TMT-Based Proteomics Analysis Revealed the Protein Changes in Perirenal Fat from Obese Rabbits
Abstract
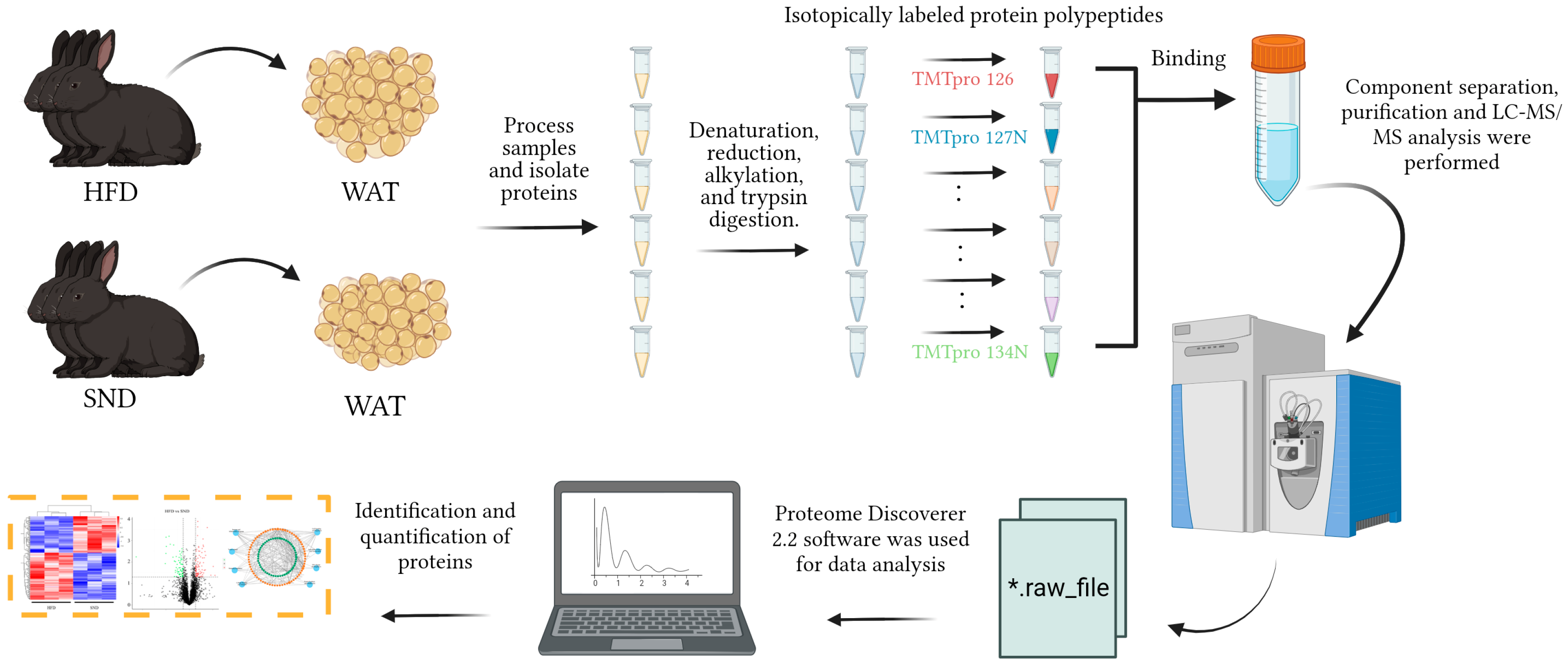
:1. Introduction
2. Results
2.1. Body-Weight and Biochemical-Indicator Disparity in the HFD and SND Groups
2.2. Quality Control of TMT Proteomic Sequencing
2.3. Identification of Differentially Expressed Proteins (DEPs)
2.4. Biological Information Analysis for DEPs
2.5. Network Analysis of PPI
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Measuring Blood Markers
4.3. Total Protein Extraction
4.4. TMT Labeling of Peptides
4.5. Separation of Fractions
4.6. LC-MS/MS Analysis
4.7. The Identification and Quantitation of Protein
4.8. The Functional Analysis of DEPs
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Gadde, K.M.; Martin, C.K.; Berthoud, H.R.; Heymsfield, S.B. Obesity: Pathophysiology and Management. J. Am. Coll. Cardiol. 2018, 71, 69–84. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Qiu, T.; Li, L.; Yu, R.; Chen, X.; Li, C.; Proud, C.G.; Jiang, T. Pathophysiology of obesity and its associated diseases. Acta Pharm. Sin. B 2023, 13, 2403–2424. [Google Scholar] [CrossRef] [PubMed]
- Franchini, F.; Palatucci, G.; Colao, A.; Ungaro, P.; Macchia, P.E.; Nettore, I.C. Obesity and Thyroid Cancer Risk: An Update. Int. J. Environ. Res. Public Health 2022, 19, 1116. [Google Scholar] [CrossRef] [PubMed]
- Ortega, F.B.; Lavie, C.J.; Blair, S.N. Obesity and Cardiovascular Disease. Circ. Res. 2016, 118, 1752–1770. [Google Scholar] [CrossRef]
- Moore, K.J.; Shah, R. Introduction to the Obesity, Metabolic Syndrome, and CVD Compendium. Circ. Res. 2020, 126, 1475–1476. [Google Scholar] [CrossRef]
- Singh, H.; Bajaj, P.; Kalotra, S.; Bhandari, A.; Kaur, T.; Singh, A.P.; Kaur, G. Tinospora cordifolia ameliorates brain functions impairments associated with high fat diet induced obesity. Neurochem. Int. 2021, 143, 104937. [Google Scholar] [CrossRef] [PubMed]
- Boutari, C.; Mantzoros, C.S. A 2022 update on the epidemiology of obesity and a call to action: As its twin COVID-19 pandemic appears to be receding, the obesity and dysmetabolism pandemic continues to rage on. Metab. Clin. Exp. 2022, 133, 155217. [Google Scholar] [CrossRef]
- Wise, J. Less than 10% of obese adults accept that they have a serious weight problem, survey finds. BMJ 2014, 349, g6825. [Google Scholar] [CrossRef]
- Di Cesare, M.; Bentham, J.; Stevens, G.A.; Bjertness, E.; Bjertness, M.B.; Ekelund, U.; Graff-Iversen, S.; Kolle, E.; Krokstad, S.; Steene-Johannessen, J. Trends in adult body-mass index in 200 countries from 1975 to 2014: A pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet 2016, 387, 1377–1396. [Google Scholar]
- Bredella, M.A. Sex Differences in Body Composition. Adv. Exp. Med. Biol. 2017, 1043, 9–27. [Google Scholar]
- Marcelin, G.; Gautier, E.L.; Clément, K. Adipose Tissue Fibrosis in Obesity: Etiology and Challenges. Annu. Rev. Physiol. 2022, 84, 135–155. [Google Scholar] [CrossRef]
- Hoyenga, K.B.; Hoyenga, K.T. Gender and energy balance: Sex differences in adaptations for feast and famine. Physiol. Behav. 1982, 28, 545–563. [Google Scholar] [CrossRef] [PubMed]
- Elffers, T.W.; de Mutsert, R.; Lamb, H.J.; de Roos, A.; Willems van Dijk, K.; Rosendaal, F.R.; Jukema, J.W.; Trompet, S. Body fat distribution, in particular visceral fat, is associated with cardiometabolic risk factors in obese women. PLoS ONE 2017, 12, e0185403. [Google Scholar] [CrossRef] [PubMed]
- Gérard, C.; Brown, K.A. Obesity and breast cancer–Role of estrogens and the molecular underpinnings of aromatase regulation in breast adipose tissue. Mol. Cell. Endocrinol. 2018, 466, 15–30. [Google Scholar] [PubMed]
- Neumark-Sztainer, D.; Falkner, N.; Story, M.; Perry, C.; Hannan, P.J.; Mulert, S. Weight-teasing among adolescents: Correlations with weight status and disordered eating behaviors. Int. J. Obes. Relat. Metab. Disord. J. Int. Assoc. Study Obes. 2002, 26, 123–131. [Google Scholar] [CrossRef]
- Latner, J.D.; Stefano, E.C. Obesity Stigmatization and the Importance of the Research of A.J. Stunkard. Curr. Obes. Rep. 2016, 5, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Van Marken Lichtenbelt, W. Brown Adipose Tissue and the Regulation of Nonshivering Thermogenesis. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 547–552. [Google Scholar] [CrossRef]
- Vishvanath, L.; Gupta, R.K. Contribution of adipogenesis to healthy adipose tissue expansion in obesity. J. Clin. Investig. 2019, 129, 4022–4031. [Google Scholar] [CrossRef]
- Zamboni, M.; Nori, N.; Brunelli, A.; Zoico, E. How does adipose tissue contribute to inflammageing? Exp. Gerontol. 2021, 143, 111162. [Google Scholar] [CrossRef]
- Obradovic, M.; Sudar-Milovanovic, E.; Soskic, S.; Essack, M.; Arya, S.; Stewart, A.J.; Gojobori, T.; Isenovic, E.R. Leptin and Obesity: Role and Clinical Implication. Front. Endocrinol. 2021, 12, 585887. [Google Scholar] [CrossRef]
- Benbaibeche, H.; Bounihi, A.; Koceir, E.A. Leptin level as a biomarker of uncontrolled eating in obesity and overweight. Ir. J. Med. Sci. 2021, 190, 155–161. [Google Scholar] [CrossRef]
- Friedman, J.M.; Halaas, J.L. Leptin and the regulation of body weight in mammals. Nature 1998, 395, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in inflammation and metabolic disease. Nat. Reviews. Immunol. 2011, 11, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Lewis, G.F.; Carpentier, A.; Adeli, K.; Giacca, A. Disordered fat storage and mobilization in the pathogenesis of insulin resistance and type 2 diabetes. Endocr. Rev. 2002, 23, 201–229. [Google Scholar] [CrossRef] [PubMed]
- Kieffer, T.J.; Heller, R.S.; Leech, C.A.; Holz, G.G.; Habener, J.F. Leptin suppression of insulin secretion by the activation of ATP-sensitive K+ channels in pancreatic beta-cells. Diabetes 1997, 46, 1087–1093. [Google Scholar] [CrossRef]
- Pereira, S.; Cline, D.L.; Glavas, M.M.; Covey, S.D.; Kieffer, T.J. Tissue-Specific Effects of Leptin on Glucose and Lipid Metabolism. Endocr. Rev. 2021, 42, 1–28. [Google Scholar] [CrossRef]
- Zhou, C.; Zou, Y.; Huang, J.; Zhao, Z.; Zhang, Y.; Wei, Y.; Ye, K. TMT-Based Quantitative Proteomic Analysis of Intestinal Organoids Infected by Listeria monocytogenes Strains with Different Virulence. Int. J. Mol. Sci. 2022, 23, 6231. [Google Scholar] [CrossRef]
- Pagel, O.; Loroch, S.; Sickmann, A.; Zahedi, R.P. Current strategies and findings in clinically relevant post-translational modification-specific proteomics. Expert Rev. Proteom. 2015, 12, 235–253. [Google Scholar] [CrossRef]
- Friedrich, C.; Schallenberg, S.; Kirchner, M.; Ziehm, M.; Niquet, S.; Haji, M.; Beier, C.; Neudecker, J.; Klauschen, F.; Mertins, P. Comprehensive micro-scaled proteome and phosphoproteome characterization of archived retrospective cancer repositories. Nat. Commun. 2021, 12, 3576. [Google Scholar] [CrossRef]
- Yu, X.T.; Wang, F.; Ding, J.T.; Cai, B.; Xing, J.J.; Guo, G.H.; Guo, F. Tandem mass tag-based serum proteomic profiling revealed diabetic foot ulcer pathogenesis and potential therapeutic targets. Bioengineered 2022, 13, 3171–3182. [Google Scholar] [CrossRef]
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet 2017, 390, 2627–2642. [Google Scholar] [CrossRef] [PubMed]
- Danaei, G.; Ding, E.L.; Mozaffarian, D.; Taylor, B.; Rehm, J.; Murray, C.J.; Ezzati, M. The preventable causes of death in the United States: Comparative risk assessment of dietary, lifestyle, and metabolic risk factors. PLoS Med. 2009, 6, e1000058. [Google Scholar] [CrossRef]
- Lavie, C.J.; Arena, R.; Alpert, M.A.; Milani, R.V.; Ventura, H.O. Management of cardiovascular diseases in patients with obesity. Nat. Rev. Cardiol. 2017, 15, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Seneviratne, S.N.; Rajindrajith, S. Fetal programming of obesity and type 2 diabetes. World J. Diabetes 2022, 13, 482–497. [Google Scholar] [CrossRef]
- Locke, A.E.; Kahali, B.; Berndt, S.I.; Justice, A.E.; Pers, T.H.; Day, F.R.; Powell, C.; Vedantam, S.; Buchkovich, M.L.; Yang, J.; et al. Genetic studies of body mass index yield new insights for obesity biology. Nature 2015, 518, 197–206. [Google Scholar] [CrossRef]
- Kawai, T.; Autieri, M.V.; Scalia, R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am. J. Physiol. Cell Physiol. 2021, 320, C375–C391. [Google Scholar] [CrossRef]
- Fantuzzi, G. Adipose tissue, adipokines, and inflammation. J. Allergy Clin. Immunol. 2005, 115, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Johanna, C.; Purdy, J.J.S. The Hematologic Consequences of Obesity. Eur. J. Haematol. 2021, 106, 306–319. [Google Scholar]
- Haenisch, M.; Nguyen, T.; Fihn, C.A.; Goldstein, A.S.; Amory, J.K.; Treuting, P.; Brabb, T.; Paik, J. Investigation of an ALDH1A1-specific inhibitor for suppression of weight gain in a diet-induced mouse model of obesity. Int. J. Obes. 2021, 45, 1542–1552. [Google Scholar] [CrossRef]
- Omran, Z. Novel Disulfiram Derivatives as ALDH1a1-Selective Inhibitors. Molecules 2022, 27, 480. [Google Scholar] [CrossRef]
- Liu, C.; Qiang, J.; Deng, Q.; Xia, J.; Deng, L.; Zhou, L.; Wang, D.; He, X.; Liu, Y.; Zhao, B.; et al. ALDH1A1 Activity in Tumor-Initiating Cells Remodels Myeloid-Derived Suppressor Cells to Promote Breast Cancer Progression. Cancer Res. 2021, 81, 5919–5934. [Google Scholar] [CrossRef] [PubMed]
- Valerio, C.; Erika, T.; Sandra, D.; Antonio, G.; Lucia, M.; Marina, Z. Stemness marker ALDH1A1 promotes tumor angiogenesis via retinoic acid/HIF-1α/VEGF signalling in MCF-7 breast cancer cells. J. Exp. Clin. Cancer Res. CR 2018, 37, 311. [Google Scholar]
- Nazari, A.; Khorramdelazad, H.; Hassanshahi, G.; Day, A.S.; Sardoo, A.M.; Fard, E.T.; Abedinzadeh, M.; Nadimi, A.E. S100A12 in renal and cardiovascular diseases. Life Sci. 2017, 191, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Ummugulsum, C.; Muammer, B.; Asuman, G.; Adnan, K. Serum Heat Shock Protein 70, S100A12 and Matrix Gla Protein in Childhood Obesity and Metabolic Syndrome. Van Med. J. 2016, 23, 307–312. [Google Scholar]
- Ghosh, S.; Stepicheva, N.; Yazdankhah, M.; Shang, P.; Watson, A.M.; Hose, S.; Liu, H.; Weiss, J.; Zigler, J.S., Jr.; Valapala, M.; et al. The role of lipocalin-2 in age-related macular degeneration (AMD). Cell. Mol. Life Sci. CMLS 2020, 77, 835–851. [Google Scholar] [CrossRef] [PubMed]
- Gupta, U.; Ghosh, S.; Wallace, C.T.; Shang, P.; Xin, Y.; Nair, A.P.; Yazdankhah, M.; Strizhakova, A.; Ross, M.A.; Liu, H.; et al. Increased LCN2 (lipocalin 2) in the RPE decreases autophagy and activates inflammasome-ferroptosis processes in a mouse model of dry AMD. Autophagy 2023, 19, 92–111. [Google Scholar] [CrossRef]
- Jeong, E.A.; Lee, J.; Shin, H.J.; Lee, J.Y.; Kim, K.E.; An, H.S.; Kim, D.R.; Choi, K.Y.; Lee, K.H.; Roh, G.S. Tonicity-responsive enhancer-binding protein promotes diabetic neuroinflammation and cognitive impairment via upregulation of lipocalin-2. J. Neuroinflammation 2021, 18, 278. [Google Scholar] [CrossRef]
- Samuels, J.; Nelson, D.; Ignatowicz, A.; Shashidharamurthy, R.; Rayalam, S. Lipocalin2, an innate immune protein, modulates iNOS and arginase-1 in macrophages to promote the anti-inflammatory function. J. Immunol. 2017, 198, 206.16. [Google Scholar]
- Ghaleb, A.M.; Bialkowska, A.B.; Snider, A.J.; Gnatenko, D.V.; Hannun, Y.A.; Yang, V.W.; Schmidt, V.A. IQ Motif-Containing GTPase-Activating Protein 2 (IQGAP2) Is a Novel Regulator of Colonic Inflammation in Mice. PLoS ONE 2015, 10, e0129314. [Google Scholar] [CrossRef]
- Xu, L.; Shao, Y.; Ren, L.; Liu, X.; Li, Y.; Xu, J.; Ye, Y. IQGAP2 Inhibits Migration and Invasion of Gastric Cancer Cells via Elevating SHIP2 Phosphatase Activity. Int. J. Mol. Sci. 2020, 21, 1968. [Google Scholar] [CrossRef]
- Wang, S.; Song, R.; Wang, Z.; Jing, Z.; Wang, S.; Ma, J. S100A8/A9 in Inflammation. Front. Immunol. 2018, 9, 1298. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; He, H.; Wang, W.; Wang, H.; Mo, S.; Fu, R.; Liu, X.; Song, Q.; Xia, Z.; Wang, L. Malignant clonal evolution drives multiple myeloma cellular ecological diversity and microenvironment reprogramming. Mol. Cancer 2022, 21, 182. [Google Scholar] [CrossRef] [PubMed]
- Meurer, S.K.; Weiskirchen, R. Endoglin: An ‘Accessory’ Receptor Regulating Blood Cell Development and Inflammation. Int. J. Mol. Sci. 2020, 21, 9247. [Google Scholar] [CrossRef] [PubMed]
- Tossetta, G.; Piani, F.; Borghi, C.; Marzioni, D. Role of CD93 in Health and Disease. Cells 2023, 12, 1778. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhu, H.; Gao, F.; Tang, Y.; Zhu, Y.; Sun, Z.; Wang, J. Upregulation of the long non-coding RNA CBR3-AS1 predicts tumor prognosis and contributes to breast cancer progression. Gene 2019, 2, 100014. [Google Scholar] [CrossRef]
- Wang, Y.W.; Cheng, H.L.; Ding, Y.R.; Chou, L.H.; Chow, N.H. EMP1, EMP 2, and EMP3 as novel therapeutic targets in human cancer. Biochim. Biophys. Acta. Rev. Cancer 2017, 1868, 199–211. [Google Scholar] [CrossRef]
- Michaud, D.S.; Siddiq, A.; Cox, D.G.; Backes, D.M.; Calboli, F.C.; Sughrue, M.E.; Gaziano, J.M.; Ma, J.; Stampfer, M.; Tworoger, S.S.; et al. Mannose-binding lectin 2 gene and risk of adult glioma. PLoS ONE 2013, 8, e61117. [Google Scholar] [CrossRef]
- Anczuków, O.; Akerman, M.; Cléry, A.; Wu, J.; Shen, C.; Shirole, N.H.; Raimer, A.; Sun, S.; Jensen, M.A.; Hua, Y.; et al. SRSF1-Regulated Alternative Splicing in Breast Cancer. Mol. Cell 2015, 60, 105–117. [Google Scholar] [CrossRef]
- Liu, Y.J.; Zeng, S.H.; Qian, W.H.; Tao, M.X.; Zhu, Y.Y.; Li, J.P. DNTTIP2 Expression is Associated with Macrophage Infiltration and Malignant Characteristics in Low-Grade Glioma. Pharmacogenomics Pers. Med. 2022, 15, 261–275. [Google Scholar] [CrossRef]
- Muñoz-Garcia, J.; Cochonneau, D.; Télétchéa, S.; Moranton, E.; Lanoe, D.; Brion, R.; Lézot, F.; Heymann, M.F.; Heymann, D. The twin cytokines interleukin-34 and CSF-1: Masterful conductors of macrophage homeostasis. Theranostics 2021, 11, 1568–1593. [Google Scholar] [CrossRef]
- Huang, W.K.; Chen, Y.; Su, H.; Chen, T.Y.; Gao, J.; Liu, Y.; Yeh, C.N.; Li, S. ARHGAP25 Inhibits Pancreatic Adenocarcinoma Growth by Suppressing Glycolysis via AKT/mTOR Pathway. Int. J. Biol. Sci. 2021, 17, 1808–1820. [Google Scholar] [CrossRef]
- Tanouchi, A.; Taniuchi, K.; Furihata, M.; Naganuma, S.; Dabanaka, K.; Kimura, M.; Watanabe, R.; Kohsaki, T.; Shimizu, T.; Saito, M.; et al. CCDC88A, a prognostic factor for human pancreatic cancers, promotes the motility and invasiveness of pancreatic cancer cells. J. Exp. Clin. Cancer Res. 2016, 35, 190. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ge, J.; Li, Y.; Cai, Y.; Zheng, Q.; Huang, N.; Gu, Y.; Han, Q.; Li, Y.; Sun, R.; et al. Integrative lipidomic and transcriptomic study unravels the therapeutic effects of saikosaponins A and D on non-alcoholic fatty liver disease. Acta Pharm. Sin. B 2021, 11, 3527–3541. [Google Scholar] [CrossRef]
- Dean, J.M.; Lodhi, I.J. Structural and functional roles of ether lipids. Protein Cell 2018, 9, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Prentki, M.; Madiraju, S.R. Glycerolipid metabolism and signaling in health and disease. Endocr. Rev. 2008, 29, 647–676. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Z.; Zhang, K.; Zhou, Q.; Chen, S.; Zheng, H.; Wang, G.; Cai, S.; Wang, F.; Li, S. Multi-Omics Characterization of a Glycerolipid Metabolism-Related Gene Enrichment Score in Colon Cancer. Front. Oncol. 2022, 12, 881953. [Google Scholar] [CrossRef]
- Levine, B.; Mizushima, N.; Virgin, H.W. Autophagy in immunity and inflammation. Nature 2011, 469, 323–335. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Y.; Wang, X.; Huang, H.; Bao, J.; Zhong, W.; Li, A. PTEN alleviates maladaptive repair of renal tubular epithelial cells by restoring CHMP2A-mediated phagosome closure. Cell Death Dis. 2021, 12, 1087. [Google Scholar] [CrossRef]
- Xiao, W.L.; Di, Z.; Yulan, J.; Qianhui, Z.; Ying, L.; Wanyu, S.; Yongzhan, B. Salvia miltiorrhiza polysaccharides alleviate florfenicol-induced inflammation and oxidative stress in chick livers by regulating phagosome signaling pathway. Ecotoxicol. Environ. Saf. 2022, 249, 114428. [Google Scholar]
- Alberti, A.; Schuelter-Trevisol, F.; Iser, B.P.M.; Traebert, E.; Freiberger, V.; Ventura, L.; Rezin, G.T.; da Silva, B.B.; Meneghetti Dallacosta, F.; Grigollo, L.; et al. Obesity in people with diabetes in COVID-19 times: Important considerations and precautions to be taken. World J. Clin. Cases 2021, 9, 5358–5371. [Google Scholar] [CrossRef]
- Kawano, Y.; Nakae, J.; Watanabe, N.; Kikuchi, T.; Tateya, S.; Tamori, Y.; Kaneko, M.; Abe, T.; Onodera, M.; Itoh, H. Colonic Pro-inflammatory Macrophages Cause Insulin Resistance in an Intestinal Ccl2/Ccr2-Dependent Manner. Cell Metab. 2016, 24, 295–310. [Google Scholar] [CrossRef]
- Rohm, T.V.; Fuchs, R.; Müller, R.L.; Keller, L.; Baumann, Z.; Bosch, A.J.T.; Schneider, R.; Labes, D.; Langer, I.; Pilz, J.B.; et al. Obesity in Humans Is Characterized by Gut Inflammation as Shown by Pro-Inflammatory Intestinal Macrophage Accumulation. Front. Immunol. 2021, 12, 668654. [Google Scholar] [CrossRef] [PubMed]
- Hannun, Y.A.; Obeid, L.M. Sphingolipids and their metabolism in physiology and disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Trayssac, M.; Hannun, Y.A.; Obeid, L.M. Role of sphingolipids in senescence: Implication in aging and age-related diseases. J. Clin. Investig. 2018, 128, 2702–2712. [Google Scholar] [CrossRef] [PubMed]
- Green, C.D.; Maceyka, M.; Cowart, L.A.; Spiegel, S. Sphingolipids in metabolic disease: The good, the bad, and the unknown. Cell Metab. 2021, 33, 1293–1306. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Ma, J.; Li, M.; Zhang, Y.; Jiang, B.; Zhao, X.; Huai, C.; Shen, L.; Zhang, N.; He, L.; et al. Cytochrome P450 Enzymes and Drug Metabolism in Humans. Int. J. Mol. Sci. 2021, 22, 12808. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.; Binns, D.; Chang, H.Y.; Fraser, M.; Li, W.; McAnulla, C.; McWilliam, H.; Maslen, J.; Mitchell, A.; Nuka, G.; et al. InterProScan 5: Genome-scale protein function classification. Bioinformatics 2014, 30, 1236–1240. [Google Scholar] [CrossRef]
- Huang, W.; Sherman, B.T.; Lempicki, R.A. Bioinformatics enrichment tools: Paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009, 37, 1–13. [Google Scholar] [CrossRef]
- Franceschini, A.; Szklarczyk, D.; Frankild, S.; Kuhn, M.; Simonovic, M.; Roth, A.; Lin, J.; Minguez, P.; Bork, P.; von Mering, C.; et al. STRING v9.1: Protein-protein interaction networks, with increased coverage and integration. Nucleic Acids Res. 2013, 41, D808–D815. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, G.; Shao, J.; Tang, T.; Wang, M.; Wang, J.; Jia, X.; Lai, S. TMT-Based Proteomics Analysis Revealed the Protein Changes in Perirenal Fat from Obese Rabbits. Int. J. Mol. Sci. 2023, 24, 17167. https://doi.org/10.3390/ijms242417167
Jiang G, Shao J, Tang T, Wang M, Wang J, Jia X, Lai S. TMT-Based Proteomics Analysis Revealed the Protein Changes in Perirenal Fat from Obese Rabbits. International Journal of Molecular Sciences. 2023; 24(24):17167. https://doi.org/10.3390/ijms242417167
Chicago/Turabian StyleJiang, Genglong, Jiahao Shao, Tao Tang, Meigui Wang, Jie Wang, Xianbo Jia, and Songjia Lai. 2023. "TMT-Based Proteomics Analysis Revealed the Protein Changes in Perirenal Fat from Obese Rabbits" International Journal of Molecular Sciences 24, no. 24: 17167. https://doi.org/10.3390/ijms242417167
APA StyleJiang, G., Shao, J., Tang, T., Wang, M., Wang, J., Jia, X., & Lai, S. (2023). TMT-Based Proteomics Analysis Revealed the Protein Changes in Perirenal Fat from Obese Rabbits. International Journal of Molecular Sciences, 24(24), 17167. https://doi.org/10.3390/ijms242417167




