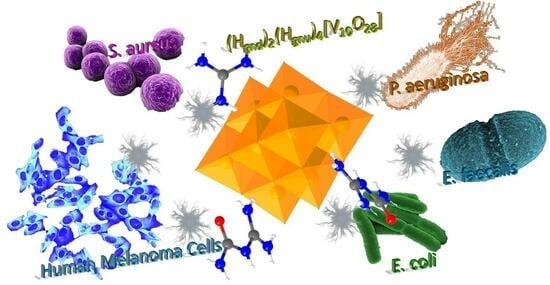
Decavanadate-Bearing Guanidine Derivatives Developed as Antimicrobial and Antitumor Species
Abstract
:1. Introduction
2. Results and Discussion
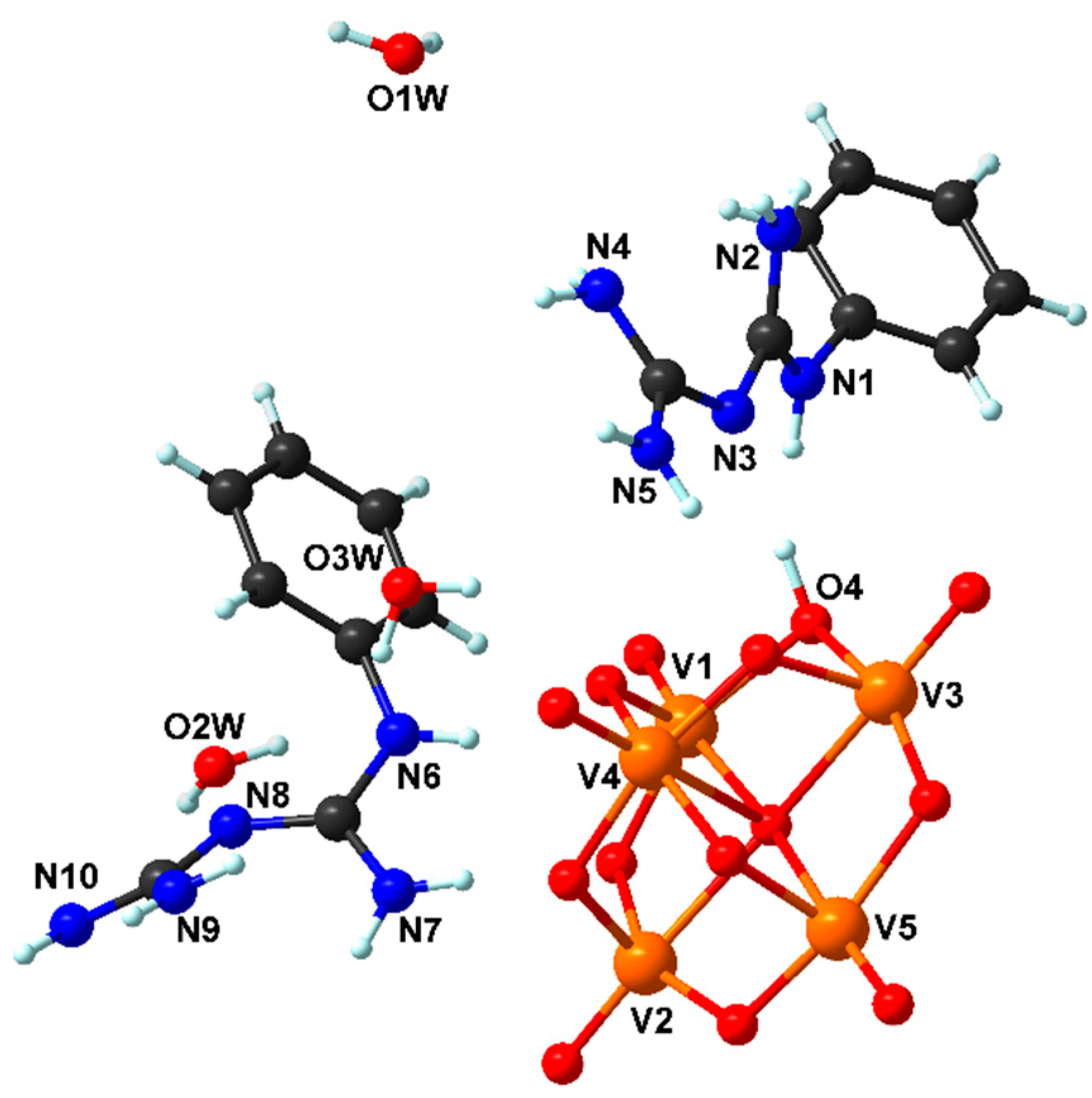
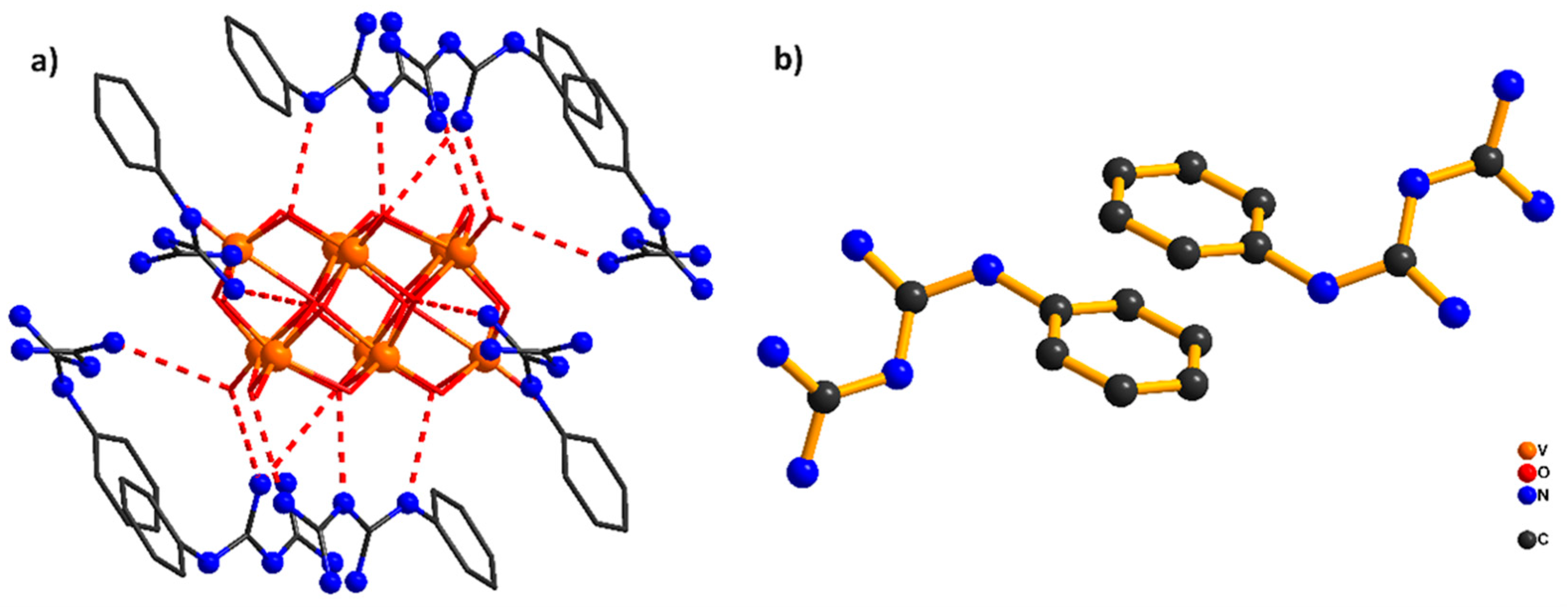
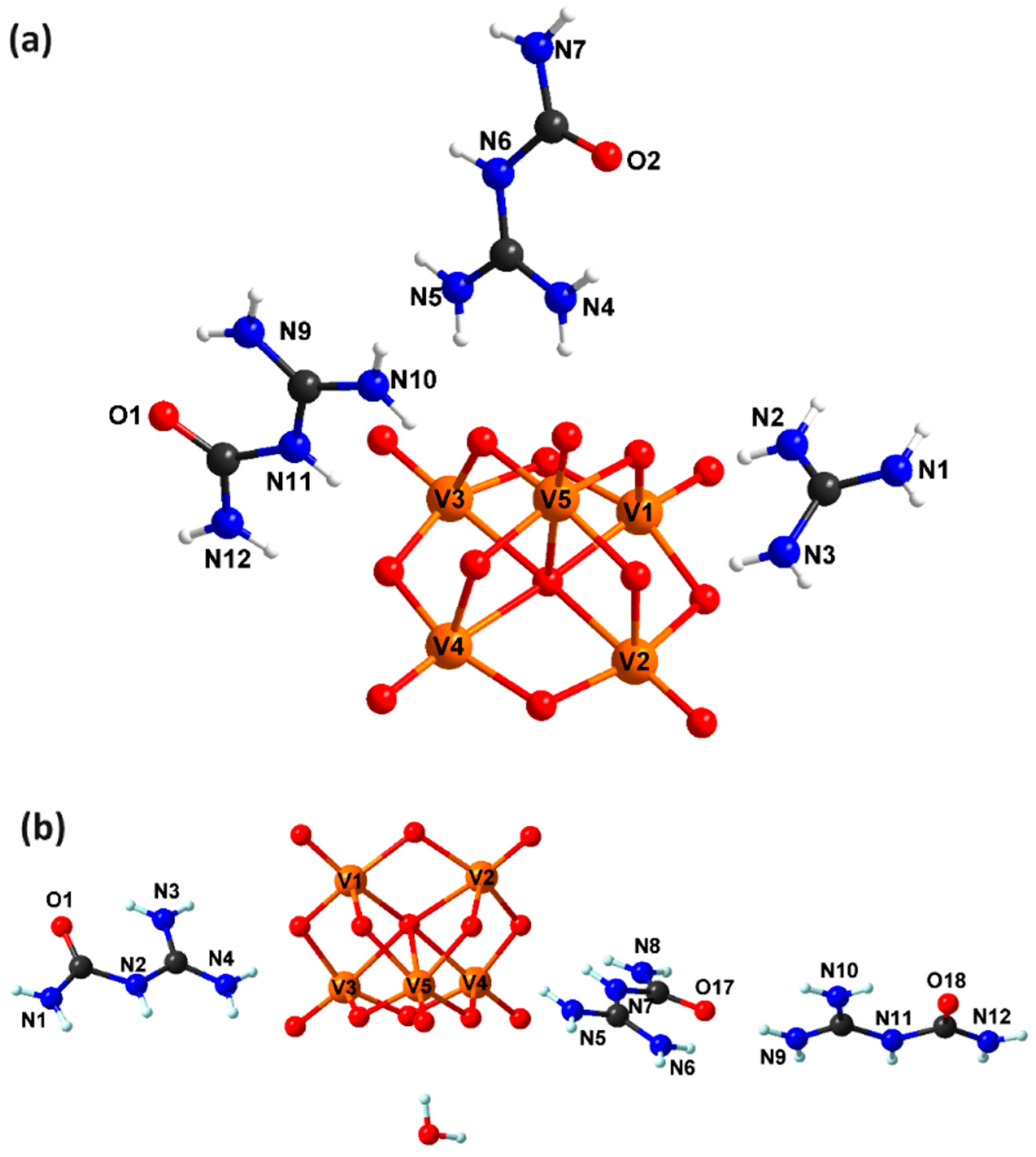
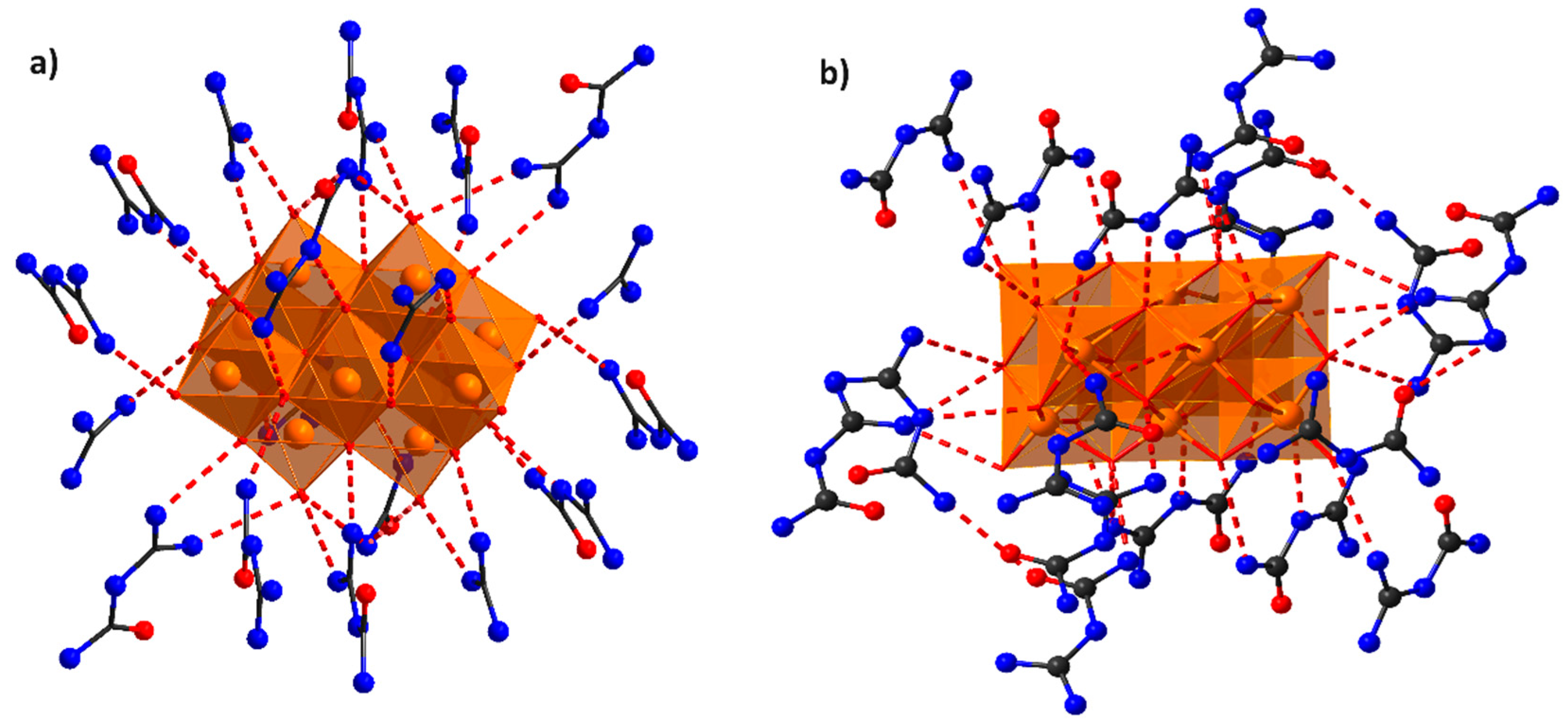
2.1. Description of the Crystal Structure of Compounds
2.2. Physicochemical Characterization of Decavanadates
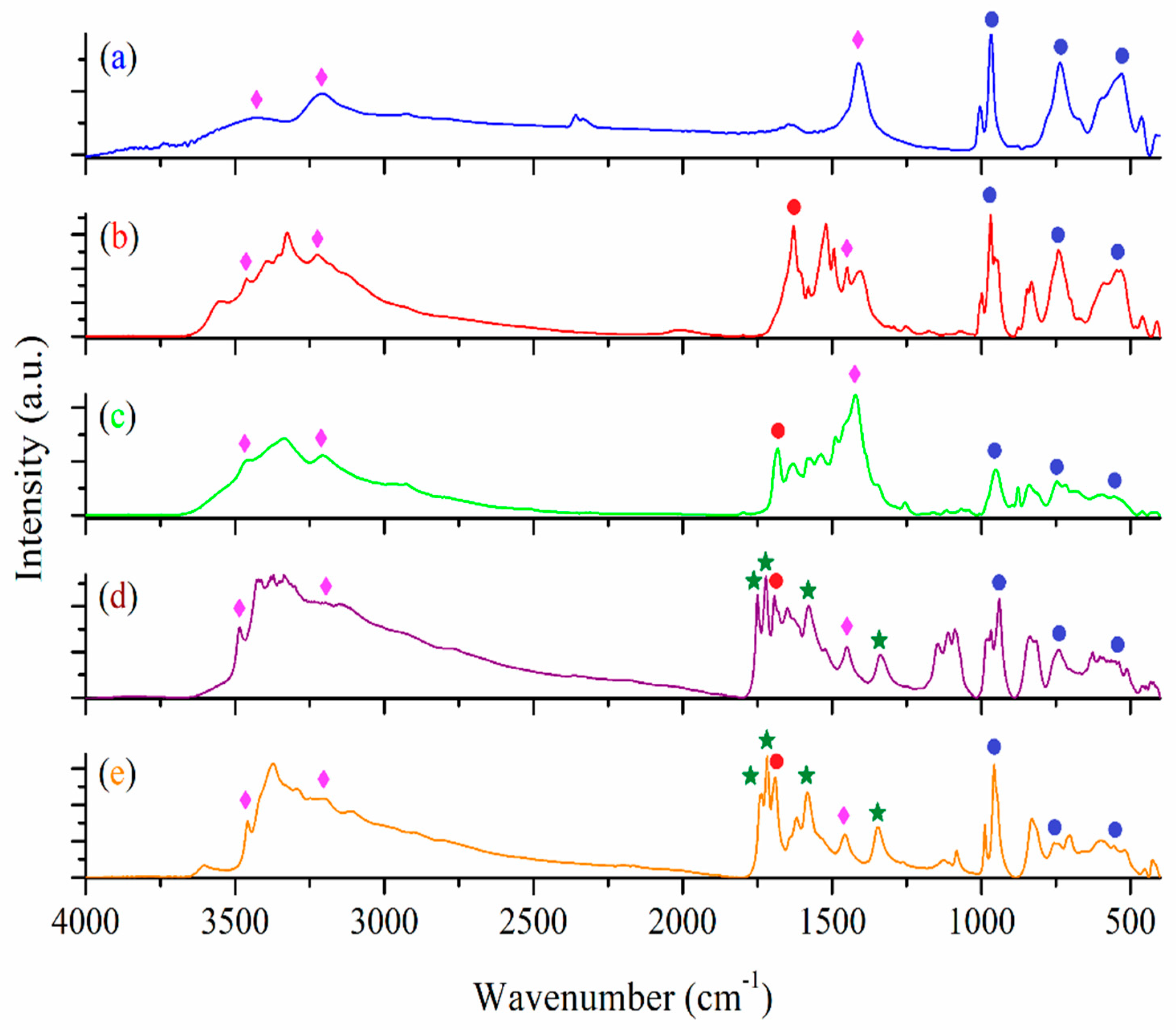
2.2.1. FT-IR Spectra
2.2.2. UV-Vis Spectra
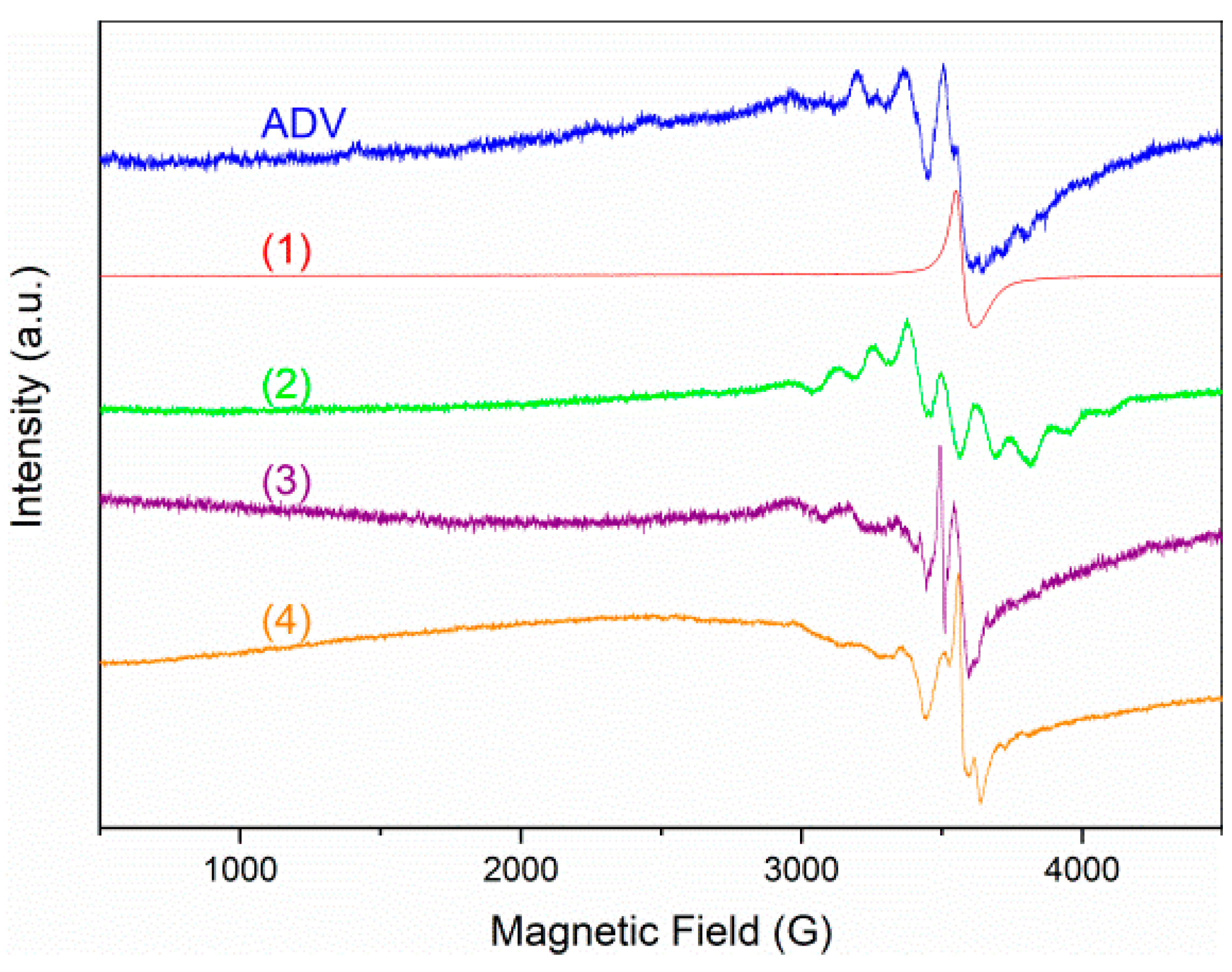
2.2.3. Solid-State EPR Spectroscopy
2.3. Antibacterial and Antitumor Activities
2.3.1. Antibacterial Activity
2.3.2. Antitumor Activity
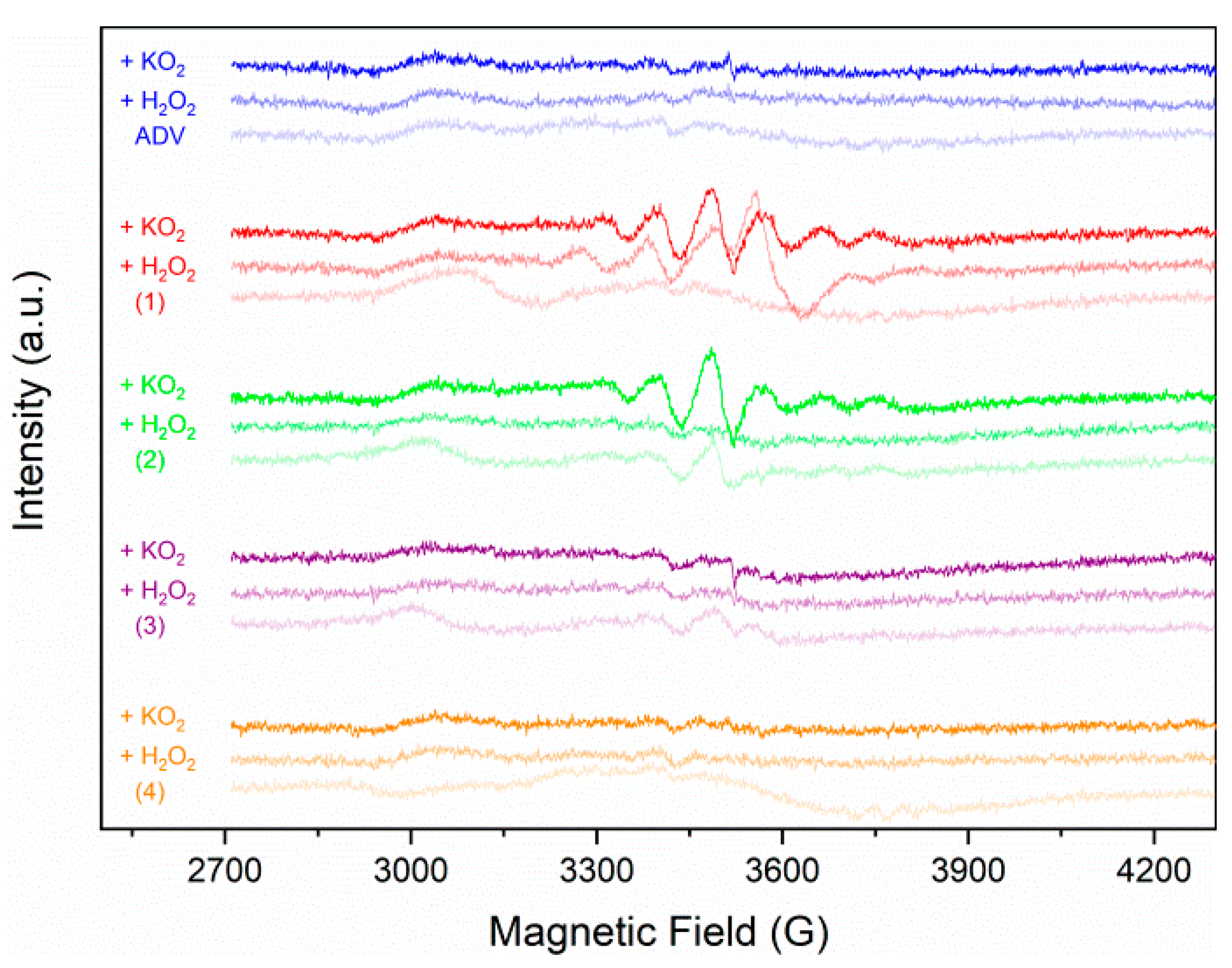
2.3.3. Solution EPR Spectroscopy
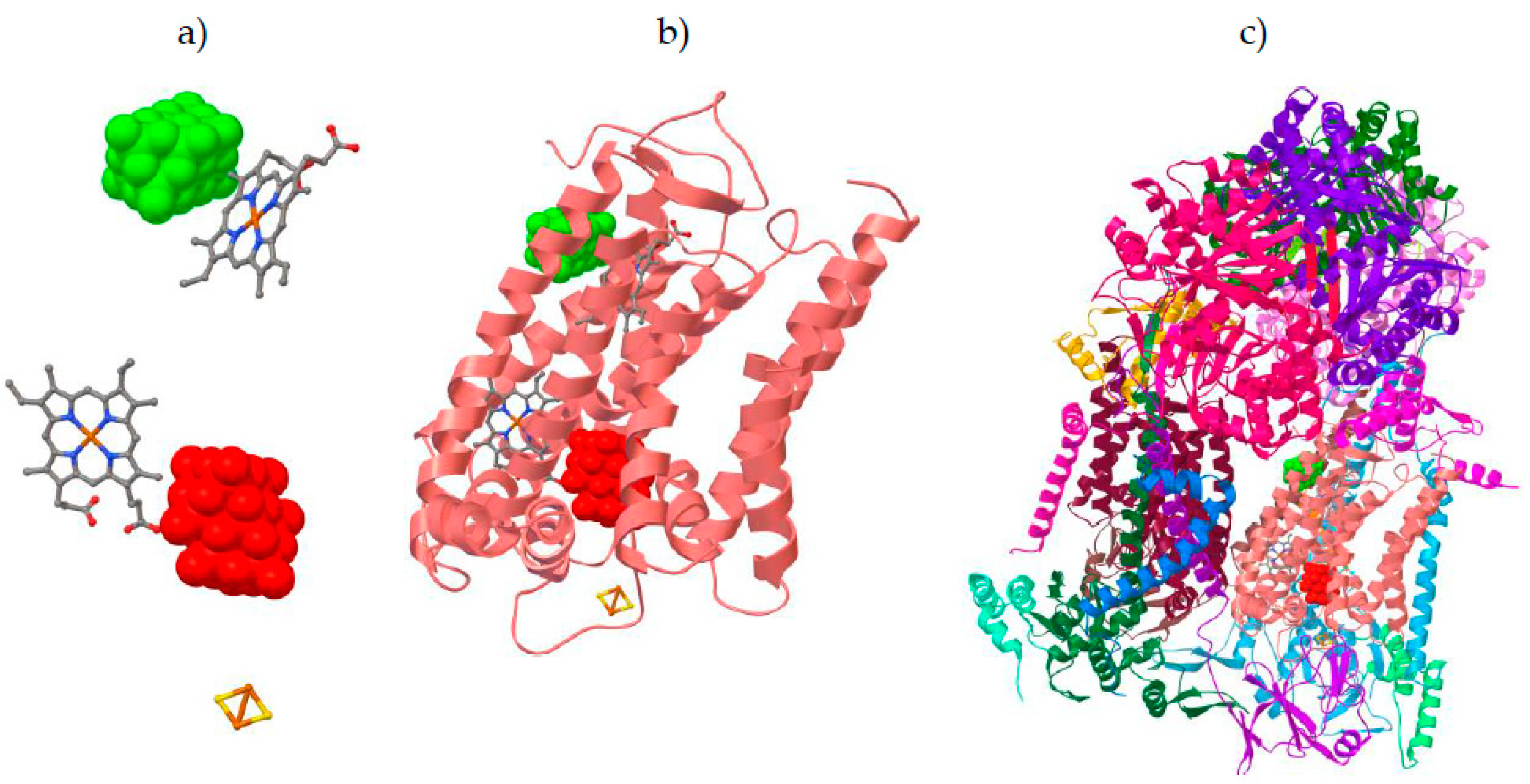
2.4. Molecular Docking Studies
3. Materials and Methods
3.1. Materials and Physical Measurements
3.2. Synthesis of Compounds
3.3. Antibacterial Assay
3.4. In Vitro Cytotoxicity Assay
3.5. Computational Strategy
Docking Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schrader, S.M.; Botella, H.; Vaubourgeix, J. Reframing antimicrobial resistance as a continuous spectrum of manifestations. Curr. Opin. Microbiol. 2023, 72, 102259. [Google Scholar] [CrossRef]
- Rogers, P.D.; Lee, R.E. Editorial overview: Recent advances in antimicrobial drug discovery and resistance. Curr. Opin. Microbiol. 2023, 71, 102242. [Google Scholar] [CrossRef]
- Ye, J.; Chen, X. Current Promising Strategies against Antibiotic-Resistant Bacterial Infections. Antibiotics 2023, 12, 67. [Google Scholar] [CrossRef]
- Olar, R.; Badea, M.; Chifiriuc, M.C. Metal Complexes—A Promising Approach to Target Biofilm Associated Infections. Molecules 2022, 27, 758. [Google Scholar] [CrossRef]
- Mishra, S.; Gupta, A.; Upadhye, V.; Singh, S.C.; Sinha, R.P.; Häder, D.-P. Therapeutic Strategies against Biofilm Infections. Life 2023, 13, 172. [Google Scholar] [CrossRef]
- Diban, F.; Di Lodovico, S.; Di Fermo, P.; D’Ercole, S.; D’Arcangelo, S.; Di Giulio, M.; Cellini, L. Biofilms in Chronic Wound Infections: Innovative Antimicrobial Approaches Using the In Vitro Lubbock Chronic Wound Biofilm Model. Int. J. Mol. Sci. 2023, 24, 1004. [Google Scholar] [CrossRef]
- Paprocka, R.; Wiese-Szadkowska, M.; Janciauskiene, S.; Kosmalski, T.; Kulik, M.; Helmin-Basa, A. Latest developments in metal complexes as anticancer agents. Coord. Chem. Rev. 2022, 452, 214307. [Google Scholar] [CrossRef]
- Lucaciu, R.L.; Hangan, A.C.; Sevastre, B.; Oprean, L.S. Metallo-Drugs in Cancer Therapy: Past, Present and Future. Molecules 2022, 27, 6485. [Google Scholar] [CrossRef] [PubMed]
- Tirsoaga, A.; Cojocaru, V.; Badea, M.; Badea, I.A.; Rostas, A.M.; Stoica, R.; Bacalum, M.; Chifiriuc, M.C.; Olar, R. Copper (II) Species with Improved Anti-Melanoma and Antibacterial Activity by Inclusion in Cyclodextrin. Int. J. Mol. Sci. 2023, 24, 2688. [Google Scholar] [CrossRef] [PubMed]
- Bijelic, A.; Aureliano, M.; Rompel, A. The antibacterial activity of polyoxometalates: Structures, antibiotic effects and future perspectives. Chem. Commun. 2018, 54, 1153–1169. [Google Scholar] [CrossRef]
- Aureliano, M.; Gumerova, N.I.; Sciortino, G.; Garribba, E.; Rompel, A.; Crans, D.C. Polyoxovanadates with emerging biomedical activities. Coord. Chem. Rev. 2021, 447, 214143. [Google Scholar] [CrossRef]
- Wang, X.; Wei, S.; Zhao, C.; Li, X.; Jin, J.; Shi, X.; Su, Z.; Li, J.; Wang, J. Promising application of polyoxometalates in the treatment of cancer, infectious diseases and Alzheimer’s disease. J. Biol. Inorg. Chem. 2022, 27, 405–419. [Google Scholar] [CrossRef]
- Bošnjaković-Pavlović, N.; Prévost, J.; Spasojević-de Biré, A. Crystallographic statistical study of decavanadate anion based-structures: Toward a prediction of noncovalent interactions. Cryst. Growth Des. 2011, 11, 3778–3789. [Google Scholar] [CrossRef]
- Pereira, M.J.; Carvalho, E.; Eriksson, J.W.; Crans, D.C.; Aureliano, M. Effects of decavanadate and insulin enhancing vanadium compounds on glucose uptake in isolated rat adipocytes. J. Inorg. Biochem. 2009, 103, 1687–1692. [Google Scholar] [CrossRef] [PubMed]
- García-Vicente, S.; Yraola, F.; Marti, L.; González-Muñoz, E.; García-Barrado, M.J.; Cantó, C.; Abella, A.; Bour, S.; Artuch, R.; Sierra, C.; et al. Oral insulin-mimetic compounds that act independently of insulin. Diabetes 2007, 56, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Treviño, S.; González-Vergara, E. Metformin-decavanadate treatment ameliorates hyperglycemia and redox balance of the liver and muscle in a rat model of alloxan-induced diabetes. New J. Chem. 2019, 43, 17850–17862. [Google Scholar] [CrossRef]
- Marques-da-Silva, D.; Fraqueza, G.; Lagoa, R.; Vannathan, A.A.; Mal, S.S.; Aureliano, M. Polyoxovanadate inhibition of Escherichia coli growth shows a reverse correlation with Ca2+-ATPase inhibition. New J. Chem. 2019, 43, 17577–17587. [Google Scholar] [CrossRef]
- Abd-Elmonsef Mahmoud, G.; Ibrahim, A.B.M.; Mayer, P. (NH4)2[Ni(H2O)6]2V10O28⋅4H2O; Structural Analysis and Bactericidal Activity against Pathogenic Gram Negative Bacteria. Chem. Select. 2021, 6, 3782–3787. [Google Scholar] [CrossRef]
- Mamdouh, A.-A.; Ibrahim, A.B.M.; Reyad, N.E.-H.A.; Elsayed, T.R.; Santos, I.C.; Paulo, A.; Mahfouz, R.M. (NH4)2[Co(H2O)6]2V10O28·4H2O Vs. (NH4)2[Ni(H2O)6]2V10O28·4H2O: Structural, Spectral and Thermal Analyses and Evaluation of Their Antibacterial Activities. J. Clust. Sci. 2022, 34, 1535–1546. [Google Scholar] [CrossRef]
- Mamdouh, A.-A.; Ibrahim, A.B.M.; Reyad, N.E.-H.A.; Elsayed, T.R.; Cordeiro Santos, I.; Paulo, A.; Mahfouz, R.M. Monoclinic- vs. triclinic-(NH4)2[Mg(H2O)6]2V10O28•4H2O: Structural studies and variation in antibacterial activities with the polymorph type. J. Mol. Struct. 2022, 1253, 132247. [Google Scholar] [CrossRef]
- Jammazi, D.; Ratel-Ramond, N.; Rzaigui, M.; Akriche, S. Trapped mixed [(water)4–(ammonium)44+ octamer in a 3D-binodal (4,8)-connected decavanadate core with hexamethylenetetramine: Synthesis, structure, photophysical and antimicrobial properties. Polyhedron 2019, 168, 146–154. [Google Scholar] [CrossRef]
- Missina, M.; Gavinho, B.; Postal, K.; Santana, F.S.; Valdameri, G.; de Souza, E.M.; Hughes, D.L.; Ramirez, M.I.; Soares, J.F.; Nunes, G.G. Effects of decavanadate salts with organic and inorganic cations on Escherichia Coli, Giardia Intestinalis, and vero cells. Inorg. Chem. 2018, 57, 11930–11941. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.; Sharma, P.K.; Anjuli; Chibber, S.; Siddiqi, Z.A. Isolation of a Decavanadate Cluster [H2V10O28][4-picH]4·2H2O (4-pic = 4-picoline): Crystal Structure, Electrochemical Characterization, Genotoxic and Antimicrobial Studies. J. Clust. Sci. 2014, 25, 1435–1447. [Google Scholar] [CrossRef]
- Samart, N.; Arhouma, Z.; Kumar, S.; Murakami, H.A.; Crick, D.C.; Crans, D.C. Decavanadate inhibits microbacterial growth more potently than other oxovanadates. Front. Chem. 2018, 6, 519. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wu, G.; Long, D.; Liu, Y. Preparation, characterization and antibacterial activity of chitosan–Ca3V10O28 complex membrane. Carbohydr. Polym. 2006, 64, 92–97. [Google Scholar] [CrossRef]
- Zhai, F.; Wang, X.; Li, D.; Zhang, H.; Li, R.; Song, L. Synthesis and biological evaluation of decavanadate Na4Co(H2O)6V10O28·18H2O. Biomed. Pharmacother. 2009, 63, 51–55. [Google Scholar] [CrossRef]
- Ksiksi, R.; Abdelkafi-Koubaa, Z.; Mlayah-Bellalouna, S.; Aissaoui, D.; Marrakchi, N.; Srairi-Abid, N.; Zid, F.M.; Graia, M. Synthesis, structural characterization and antitumoral activity of (NH4)4Li2V10O28.10H2O compound. J. Mol. Struct. 2021, 1229, 129492. [Google Scholar] [CrossRef]
- Louati, M.; Ksiksi, R.; Elbini-Dhouib, I.; Mlayah-Bellalouna, S.; Doghri, R.; Srairi-Abid, N.; Zid, M.-F. Synthesis, structure and characterization of a novel decavanadate, Mg(H2O)6(C4N2H7)4V10O28·4H2O, with a potential antitumor activity. J. Mol. Struct. 2021, 1242, 130711. [Google Scholar] [CrossRef]
- Li, Y.-T.; Zhu, C.-Y.; Wu, Z.-Y.; Jiang, M.; Yan, C.-W. Synthesis, crystal structures and anticancer activities of two decavanadate compounds. Transit. Met. Chem. 2010, 35, 597–603. [Google Scholar] [CrossRef]
- Galani, A.; Tsitsias, V.; Stellas, D.; Psycharis, V.; Raptopoulou, C.P.; Karaliota, A. Two novel compounds of vanadium and molybdenum with carnitine exhibiting potential pharmacological use. J. Inorg. Biochem. 2015, 142, 109–117. [Google Scholar] [CrossRef]
- Kioseoglou, E.; Gabriel, C.; Petanidis, S.; Psycharis, V.; Raptopoulou, C.P.; Terzis, A.; Salifoglou, A. Binary decavanadate-betaine composite materials of potential anticarcinogenic activity. Z. Anorg. Allg. Chem. 2013, 639, 1407–1416. [Google Scholar] [CrossRef]
- De Sousa-Coelho, A.L.; Aureliano, M.; Fraqueza, G.; Serrão, G.; Gonçalves, J.; Sánchez-Lombardo, I.; Link, W.; Ferreira, B.I. Decavanadate and metformin-decavanadate effects in human melanoma cells. J. Inorg. Biochem. 2022, 235, 111915. [Google Scholar] [CrossRef] [PubMed]
- Ksiksi, R.; Essid, A.; Kouka, S.; Boujelbane, F.; Daoudi, M.; Srairi-Abid, N.; Zid, M.F. Synthesis and characterization of a tetra-(benzylammonium) dihydrogen decavanadate dihydrate compound inhibiting MDA-MB-231 human breast cancer cells proliferation and migration. J. Mol. Struct. 2022, 1250, 131929. [Google Scholar] [CrossRef]
- Favre, D.; Harmon, J.F.; Zhang, A.; Miller, M.S.; Kaltashov, I.A. Decavanadate interactions with the elements of the SARS-CoV-2 spike protein highlight the potential role of electrostatics in disrupting the infectivity cycle. J. Inorg. Biochem. 2022, 234, 111899. [Google Scholar] [CrossRef]
- Saczewski, F.; Balewski, Ł. Biological activities of guanidine compounds. Expert Opin. Ther. Pat. 2009, 19, 1417–1448. [Google Scholar] [CrossRef]
- Ji, Y.; Yang, X.; Ji, Z.; Zhu, L.; Ma, N.; Chen, D.; Jia, X.; Tang, J.; Cao, Y. DFT-Calculated IR Spectrum Amide I, II, and III Band Contributions of N-Methylacetamide Fine Components. ACS Omega 2020, 5, 8572–8578. [Google Scholar] [CrossRef]
- Omri, I.; Mhiri, T.; Graia, M. Novel decavanadate cluster complex (HImz)12(V10O28)2·3H2O: Synthesis, characterization, crystal structure, optical and thermal properties. J. Mol. Struct. 2015, 1098, 324–331. [Google Scholar] [CrossRef]
- Magon, C.J.; Lima, J.F.; Donoso, J.P.; Lavayen, V.; Benavente, E.; Navas, D.; Gonzalez, G. Deconvolution of the EPR spectra of vanadium oxide nanotubes. J. Magn. Reson. 2012, 222, 26–33. [Google Scholar] [CrossRef]
- Brückner, A. In situ electron paramagnetic resonance: A unique tool for analyzing structure–reactivity relationships in heterogeneous catalysis. Chem. Soc. Rev. 2010, 39, 4673–4684. [Google Scholar] [CrossRef]
- Suyama, K.; Kesamaru, H.; Okubo, T.; Kasatani, K.; Tomohara, K.; Matsushima, A.; Nose, T. High cytotoxicity of a degraded TBBPA, dibromobisphenol A, through apoptotic and necrosis pathways. Heliyon 2023, 16, e13003. [Google Scholar] [CrossRef]
- Ciorîță, A.; Suciu, M.; Macavei, S.; Kacso, I.; Lung, I.; Soran, M.-L.; Pârvu, M. Green Synthesis of Ag-MnO2 Nanoparticles using Chelidonium majus and Vinca minor Extracts and Their In Vitro Cytotoxicity. Molecules 2020, 25, 819. [Google Scholar] [CrossRef] [PubMed]
- Xia, D.; Esser, L.; Tang, W.-K.; Zhou, F.; Zhou, Y.; Yu, L.; Yu, C.-A. Structural analysis of cytochrome bc1 complexes: Implications to the mechanism of function. Biochimica Biophysica Acta 2013, 1827, 1278–1294. [Google Scholar] [CrossRef] [PubMed]
- Sarewicz, M.; Osyczka, A. Electronic connection between the quinone and cytochrome c redox pools and its role in regulation of mitochondrial electron transport and redox signaling. Physiol. Rev. 2015, 95, 219–243. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Vazquez, E.J.; Moghaddas, S.; Hoppel, C.L.; Lesnefsky, E.J. Production of reactive oxygen species by mitochondria: Central role of complex III. J. Biol. Chem. 2003, 278, 36027–36031. [Google Scholar] [CrossRef] [PubMed]
- Rosell-Hidalgo, A.; Moore, L.A.; Ghafourian, T. Prediction of grug-induced mitochondiral dysfunction using succinate-cytochrome c reductase activity, QSAR and molecular docking. Toxicology 2023, 485, 153412. [Google Scholar] [CrossRef]
- Soares, S.S.; Gutiérrez-Merino, C.; Aureliano, M. Decavanadate induces mitochondrial membrane depolarization and inhibits oxygen consumption. J. Inorg. Biochem. 2007, 101, 789–796. [Google Scholar] [CrossRef]
- Huang, L.S.; Cobessi, D.; Tung, E.; Berry, E.A. Binding of the respiratory chain inhibitor antimycin to the mitochondrial bc1 complex: A new crystal structure reveals an altered intramolecular hydrogen-bonding pattern. J. Mol. Biol. 2005, 351, 573–597. [Google Scholar] [CrossRef]
- Kim, H.J.; Khalimonchuk, O.; Smith, P.M.; Winge, D.R. Structure, function and assembly of heme centers in mitochondrial respiratory complexes. Biochimia Biophysica Acta Mol. Cell Res. 2012, 1823, 1604–1616. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXL-97, Program for Crystal Structure Refinement; University of Göttingen: Göttingen, Germany, 1998. [Google Scholar]
- Nekvapil, F.; Ganea, I.-V.; Ciorîță, A.; Hirian, R.; Ogresta, L.; Glamuzina, B.; Roba, C.; Cintă Pinzaru, S. Wasted Biomaterials from Crustaceans as a Compliant Natural Product Regarding Microbiological, Antibacterial Properties and Heavy Metal Content for Reuse in Blue Bioeconomy: A Preliminary Study. Materials 2021, 14, 4558. [Google Scholar] [CrossRef]
- Walencka, E.; Różalska, S.; Sadowska, B.; Różalska, B. The influence of Lactobacillus acidophilus-derived surfactants on staphylococcal adhesion and biofilm formation. Folia Microbiol. 2008, 53, 61–66. [Google Scholar] [CrossRef]
- Ciorîță, A.; Zăgrean-Tuza, C.; Mot, A.C.; Carpa, R.; Pârvu, M. The Phytochemical Analysis of Vinca L. Species Leaf Extracts Is Correlated with the Antioxidant, Antibacterial, and Antitumor Effects. Molecules 2021, 26, 3040. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J. Comp. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Eberhardt, J.; Santos Martins, D.; Tillack, A.F.; Forli, S. AutoDock Vina 1.2.0: New Docking Methods, Expanded Force Field and Python Bindings. J. Chem. Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef] [PubMed]
- Sanner, M.F. Python: A programming language for software integration and development. J. Mol. Graphics Mod. 1999, 17, 57–61. [Google Scholar] [CrossRef]



| Compound | Absorption Maxima (nm) | |
|---|---|---|
| π→π* | LMCT | |
| ADV | - | 280, 350 |
| (1) | 260 | 390, 450 |
| (2) | 265 | 395, 425 |
| (3) | 215, 280 | 335, 395 |
| (4) | 265 | 365, 450 |
| Cell | ADV | (1) | (2) | (3) | (4) |
|---|---|---|---|---|---|
| IC50 (µM) | |||||
| A375 | 57.5 | 17.6 | 39.01 | 34.6 | 59.6 |
| BJ | 3.63 | 8.04 | 4.43 | 7.21 | 6.56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dumitrescu, A.; Maxim, C.; Badea, M.; Rostas, A.M.; Ciorîță, A.; Tirsoaga, A.; Olar, R. Decavanadate-Bearing Guanidine Derivatives Developed as Antimicrobial and Antitumor Species. Int. J. Mol. Sci. 2023, 24, 17137. https://doi.org/10.3390/ijms242417137
Dumitrescu A, Maxim C, Badea M, Rostas AM, Ciorîță A, Tirsoaga A, Olar R. Decavanadate-Bearing Guanidine Derivatives Developed as Antimicrobial and Antitumor Species. International Journal of Molecular Sciences. 2023; 24(24):17137. https://doi.org/10.3390/ijms242417137
Chicago/Turabian StyleDumitrescu, Andreea, Catalin Maxim, Mihaela Badea, Arpad Mihai Rostas, Alexandra Ciorîță, Alina Tirsoaga, and Rodica Olar. 2023. "Decavanadate-Bearing Guanidine Derivatives Developed as Antimicrobial and Antitumor Species" International Journal of Molecular Sciences 24, no. 24: 17137. https://doi.org/10.3390/ijms242417137
APA StyleDumitrescu, A., Maxim, C., Badea, M., Rostas, A. M., Ciorîță, A., Tirsoaga, A., & Olar, R. (2023). Decavanadate-Bearing Guanidine Derivatives Developed as Antimicrobial and Antitumor Species. International Journal of Molecular Sciences, 24(24), 17137. https://doi.org/10.3390/ijms242417137