Molecular Mechanisms of IL18 in Disease
Abstract
:1. Introduction
2. Microarray and Ingenuity Pathway Analysis (IPA)
3. IL18 and Cancer
4. Cancer-Related Genes in Il18−/− Mice
5. IL18 and Energy Metabolism
6. Metabolism-Related Genes in Il18−/− Mice
7. IL18 and Psychiatric Disorders
8. Psychiatric and Brain Disorder-Related Genes in Il18−/− Mice
9. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Okamura, H.; Tsutsi, H.; Komatsu, T.; Yutsudo, M.; Hakura, A.; Tanimoto, T.; Torigoe, K.; Okura, T.; Nukada, Y.; Hattori, K.; et al. Cloning of a new cytokine that induces IFN-gamma production by T cells. Nature 1995, 378, 88–91. [Google Scholar] [CrossRef] [PubMed]
- Ghayur, T.; Banerjee, S.; Hugunin, M.; Butler, D.; Herzog, L.; Carter, A.; Quintal, L.; Sekut, L.; Talanian, R.; Paskind, M.; et al. Caspase-1 processes IFN-gamma-inducing factor and regulates LPS-induced IFN-gamma production. Nature 1997, 386, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Okamura, H.; Tsutsui, H.; Kashiwamura, S.; Yoshimoto, T.; Nakanishi, K. Interleukin-18: A novel cytokine that augments both innate and acquired immunity. Adv. Immunol. 1998, 70, 281–312. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, S.; Uehara, A.; Nochi, T.; Yamaguchi, T.; Ueda, H.; Sugiyama, A.; Hanzawa, K.; Kumagai, K.; Okamura, H.; Takada, H. Neutrophil proteinase 3-mediated induction of bioactive IL-18 secretion by human oral epithelial cells. J. Immunol. 2001, 167, 6568–6575. [Google Scholar] [CrossRef] [PubMed]
- Tsutsui, H.; Kayagaki, N.; Kuida, K.; Nakano, H.; Hayashi, N.; Takeda, K.; Matsui, K.; Kashiwamura, S.; Hada, T.; Akira, S.; et al. Caspase-1-independent, Fas/Fas ligand-mediated IL-18 secretion from macrophages causes acute liver injury in mice. Immunity 1999, 11, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Yamanishi, K.; Miyauchi, M.; Mukai, K.; Hashimoto, T.; Uwa, N.; Seino, H.; Li, W.; Gamachi, N.; Hata, M.; Kuwahara-Otani, S.; et al. Exploring Molecular Mechanisms Involved in the Development of the Depression-Like Phenotype in Interleukin-18-Deficient Mice. BioMed Res. Int. 2021, 2021, 9975865. [Google Scholar] [CrossRef]
- Prinz, M.; Hanisch, U.K. Murine microglial cells produce and respond to interleukin-18. J. Neurochem. 1999, 72, 2215–2218. [Google Scholar] [CrossRef]
- Yamanishi, K.; Maeda, S.; Kuwahara-Otani, S.; Watanabe, Y.; Yoshida, M.; Ikubo, K.; Okuzaki, D.; El-Darawish, Y.; Li, W.; Nakasho, K.; et al. Interleukin-18-deficient mice develop dyslipidemia resulting in nonalcoholic fatty liver disease and steatohepatitis. Transl. Res. J. Lab. Clin. Med. 2016, 173, 101–114.e17. [Google Scholar] [CrossRef]
- Yamanishi, K.; Maeda, S.; Kuwahara-Otani, S.; Hashimoto, T.; Ikubo, K.; Mukai, K.; Nakasho, K.; Gamachi, N.; El-Darawish, Y.; Li, W.; et al. Deficiency in interleukin-18 promotes differentiation of brown adipose tissue resulting in fat accumulation despite dyslipidemia. J. Transl. Med. 2018, 16, 314. [Google Scholar] [CrossRef]
- Yamanishi, K.; Doe, N.; Mukai, K.; Ikubo, K.; Hashimoto, T.; Uwa, N.; Sumida, M.; El-Darawish, Y.; Gamachi, N.; Li, W.; et al. Interleukin-18-deficient mice develop hippocampal abnormalities related to possible depressive-like behaviors. Neuroscience 2019, 408, 147–160. [Google Scholar] [CrossRef]
- Kokai, M.; Kashiwamura, S.; Okamura, H.; Ohara, K.; Morita, Y. Plasma interleukin-18 levels in patients with psychiatric disorders. J. Immunother. 2002, 25 (Suppl. S1), S68–S71. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Li, W.; Yoshiya, S.; Xu, Y.; Hata, M.; El-Darawish, Y.; Markova, T.; Yamanishi, K.; Yamanishi, H.; Tahara, H.; et al. Augmentation of Immune Checkpoint Cancer Immunotherapy with IL18. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2016, 22, 2969–2980. [Google Scholar] [CrossRef] [PubMed]
- El-Darawish, Y.; Li, W.; Yamanishi, K.; Pencheva, M.; Oka, N.; Yamanishi, H.; Matsuyama, T.; Tanaka, Y.; Minato, N.; Okamura, H. Frontline Science: IL-18 primes murine NK cells for proliferation by promoting protein synthesis, survival, and autophagy. J. Leukoc. Biol. 2018, 104, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Oka, N.; Markova, T.; Tsuzuki, K.; Li, W.; El-Darawish, Y.; Pencheva-Demireva, M.; Yamanishi, K.; Yamanishi, H.; Sakagami, M.; Tanaka, Y.; et al. IL-12 regulates the expansion, phenotype, and function of murine NK cells activated by IL-15 and IL-18. Cancer Immunol. Immunother. CII 2020, 69, 1699–1712. [Google Scholar] [CrossRef] [PubMed]
- Netea, M.G.; Joosten, L.A.; Lewis, E.; Jensen, D.R.; Voshol, P.J.; Kullberg, B.J.; Tack, C.J.; van Krieken, H.; Kim, S.H.; Stalenhoef, A.F.; et al. Deficiency of interleukin-18 in mice leads to hyperphagia, obesity and insulin resistance. Nat. Med. 2006, 12, 650–656. [Google Scholar] [CrossRef]
- Lisboa, S.F.; Issy, A.C.; Biojone, C.; Montezuma, K.; Fattori, V.; Del-Bel, E.A.; Guimarães, F.S.; Cunha, F.Q.; Verri, W.A.; Joca, S.R.L. Mice lacking interleukin-18 gene display behavioral changes in animal models of psychiatric disorders: Possible involvement of immunological mechanisms. J. Neuroimmunol. 2018, 314, 58–66. [Google Scholar] [CrossRef]
- Yamanishi, K.; Hashimoto, T.; Miyauchi, M.; Mukai, K.; Ikubo, K.; Uwa, N.; Watanabe, Y.; Ikawa, T.; Okuzaki, D.; Okamura, H.; et al. Analysis of genes linked to depressive-like behaviors in interleukin-18-deficient mice: Gene expression profiles in the brain. Biomed. Rep. 2020, 12, 3–10. [Google Scholar] [CrossRef]
- Yamanishi, K.; Doe, N.; Sumida, M.; Watanabe, Y.; Yoshida, M.; Yamamoto, H.; Xu, Y.; Li, W.; Yamanishi, H.; Okamura, H.; et al. Hepatocyte nuclear factor 4 alpha is a key factor related to depression and physiological homeostasis in the mouse brain. PLoS ONE 2015, 10, e0119021. [Google Scholar] [CrossRef]
- Ikubo, K.; Yamanishi, K.; Doe, N.; Hashimoto, T.; Sumida, M.; Watanabe, Y.; El-Darawish, Y.; Li, W.; Okamura, H.; Yamanishi, H.; et al. Molecular analysis of the mouse brain exposed to chronic mild stress: The influence of hepatocyte nuclear factor 4α on physiological homeostasis. Mol. Med. Rep. 2017, 16, 301–309. [Google Scholar] [CrossRef]
- Ahmed, A.; Klotz, R.; Köhler, S.; Giese, N.; Hackert, T.; Springfeld, C.; Jäger, D.; Halama, N. Immune features of the peritumoral stroma in pancreatic ductal adenocarcinoma. Front. Immunol. 2022, 13, 947407. [Google Scholar] [CrossRef]
- Bellone, G.; Smirne, C.; Mauri, F.A.; Tonel, E.; Carbone, A.; Buffolino, A.; Dughera, L.; Robecchi, A.; Pirisi, M.; Emanuelli, G. Cytokine expression profile in human pancreatic carcinoma cells and in surgical specimens: Implications for survival. Cancer Immunol. Immunother. CII 2006, 55, 684–698. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Fan, G.; Zhuo, Q.; Dai, W.; Ye, Z.; Ji, S.; Xu, W.; Liu, W.; Hu, Q.; Zhang, Z.; et al. Pin1 promotes pancreatic cancer progression and metastasis by activation of NF-κB-IL-18 feedback loop. Cell Prolif. 2020, 53, e12816. [Google Scholar] [CrossRef] [PubMed]
- Usul Afsar, Ç.; Karabulut, M.; Karabulut, S.; Alis, H.; Gonenc, M.; Dagoglu, N.; Serilmez, M.; Tas, F. Circulating interleukin-18 (IL-18) is a predictor of response to gemcitabine based chemotherapy in patients with pancreatic adenocarcinoma. J. Infect. Chemother. Off. J. Jpn. Soc. Chemother. 2017, 23, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Carbone, A.; Vizio, B.; Novarino, A.; Mauri, F.A.; Geuna, M.; Robino, C.; Brondino, G.; Prati, A.; Giacobino, A.; Campra, D.; et al. IL-18 paradox in pancreatic carcinoma: Elevated serum levels of free IL-18 are correlated with poor survival. J. Immunother. 2009, 32, 920–931. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Zhao, X.; Zhu, N.; Zhao, M.; Hu, Q.; Ni, Y. The balance of serum IL-18/IL-37 levels is disrupted during the development of oral squamous cell carcinoma. Surg. Oncol. 2020, 32, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Robertson, M.J.; Kirkwood, J.M.; Logan, T.F.; Koch, K.M.; Kathman, S.; Kirby, L.C.; Bell, W.N.; Thurmond, L.M.; Weisenbach, J.; Dar, M.M. A dose-escalation study of recombinant human interleukin-18 using two different schedules of administration in patients with cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2008, 14, 3462–3469. [Google Scholar] [CrossRef]
- Tarhini, A.A.; Millward, M.; Mainwaring, P.; Kefford, R.; Logan, T.; Pavlick, A.; Kathman, S.J.; Laubscher, K.H.; Dar, M.M.; Kirkwood, J.M. A phase 2, randomized study of SB-485232, rhIL-18, in patients with previously untreated metastatic melanoma. Cancer 2009, 115, 859–868. [Google Scholar] [CrossRef]
- Robertson, M.J.; Mier, J.W.; Logan, T.; Atkins, M.; Koon, H.; Koch, K.M.; Kathman, S.; Pandite, L.N.; Oei, C.; Kirby, L.C.; et al. Clinical and biological effects of recombinant human interleukin-18 administered by intravenous infusion to patients with advanced cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2006, 12 Pt 1, 4265–4273. [Google Scholar] [CrossRef]
- Zhou, T.; Damsky, W.; Weizman, O.E.; McGeary, M.K.; Hartmann, K.P.; Rosen, C.E.; Fischer, S.; Jackson, R.; Flavell, R.A.; Wang, J.; et al. IL-18BP is a secreted immune checkpoint and barrier to IL-18 immunotherapy. Nature 2020, 583, 609–614. [Google Scholar] [CrossRef]
- Liu, W.; Han, B.; Sun, B.; Gao, Y.; Huang, Y.; Hu, M. Overexpression of interleukin-18 induces growth inhibition, apoptosis and gene expression changes in a human tongue squamous cell carcinoma cell line. J. Int. Med. Res. 2012, 40, 537–544. [Google Scholar] [CrossRef]
- Zhang, J.; Pan, C.; Xu, T.; Niu, Z.; Ma, C.; Xu, C. Interleukin 18 augments growth ability via NF-κB and p38/ATF2 pathways by targeting cyclin B1, cyclin B2, cyclin A2, and Bcl-2 in BRL-3A rat liver cells. Gene 2015, 563, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.K.; Park, Y.; Song, S.B.; Cheon, S.Y.; Park, S.; Houh, Y.; Ha, S.; Kim, H.J.; Park, J.M.; Kim, T.S.; et al. Erythroid differentiation regulator 1, an interleukin 18-regulated gene, acts as a metastasis suppressor in melanoma. J. Investig. Dermatol. 2011, 131, 2096–2104. [Google Scholar] [CrossRef] [PubMed]
- Houh, Y.K.; Kim, K.E.; Park, H.J.; Cho, D. Roles of Erythroid Differentiation Regulator 1 (Erdr1) on Inflammatory Skin Diseases. Int. J. Mol. Sci. 2016, 17, 2059. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.K.; Houh, Y.K.; Ha, S.; Yang, Y.; Kim, D.; Kim, T.S.; Yoon, S.R.; Bang, S.I.; Cho, B.J.; Lee, W.J.; et al. Recombinant Erdr1 suppresses the migration and invasion ability of human gastric cancer cells, SNU-216, through the JNK pathway. Immunol. Lett. 2013, 150, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Jung, M.K.; Park, H.J.; Kim, K.E.; Cho, D. Erdr1 Suppresses Murine Melanoma Growth via Regulation of Apoptosis. Int. J. Mol. Sci. 2016, 17, 107. [Google Scholar] [CrossRef]
- Helmbach, H.; Rossmann, E.; Kern, M.A.; Schadendorf, D. Drug-resistance in human melanoma. Int. J. Cancer 2001, 93, 617–622. [Google Scholar] [CrossRef] [PubMed]
- Fofaria, N.M.; Srivastava, S.K. Critical role of STAT3 in melanoma metastasis through anoikis resistance. Oncotarget 2014, 5, 7051–7064. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Pardoll, D.; Jove, R. STATs in cancer inflammation and immunity: A leading role for STAT3. Nat. Rev. Cancer 2009, 9, 798–809. [Google Scholar] [CrossRef]
- Lee, H.R.; Huh, S.Y.; Hur, D.Y.; Jeong, H.; Kim, T.S.; Kim, S.Y.; Park, S.B.; Yang, Y.; Bang, S.I.; Park, H.; et al. ERDR1 enhances human NK cell cytotoxicity through an actin-regulated degranulation-dependent pathway. Cell. Immunol. 2014, 292, 78–84. [Google Scholar] [CrossRef]
- Liu, Y.R.; Hu, Y.; Zeng, Y.; Li, Z.X.; Zhang, H.B.; Deng, J.L.; Wang, G. Neurexophilin and PC-esterase domain family member 4 (NXPE4) and prostate androgen-regulated mucin-like protein 1 (PARM1) as prognostic biomarkers for colorectal cancer. J. Cell. Biochem. 2019, 120, 18041–18052. [Google Scholar] [CrossRef]
- Yassin, M.; Kissow, H.; Vainer, B.; Joseph, P.D.; Hay-Schmidt, A.; Olsen, J.; Pedersen, A.E. Cytoglobin affects tumorigenesis and the expression of ulcerative colitis-associated genes under chemically induced colitis in mice. Sci. Rep. 2018, 8, 6905. [Google Scholar] [CrossRef]
- Yang, C.; Wang, T.; Zhu, S.; Zong, Z.; Luo, C.; Zhao, Y.; Liu, J.; Li, T.; Liu, X.; Liu, C.; et al. Nicotinamide N-Methyltransferase Remodeled Cell Metabolism and Aggravated Proinflammatory Responses by Activating STAT3/IL1β/PGE(2) Pathway. ACS Omega 2022, 7, 37509–37519. [Google Scholar] [CrossRef] [PubMed]
- You, Z.; Liu, Y.; Liu, X. Nicotinamide N-methyltransferase enhances the progression of prostate cancer by stabilizing sirtuin 1. Oncol. Lett. 2018, 15, 9195–9201. [Google Scholar] [CrossRef] [PubMed]
- Hrašovec, S.; Hauptman, N.; Glavač, D.; Jelenc, F.; Ravnik-Glavač, M. TMEM25 is a candidate biomarker methylated and down-regulated in colorectal cancer. Dis. Markers 2013, 34, 93–104. [Google Scholar] [CrossRef]
- Doolan, P.; Clynes, M.; Kennedy, S.; Mehta, J.P.; Germano, S.; Ehrhardt, C.; Crown, J.; O’Driscoll, L. TMEM25, REPS2 and Meis 1: Favourable prognostic and predictive biomarkers for breast cancer. Tumour Biol. J. Int. Soc. Oncodevelopmental Biol. Med. 2009, 30, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Katoh, M.; Katoh, M. Identification and characterization of human TMEM25 and mouse Tmem25 genes in silico. Oncol. Rep. 2004, 12, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Chakravarti, D.; Hu, B.; Mao, X.; Rashid, A.; Li, J.; Li, J.; Liao, W.T.; Whitley, E.M.; Dey, P.; Hou, P.; et al. Telomere dysfunction activates YAP1 to drive tissue inflammation. Nat. Commun. 2020, 11, 4766. [Google Scholar] [CrossRef]
- Angèle, S.; Hall, J. The ATM gene and breast cancer: Is it really a risk factor? Mutat. Res. 2000, 462, 167–178. [Google Scholar] [CrossRef]
- Renwick, A.; Thompson, D.; Seal, S.; Kelly, P.; Chagtai, T.; Ahmed, M.; North, B.; Jayatilake, H.; Barfoot, R.; Spanova, K.; et al. ATM mutations that cause ataxia-telangiectasia are breast cancer susceptibility alleles. Nat. Genet. 2006, 38, 873–875. [Google Scholar] [CrossRef]
- Hsu, F.C.; Roberts, N.J.; Childs, E.; Porter, N.; Rabe, K.G.; Borgida, A.; Ukaegbu, C.; Goggins, M.G.; Hruban, R.H.; Zogopoulos, G.; et al. Risk of Pancreatic Cancer Among Individuals With Pathogenic Variants in the ATM Gene. JAMA Oncol. 2021, 7, 1664–1668. [Google Scholar] [CrossRef]
- Martino, C.; Pandya, D.; Lee, R.; Levy, G.; Lo, T.; Lobo, S.; Frank, R.C. ATM-Mutated Pancreatic Cancer: Clinical and Molecular Response to Gemcitabine/Nab-Paclitaxel After Genome-Based Therapy Resistance. Pancreas 2020, 49, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.W.; Cha, J.M.; Kwak, M.S. Identification of Potential Biomarkers and Biological Pathways for Poor Clinical Outcome in Mucinous Colorectal Adenocarcinoma. Cancers 2021, 13, 3280. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.H.; Hsu, C.W.; Hsueh, C.; Wang, C.L.; Wu, Y.C.; Wu, C.C.; Liu, C.C.; Yu, J.S.; Chang, Y.S.; Yu, C.J. Identification and Characterization of Potential Biomarkers by Quantitative Tissue Proteomics of Primary Lung Adenocarcinoma. Mol. Cell. Proteom. MCP 2016, 15, 2396–2410. [Google Scholar] [CrossRef] [PubMed]
- Sadhra, S.; Kurmi, O.P.; Sadhra, S.S.; Lam, K.B.; Ayres, J.G. Occupational COPD and job exposure matrices: A systematic review and meta-analysis. Int. J. Chronic Obstr. Pulm. Dis. 2017, 12, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Reddy, R.B.; Khora, S.S.; Suresh, A. Molecular prognosticators in clinically and pathologically distinct cohorts of head and neck squamous cell carcinoma-A meta-analysis approach. PLoS ONE 2019, 14, e0218989. [Google Scholar] [CrossRef]
- Li, S.; Wang, C.; Liu, X.; Hua, S.; Liu, X. The roles of AXIN2 in tumorigenesis and epigenetic regulation. Fam. Cancer 2015, 14, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Park, S.Y.; Lee, S.A.; Park, M.G.; Yu, S.K.; Lee, M.H.; Park, M.R.; Kim, S.G.; Oh, J.S.; Lee, S.Y.; et al. MicroRNA-205 suppresses the oral carcinoma oncogenic activity via down-regulation of Axin-2 in KB human oral cancer cell. Mol. Cell. Biochem. 2014, 387, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Ying, Y.; Tao, Q. Epigenetic disruption of the WNT/beta-catenin signaling pathway in human cancers. Epigenetics 2009, 4, 307–312. [Google Scholar] [CrossRef]
- Lustig, B.; Jerchow, B.; Sachs, M.; Weiler, S.; Pietsch, T.; Karsten, U.; van de Wetering, M.; Clevers, H.; Schlag, P.M.; Birchmeier, W.; et al. Negative feedback loop of Wnt signaling through upregulation of conductin/axin2 in colorectal and liver tumors. Mol. Cell. Biol. 2002, 22, 1184–1193. [Google Scholar] [CrossRef]
- Kim, W.K.; Byun, W.S.; Chung, H.J.; Oh, J.; Park, H.J.; Choi, J.S.; Lee, S.K. Esculetin suppresses tumor growth and metastasis by targeting Axin2/E-cadherin axis in colorectal cancer. Biochem. Pharmacol. 2018, 152, 71–83. [Google Scholar] [CrossRef]
- Schulten, H.J.; Hussein, D.; Al-Adwani, F.; Karim, S.; Al-Maghrabi, J.; Al-Sharif, M.; Jamal, A.; Bakhashab, S.; Weaver, J.; Al-Ghamdi, F.; et al. Microarray expression profiling identifies genes, including cytokines, and biofunctions, as diapedesis, associated with a brain metastasis from a papillary thyroid carcinoma. Am. J. Cancer Res. 2016, 6, 2140–2161. [Google Scholar] [PubMed]
- Terlizzi, M.; Colarusso, C.; De Rosa, I.; De Rosa, N.; Somma, P.; Curcio, C.; Sanduzzi, A.; Micheli, P.; Molino, A.; Saccomanno, A.; et al. Circulating and tumor-associated caspase-4: A novel diagnostic and prognostic biomarker for non-small cell lung cancer. Oncotarget 2018, 9, 19356–19367. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ni, F.; Yu, F.; Cui, Z.; Zhu, X.; Chen, J. Prognostic significance of mRNA expression of CASPs in gastric cancer. Oncol. Lett. 2019, 18, 4535–4554. [Google Scholar] [CrossRef] [PubMed]
- Shibamoto, M.; Hirata, H.; Eguchi, H.; Sawada, G.; Sakai, N.; Kajiyama, Y.; Mimori, K. The loss of CASP4 expression is associated with poor prognosis in esophageal squamous cell carcinoma. Oncol. Lett. 2017, 13, 1761–1766. [Google Scholar] [CrossRef]
- Zahalka, A.H.; Arnal-Estapé, A.; Maryanovich, M.; Nakahara, F.; Cruz, C.D.; Finley, L.W.S.; Frenette, P.S. Adrenergic nerves activate an angio-metabolic switch in prostate cancer. Science 2017, 358, 321–326. [Google Scholar] [CrossRef]
- Yin, Q.Q.; Xu, L.H.; Zhang, M.; Xu, C. Muscarinic acetylcholine receptor M1 mediates prostate cancer cell migration and invasion through hedgehog signaling. Asian J. Androl. 2018, 20, 608–614. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, L.L.; Huan, H.B.; Wen, X.D.; Yang, D.P.; Chen, D.F.; Xia, F. Activation of muscarinic acetylcholine receptor 1 promotes invasion of hepatocellular carcinoma by inducing epithelial-mesenchymal transition. Anti-Cancer Drugs 2020, 31, 908–917. [Google Scholar] [CrossRef]
- Renz, B.W.; Tanaka, T.; Sunagawa, M.; Takahashi, R.; Jiang, Z.; Macchini, M.; Dantes, Z.; Valenti, G.; White, R.A.; Middelhoff, M.A.; et al. Cholinergic Signaling via Muscarinic Receptors Directly and Indirectly Suppresses Pancreatic Tumorigenesis and Cancer Stemness. Cancer Discov. 2018, 8, 1458–1473. [Google Scholar] [CrossRef]
- Tang, H.; Guo, Q.; Zhang, C.; Zhu, J.; Yang, H.; Zou, Y.L.; Yan, Y.; Hong, D.; Sou, T.; Yan, X.M. Identification of an intermediate signature that marks the initial phases of the colorectal adenoma-carcinoma transition. Int. J. Mol. Med. 2010, 26, 631–641. [Google Scholar] [CrossRef]
- Yang, C.A.; Huang, H.Y.; Chang, Y.S.; Lin, C.L.; Lai, I.L.; Chang, J.G. DNA-Sensing and Nuclease Gene Expressions as Markers for Colorectal Cancer Progression. Oncology 2017, 92, 115–124. [Google Scholar] [CrossRef]
- Cai, H.; Yan, L.; Liu, N.; Xu, M.; Cai, H. IFI16 promotes cervical cancer progression by upregulating PD-L1 in immunomicroenvironment through STING-TBK1-NF-kB pathway. Biomed. Pharmacother. = Biomed. Pharmacother. 2020, 123, 109790. [Google Scholar] [CrossRef] [PubMed]
- Unterholzner, L.; Keating, S.E.; Baran, M.; Horan, K.A.; Jensen, S.B.; Sharma, S.; Sirois, C.M.; Jin, T.; Latz, E.; Xiao, T.S.; et al. IFI16 is an innate immune sensor for intracellular DNA. Nat. Immunol. 2010, 11, 997–1004. [Google Scholar] [CrossRef] [PubMed]
- Kerur, N.; Veettil, M.V.; Sharma-Walia, N.; Bottero, V.; Sadagopan, S.; Otageri, P.; Chandran, B. IFI16 acts as a nuclear pathogen sensor to induce the inflammasome in response to Kaposi Sarcoma-associated herpesvirus infection. Cell Host Microbe 2011, 9, 363–375. [Google Scholar] [CrossRef]
- Lin, W.; Zhao, Z.; Ni, Z.; Zhao, Y.; Du, W.; Chen, S. IFI16 restoration in hepatocellular carcinoma induces tumour inhibition via activation of p53 signals and inflammasome. Cell Prolif. 2017, 50, e12392. [Google Scholar] [CrossRef]
- Song, A.; Chen, Y.F.; Thamatrakoln, K.; Storm, T.A.; Krensky, A.M. RFLAT-1: A new zinc finger transcription factor that activates RANTES gene expression in T lymphocytes. Immunity 1999, 10, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Tetreault, M.P.; Yang, Y.; Katz, J.P. Krüppel-like factors in cancer. Nat. Rev. Cancer 2013, 13, 701–713. [Google Scholar] [CrossRef]
- Fernandez-Zapico, M.E.; Lomberk, G.A.; Tsuji, S.; DeMars, C.J.; Bardsley, M.R.; Lin, Y.H.; Almada, L.L.; Han, J.J.; Mukhopadhyay, D.; Ordog, T.; et al. A functional family-wide screening of SP/KLF proteins identifies a subset of suppressors of KRAS-mediated cell growth. Biochem. J. 2011, 435, 529–537. [Google Scholar] [CrossRef]
- Wang, Q.; Peng, R.; Wang, B.; Wang, J.; Yu, W.; Liu, Y.; Shi, G. Transcription factor KLF13 inhibits AKT activation and suppresses the growth of prostate carcinoma cells. Cancer Biomark. Sect. A Dis. Markers 2018, 22, 533–541. [Google Scholar] [CrossRef]
- Yao, W.; Jiao, Y.; Zhou, Y.; Luo, X. KLF13 suppresses the proliferation and growth of colorectal cancer cells through transcriptionally inhibiting HMGCS1-mediated cholesterol biosynthesis. Cell Biosci. 2020, 10, 76. [Google Scholar] [CrossRef]
- Nemer, M.; Horb, M.E. The KLF family of transcriptional regulators in cardiomyocyte proliferation and differentiation. Cell Cycle 2007, 6, 117–121. [Google Scholar] [CrossRef]
- Henson, B.J.; Gollin, S.M. Overexpression of KLF13 and FGFR3 in oral cancer cells. Cytogenet. Genome Res. 2010, 128, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhong, Y.; Yang, B.; Zhu, Y.; Zhu, X.; Xia, Z.; Xu, J.; Xu, L. LINC00958 facilitates cervical cancer cell proliferation and metastasis by sponging miR-625-5p to upregulate LRRC8E expression. J. Cell. Biochem. 2020, 121, 2500–2509. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Wang, S.; Shi, Y. LncRNA PCAT6 activated by SP1 facilitates the progression of breast cancer by the miR-326/LRRC8E axis. Anti-Cancer Drugs 2022, 33, 178–190. [Google Scholar] [CrossRef]
- Sullivan, J.P.; Minna, J.D. Tumor oncogenotypes and lung cancer stem cell identity. Cell Stem Cell 2010, 7, 2–4. [Google Scholar] [CrossRef] [PubMed]
- Ceder, J.A.; Aalders, T.W.; Schalken, J.A. Label retention and stem cell marker expression in the developing and adult prostate identifies basal and luminal epithelial stem cell subpopulations. Stem Cell Res. Ther. 2017, 8, 95. [Google Scholar] [CrossRef]
- Dall, G.V.; Vieusseux, J.L.; Korach, K.S.; Arao, Y.; Hewitt, S.C.; Hamilton, K.J.; Dzierzak, E.; Boon, W.C.; Simpson, E.R.; Ramsay, R.G.; et al. SCA-1 Labels a Subset of Estrogen-Responsive Bipotential Repopulating Cells within the CD24(+) CD49f(hi) Mammary Stem Cell-Enriched Compartment. Stem Cell Rep. 2017, 8, 417–431. [Google Scholar] [CrossRef]
- Batts, T.D.; Machado, H.L.; Zhang, Y.; Creighton, C.J.; Li, Y.; Rosen, J.M. Stem cell antigen-1 (sca-1) regulates mammary tumor development and cell migration. PLoS ONE 2011, 6, e27841. [Google Scholar] [CrossRef]
- Li, S.; Zhuang, Z.; Wu, T.; Lin, J.C.; Liu, Z.X.; Zhou, L.F.; Dai, T.; Lu, L.; Ju, H.Q. Nicotinamide nucleotide transhydrogenase-mediated redox homeostasis promotes tumor growth and metastasis in gastric cancer. Redox Biol. 2018, 18, 246–255. [Google Scholar] [CrossRef]
- Chortis, V.; Taylor, A.E.; Doig, C.L.; Walsh, M.D.; Meimaridou, E.; Jenkinson, C.; Rodriguez-Blanco, G.; Ronchi, C.L.; Jafri, A.; Metherell, L.A.; et al. Nicotinamide Nucleotide Transhydrogenase as a Novel Treatment Target in Adrenocortical Carcinoma. Endocrinology 2018, 159, 2836–2849. [Google Scholar] [CrossRef]
- Ward, N.P.; Kang, Y.P.; Falzone, A.; Boyle, T.A.; DeNicola, G.M. Nicotinamide nucleotide transhydrogenase regulates mitochondrial metabolism in NSCLC through maintenance of Fe-S protein function. J. Exp. Med. 2020, 217, e20191689. [Google Scholar] [CrossRef]
- Claudio, J.O.; Zhu, Y.X.; Benn, S.J.; Shukla, A.H.; McGlade, C.J.; Falcioni, N.; Stewart, A.K. HACS1 encodes a novel SH3-SAM adaptor protein differentially expressed in normal and malignant hematopoietic cells. Oncogene 2001, 20, 5373–5377. [Google Scholar] [CrossRef] [PubMed]
- Yamada, H.; Yanagisawa, K.; Tokumaru, S.; Taguchi, A.; Nimura, Y.; Osada, H.; Nagino, M.; Takahashi, T. Detailed characterization of a homozygously deleted region corresponding to a candidate tumor suppressor locus at 21q11-21 in human lung cancer. Genes Chromosomes Cancer 2008, 47, 810–818. [Google Scholar] [CrossRef] [PubMed]
- Noll, J.E.; Hewett, D.R.; Williams, S.A.; Vandyke, K.; Kok, C.; To, L.B.; Zannettino, A.C. SAMSN1 is a tumor suppressor gene in multiple myeloma. Neoplasia 2014, 16, 572–585. [Google Scholar] [CrossRef]
- Kanda, M.; Shimizu, D.; Sueoka, S.; Nomoto, S.; Oya, H.; Takami, H.; Ezaka, K.; Hashimoto, R.; Tanaka, Y.; Kobayashi, D.; et al. Prognostic relevance of SAMSN1 expression in gastric cancer. Oncol. Lett. 2016, 12, 4708–4716. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Zhang, L.; Xu, T.; Zhou, J.; Qin, R.; Chen, C.; Zou, Y.; Fu, D.; Hu, G.; Chen, J.; et al. SAMSN1 is highly expressed and associated with a poor survival in glioblastoma multiforme. PLoS ONE 2013, 8, e81905. [Google Scholar] [CrossRef] [PubMed]
- Sueoka, S.; Kanda, M.; Sugimoto, H.; Shimizu, D.; Nomoto, S.; Oya, H.; Takami, H.; Ezaka, K.; Hashimoto, R.; Tanaka, Y.; et al. Suppression of SAMSN1 Expression is Associated with the Malignant Phenotype of Hepatocellular Carcinoma. Ann. Surg. Oncol. 2015, 22 (Suppl. S3), S1453–S1460. [Google Scholar] [CrossRef]
- Zhuang, H.; Han, J.; Cheng, L.; Liu, S.L. A Positive Causal Influence of IL-18 Levels on the Risk of T2DM: A Mendelian Randomization Study. Front. Genet. 2019, 10, 295. [Google Scholar] [CrossRef] [PubMed]
- Moriwaki, Y.; Yamamoto, T.; Shibutani, Y.; Aoki, E.; Tsutsumi, Z.; Takahashi, S.; Okamura, H.; Koga, M.; Fukuchi, M.; Hada, T. Elevated levels of interleukin-18 and tumor necrosis factor-alpha in serum of patients with type 2 diabetes mellitus: Relationship with diabetic nephropathy. Metab. Clin. Exp. 2003, 52, 605–608. [Google Scholar] [CrossRef]
- Zaharieva, E.; Kamenov, Z.; Velikova, T.; Tsakova, A.; El-Darawish, Y.; Okamura, H. Interleukin-18 serum level is elevated in type 2 diabetes and latent autoimmune diabetes. Endocr. Connect. 2018, 7, 179–185. [Google Scholar] [CrossRef]
- Fischer, C.P.; Perstrup, L.B.; Berntsen, A.; Eskildsen, P.; Pedersen, B.K. Elevated plasma interleukin-18 is a marker of insulin-resistance in type 2 diabetic and non-diabetic humans. Clin. Immunol. 2005, 117, 152–160. [Google Scholar] [CrossRef]
- Nedeva, I.; Gateva, A.; Assyov, Y.; Karamfilova, V.; Hristova, J.; Yamanishi, K.; Kamenov, Z.; Okamura, H. IL-18 Serum Levels in Patients with Obesity, Prediabetes and Newly Diagnosed Type 2 Diabetes. Iran. J. Immunol. IJI 2022, 19, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Kabakchieva, P.; Gateva, A.; Velikova, T.; Georgiev, T.; Yamanishi, K.; Okamura, H.; Kamenov, Z. Elevated levels of interleukin-18 are associated with several indices of general and visceral adiposity and insulin resistance in women with polycystic ovary syndrome. Arch. Endocrinol. Metab. 2022, 66, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Hung, J.; McQuillan, B.M.; Chapman, C.M.; Thompson, P.L.; Beilby, J.P. Elevated interleukin-18 levels are associated with the metabolic syndrome independent of obesity and insulin resistance. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1268–1273. [Google Scholar] [CrossRef] [PubMed]
- Olusi, S.O.; Al-Awadhi, A.; Abraham, M. Relations of serum interleukin 18 levels to serum lipid and glucose concentrations in an apparently healthy adult population. Horm. Res. 2003, 60, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Esposito, K.; Pontillo, A.; Ciotola, M.; Di Palo, C.; Grella, E.; Nicoletti, G.; Giugliano, D. Weight loss reduces interleukin-18 levels in obese women. J. Clin. Endocrinol. Metab. 2002, 87, 3864–3866. [Google Scholar] [CrossRef] [PubMed]
- Membrez, M.; Ammon-Zufferey, C.; Philippe, D.; Aprikian, O.; Monnard, I.; Macé, K.; Darimont, C. Interleukin-18 protein level is upregulated in adipose tissue of obese mice. Obesity 2009, 17, 393–395. [Google Scholar] [CrossRef]
- Valentin-Vega, Y.A.; Kastan, M.B. A new role for ATM: Regulating mitochondrial function and mitophagy. Autophagy 2012, 8, 840–841. [Google Scholar] [CrossRef]
- Biton, S.; Ashkenazi, A. NEMO and RIP1 control cell fate in response to extensive DNA damage via TNF-α feedforward signaling. Cell 2011, 145, 92–103. [Google Scholar] [CrossRef]
- Ching, J.K.; Spears, L.D.; Armon, J.L.; Renth, A.L.; Andrisse, S.; Collins, R.L.t.; Fisher, J.S. Impaired insulin-stimulated glucose transport in ATM-deficient mouse skeletal muscle. Appl. Physiol. Nutr. Metab. = Physiol. Appl. Nutr. Metab. 2013, 38, 589–596. [Google Scholar] [CrossRef]
- Suzuki, A.; Kusakai, G.; Kishimoto, A.; Shimojo, Y.; Ogura, T.; Lavin, M.F.; Esumi, H. IGF-1 phosphorylates AMPK-alpha subunit in ATM-dependent and LKB1-independent manner. Biochem. Biophys. Res. Commun. 2004, 324, 986–992. [Google Scholar] [CrossRef]
- Miles, P.D.; Treuner, K.; Latronica, M.; Olefsky, J.M.; Barlow, C. Impaired insulin secretion in a mouse model of ataxia telangiectasia. Am. J. Physiology. Endocrinol. Metab. 2007, 293, E70–E74. [Google Scholar] [CrossRef] [PubMed]
- Barlow, C.; Hirotsune, S.; Paylor, R.; Liyanage, M.; Eckhaus, M.; Collins, F.; Shiloh, Y.; Crawley, J.N.; Ried, T.; Tagle, D.; et al. Atm-deficient mice: A paradigm of ataxia telangiectasia. Cell 1996, 86, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.G.; Finck, B.N.; Ren, J.; Standley, K.N.; Takagi, M.; Maclean, K.H.; Bernal-Mizrachi, C.; Muslin, A.J.; Kastan, M.B.; Semenkovich, C.F. ATM-dependent suppression of stress signaling reduces vascular disease in metabolic syndrome. Cell Metab. 2006, 4, 377–389. [Google Scholar] [CrossRef] [PubMed]
- Daugherity, E.K.; Balmus, G.; Al Saei, A.; Moore, E.S.; Abi Abdallah, D.; Rogers, A.B.; Weiss, R.S.; Maurer, K.J. The DNA damage checkpoint protein ATM promotes hepatocellular apoptosis and fibrosis in a mouse model of non-alcoholic fatty liver disease. Cell Cycle 2012, 11, 1918–1928. [Google Scholar] [CrossRef] [PubMed]
- Blackford, A.N.; Jackson, S.P. ATM, ATR, and DNA-PK: The Trinity at the Heart of the DNA Damage Response. Mol. Cell 2017, 66, 801–817. [Google Scholar] [CrossRef]
- Ahrens, M.; Ammerpohl, O.; von Schönfels, W.; Kolarova, J.; Bens, S.; Itzel, T.; Teufel, A.; Herrmann, A.; Brosch, M.; Hinrichsen, H.; et al. DNA methylation analysis in nonalcoholic fatty liver disease suggests distinct disease-specific and remodeling signatures after bariatric surgery. Cell Metab. 2013, 18, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liu, H.; Hu, X.; Huang, Y.; Wang, Y.; He, Y.; Lei, Q. Identification of key genes in non-alcoholic fatty liver disease progression based on bioinformatics analysis. Mol. Med. Rep. 2018, 17, 7708–7720. [Google Scholar] [CrossRef]
- Viswanathan, P.; Sharma, Y.; Maisuradze, L.; Tchaikovskaya, T.; Gupta, S. Ataxia telangiectasia mutated pathway disruption affects hepatic DNA and tissue damage in nonalcoholic fatty liver disease. Exp. Mol. Pathol. 2020, 113, 104369. [Google Scholar] [CrossRef]
- Morishima, N.; Nakanishi, K. Proplatelet formation in megakaryocytes is associated with endoplasmic reticulum stress. Genes Cells Devoted Mol. Cell. Mech. 2016, 21, 798–806. [Google Scholar] [CrossRef]
- Han, J.; Kaufman, R.J. The role of ER stress in lipid metabolism and lipotoxicity. J. Lipid Res. 2016, 57, 1329–1338. [Google Scholar] [CrossRef]
- Hoseini, Z.; Sepahvand, F.; Rashidi, B.; Sahebkar, A.; Masoudifar, A.; Mirzaei, H. NLRP3 inflammasome: Its regulation and involvement in atherosclerosis. J. Cell. Physiol. 2018, 233, 2116–2132. [Google Scholar] [CrossRef] [PubMed]
- Piccaluga, P.P.; Navari, M.; Visani, A.; Rigotti, F.; Agostinelli, C.; Righi, S.; Diani, E.; Ligozzi, M.; Carelli, M.; Ponti, C.; et al. Interferon gamma inducible protein 16 (IFI16) expression is reduced in mantle cell lymphoma. Heliyon 2019, 5, e02643. [Google Scholar] [CrossRef]
- Stadion, M.; Schwerbel, K.; Graja, A.; Baumeier, C.; Rödiger, M.; Jonas, W.; Wolfrum, C.; Staiger, H.; Fritsche, A.; Häring, H.U.; et al. Increased Ifi202b/IFI16 expression stimulates adipogenesis in mice and humans. Diabetologia 2018, 61, 1167–1179. [Google Scholar] [CrossRef] [PubMed]
- Trammell, S.A.; Brenner, C. NNMT: A Bad Actor in Fat Makes Good in Liver. Cell Metab. 2015, 22, 200–201. [Google Scholar] [CrossRef] [PubMed]
- Roberti, A.; Fernández, A.F.; Fraga, M.F. Nicotinamide N-methyltransferase: At the crossroads between cellular metabolism and epigenetic regulation. Mol. Metab. 2021, 45, 101165. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, M.; Kanda, T.; Urai, H.; Kurokochi, A.; Kitahama, R.; Shigaki, S.; Ono, T.; Yukioka, H.; Hasegawa, K.; Tokuyama, H.; et al. NNMT activation can contribute to the development of fatty liver disease by modulating the NAD (+) metabolism. Sci. Rep. 2018, 8, 8637. [Google Scholar] [CrossRef]
- Kannt, A.; Pfenninger, A.; Teichert, L.; Tönjes, A.; Dietrich, A.; Schön, M.R.; Klöting, N.; Blüher, M. Association of nicotinamide-N-methyltransferase mRNA expression in human adipose tissue and the plasma concentration of its product, 1-methylnicotinamide, with insulin resistance. Diabetologia 2015, 58, 799–808. [Google Scholar] [CrossRef]
- Al-Hakeim, H.K.; Al-Rammahi, D.A.; Al-Dujaili, A.H. IL-6, IL-18, sIL-2R, and TNFα proinflammatory markers in depression and schizophrenia patients who are free of overt inflammation. J. Affect. Disord. 2015, 182, 106–114. [Google Scholar] [CrossRef]
- Du, X.; Zou, S.; Yue, Y.; Fang, X.; Wu, Y.; Wu, S.; Wang, H.; Li, Z.; Zhao, X.; Yin, M.; et al. Peripheral Interleukin-18 is negatively correlated with abnormal brain activity in patients with depression: A resting-state fMRI study. BMC Psychiatry 2022, 22, 531. [Google Scholar] [CrossRef]
- Corbo, R.M.; Businaro, R.; Scarabino, D. Leukocyte telomere length and plasma interleukin-1β and interleukin-18 levels in mild cognitive impairment and Alzheimer’s disease: New biomarkers for diagnosis and disease progression? Neural Regen. Res. 2021, 16, 1397–1398. [Google Scholar] [CrossRef]
- Ojala, J.; Alafuzoff, I.; Herukka, S.K.; van Groen, T.; Tanila, H.; Pirttilä, T. Expression of interleukin-18 is increased in the brains of Alzheimer’s disease patients. Neurobiol. Aging 2009, 30, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Motta, M.; Imbesi, R.; Di Rosa, M.; Stivala, F.; Malaguarnera, L. Altered plasma cytokine levels in Alzheimer’s disease: Correlation with the disease progression. Immunol. Lett. 2007, 114, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Orhan, F.; Fatouros-Bergman, H.; Schwieler, L.; Cervenka, S.; Flyckt, L.; Sellgren, C.M.; Engberg, G.; Erhardt, S. First-episode psychosis patients display increased plasma IL-18 that correlates with cognitive dysfunction. Schizophr. Res. 2018, 195, 406–408. [Google Scholar] [CrossRef] [PubMed]
- Sutinen, E.M.; Pirttilä, T.; Anderson, G.; Salminen, A.; Ojala, J.O. Pro-inflammatory interleukin-18 increases Alzheimer’s disease-associated amyloid-β production in human neuron-like cells. J. Neuroinflamm. 2012, 9, 199. [Google Scholar] [CrossRef] [PubMed]
- Ojala, J.O.; Sutinen, E.M.; Salminen, A.; Pirttilä, T. Interleukin-18 increases expression of kinases involved in tau phosphorylation in SH-SY5Y neuroblastoma cells. J. Neuroimmunol. 2008, 205, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Yamanishi, K.; Doe, N.; Mukai, K.; Hashimoto, T.; Gamachi, N.; Hata, M.; Watanabe, Y.; Yamanishi, C.; Yagi, H.; Okamura, H.; et al. Acute stress induces severe neural inflammation and overactivation of glucocorticoid signaling in interleukin-18-deficient mice. Transl. Psychiatry 2022, 12, 404. [Google Scholar] [CrossRef] [PubMed]
- Herzog, K.H.; Chong, M.J.; Kapsetaki, M.; Morgan, J.I.; McKinnon, P.J. Requirement for Atm in ionizing radiation-induced cell death in the developing central nervous system. Science 1998, 280, 1089–1091. [Google Scholar] [CrossRef]
- Chong, M.J.; Murray, M.R.; Gosink, E.C.; Russell, H.R.; Srinivasan, A.; Kapsetaki, M.; Korsmeyer, S.J.; McKinnon, P.J. Atm and Bax cooperate in ionizing radiation-induced apoptosis in the central nervous system. Proc. Natl. Acad. Sci. USA 2000, 97, 889–894. [Google Scholar] [CrossRef]
- Lee, Y.; Barnes, D.E.; Lindahl, T.; McKinnon, P.J. Defective neurogenesis resulting from DNA ligase IV deficiency requires Atm. Genes Dev. 2000, 14, 2576–2580. [Google Scholar] [CrossRef]
- Allen, D.M.; van Praag, H.; Ray, J.; Weaver, Z.; Winrow, C.J.; Carter, T.A.; Braquet, R.; Harrington, E.; Ried, T.; Brown, K.D.; et al. Ataxia telangiectasia mutated is essential during adult neurogenesis. Genes Dev. 2001, 15, 554–566. [Google Scholar] [CrossRef]
- Kajiwara, Y.; McKenzie, A.; Dorr, N.; Gama Sosa, M.A.; Elder, G.; Schmeidler, J.; Dickstein, D.L.; Bozdagi, O.; Zhang, B.; Buxbaum, J.D. The human-specific CASP4 gene product contributes to Alzheimer-related synaptic and behavioural deficits. Hum. Mol. Genet. 2016, 25, 4315–4327. [Google Scholar] [CrossRef] [PubMed]
- Hopper, S.; Pavey, G.M.; Gogos, A.; Dean, B. Widespread Changes in Positive Allosteric Modulation of the Muscarinic M1 Receptor in Some Participants With Schizophrenia. Int. J. Neuropsychopharmacol. 2019, 22, 640–650. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Matsunaga, H.; Kimura, M.; Tatsumi, K.; Hidaka, Y.; Takano, T.; Uema, T.; Takeda, M.; Amino, N. Autoantibodies against four kinds of neurotransmitter receptors in psychiatric disorders. J. Neuroimmunol. 2003, 141, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Dean, B.; McLeod, M.; Keriakous, D.; McKenzie, J.; Scarr, E. Decreased muscarinic1 receptors in the dorsolateral prefrontal cortex of subjects with schizophrenia. Mol. Psychiatry 2002, 7, 1083–1091. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, M.; Matsui, M.; Watanabe, M. Preferential localization of muscarinic M1 receptor on dendritic shaft and spine of cortical pyramidal cells and its anatomical evidence for volume transmission. J. Neurosci. Off. J. Soc. Neurosci. 2010, 30, 4408–4418. [Google Scholar] [CrossRef]
- Levey, A.I. Muscarinic acetylcholine receptor expression in memory circuits: Implications for treatment of Alzheimer disease. Proc. Natl. Acad. Sci. USA 1996, 93, 13541–13546. [Google Scholar] [CrossRef]
- Stanco, A.; Pla, R.; Vogt, D.; Chen, Y.; Mandal, S.; Walker, J.; Hunt, R.F.; Lindtner, S.; Erdman, C.A.; Pieper, A.A.; et al. NPAS1 represses the generation of specific subtypes of cortical interneurons. Neuron 2014, 84, 940–953. [Google Scholar] [CrossRef]
- Winden, K.D.; Oldham, M.C.; Mirnics, K.; Ebert, P.J.; Swan, C.H.; Levitt, P.; Rubenstein, J.L.; Horvath, S.; Geschwind, D.H. The organization of the transcriptional network in specific neuronal classes. Mol. Syst. Biol. 2009, 5, 291. [Google Scholar] [CrossRef]
- Morais-Silva, G.; Nam, H.; Campbell, R.R.; Basu, M.; Pagliusi, M.; Fox, M.E.; Chan, S.; Iñiguez, S.D.; Ament, S.; Marin, M.T.; et al. Molecular, circuit, and stress response characterization of Ventral Pallidum Npas1-neurons. bioRxiv 2022. [Google Scholar] [CrossRef]
- Kocinaj, A.; Chaudhury, T.; Uddin, M.S.; Junaid, R.R.; Ramsden, D.B.; Hondhamuni, G.; Klamt, F.; Parsons, L.; Parsons, R.B. High Expression of Nicotinamide N-Methyltransferase in Patients with Sporadic Alzheimer’s Disease. Mol. Neurobiol. 2021, 58, 1769–1781. [Google Scholar] [CrossRef]
- Bromberg, A.; Lerer, E.; Udawela, M.; Scarr, E.; Dean, B.; Belmaker, R.H.; Ebstein, R.; Agam, G. Nicotinamide-N-methyltransferase (NNMT) in schizophrenia: Genetic association and decreased frontal cortex mRNA levels. Int. J. Neuropsychopharmacol. 2012, 15, 727–737. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Liu, F.; Yang, L.; Fang, Z.; He, J.; Wang, W.; You, P. Lower serum nicotinamide N-methyltransferase levels in patients with bipolar disorder during acute episodes compared to healthy controls: A cross-sectional study. BMC Psychiatry 2020, 20, 33. [Google Scholar] [CrossRef] [PubMed]
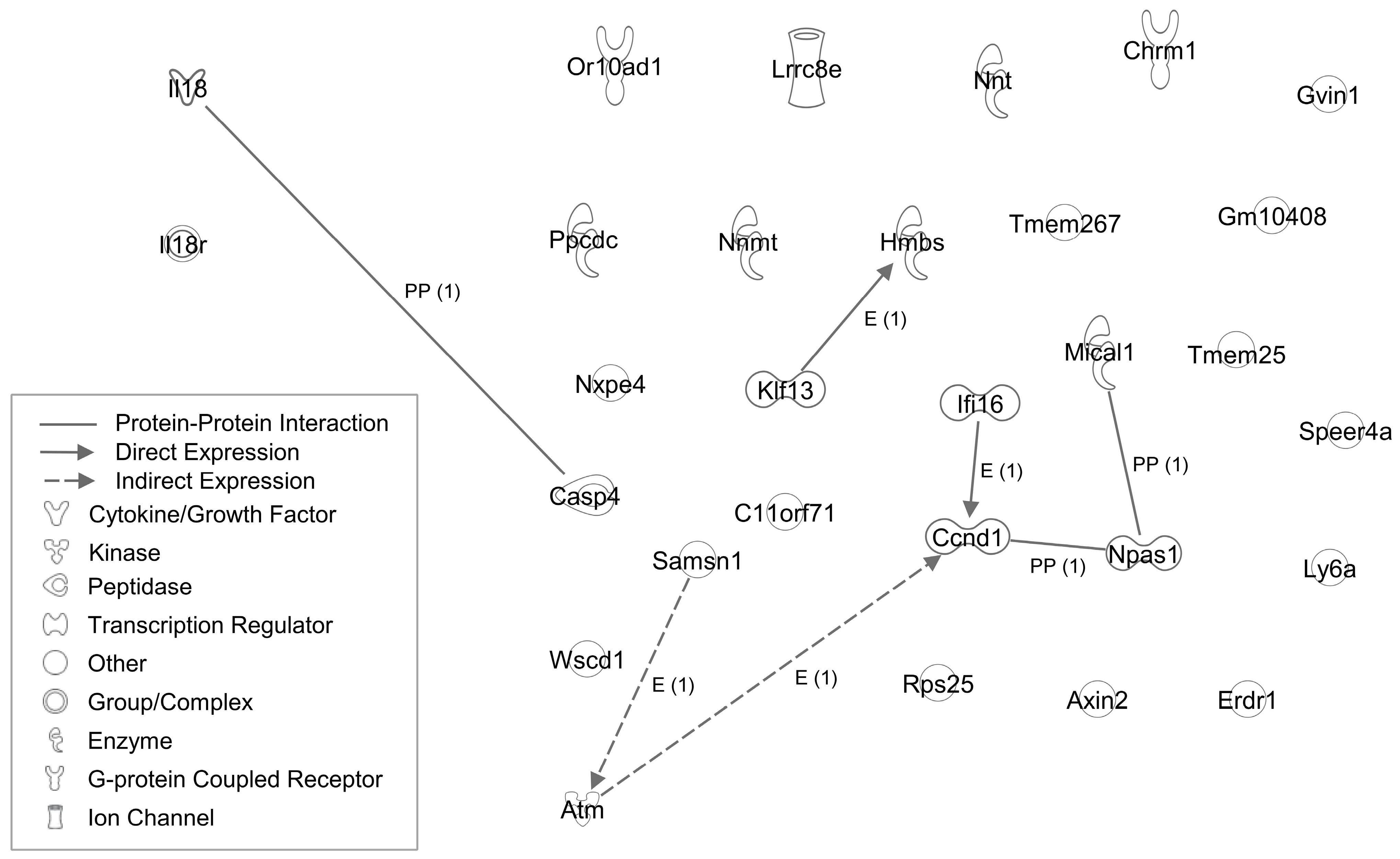
| Symbol | Entrez Gene Name | Cancer |
|---|---|---|
| Down-regulated genes | ||
| Nxpe4 | neurexophilin and PC-esterase domain family member 4 | colon cancer adenocarcinoma, carcinoma, melanoma |
| Ifi16 | interferon gamma inducible protein 16 | colorectal cancer, hepatocellular carcinoma |
| Ccnd1 | cyclin D1 | tongue squamous cell carcinoma |
| Nnmt | nicotinamide N-methyltransferase | prostate cancer |
| Tmem25 | transmembrane protein 25 | breast cancer, colon cancer |
| Rps25 | ribosomal protein S25 | colon rectal cancer, adenocarcinoma, T-cell leukemia |
| Ppcdc | phosphopantothenoylcysteine decarboxylase | endometrioid carcinoma, melanoma |
| Axin2 | axin 2 | colorectal cancer, liver cancer, gastric cancer |
| Lrrc8e | leucine rich repeat containing 8 VRAC subunit E | breast cancer |
| Atm | ATM serine/threonine kinase | pancreatic cancer |
| Samsn1 | SAM domain, SH3 domain and nuclear localization signals 1 | lung cancer, myeloma, gastric cancer, glioblastoma, HCC |
| Ly6a | lymphocyte antigen 6 complex, locus A | tumor progression |
| Casp4 | caspase 4 | lung cancer, gastric cancer, esophageal squamous cell carcinoma |
| Up-regulated genes | ||
| C11orf71 | chromosome 11 open reading frame 71 | N.A. |
| Wscd1 | WSC domain containing 1 | N.A. |
| Npas1 | neuronal PAS domain protein 1 | alveolar rhabdomyosarcoma, soft tissue sarcoma cancer |
| Hmbs | hydroxymethylbilane synthase | N.A. |
| Or10ad1 | olfactory receptor family 10 subfamily AD member 1 | carcinoma, melanoma |
| Klf13 | Kruppel like factor 13 | oral cancer, pancreatic cancer, prostate cancer, colorectal cancer, oral squamous cell carcinoma |
| Mical1 | microtubule associated monooxygenase, calponin and LIM domain containing 1 | N.A. |
| Tmem267 | transmembrane protein 267 | liver cancer, colon caner |
| Chrm1 | cholinergic receptor muscarinic 1 | prostate cancer, pancreatic tumor |
| Nnt | nicotinamide nucleotide transhydrogenase | gastric cancer, adrenocortical carcinoma, lung tumor |
| Erdr1 | erythroid differentiation regulator 1 | melanoma, gastric cancers, leukemia cell cancer |
| Symbol | Entrez Gene Name | Function |
|---|---|---|
| Down-regulated genes | ||
| Ifi16 | interferon gamma inducible protein 16 | Glucose metabolism |
| Ccnd1 | cyclin D1 | Glucose metabolism, lipid metabolism |
| Nnmt | nicotinamide N-methyltransferase | Glucose metabolism |
| Atm | ATM serine/threonine kinase | Glucose metabolism, lipid metabolism |
| Casp4 | caspase 4 | Glucose metabolism |
| Up-regulated genes | ||
| Hmbs | hydroxymethylbilane synthase | Glucose metabolism |
| Chrm1 | cholinergic receptor muscarinic 1 | Glucose metabolism, lipid metabolism |
| Symbol | Entrez Gene Name | Function and Diseases |
|---|---|---|
| Down-regulated genes | ||
| Nnmt | nicotinamide N-methyltransferase | Psychological disorders |
| Atm | ATM serine/threonine kinase | Learning, cognitive impairment |
| Casp4 | caspase 4 | Dementia, Alzheimer’s disease |
| Up-regulated genes | ||
| Npas1 | neuronal PAS domain protein 1 | Learning, memory |
| Chrm1 | cholinergic receptor muscarinic 1 | Major depressive disorder, learning, memory, dementia, cognitive impairment, Alzheimer’s disease, psychological disorders |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamanishi, K.; Hata, M.; Gamachi, N.; Watanabe, Y.; Yamanishi, C.; Okamura, H.; Matsunaga, H. Molecular Mechanisms of IL18 in Disease. Int. J. Mol. Sci. 2023, 24, 17170. https://doi.org/10.3390/ijms242417170
Yamanishi K, Hata M, Gamachi N, Watanabe Y, Yamanishi C, Okamura H, Matsunaga H. Molecular Mechanisms of IL18 in Disease. International Journal of Molecular Sciences. 2023; 24(24):17170. https://doi.org/10.3390/ijms242417170
Chicago/Turabian StyleYamanishi, Kyosuke, Masaki Hata, Naomi Gamachi, Yuko Watanabe, Chiaki Yamanishi, Haruki Okamura, and Hisato Matsunaga. 2023. "Molecular Mechanisms of IL18 in Disease" International Journal of Molecular Sciences 24, no. 24: 17170. https://doi.org/10.3390/ijms242417170
APA StyleYamanishi, K., Hata, M., Gamachi, N., Watanabe, Y., Yamanishi, C., Okamura, H., & Matsunaga, H. (2023). Molecular Mechanisms of IL18 in Disease. International Journal of Molecular Sciences, 24(24), 17170. https://doi.org/10.3390/ijms242417170







