Gypsum-Related Impact on Antibiotic-Loaded Composite Based on Highly Porous Hydroxyapatite—Advantages and Disadvantages
Abstract
:1. Introduction
2. Results and Discussion
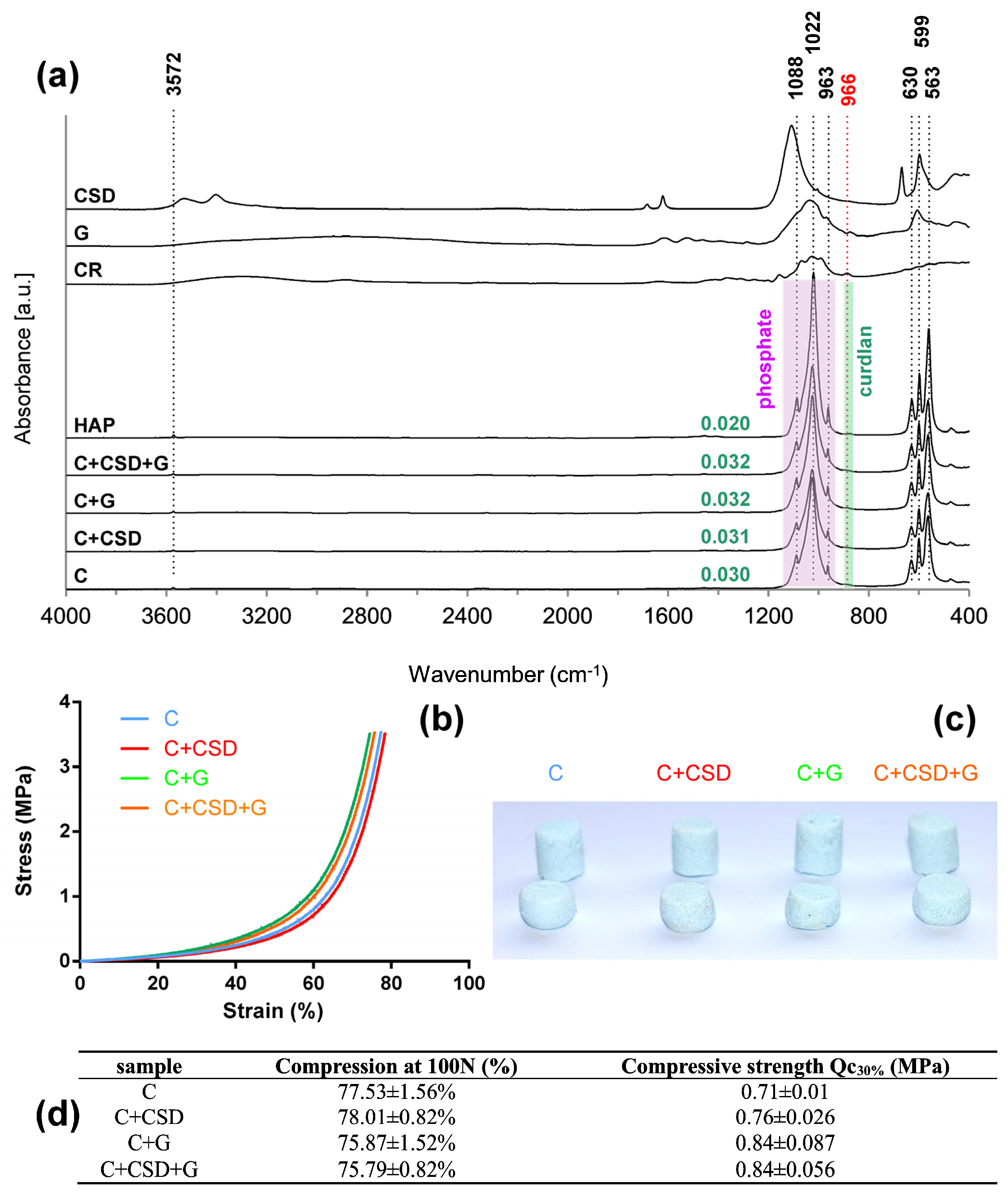
2.1. Composite Characterization
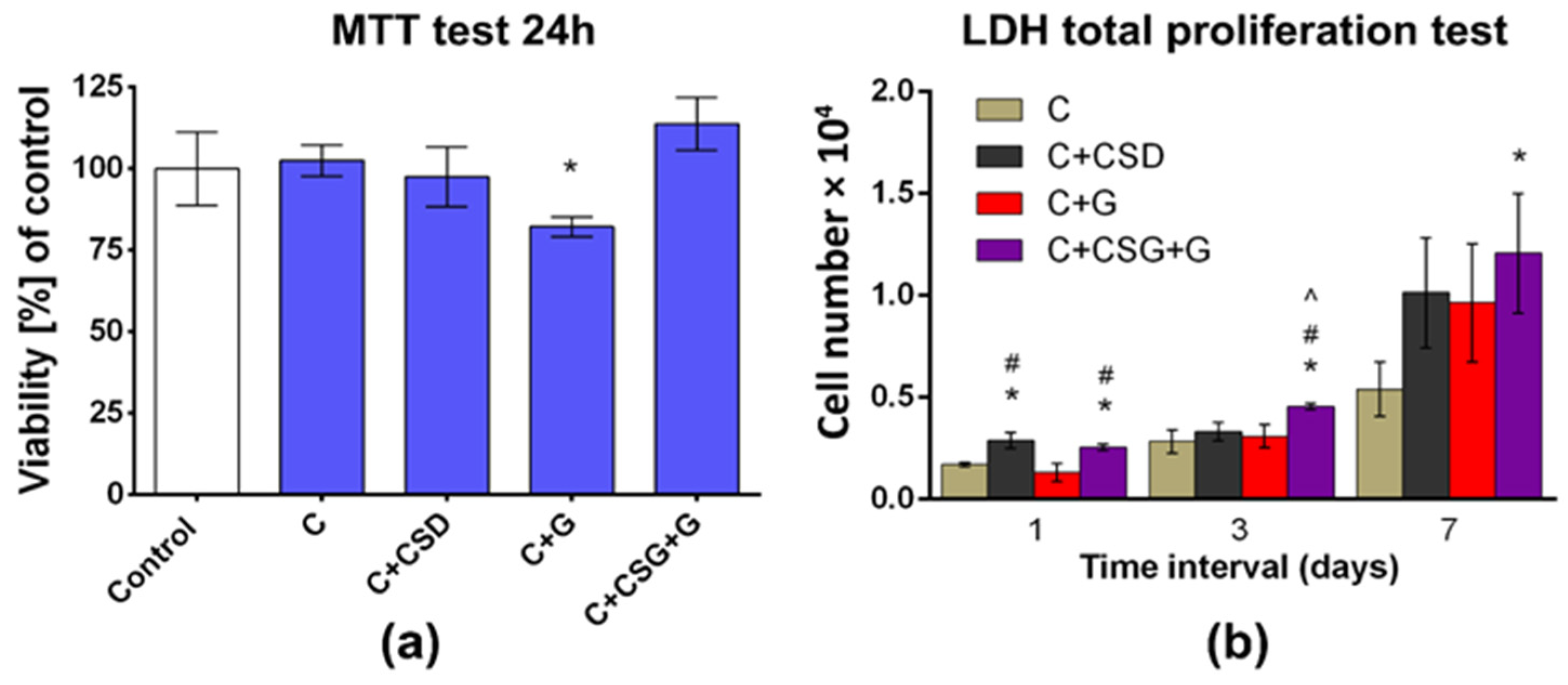
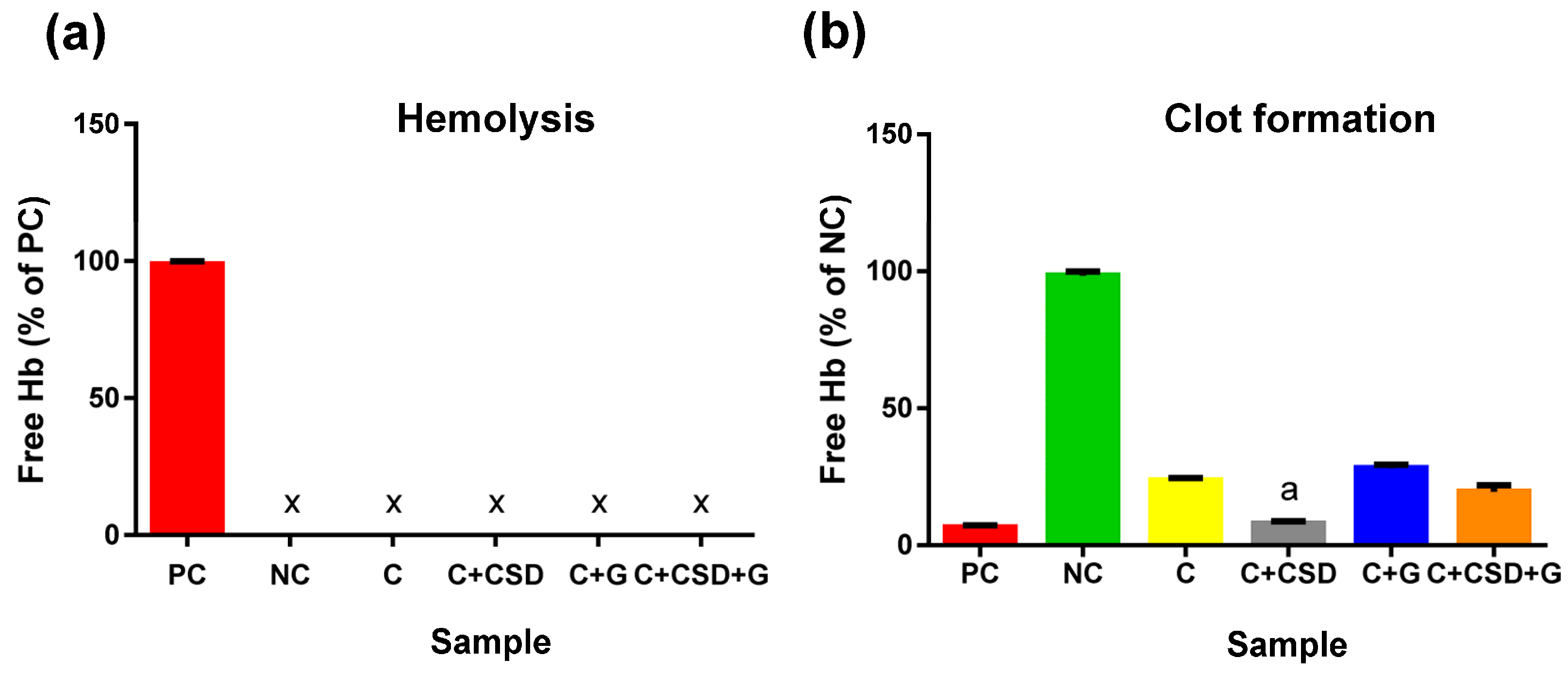
2.2. Biocompatibility
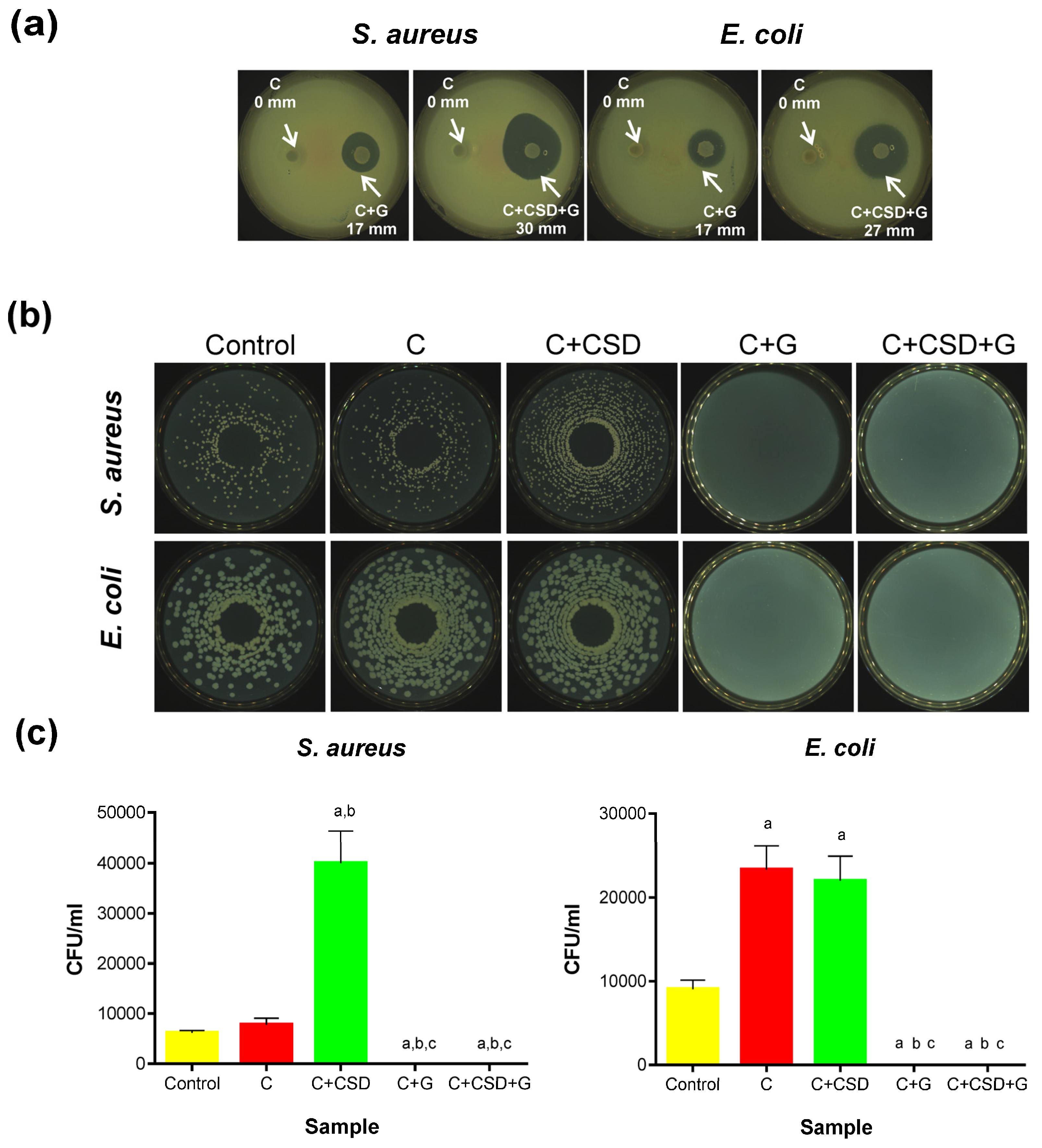
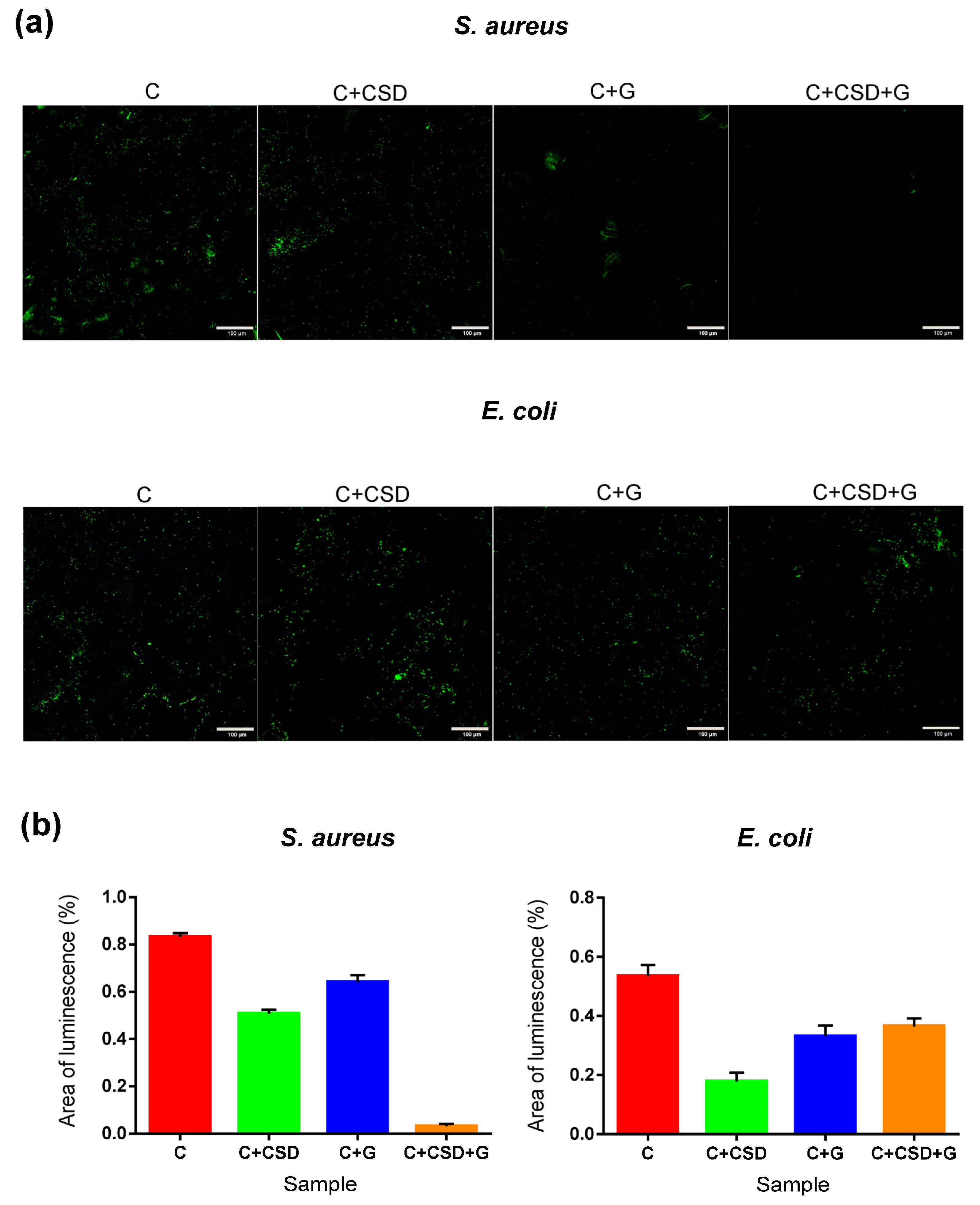
2.3. Antibacterial Activity
3. Materials and Methods
3.1. HA Preparation
3.2. Composite Preparation
3.3. Composite Characterization
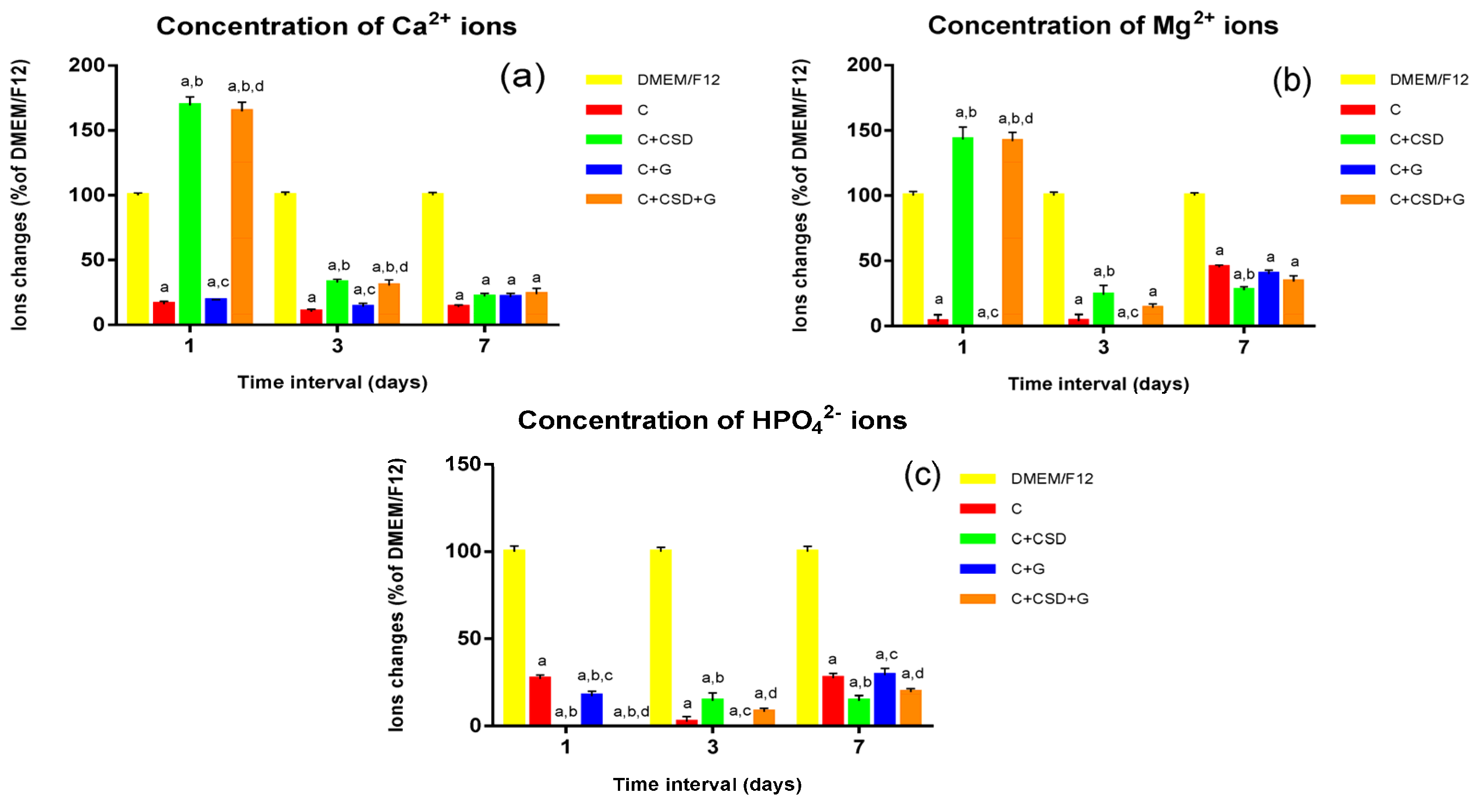
3.4. Evaluation of Ionic Reactivity of Composites in Culture Medium (DMEM/F12)
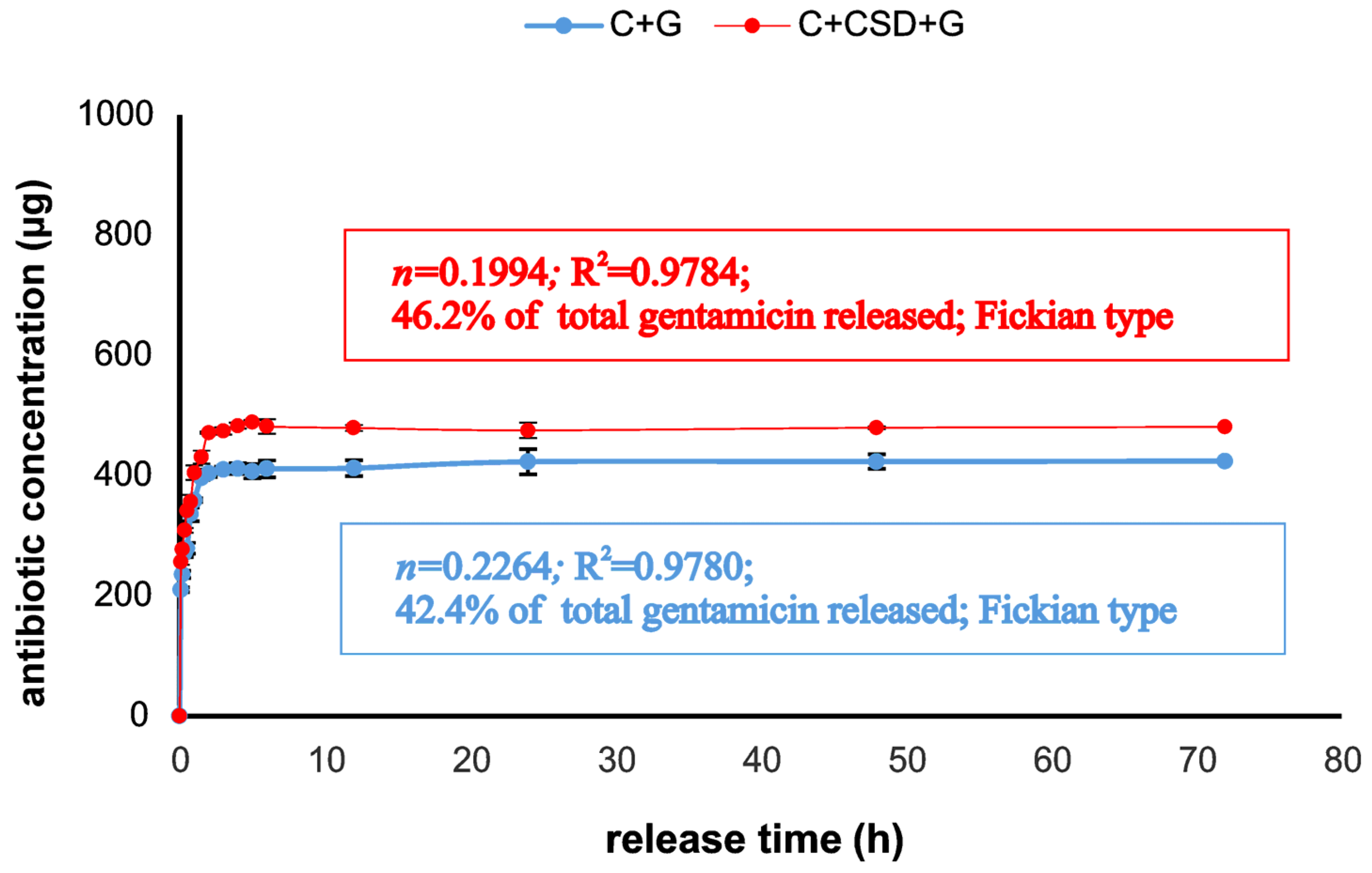
3.5. Gentamicin Release
3.6. Cell Culture Experiments
3.7. Hemolysis and Blood Clot Formation Tests
3.8. Antibacterial Activity
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Szałaj, U.; Chodara, A.; Gierlotka, S.; Wojnarowicz, J.; Łojkowski, W. Enhanced Release of Calcium Ions from Hydroxyapatite Nanoparticles with an Increase in Their Specific Surface Area. Materials 2023, 16, 6397. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, S.; Maleki-Dizaj, S.; Shahi, S.; Mahdilouy, M. Synthesis, Characterization, and Evaluation of Nano-Hydroxyapatite Based Experimental Calcium Silicate Cement As a Root Repair Material. J. Oral Res. 2022, 11, 1–13. [Google Scholar] [CrossRef]
- Klimek, K.; Belcarz, A.; Pazik, R.; Sobierajska, P.; Han, T.; Wiglusz, R.J.; Ginalska, G. “False” cytotoxicity of ions-adsorbing hydroxyapatite—Corrected method of cytotoxicity evaluation for ceramics of high specific surface area. Mater. Sci. Eng. C 2016, 65, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Kito, H.; Ohya, S. Role of K+ and Ca2+-permeable channels in osteoblast functions. Int. J. Mol. Sci. 2021, 22, 10459. [Google Scholar] [CrossRef] [PubMed]
- Jung, G.Y.; Park, Y.J.; Han, J.S. Effects of HA released calcium ion on osteoblast differentiation. J. Mater. Sci. Mater. Med. 2010, 21, 1649–1654. [Google Scholar] [CrossRef]
- An, S. The emerging role of extracellular Ca2+ in osteo/odontogenic differentiation and the involvement of intracellular Ca2+ signaling: From osteoblastic cells to dental pulp cells and odontoblasts. J. Cell. Physiol. 2019, 234, 2169–2193. [Google Scholar] [CrossRef]
- Nicoara, A.I.; Voineagu, T.G.; Alecu, A.E.; Vasile, B.S.; Maior, I.; Cojocaru, A.; Trusca, R.; Popescu, R.C. Fabrication and Characterisation of Calcium Sulphate Hemihydrate Enhanced with Zn- or B-Doped Hydroxyapatite Nanoparticles for Hard Tissue Restoration. Nanomaterials 2023, 13, 2219. [Google Scholar] [CrossRef]
- Lazáry, Á.; Balla, B.; Kósa, J.P.; Bácsi, K.; Nagy, Z.; Takács, I.; Varga, P.P.; Speer, G.; Lakatos, P. Effect of gypsum on proliferation and differentiation of MC3T3-E1 mouse osteoblastic cells. Biomaterials 2007, 28, 393–399. [Google Scholar] [CrossRef]
- He, W.; Wu, Z.; Wu, Y.; Zhong, Z.; Hong, Y. Construction of the Gypsum-Coated Scaffolds for In Situ Bone Regeneration. ACS Appl. Mater. Interfaces 2021, 13, 31527–31541. [Google Scholar] [CrossRef]
- Naini, A.; Sudiana, I.K.; Rubianto, M.; Kresnoadi, U.; Latief, F.D.E. Effects of hydroxyapatite gypsum puger scaffold applied to rat alveolar bone sockets on osteoclasts, osteoblasts and the trabecular bone area. Dent. J. 2019, 52, 13–17. [Google Scholar] [CrossRef]
- Liu, K.; Han, S.; Gao, W.; Tang, Y.; Han, X.; Liu, Z.; Bao, L.; Zhi, M.; Wang, H.; Wang, Y.; et al. Changes of Mineralogical Properties and Biological Activities of Gypsum and Its Calcined Products with Different Phase Structures. Evid.-Based Complement. Altern. Med. 2021, 2021, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kumar, C.Y.; Nalini, K.B.; Menon, J.; Patro, D.K.; Banerji, B.H. Calcium sulfate as bone graft substitute in the treatment of osseous bone defects, a prospective study. J. Clin. Diagn. Res. 2013, 7, 2926–2928. [Google Scholar] [CrossRef]
- Naini, A.; Rubianto, M.; Latief, F.D.E.; Gunadi, A.; Kristiana, D.; Hendrijantini, N.; Sudiana, K. Inflammatory and Immunogenic Response of the Tissue after Application of Freeze-Dried Hydroxyapatite Gypsum Puger Scaffold Compared to Freeze-dried Hydroxyapatite Bovine Scaffold. Contemp. Clin. Dent. 2020, 11, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Zalewska, J.; Przekora, A.; Pałka, K.; Belcarz, A. Gypsum-related compensation of ions uptake by highly porous hydroxyapatite ceramics—Consequences for osteoblasts growth and proliferation. Biomater. Adv. 2022, 133, 112665. [Google Scholar] [CrossRef] [PubMed]
- Lew, D.P.; Waldvogel, F.A. Osteomyelitis. N. Engl. J. Med. 1997, 336, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- Berry, E.D.; Siragusa, G.R. Hydroxyapatite adherence as a means to concentrate bacteria. Appl. Environ. Microbiol. 1997, 63, 4069–4074. [Google Scholar] [CrossRef] [PubMed]
- Eliaz, N.; Ritman-Hertz, O.; Aronov, D.; Weinberg, E.; Shenhar, Y.; Rosenman, G.; Weinreb, M.; Ron, E. The effect of surface treatments on the adhesion of electrochemically deposited hydroxyapatite coating to titanium and on its interaction with cells and bacteria. J. Mater. Sci. Mater. Med. 2011, 22, 1741–1752. [Google Scholar] [CrossRef]
- Pitz, W. Effect of Elemental Sulphur and of Calcium Sulphate on Certain of the Higher and lower forms of plant life. J. Agric. Res. V 1916, 5, 771–780. [Google Scholar]
- King, M.M.; Kayastha, B.B.; Franklin, M.J.; Patrauchan, M.A. Calcium Regulation of Bacterial Virulence. Adv. Exp. Med. Biol. 2020, 1131, 827–855. [Google Scholar] [CrossRef]
- Dominguez, D.C. Calcium signalling in bacteria. Mol. Microbiol. 2004, 54, 291–297. [Google Scholar] [CrossRef]
- Keren-Paz, A.; Maan, H.; Karunker, I.; Olender, T.; Kapishnikov, S.; Dersch, S.; Kartvelishvily, E.; Wolf, S.G.; Gal, A.; Graumann, P.L.; et al. The roles of intracellular and extracellular calcium in Bacillus subtilis biofilms. iScience 2022, 25, 104308. [Google Scholar] [CrossRef] [PubMed]
- Butini, M.E.; Cabric, S.; Trampuz, A.; Di Luca, M. In vitro anti-biofilm activity of a biphasic gentamicin-loaded calcium sulfate/hydroxyapatite bone graft substitute. Colloids Surf. B Biointerfaces 2018, 161, 252–260. [Google Scholar] [CrossRef]
- Mistry, S.; Burman, S.; Roy, S.; Maitra, N.J.; Roy, R.; Chanda, A. Biological analysis of an innovative biodegradable antibiotic eluting bioactive glass/gypsum composite bone cement for treating experimental chronic MRSA osteomyelitis. J. Pharm. Anal. 2022, 12, 164–177. [Google Scholar] [CrossRef] [PubMed]
- Zelmer, A.R.; Nelson, R.; Richter, K.; Atkins, G.J. Can intracellular Staphylococcus aureus in osteomyelitis be treated using current antibiotics? A systematic review and narrative synthesis. Bone Res. 2022, 10, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Gogia, J.; Meehan, J.; Di Cesare, P.; Jamali, A. Local Antibiotic Therapy in Osteomyelitis. Semin. Plast. Surg. 2009, 23, 100–107. [Google Scholar] [CrossRef]
- Belcarz, A.; Ginalska, G.; Pycka, T.; Zima, A.; Ślósarczyk, A.; Polkowska, I.; Paszkiewicz, Z.; Piekarczyk, W. Application of β-1,3-glucan in production of ceramics-based elastic composite for bone repair. Cent. Eur. J. Biol. 2013, 8, 534–548. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release II. Fickian and anomalous release from swellable devices. J. Control. Release 1987, 5, 37–42. [Google Scholar] [CrossRef]
- Kazimierczak, P.; Wessely-Szponder, J.; Palka, K.; Barylyak, A.; Zinchenko, V.; Przekora, A. Hydroxyapatite or Fluorapatite—Which Bioceramic Is Better as a Base for the Production of Bone Scaffold?—A Comprehensive Comparative Study. Int. J. Mol. Sci. 2023, 24, 5576. [Google Scholar] [CrossRef]
- IOS 10993-5:2009; Biological Evaluation of Medical Devices-Part 5: Tests for In Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009; pp. 1–42.
- Rauschmann, M.A.; Wichelhaus, T.A.; Stirnal, V.; Dingeldein, E.; Zichner, L.; Schnettler, R.; Alt, V. Nanocrystalline hydroxyapatite and calcium sulphate as biodegradable composite carrier material for local delivery of antibiotics in bone infections. Biomaterials 2005, 26, 2677–2684. [Google Scholar] [CrossRef]
- Ghorbanzadeh, A.; Aminsobhani, M.; Khoshzaban, A.; Abbaszadeh, A.; Bolhari, B.; Ghorbanzadeh, A.; Shamshiri, A.R. Cytotoxic Effects and Osteogenic Activity of Calcium Sulfate with and without Recombinant Human Bone Morphogenetic Protein 2 and Nano-Hydroxyapatite Adjacent to MG-63 Cell Line. J. Dent. 2015, 12, 352–363. [Google Scholar]
- AATCC Test Method 100-2004; Antibacterial Finishes on Textile Materials: Assessment of Antibacterial Finishes on Textile Materials. AATCC Tech. Manual/2012; AATCC: Research Triangle Park, NC, USA, 2011; pp. 147–150.
- Shi, Y.; Liu, J.; Yan, Q.; You, X.; Yang, S.; Jiang, Z. In vitro digestibility and prebiotic potential of curdlan (1 → 3)-β-D-glucan oligosaccharides in Lactobacillus species. Carbohydr. Polym. 2018, 188, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Norris, V.; Chen, M.; Goldberg, M.; Voskuil, J.; McGurk, G.; Holland, B. Calcium in bacteria: A solution to which problem? Mol. Microbiol. 1991, 5, 775–778. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.J. Calcium and Bacteria. Adv. Microb. Physiol. 1995, 37, 83–133. [Google Scholar] [CrossRef] [PubMed]
- Belcarz, A.; Janczarek, M.; Kolacz, K.; Urbanik-Sypniewska, T.; Ginalska, G. Do Ca2+-chelating polysaccharides reduce calcium ion release from gypsum-based biomaterials? Cent. Eur. J. Biol. 2013, 8, 735–746. [Google Scholar] [CrossRef]
- Belcarz, A.; Zima, A.; Ginalska, G. Biphasic mode of antibacterial action of aminoglycoside antibiotics-loaded elastic hydroxyapatite-glucan composite. Int. J. Pharm. 2013, 454, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Sabee, M.M.S.M.; Awang, M.S.; Bustami, Y.; Hamid, Z.A.A. Gentamicin loaded PLA microspheres susceptibility against staphylococcus aureus and escherichia coli by kirby-bauer and micro-dilution methods. AIP Conf. Proc. 2020, 2267, 020032. [Google Scholar] [CrossRef]
- Jiang, N.; Dusane, D.H.; Brooks, J.R.; Delury, C.P.; Aiken, S.S.; Laycock, P.A.; Stoodley, P. Antibiotic loaded β-tricalcium phosphate/calcium sulfate for antimicrobial potency, prevention and killing efficacy of Pseudomonas aeruginosa and Staphylococcus aureus biofilms. Sci. Rep. 2021, 11, 1446. [Google Scholar] [CrossRef]
- Kolmas, J.; Pajor, K.; Pajchel, L.; Przekora, A.; Ginalska, G.; Oledzka, E.; Sobczak, M. Fabrication and physicochemical characterization of porous composite microgranules with selenium oxyanions and risedronate sodium for potential applications in bone tumors. Int. J. Nanomed. 2017, 12, 5633–5642. [Google Scholar] [CrossRef]


| Sample Code | % of CSD in Ceramics Content | Amount of Compound (g) | Amount of Liquid (mL) | |||
|---|---|---|---|---|---|---|
| CSD Particles (0.1–0.2 mm) | HA Particles (≤0.4 mm) | Curdlan | DI H2O | Gentamicin (10 mg/mL) | ||
| C | 0 | - | 8.8 | 3 | 20 | - |
| C + CSD | 1.75 | 0.154 | 8.646 | 3 | 20 | - |
| C + G | 0 | - | 8.8 | 3 | 18.82 | 1.18 |
| C + CSD + G | 1.75 | 0.154 | 8.646 | 3 | 18.82 | 1.18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zalewska, J.; Vivcharenko, V.; Belcarz, A. Gypsum-Related Impact on Antibiotic-Loaded Composite Based on Highly Porous Hydroxyapatite—Advantages and Disadvantages. Int. J. Mol. Sci. 2023, 24, 17178. https://doi.org/10.3390/ijms242417178
Zalewska J, Vivcharenko V, Belcarz A. Gypsum-Related Impact on Antibiotic-Loaded Composite Based on Highly Porous Hydroxyapatite—Advantages and Disadvantages. International Journal of Molecular Sciences. 2023; 24(24):17178. https://doi.org/10.3390/ijms242417178
Chicago/Turabian StyleZalewska, Justyna, Vladyslav Vivcharenko, and Anna Belcarz. 2023. "Gypsum-Related Impact on Antibiotic-Loaded Composite Based on Highly Porous Hydroxyapatite—Advantages and Disadvantages" International Journal of Molecular Sciences 24, no. 24: 17178. https://doi.org/10.3390/ijms242417178
APA StyleZalewska, J., Vivcharenko, V., & Belcarz, A. (2023). Gypsum-Related Impact on Antibiotic-Loaded Composite Based on Highly Porous Hydroxyapatite—Advantages and Disadvantages. International Journal of Molecular Sciences, 24(24), 17178. https://doi.org/10.3390/ijms242417178







