu, J.; Liu, Y.; Xu, A. Seawater Accelerated the Aging of Polystyrene and Enhanced Its Toxic Effects on Caenorhabditis elegans. Int. J. Mol. Sci. 2023, 24, 17219. https://doi.org/10.3390/ijms242417219
Zhou T, Wu J, Liu Y, Xu A. Seawater Accelerated the Aging of Polystyrene and Enhanced Its Toxic Effects on Caenorhabditis elegans. International Journal of Molecular Sciences. 2023; 24(24):17219. https://doi.org/10.3390/ijms242417219
Chicago/Turabian StyleZhou, Tong, Jiajie Wu, Yun Liu, and An Xu. 2023. "Seawater Accelerated the Aging of Polystyrene and Enhanced Its Toxic Effects on Caenorhabditis elegans" International Journal of Molecular Sciences 24, no. 24: 17219. https://doi.org/10.3390/ijms242417219
APA StyleZhou, T., Wu, J., Liu, Y., & Xu, A. (2023). Seawater Accelerated the Aging of Polystyrene and Enhanced Its Toxic Effects on Caenorhabditis elegans. International Journal of Molecular Sciences, 24(24), 17219. https://doi.org/10.3390/ijms242417219





