Olanzapine Effects on Parvalbumin/GAD67 Cell Numbers in Layers/Subregions of Dorsal Hippocampus of Chronically Socially Isolated Rats
Abstract
:1. Introduction
2. Results
2.1. Behavior Testing—Forced Swim Test
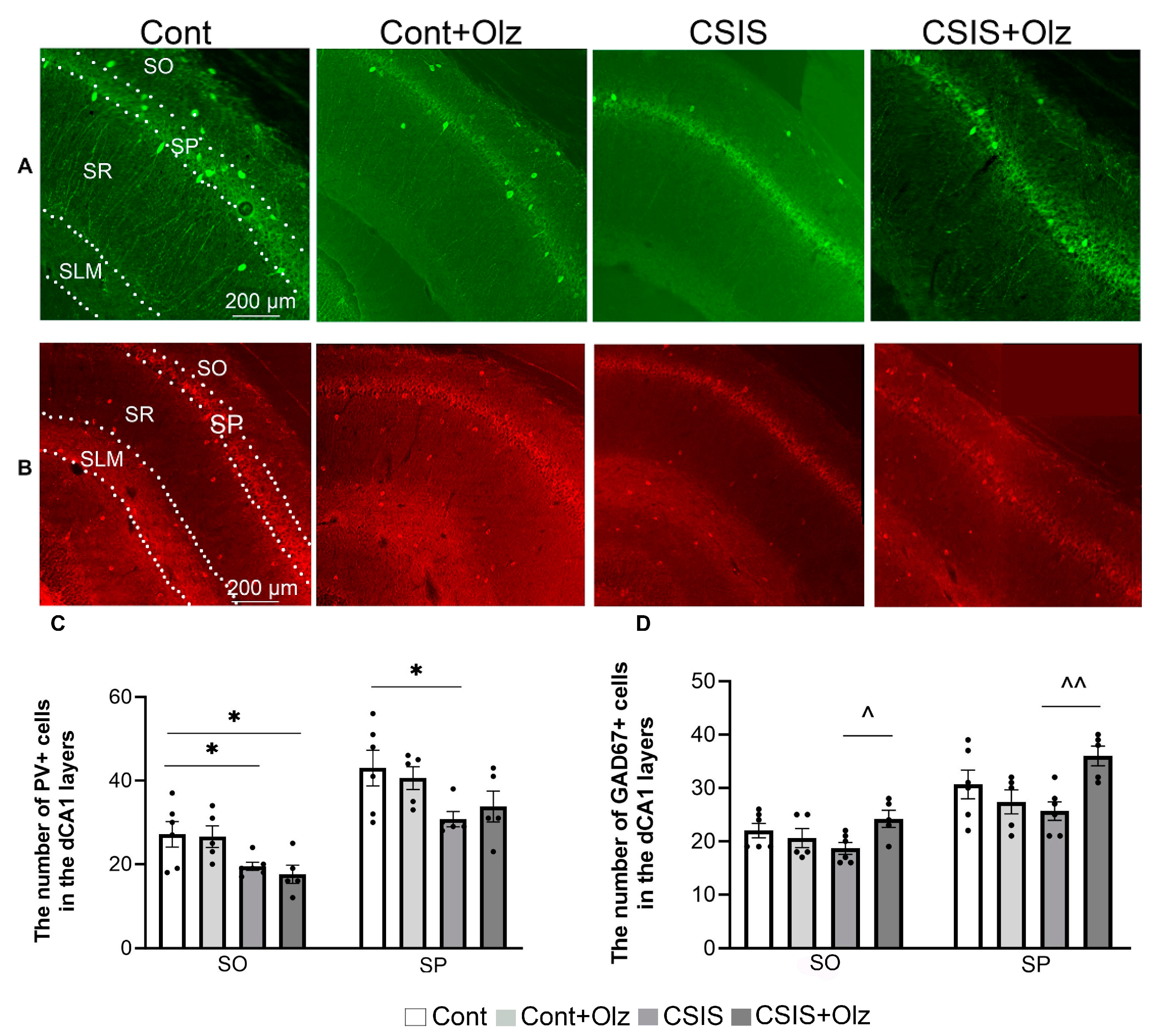
2.2. Layer-Specific Distribution of the Number of PV+ and GAD67+ Cells in the dCA1 Subregion
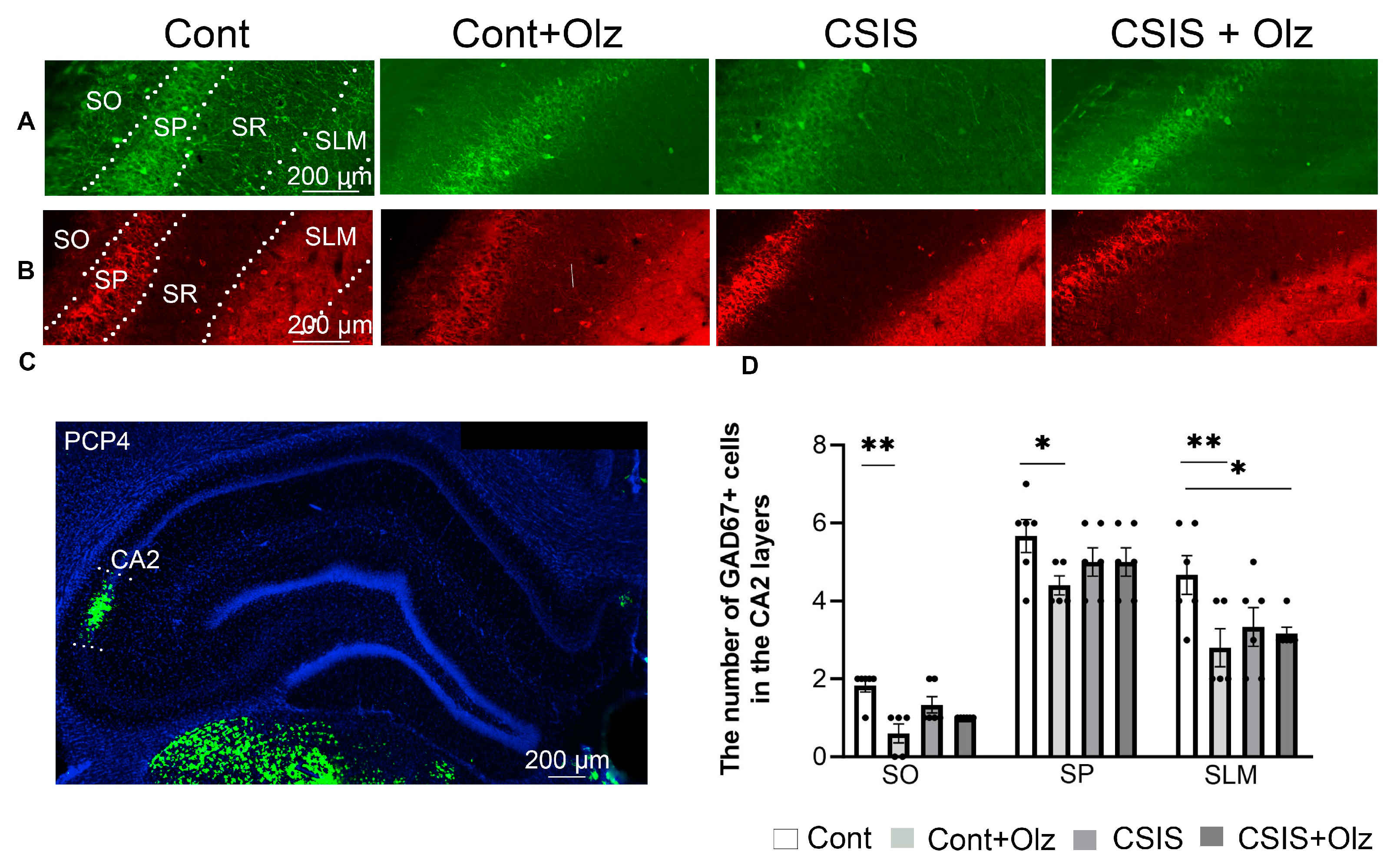
2.3. Layer-Specific Distribution of the Number of PV+ and GAD67+ Cells in the CA2 Subregion
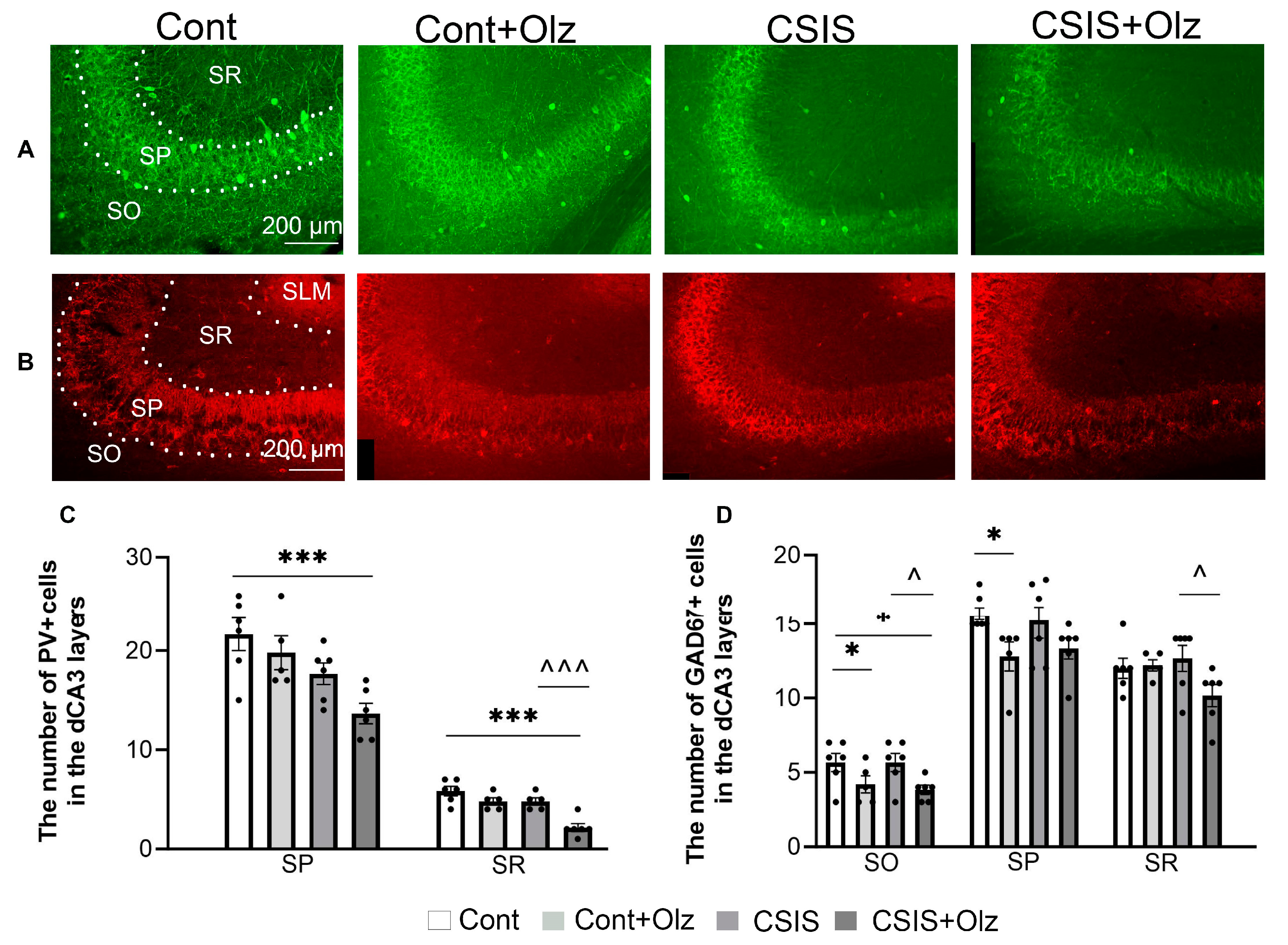
2.4. Layer-Specific Distribution of the Number of PV+ and GAD67+ Cells in the dCA3 Subregion
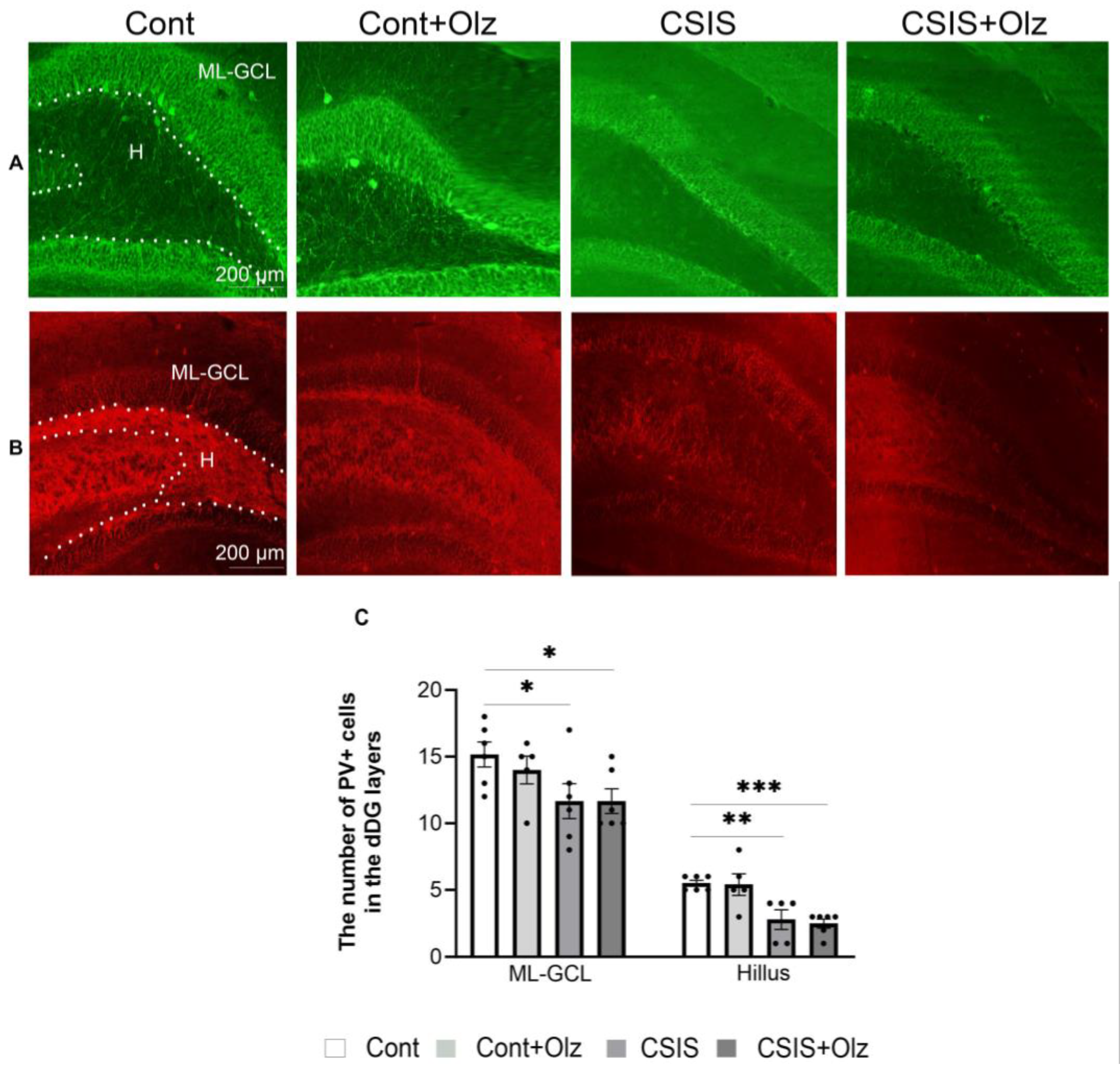
2.5. The Number of PV+ and GAD67+ Cells in the ML-GCL and H of the dDG Subregion
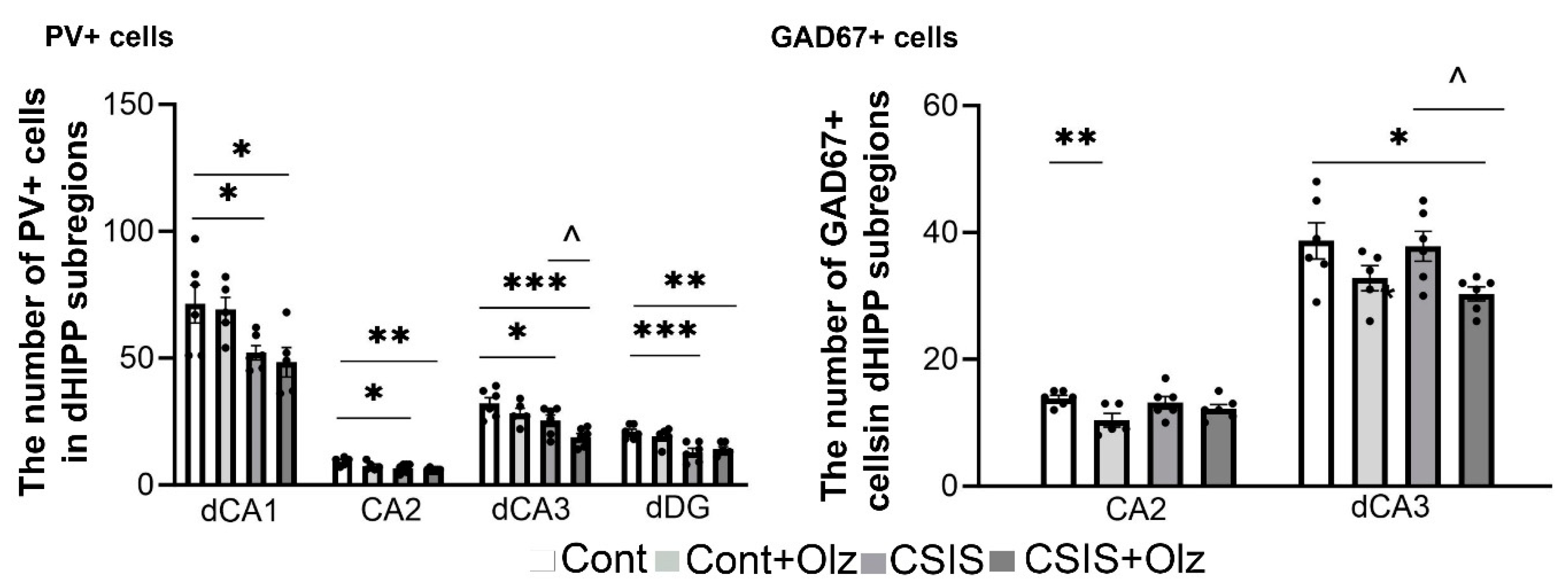
2.6. The Number of PV+ and GAD67+ Cells in dCA1, CA2, dCA3, and dDG Subregions
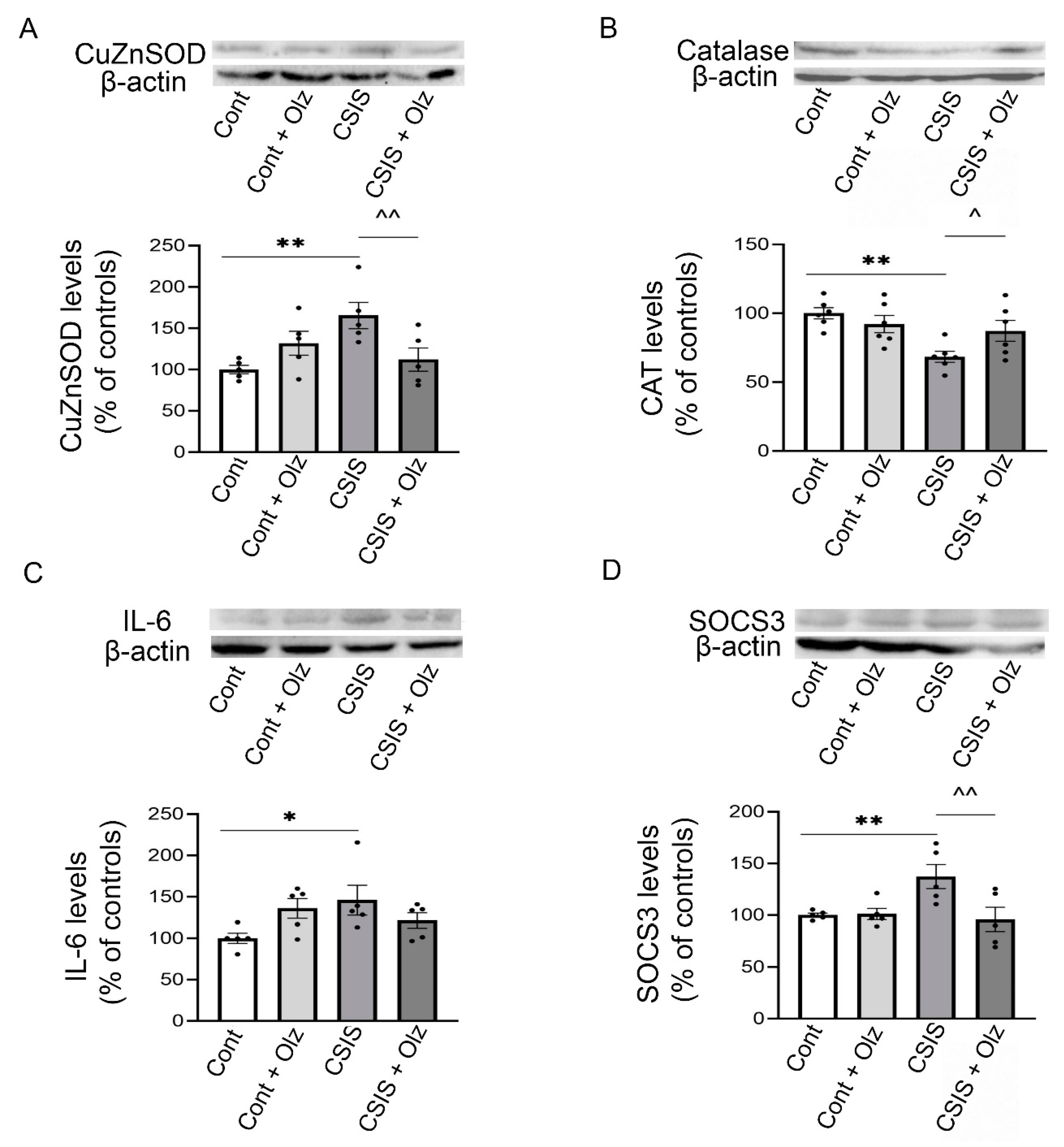
2.7. Western Blot Analyses
3. Discussion
4. Material and Methods
4.1. Animals
4.2. Olanzapine Solution
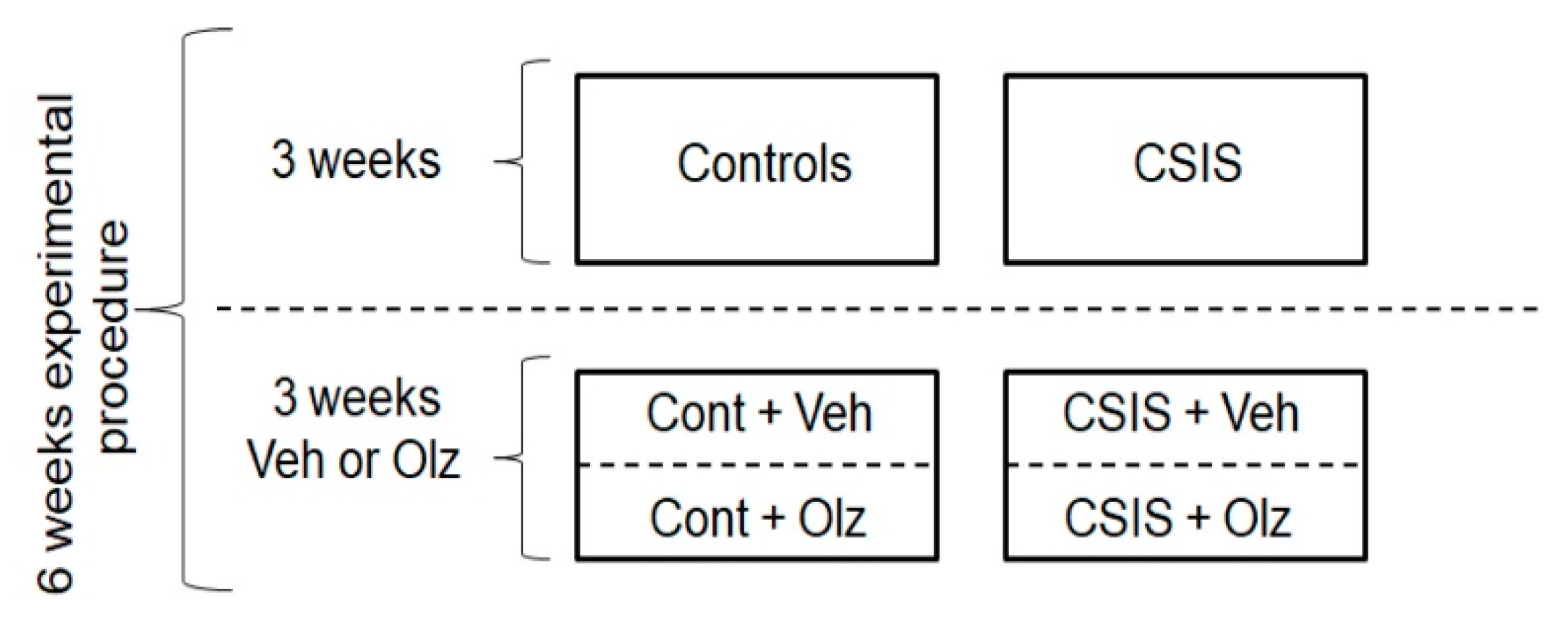
4.3. Study Design
4.4. Behavioral Test
Forced Swim Test
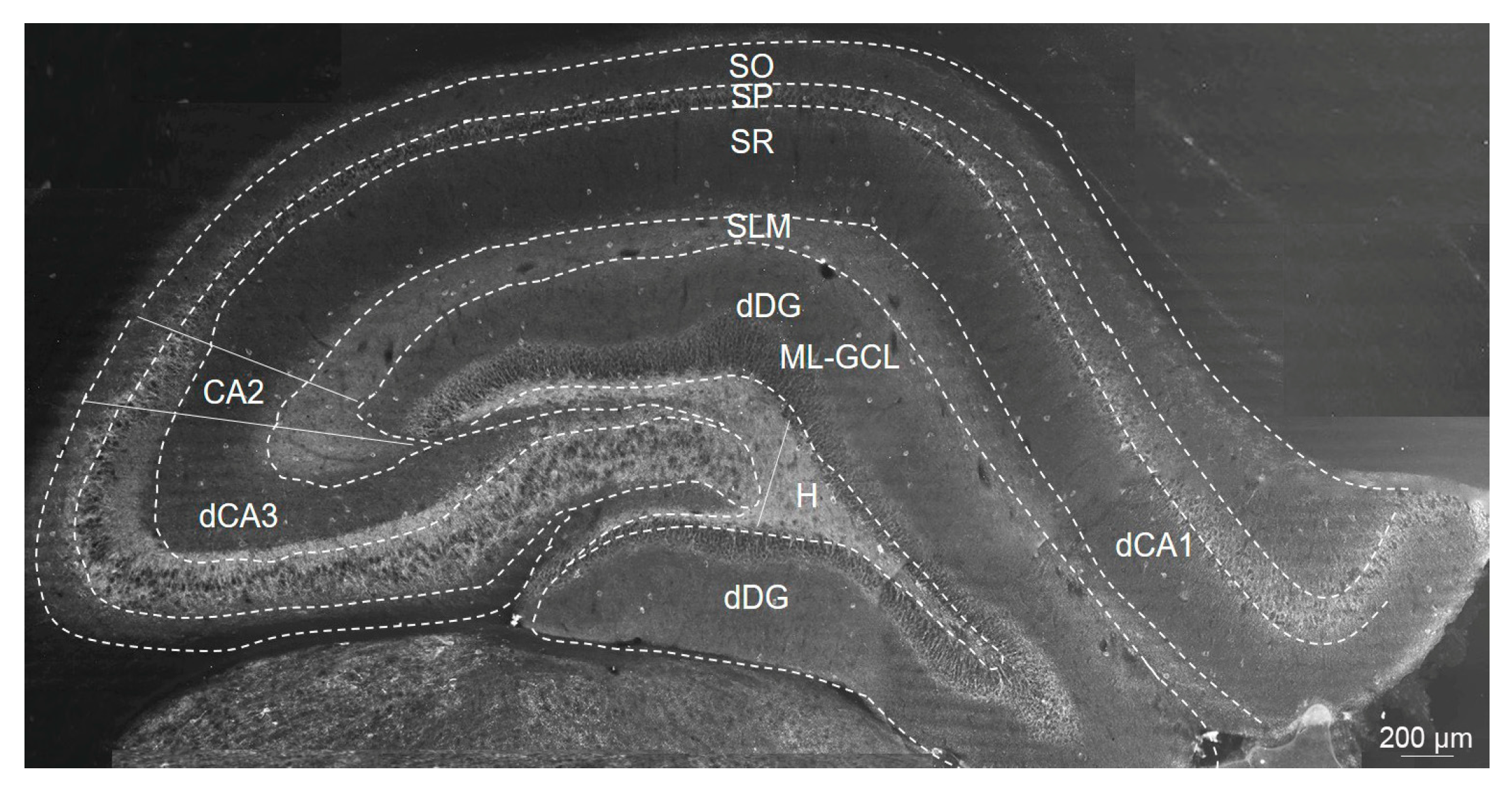
4.5. Immunofluorescence
4.6. Preparation of Cytosolic Fractions from the Rat Hippocampus
4.7. Western Blot Analysis
4.8. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Möhler, H. The GABA System in Anxiety and Depression and Its Therapeutic Potential. Neuropharmacology 2012, 62, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Wassef, A.; Baker, J.; Kochan, L.D. GABA and Schizophrenia: A Review of Basic Science and Clinical Studies. J. Clin. Psychopharmacol. 2003, 23, 601–640. [Google Scholar] [CrossRef] [PubMed]
- Kepecs, A.; Fishell, G. Interneuron Cell Types Are Fit to Function. Nature 2014, 505, 318–326. [Google Scholar] [CrossRef]
- Erlander, M.G.; Tillakaratne, N.J.K.; Feldblum, S.; Patel, N.; Tobin, A.J. Two Genes Encode Distinct Glutamate Decarboxylases. Neuron 1991, 7, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Freund, T.F.; Buzsáki, G. Interneurons of the Hippocampus. Hippocampus 1996, 6, 347–470. [Google Scholar] [CrossRef]
- Freund, T.F. Interneuron Diversity Series: Rhythm and Mood in Perisomatic Inhibition. Trends Neurosci. 2003, 26, 489–495. [Google Scholar] [CrossRef]
- Markram, H.; Toledo-Rodriguez, M.; Wang, Y.; Gupta, A.; Silberberg, G.; Wu, C. Interneurons of the Neocortical Inhibitory System. Nat. Rev. Neurosci. 2004, 5, 793–807. [Google Scholar] [CrossRef]
- Kosaka, T.; Katsumaru, H.; Hama, K.; Wu, J.Y.; Heizmann, C.W. GABAergic Neurons Containing the Ca2+-Binding Protein Parvalbumin in the Rat Hippocampus and Dentate Gyrus. Brain Res. 1987, 419, 119–130. [Google Scholar] [CrossRef]
- Nomura, T.; Fukuda, T.; Aika, Y.; Heizmann, C.W.; Emson, P.C.; Kobayashi, T.; Kosaka, T. Laminar Distribution of Non-Principal Neurons in the Rat Hippocampus, with Special Reference to Their Compositional Difference among Layers. Brain Res. 1997, 764, 197–204. [Google Scholar] [CrossRef]
- Czeh, B.; Simon, M.; Van Der Hart, M.G.C.; Schmelting, B.; Hesselink, M.B.; Fuchs, E. Chronic Stress Decreases the Number of Parvalbumin-Immunoreactive Interneurons in the Hippocampus: Prevention by Treatment with a Substance P Receptor (NK1) Antagonist. Neuropsychopharmacology 2005, 30, 67–79. [Google Scholar] [CrossRef]
- Czéh, B.; Varga, Z.K.K.; Henningsen, K.; Kovács, G.L.; Miseta, A.; Wiborg, O. Chronic Stress Reduces the Number of GABAergic Interneurons in the Adult Rat Hippocampus, Dorsal-Ventral and Region-Specific Differences. Hippocampus 2015, 25, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Filipović, D.; Zlatković, J.; Gass, P.; Inta, D. The Differential Effects of Acute vs. Chronic Stress and Their Combination on Hippocampal Parvalbumin and Inducible Heat Shock Protein 70 Expression. Neuroscience 2013, 236, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Harte, M.K.; Powell, S.B.; Swerdlow, N.R.; Geyer, M.A.; Reynolds, G.P. Deficits in Parvalbumin and Calbindin Immunoreactive Cells in the Hippocampus of Isolation Reared Rats. J. Neural Transm. 2007, 114, 893–898. [Google Scholar] [CrossRef] [PubMed]
- Perić, I.; Stanisavljević, A.; Inta, D.; Gass, P.; Undine, E.L.; Borgwardt, S.; Filipović, D. Tianeptine Antagonizes the Reduction of PV+ and GAD67 Cells Number in Dorsal Hippocampus of Socially Isolated Rats. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2019, 89, 386–399. [Google Scholar] [CrossRef] [PubMed]
- Perić, I.; Stanisavljević, A.; Gass, P.; Filipović, D. Fluoxetine Exerts Subregion/Layer Specific Effects on Parvalbumin/GAD67 Protein Expression in the Dorsal Hippocampus of Male Rats Showing Social Isolation-Induced Depressive-like Behaviour. Brain Res. Bull. 2021, 173, 174–183. [Google Scholar] [CrossRef]
- Konradi, C.; Zimmerman, E.I.; Yang, C.K.; Lohmann, K.M.; Gresch, P.; Pantazopoulos, H.; Berretta, S.; Heckers, S. Hippocampal Interneurons in Bipolar Disorder. Arch. Gen. Psychiatry 2011, 68, 340–350. [Google Scholar] [CrossRef]
- Kann, O. The Interneuron Energy Hypothesis: Implications for Brain Disease. Neurobiol. Dis. 2016, 90, 75–85. [Google Scholar] [CrossRef]
- Fanselow, M.S.; Dong, H.W. Are the Dorsal and Ventral Hippocampus Functionally Distinct Structures? Neuron 2010, 65, 7–19. [Google Scholar] [CrossRef]
- Ueno, H.; Suemitsu, S.; Murakami, S.; Kitamura, N.; Wani, K.; Okamoto, M.; Matsumoto, Y.; Ishihara, T. Region-specific impairments in parvalbumin interneurons in social isolation-reared mice. Neuroscience 2017, 359, 196–208. [Google Scholar] [CrossRef]
- Dowlati, Y.; Herrmann, N.; Swardfager, W.; Liu, H.; Sham, L.; Reim, E.K.; Lanctôt, K.L. A Meta-Analysis of Cytokines in Major Depression. Biol. Psychiatry 2010, 67, 446–457. [Google Scholar] [CrossRef]
- Babon, J.J.; Varghese, L.N.; Nicola, N.A. Inhibition of IL-6 Family Cytokines by SOCS3. Semin. Immunol. 2014, 26, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Perić, I.; Stanisavljević, A.; Gass, P.; Filipović, D. Fluoxetine Reverses Behavior Changes in Socially Isolated Rats: Role of the Hippocampal GSH-Dependent Defense System and Proinflammatory Cytokines. Eur. Arch. Psychiatry Clin. Neurosci. 2017, 267, 737–749. [Google Scholar] [CrossRef]
- Citrome, L.; McEvoy, J.P.; Todtenkopf, M.S.; McDonnell, D.; Weiden, P.J. A Commentary on the Efficacy of Olanzapine for the Treatment of Schizophrenia: The Past, Present, and Future. Neuropsychiatry Dis. Treat. 2019, 15, 2559–2569. [Google Scholar] [CrossRef] [PubMed]
- Jackson, W.C.; Manning, J.S.; Connor, P.D.; Deardorff, O.G. Retrospective Study of Olanzapine in Depressive and Anxious States in Primary Care. Prim. Care Companion J. Clin. Psychiatry 2004, 6, 199–202. [Google Scholar] [CrossRef]
- Gao, K.; Sheehan, D.V.; Calabrese, J.R. Atypical Antipsychotics in Primary Generalized Anxiety Disorder or Comorbid with Mood Disorders. Expert Rev. Neurother. 2009, 9, 1147–1158. [Google Scholar] [CrossRef] [PubMed]
- Olanzapine | Ligand page | IUPHAR/BPS Guide to PHARMACOLOGY. Available online: https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?tab=biology&ligandId=47 (accessed on 31 October 2023).
- Nelson, J.C.; Papakostas, G.I. Atypical Antipsychotic Augmentation in Major Depressive Disorder: A Meta-Analysis of Placebo-Controlled Randomized Trials. Am. J. Psychiatry 2009, 166, 980–991. [Google Scholar] [CrossRef]
- Valenstein, M. What Happened to Lithium? Antidepressant Augmentation in Clinical Settings. Am. J. Psychiatry 2006, 163, 1219. [Google Scholar] [CrossRef]
- Locchi, F.; Dall’Olio, R.; Gandolfi, O.; Rimondini, R. Olanzapine Counteracts Stress-Induced Anxiety-like Behavior in Rats. Neurosci. Lett. 2008, 438, 146–149. [Google Scholar] [CrossRef]
- Sigel, E.; Buhr, A. The Benzodiazepine Binding Site of GABAA Receptors. Trends Pharmacol. Sci. 1997, 18, 425–429. [Google Scholar] [CrossRef]
- Esmaeili, A.; Ghaedi, K. GABAA Receptors as Novel Drug Targets for Treatment of Mental Disorders. Arch. Adv. Biosci. 2010, 1, 50–61. [Google Scholar] [CrossRef]
- Heinrich, L.M.; Gullone, E. The Clinical Significance of Loneliness: A Literature Review. Clin. Psychol. Rev. 2006, 26, 695–718. [Google Scholar] [CrossRef] [PubMed]
- Möller, M.; Du Preez, J.L.; Viljoen, F.P.; Berk, M.; Emsley, R.; Harvey, B.H. Social Isolation Rearing Induces Mitochondrial, Immunological, Neurochemical and Behavioural Deficits in Rats, and Is Reversed by Clozapine or N-Acetyl Cysteine. Brain Behav. Immun. 2013, 30, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Brenes, J.C.; Fornaguera, J. The Effect of Chronic Fluoxetine on Social Isolation-Induced Changes on Sucrose Consumption, Immobility Behavior, and on Serotonin and Dopamine Function in Hippocampus and Ventral Striatum. Behav. Brain Res. 2009, 198, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Cacioppo, J.T.; Cacioppo, S.; Capitanio, J.P.; Cole, S.W. The Neuroendocrinology of Social Isolation. Annu. Rev. Psychol. 2015, 66, 733–767. [Google Scholar] [CrossRef]
- Filipović, D.; Todorović, N.; Bernardi, R.E.; Gass, P. Oxidative and Nitrosative Stress Pathways in the Brain of Socially Isolated Adult Male Rats Demonstrating Depressive- and Anxiety-like Symptoms. Brain Struct. Funct. 2017, 222, s00429–s016. [Google Scholar] [CrossRef]
- Mumtaz, F.; Khan, M.I.; Zubair, M.; Dehpour, A.R. Neurobiology and Consequences of Social Isolation Stress in Animal Model—A Comprehensive Review. Biomed. Pharmacother. 2018, 105, 1205–1222. [Google Scholar] [CrossRef] [PubMed]
- Pryce, C.R.; Fuchs, E. Chronic Psychosocial Stressors in Adulthood: Studies in Mice, Rats and Tree Shrews. Neurobiol. Stress 2017, 6, 94–103. [Google Scholar] [CrossRef]
- Steullet, P.; Cabungcal, J.H.; Coyle, J.; Didriksen, M.; Gill, K.; Grace, A.A.; Hensch, T.K.; Lamantia, A.S.; Lindemann, L.; Maynard, T.M.; et al. Oxidative Stress-Driven Parvalbumin Interneuron Impairment as a Common Mechanism in Models of Schizophrenia. Mol. Psychiatry 2017, 22, 936–943. [Google Scholar] [CrossRef] [PubMed]
- Filipović, D.; Novak, B.; Xiao, J.; Yan, Y.; Yeoh, K.; Turck, C.W. Chronic Fluoxetine Treatment of Socially Isolated Rats Modulates Prefrontal Cortex Proteome. Neuroscience 2022, 501, 52–71. [Google Scholar] [CrossRef]
- Đorđević, J.; Đorđević, A.; Adžić, M.; Radojčić, M.B. Effects of Chronic Social Isolation on Wistar Rat Behavior and Brain Plasticity Markers. Neuropsychobiology 2012, 66, 112–119. [Google Scholar] [CrossRef]
- Stanisavljević, A.; Perić, I.; Gass, P.; Inta, D.; Lang, U.E.; Borgwardt, S.; Filipović, D. Brain Sub/Region-Specific Effects of Olanzapine on c-Fos Expression of Chronically Socially Isolated Rats. Neuroscience 2019, 396, 46–65. [Google Scholar] [CrossRef]
- Đorđević, J.; Mitić, M.; Lukić, I.; Adžić, M. Maternal Deprivation of Rat Pups Reduces Body Weight and Alters Behavior in Adulthood in a Gender-Specific Manner. Arch. Biol. Sci 2015, 67, 131–138. [Google Scholar] [CrossRef]
- Filipović, D.; Stanisavljević, A.; Jasnić, N.; Bernardi, R.E.; Inta, D.; Perić, I.; Gass, P. Chronic Treatment with Fluoxetine or Clozapine of Socially Isolated Rats Prevents Subsector-Specific Reduction of Parvalbumin Immunoreactive Cells in the Hippocampus. Neuroscience 2018, 371, 384–394. [Google Scholar] [CrossRef] [PubMed]
- Zierhut, K.C.; Graßmann, R.; Kaufmann, J.; Steiner, J.; Bogerts, B.; Schiltz, K. Hippocampal CA1 Deformity Is Related to Symptom Severity and Antipsychotic Dosage in Schizophrenia. Brain 2013, 136, 804–814. [Google Scholar] [CrossRef]
- Koh, M.T.; Haberman, R.P.; Foti, S.; McCown, T.J.; Gallagher, M. Treatment Strategies Targeting Excess Hippocampal Activity Benefit Aged Rats with Cognitive Impairment. Neuropsychopharmacology 2010, 35, 1016–1025. [Google Scholar] [CrossRef]
- Calabrese, F.; Brivio, P.; Gruca, P.; Lason-Tyburkiewicz, M.; Papp, M.; Riva, M.A. Chronic Mild Stress-Induced Alterations of Local Protein Synthesis: A Role for Cognitive Impairment. ACS Chem. Neurosci. 2017, 8, 817–825. [Google Scholar] [CrossRef]
- Czéh, B.; Welt, T.; Fischer, A.K.; Erhardt, A.; Schmitt, W.; Müller, M.B.; Toschi, N.; Fuchs, E.; Keck, M.E. Chronic Psychosocial Stress and Concomitant Repetitive Transcranial Magnetic Stimulation: Effects on Stress Hormone Levels and Adult Hippocampal Neurogenesis. Biol. Psychiatry 2002, 52, 1057–1065. [Google Scholar] [CrossRef]
- Czéh, B.; Michaelis, T.; Watanabe, T.; Frahm, J.; De Biurrun, G.; Van Kampen, M.; Bartolomucci, A.; Fuchs, E. Stress-Induced Changes in Cerebral Metabolites, Hippocampal Volume, and Cell Proliferation Are Prevented by Antidepressant Treatment with Tianeptine. Proc. Natl. Acad. Sci. USA 2001, 98, 12796–12801. [Google Scholar] [CrossRef]
- Liu, S.H.; Wang, J.; Zhu, D.Y.; Fu, Y.P.; Lukowiak, K.; Lu, Y.M. Generation of Functional Inhibitory Neurons in the Adult Rat Hippocampus. J. Neurosci. 2003, 23, 732–736. [Google Scholar] [CrossRef]
- Vaden, R.J.; Gonzalez, J.C.; Tsai, M.C.; Niver, A.J.; Fusilier, A.R.; Griffith, C.M.; Kramer, R.H.; Wadiche, J.I.; Overstreet-Wadiche, L. Parvalbumin Interneurons Provide Spillover to Newborn and Mature Dentate Granule Cells. Elife 2020, 9, e54125. [Google Scholar] [CrossRef] [PubMed]
- Segi-Nishida, E.; Suzuki, K. Regulation of Adult-Born and Mature Neurons in Stress Response and Antidepressant Action in the Dentate Gyrus of the Hippocampus. Neurosci. Res. 2022, 360, 201–205. [Google Scholar] [CrossRef]
- Filipović, D.; Zlatković, J.; Inta, D.; Bjelobaba, I.; Stojiljkovic, M.; Gass, P. Chronic Isolation Stress Predisposes the Frontal Cortex but Not the Hippocampus to the Potentially Detrimental Release of Cytochrome c from Mitochondria and the Activation of Caspase-3. J. Neurosci. Res. 2011, 89, 1461–1470. [Google Scholar] [CrossRef] [PubMed]
- Gulyás, A.I.; Buzsáki, G.; Freund, T.F.; Hirase, H. Populations of Hippocampal Inhibitory Neurons Express Different Levels of Cytochrome C. Eur. J. Neurosci. 2006, 23, 2581–2594. [Google Scholar] [CrossRef] [PubMed]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive Oxygen Species in Inflammation and Tissue Injury. Antioxid. Redox Signal. 2014, 20, 1126. [Google Scholar] [CrossRef] [PubMed]
- Dugan, L.L.; Ali, S.S.; Shekhtman, G.; Roberts, A.J.; Lucero, J.; Quick, K.L.; Behrens, M.M. IL-6 Mediated Degeneration of Forebrain GABAergic Interneurons and Cognitive Impairment in Aged Mice through Activation of Neuronal NADPH Oxidase. PLoS ONE 2009, 4, e5518. [Google Scholar] [CrossRef] [PubMed]
- Rossetti, A.C.; Paladini, M.S.; Brüning, C.A.; Spero, V.; Cattaneo, M.G.; Racagni, G.; Papp, M.; Riva, M.A.; Molteni, R. Involvement of the IL-6 Signaling Pathway in the Anti-Anhedonic Effect of the Antidepressant Agomelatine in the Chronic Mild Stress Model of Depression. Int. J. Mol. Sci. 2022, 23, 2453. [Google Scholar] [CrossRef]
- Rossetti, A.C.; Serena Paladini, M.; Colombo, M.; Gruca, P.; Lason-Tyburkiewicz, M.; Tota-Glowczyk, K.; Papp, M.; Riva, M.A.; Molteni, R. Chronic Stress Exposure Reduces Parvalbumin Expression in the Rat Hippocampus through an Imbalance of Redox Mechanisms: Restorative Effect of the Antipsychotic Lurasidone. Int. J. Neuropsychopharmacol. 2018, 21, 883–893. [Google Scholar] [CrossRef]
- Klenotich, S.J.; Seiglie, M.P.; Mcmurray, M.S.; Roitman, J.D.; Le Grange, D.; Dugad, P.; Dulawa, S.C. Olanzapine, but Not Fluoxetine, Treatment Increases Survival in Activity-Based Anorexia in Mice. Neuropsychopharmacology 2012, 37, 1620–1631. [Google Scholar] [CrossRef]
- Kapur, S.; VanderSpek, S.C.; Brownlee, B.A.; Nobrega, J.N. Erratum: Antipsychotic Dosing in Preclinical Models Is Often Unrepresentative of the Clinical Condition: A Suggested Solution Based on in Vivo Occupancy (Journal of Pharmacology and Experimental Therapeutics (2003) 305 (625–631)). J. Pharmacol. Exp. Ther. 2006, 317, 919. [Google Scholar] [CrossRef]
- Perry, P.J.; Sanger, T.; Beasley, C. Olanzapine Plasma Concentrations and Clinical Response in Acutely Ill Schizophrenic Patients. J. Clin. Psychopharmacol. 1997, 17, 472–477. [Google Scholar] [CrossRef]
- Chay, S.H.; Herman, J.L. Disposition of the Novel Anti-Schizophrenic Drug [14C]Olanzapine in Male Fischer 344 and Female CD Rats Following Single Oral Dose Administration. Arzneimittelforschung 1998, 48, 446–454. [Google Scholar] [PubMed]
- Aravagiri, M.; Teper, Y.; Marder, S.R. Pharmacokinetics and Tissue Distribution of Olanzapine in Rats. Biopharm. Drug Dispos. 1999, 20, 369–377. [Google Scholar] [CrossRef]
- Elsworth, J.D.; Morrow, B.A.; Hajszan, T.; Leranth, C.; Roth, R.H. Phencyclidine-Induced Loss of Asymmetric Spine Synapses in Rodent Prefrontal Cortex Is Reversed by Acute and Chronic Treatment with Olanzapine. Neuropsychopharmacology 2011, 36, 2054–2061. [Google Scholar] [CrossRef]
- Garzón, J.; Del Río, J. Hyperactivity Induced in Rats by Long-Term Isolation: Further Studies on a New Animal Model for the Detection of Antidepressants. Eur. J. Pharmacol. 1981, 74, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Molina-Hernández, M.; Téllez-Alcántara, N.P.; Olivera-Lopez, J.I.; Jaramillo, M.T. Olanzapine plus 17-β Estradiol Produce Antidepressant-like Actions in Rats Forced to Swim. Pharmacol. Biochem. Behav. 2009, 93, 491–497. [Google Scholar] [CrossRef]
- Porsolt, R.D.; Anton, G.; Blavet, N.; Jalfre, M. Behavioural Despair in Rats: A New Model Sensitive to Antidepressant Treatments. Eur. J. Pharmacol. 1978, 47, 379–391. [Google Scholar] [CrossRef]
- Yankelevitch-Yahav, R.; Franko, M.; Huly, A.; Doron, R. The Forced Swim Test as a Model of Depressive-like Behavior. J. Vis. Exp. 2015, 97, 52587. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 5th ed.; Academic Press: New York, NY, USA, 2005; ISBN 9780080474120. [Google Scholar]
- Botcher, N.A.; Falck, J.E.; Thomson, A.M.; Mercer, A. Distribution of Interneurons in the CA2 Region of the Rat Hippocampus. Front. Neuroanat. 2014, 8, 104. [Google Scholar] [CrossRef]
- San Antonio, A.; Liban, K.; Ikrar, T.; Tsyganovskiy, E.; Xu, X. Distinct Physiological and Developmental Properties of Hippocampal CA2 Subfield Revealed by Using Anti-Purkinje Cell Protein 4 (PCP4) Immunostaining. J. Comp. Neurol. 2014, 522, 1333–1354. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosenbrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]
| Forced Swim Test | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Immobility Time (Seconds) | Swimming Time (Seconds) | Climbing Time (Seconds) | |||||||
| Group | 0 week | 3rd weeks | 6th weeks | 0 week | 3rd weeks | 6th weeks | 0 week | 3rd weeks | 6th weeks |
| Cont | 78 ± 13 | 43 ± 11 ** | |||||||
| CSIS | 57 ± 21 | 137 ± 37 ** | 169 ± 26 *** | 182 ± 7 | 124 ± 27 * | 109 ± 17 * | 77 ± 17 | 39 ± 17 ** | 22 ± 11 *** |
| CSIS + Olz | 85 ± 17 ## | 186 ± 9 ## | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stanisavljević Ilić, A.; Đorđević, S.; Inta, D.; Borgwardt, S.; Filipović, D. Olanzapine Effects on Parvalbumin/GAD67 Cell Numbers in Layers/Subregions of Dorsal Hippocampus of Chronically Socially Isolated Rats. Int. J. Mol. Sci. 2023, 24, 17181. https://doi.org/10.3390/ijms242417181
Stanisavljević Ilić A, Đorđević S, Inta D, Borgwardt S, Filipović D. Olanzapine Effects on Parvalbumin/GAD67 Cell Numbers in Layers/Subregions of Dorsal Hippocampus of Chronically Socially Isolated Rats. International Journal of Molecular Sciences. 2023; 24(24):17181. https://doi.org/10.3390/ijms242417181
Chicago/Turabian StyleStanisavljević Ilić, Andrijana, Snežana Đorđević, Dragoš Inta, Stefan Borgwardt, and Dragana Filipović. 2023. "Olanzapine Effects on Parvalbumin/GAD67 Cell Numbers in Layers/Subregions of Dorsal Hippocampus of Chronically Socially Isolated Rats" International Journal of Molecular Sciences 24, no. 24: 17181. https://doi.org/10.3390/ijms242417181
APA StyleStanisavljević Ilić, A., Đorđević, S., Inta, D., Borgwardt, S., & Filipović, D. (2023). Olanzapine Effects on Parvalbumin/GAD67 Cell Numbers in Layers/Subregions of Dorsal Hippocampus of Chronically Socially Isolated Rats. International Journal of Molecular Sciences, 24(24), 17181. https://doi.org/10.3390/ijms242417181






