Increased Lipogenesis Is Important for Hexavalent Chromium-Transformed Lung Cells and Xenograft Tumor Growth
Abstract
1. Introduction
2. Results
2.1. Chromium(VI)-Transformed BEAS-2B Cells Have Increased Lipogenesis and Related Proteins
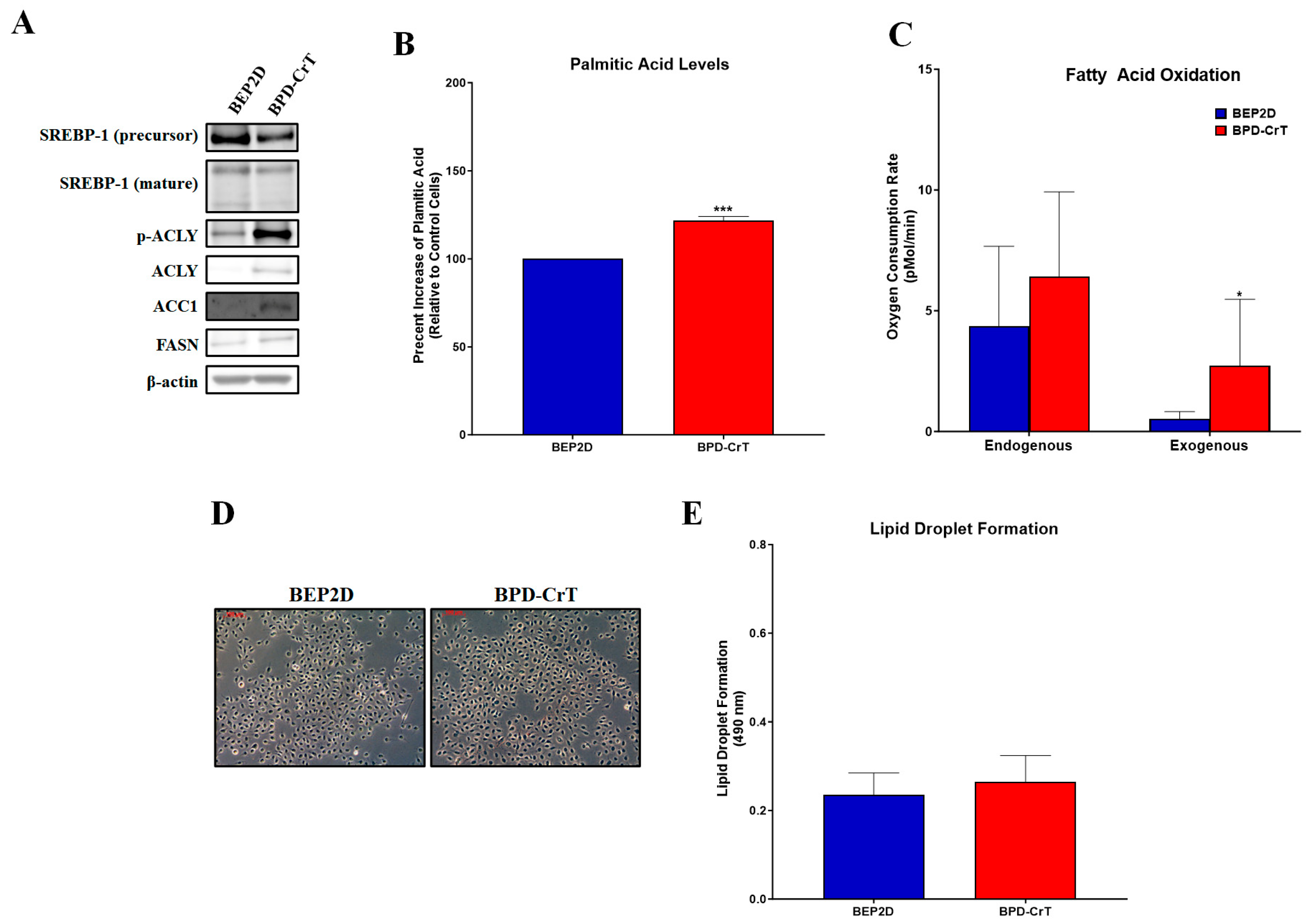
2.2. Chromium(VI)-Transformed BEP2D Cells Have Increased Lipogenesis and Related Proteins
2.3. Chromium(VI)-Transformed WTHBF-6 Cells Have Increased Lipogenesis and Related Proteins
2.4. Increased FASN Expression Is Important for Chromium(VI)-Transformed Cell Survival
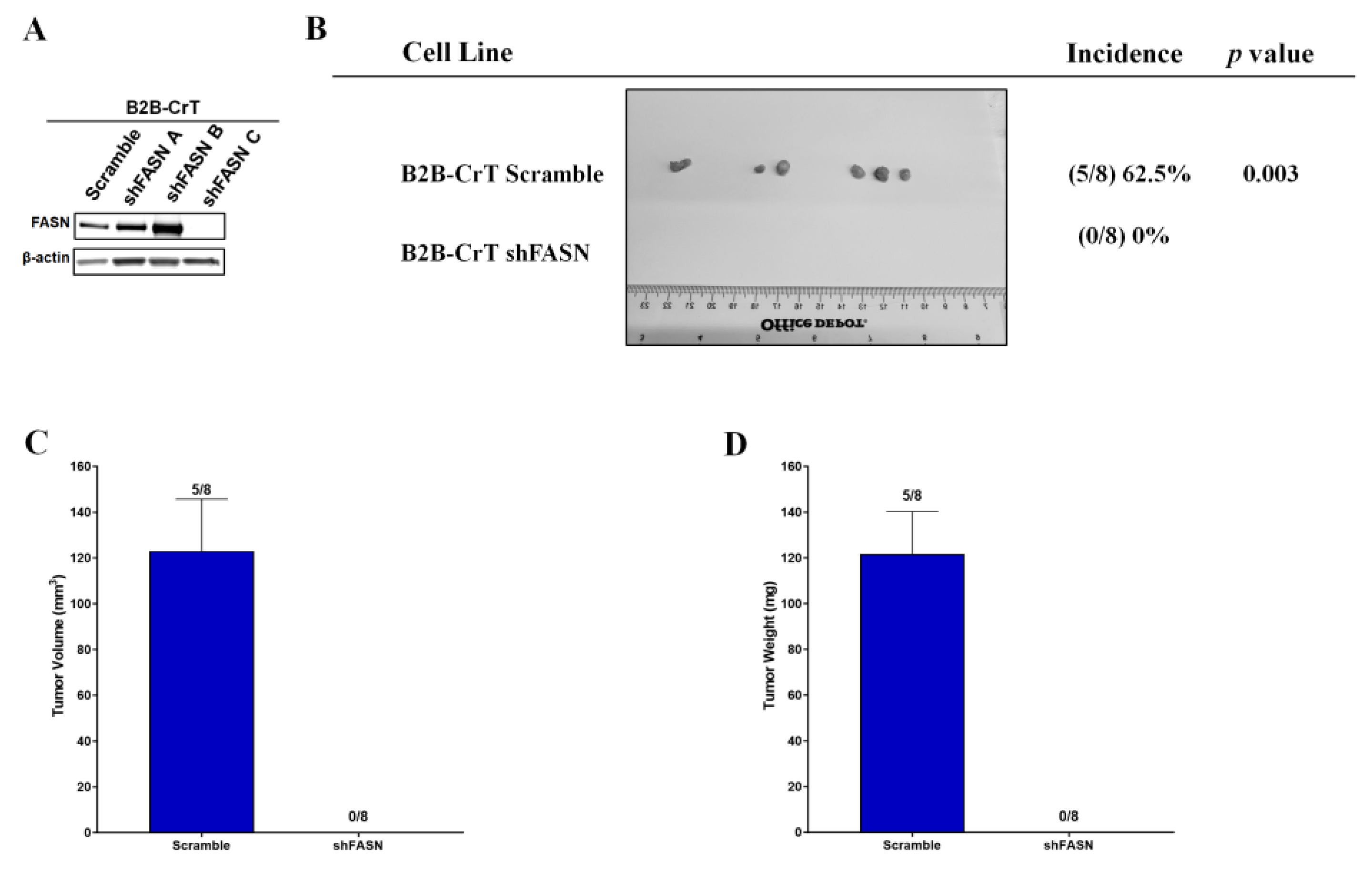
2.5. Increased FASN Expression Is Important for Chromium(VI)-Transformed Cell Tumorigenesis
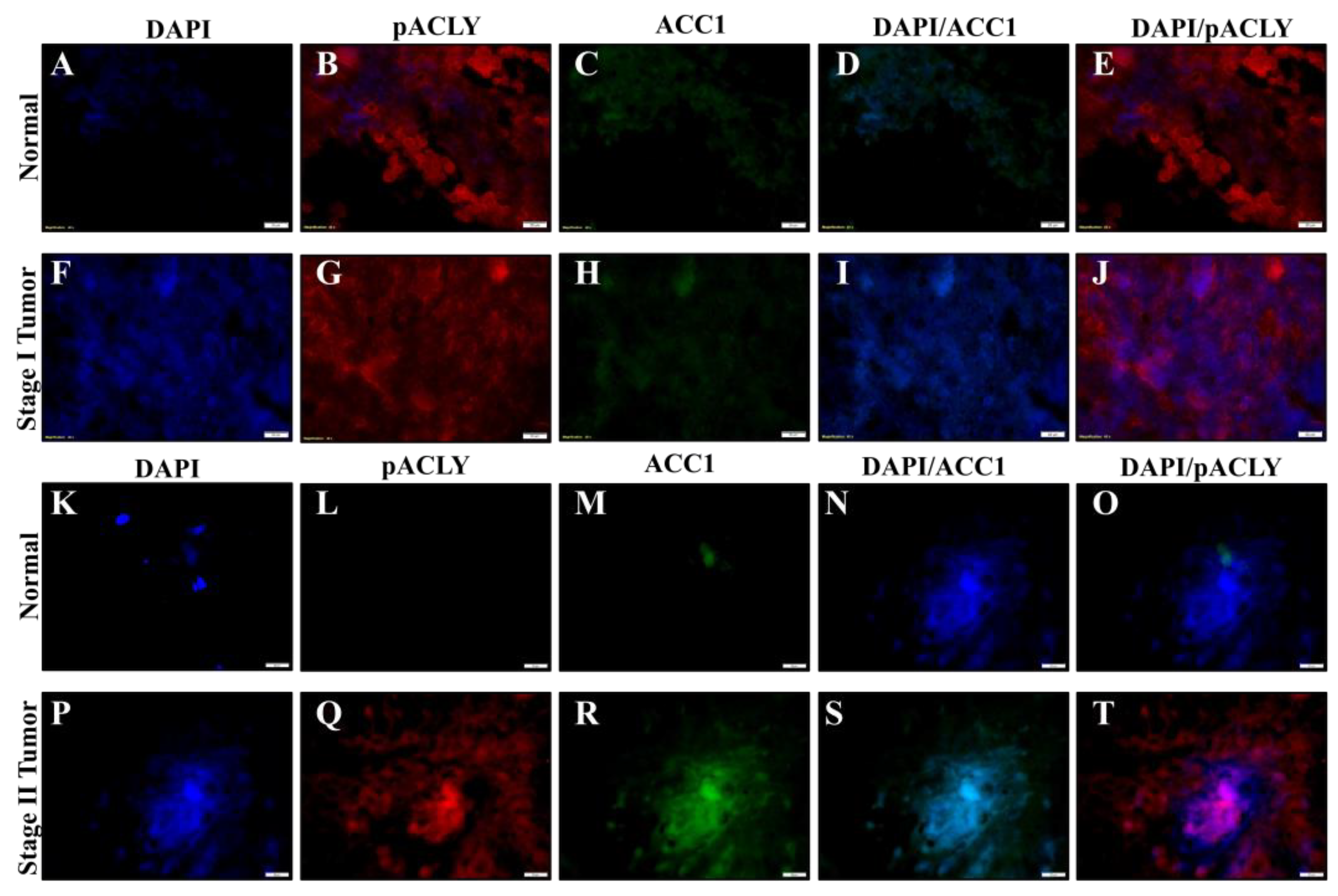
2.6. Chromium(VI)-Induced Lung Tumors Have Increased Lipogenesis Protein Expressions
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Antibodies
4.3. Cell Culture
4.4. Chromium(VI)-Transformed Cells
4.5. Seahorse Extracellular Flux Analysis
4.6. Soft Agar Assay
4.7. Growth Curve Analysis
4.8. Drug Compound Treatments
4.9. Palmitic Acid Levels
4.10. Protein Preparation and Quantification
4.11. Oil Red O Staining
4.12. Western Blot Analysis
4.13. shRNA Transfection
4.14. Xenograft Studies
4.15. Patient Lung Sample Collection
4.16. Immunofluorescence
4.17. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, D.; Dai, J.; Fai, L.Y.; Yao, H.; Son, Y.O.; Wang, L.; Pratheeshkumar, P.; Kondo, K.; Shi, X.; Zhang, Z. Constitutive activation of epidermal growth factor receptor promotes tumorigenesis of Cr(VI)-transformed cells through decreased reactive oxygen species and apoptosis resistance development. J. Biol. Chem. 2015, 290, 2213–2224. [Google Scholar] [CrossRef]
- Tsuneta, Y.; Ohsaki, Y.; Kiyonobu, K.; Mikami, H.; Abe, S.; Murao, M. Chromium content of lungs of chromate workers with lung cancer. Thorax 1980, 35, 294–297. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Son, Y.O.; Chang, Q.; Sun, L.; Hitron, J.A.; Budhraja, A.; Zhang, Z.; Ke, Z.; Chen, F.; Luo, J.; et al. NADPH oxidase activation is required in reactive oxygen species generation and cell transformation induced by hexavalent chromium. Toxicol. Sci. 2011, 123, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Holmes, A.L.; Wise, S.S.; Huang, S.; Peng, C.; Wise, J.P., Sr. Neoplastic transformation of human bronchial cells by lead chromate particles. Am. J. Respir. Cell Mol. Biol. 2007, 37, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Clementino, M.; Xie, J.; Yang, P.; Li, Y.; Lin, H.P.; Fenske, W.K.; Tao, H.; Kondo, K.; Yang, C.; Wang, Z. A Positive Feedback Loop Between c-Myc Upregulation, Glycolytic Shift, and Histone Acetylation Enhances Cancer Stem Cell-like Property and Tumorigenicity of Cr(VI)-transformed Cells. Toxicol. Sci. 2020, 177, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wise, J.T.F.; Zhang, Z.; Shi, X. Progress and prospects of free radicals in metal-induced carcinogenesis. Curr. Pharmacol. Rep. 2016, 2, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Wise, S.S.; Elmore, L.W.; Holt, S.E.; Little, J.E.; Antonucci, P.G.; Bryant, B.H.; Wise, J.P., Sr. Telomerase-mediated lifespan extension of human bronchial cells does not affect hexavalent chromium-induced cytotoxicity or genotoxicity. Mol. Cell Biochem. 2004, 255, 103–111. [Google Scholar] [CrossRef]
- Wise, S.S.; Holmes, A.L.; Wise, J.P., Sr. Hexavalent chromium-induced DNA damage and repair mechanisms. Rev. Environ. Health 2008, 23, 39–57. [Google Scholar] [CrossRef]
- Abreu, P.L.; Ferreira, L.M.R.; Alpoim, M.C. Impact of hexavalent chromium on mammalian cell bioenergetics: Phenotypic changes, molecular basis and potential relevance to chromate-induced lung cancer. Biometals 2014, 27, 409–443. [Google Scholar] [CrossRef]
- Cerveira, J.F.; Sánchez-Aragó, M.; Urbano, A.M.; Cuezva, J.M. Short-term exposure of nontumorigenic human bronchial epithelial cells to carcinogenic chromium(VI) compromises their respiratory capacity and alters their bioenergetic signature. FEBS Open Bio. 2014, 26, 594–601. [Google Scholar] [CrossRef]
- Guo, L.; Xiao, Y.; Wang, Y. Hexavalent chromium-induced alteration of proteomic landscape in human skin fibroblast cells. J. Proteome Res. 2013, 12, 3511–3518. [Google Scholar] [CrossRef]
- Wise, J.T.F.; Wang, L.; Alstott, M.C.; Ngalame, N.N.O.; Wang, Y.; Zhang, Z.; Shi, X. Investigating the Role of Mitochondrial Respiratory Dysfunction during Hexavalent Chromium-Induced Lung Carcinogenesis. J. Environ. Pathol. Toxicol. Oncol. 2018, 37, 317–329. [Google Scholar] [CrossRef]
- Roy, R.V.; Pratheeshkumar, P.; Son, Y.O.; Wang, L.; Hitron, J.A.; Divya, S.P.; Zhang, Z.; Shi, X. Different roles of ROS and Nrf2 in Cr(VI)-induced inflammatory responses in normal and Cr(VI)-transformed cells. Toxicol. Appl. Pharmacol. 2016, 307, 81–90. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Warburg, O.; Wind, F.; Negelein, E. The metabolism of tumors in the body. J. Gen. Physiol. 1927, 8, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Warburg, O. On the origin of cancer cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef]
- Warburg, O. On respiratory impairment in cancer cells. Science 1956, 124, 269–270. [Google Scholar] [CrossRef]
- Otto, A.M. Warburg effect(s)-a biographical sketch of Otto Warburg and his impacts on tumor metabolism. Cancer Metab. 2016, 4, 5. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Suo, C.; Li, S.T.; Zhang, H.; Gao, P. Metabolic reprogramming for cancer cells and their microenvironment: Beyond the Warburg Effect. Biochim. Biophys. Acta. Rev. Cancer 2018, 1870, 51–66. [Google Scholar] [CrossRef]
- Santos, C.R.; Schulze, A. Lipid metabolism in cancer. FEBS J. 2012, 279, 2610–2623. [Google Scholar] [CrossRef]
- Menendez, J.A.; Lupu, R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat. Rev. Cancer 2007, 7, 762–777. [Google Scholar] [CrossRef] [PubMed]
- Migita, T.; Narita, T.; Nomura, K.; Miyagi, E.; Inazuka, F.; Matsuura, M.; Ushijima, M.; Mashima, T.; Seimiya, H.; Satoh, Y.; et al. ATP citrate lyase: Activation and therapeutic implications in non-small cell lung cancer. Cancer Res. 2008, 68, 8547–8554. [Google Scholar] [CrossRef] [PubMed]
- Orita, H.; Coulter, J.; Lemmon, C.; Tully, E.; Vadlamudi, A.; Medghalchi, S.M.; Kuhajda, F.P.; Gabrielson, E. Selective inhibition of fatty acid synthase for lung cancer treatment. Clin. Cancer Res. 2007, 13, 7139–7145. [Google Scholar] [CrossRef] [PubMed]
- Osugi, J.; Yamaura, T.; Muto, S.; Okabe, N.; Matsumura, Y.; Hoshino, M.; Higuchi, M.; Suzuki, H.; Gotoh, M. Prognostic impact of the combination of glucose transporter 1 and ATP citrate lyase in node-negative patients with non-small lung cancer. Lung Cancer 2015, 88, 310–318. [Google Scholar] [CrossRef]
- Piyathilake, C.J.; Frost, A.R.; Manne, U.; Bell, W.C.; Weiss, H.; Heimburger, D.C.; Grizzle, W.E. The expression of fatty acid synthase (FASE) is an early event in the development and progression of squamous cell carcinoma of the lung. Hum. Pathol. 2000, 31, 1068–1073. [Google Scholar] [CrossRef]
- Zaytseva, Y.Y.; Harris, J.W.; Mitov, M.I.; Kim, J.T.; Butterfield, D.A.; Lee, E.Y.; Weiss, H.L.; Gao, T.; Evers, B.M. Increased expression of fatty acid synthase provides a survival advantage to colorectal cancer cells via upregulation of cellular respiration. Oncotarget 2015, 6, 18891–18904. [Google Scholar] [CrossRef]
- Jin, X.; Zhang, K.J.; Guo, X.; Myers, R.; Ye, Z.; Zhang, Z.P.; Li, X.F.; Yang, H.S.; Xing, J.L. Fatty acid synthesis pathway genetic variants and clinical outcome of non-small cell lung cancer patients after surgery. Asian Pac. J. Cancer Prev. 2014, 15, 7097–7103. [Google Scholar] [CrossRef]
- Visca, P.; Sebastiani, V.; Botti, C.; Diodoro, M.G.; Lasagni, R.P.; Romagnoli, F.; Brenna, A.; De Joannon, B.C.; Donnorso, R.P.; Lombardi, G.; et al. Fatty acid synthase (FAS) is a marker of increased risk of recurrence in lung carcinoma. Anticancer Res. 2004, 24, 4169–4173. [Google Scholar]
- Kitteringham, N.R.; Abdullah, A.; Walsh, J.; Randle, L.; Jenkins, R.E.; Sison, R.; Goldring, C.E.; Powell, H.; Sanderson, C.; Williams, S.; et al. Proteomic analysis of Nrf2 deficient transgenic mice reveals cellular defence and lipid metabolism as primary Nrf2-dependent pathways in the liver. J. Proteom. 2008, 73, 1612–1631. [Google Scholar] [CrossRef]
- Swinnen, J.V.; Brusselmans, K.; Verhoeven, G. Increased lipogenesis in cancer cells: New players, novel targets. Curr. Opin. Clin. Nutr. Metab. Care 2006, 9, 358–365. [Google Scholar] [CrossRef]
- Little, J.L.; Kridel, S.J. Fatty acid synthase activity in tumor cells. Subcell. Biochem. 2008, 49, 169–194. [Google Scholar] [PubMed]
- Baud, S.; Lepiniec, L. Regulation of de novo fatty acid synthesis in maturing oilseeds of Arabidopsis. Plant Physiol. Biochem. 2009, 47, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Bu, S.Y.; Mashek, M.T.; Mashek, D.G. Suppression of long-chain acyl-CoA synthetase 3 decreases hepatic de novo fatty acid synthesis through decreased transcriptional activity. J. Biol. Chem. 2009, 284, 30474–30483. [Google Scholar] [CrossRef] [PubMed]
- López, M.; Lelliott, C.J.; Vidal-Puig, A. Hypothalamic fatty acid metabolism: A housekeeping pathway that regulates food intake. Bioessays 2007, 29, 248–261. [Google Scholar] [CrossRef] [PubMed]
- Mashima, T.; Seimiya, H.; Tsuruo, T. De novo fatty-acid synthesis and related pathways as molecular targets for cancer therapy. Br. J. Cancer 2009, 100, 1369–1372. [Google Scholar] [CrossRef] [PubMed]
- Suagee, J.K.; Corl, B.A.; Crisman, M.V.; Wearn, J.G.; McCutcheon, L.J.; Geor, R.J. De novo fatty acid synthesis and NADPH generation in equine adipose and liver tissue. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2010, 155, 322–326. [Google Scholar] [CrossRef]
- Tarun, A.S.; Vaughan, A.M.; Kappe, S.H. Redefining the role of de novo fatty acid synthesis in Plasmodium parasites. Trends Parasitol. 2009, 25, 545–550. [Google Scholar] [CrossRef]
- Brusselmans, K.; Vrolix, R.; Verhoeven, G.; Swinnen, J.V. Induction of Cancer Cell Apoptosis by Flavonoids Is Associated with Their Ability to Inhibit Fatty Acid Synthase Activity. J. Biol. Chem. 2005, 280, 5636–5645. [Google Scholar] [CrossRef]
- Corominas-Faja, B.; Cuyàs, E.; Gumuzio, J.; Bosch-Barrera, J.; Leis, O.; Martin, Á.G.; Menendez, J.A. Chemical inhibition of acetyl-CoA carboxylase suppresses self-renewal growth of cancer stem cells. Oncotarget 2014, 5, 8306–8316. [Google Scholar] [CrossRef]
- Migita, T.; Okabe, S.; Ikeda, K.; Igarashi, S.; Sugawara, S.; Tomida, A.; Soga, T.; Taguchi, R.; Seimiya, H. Inhibition of ATP citrate lyase induces triglyceride accumulation with altered fatty acid composition in cancer cells. Int. J. Cancer 2014, 135, 37–47. [Google Scholar] [CrossRef]
- Relat, J.; Blancafort, A.; Oliveras, G.; Cufí, S.; Haro, D.; Marrero, P.F.; Puig, T. Different fatty acid metabolism effects of (-)-epigallocatechin-3-gallate and C75 in adenocarcinoma lung cancer. BMC Cancer 2012, 6, 280. [Google Scholar] [CrossRef]
- Xin, M.; Qiao, Z.; Li, J.; Liu, J.; Song, S.; Zhao, X.; Miao, P.; Tang, T.; Wang, L.; Liu, W.; et al. miR-22 inhibits tumor growth and metastasis by targeting ATP citrate lyase: Evidence in osteosarcoma, prostate cancer, cervical cancer and lung cancer. Oncotarget 2016, 7, 44252–44265. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, C.; Du, H.; Huang, V.; Sun, B.; Harris, J.P.; Richardson, Q.; Shen, X.; Jin, R.; Li, G.; et al. Withaferin A suppresses the up-regulation of acetyl-coA carboxylase 1 and skin tumor formation in a skin carcinogenesis mouse model. Mol. Carcinog. 2016, 55, 1739–1746. [Google Scholar] [CrossRef]
- Yang, Y.A.; Han, W.F.; Morin, P.J.; Chrest, F.J.; Pizer, E.S. Activation of fatty acid synthesis during neoplastic transformation: Role of mitogen-activated protein kinase and phosphatidylinositol 3-kinase. Exp. Cell Res. 2002, 279, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Conde, E.; Suarez-Gauthier, A.; García-García, E.; Lopez-Rios, F.; Lopez-Encuentra, A.; García-Lujan, R.; Morente, M.; Sanchez-Verde, L.; Sanchez-Cespedes, M. Specific pattern of LKB1 and phospho-acetyl-CoA carboxylase protein immunostaining in human normal tissues and lung carcinomas. Hum. Pathol. 2007, 38, 1351–1360. [Google Scholar] [CrossRef]
- Hess, D.; Igal, R.A. Genistein downregulates de novo lipid synthesis and impairs cell proliferation in human lung cancer cells. Exp. Biol. Med. 2011, 236, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Levantini, E.; Teo, J.T.; Goggi, J.; Clohessy, J.G.; Wu, C.S.; Chen, P.L.; Yang, H.; Krishnan, I.; Kocher, O.; et al. Fatty acid synthase mediates EGFR palmitoylation in EGFR mutated non-small cell lung cancer. EMBO Mol. Med. 2018, 2018, e8313. [Google Scholar] [CrossRef]
- Bollu, L.R.; Katreddy, R.R.; Blessing, A.M.; Pham, N.; Zheng, B.; Wu, X.; Weihua, Z. Intracellular activation of EGFR by fatty acid synthase dependent palmitoylation. Oncotarget 2015, 6, 34992–35003. [Google Scholar] [CrossRef]
- Ke, Y.; Reddel, R.R.; Gerwin, B.I.; Miyashita, M.; McMenamin, M.; Lechner, J.F.; Harris, C.C. Human bronchial epithelial cells with integrated SV40 virus T antigen genes retain the ability to undergo squamous differentiation. Differentiation 1988, 38, 60–66. [Google Scholar] [CrossRef]
- Willey, J.C.; Broussoud, A.; Sleemi, A.; Bennett, W.P.; Cerutti, P.; Harris, C.C. Immortalization of normal human bronchial epithelial cells by human papillomaviruses 16 or 18. Cancer Res. 1991, 51, 5370–5377. [Google Scholar]
- Ewis, A.A.; Kondo, K.; Lee, J.; Tsuyuguchi, M.; Hashimoto, M.; Yokose, T.; Mukai, K.; Kodama, T.; Shinka, T.; Monden, Y.; et al. Occupational cancer genetics: Infrequent ras oncogenes point mutations in lung cancer samples from chromate workers. Am. J. Ind. Med. 2001, 40, 92–97. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wise, J.T.F.; Kondo, K. Increased Lipogenesis Is Important for Hexavalent Chromium-Transformed Lung Cells and Xenograft Tumor Growth. Int. J. Mol. Sci. 2023, 24, 17060. https://doi.org/10.3390/ijms242317060
Wise JTF, Kondo K. Increased Lipogenesis Is Important for Hexavalent Chromium-Transformed Lung Cells and Xenograft Tumor Growth. International Journal of Molecular Sciences. 2023; 24(23):17060. https://doi.org/10.3390/ijms242317060
Chicago/Turabian StyleWise, James T. F., and Kazuya Kondo. 2023. "Increased Lipogenesis Is Important for Hexavalent Chromium-Transformed Lung Cells and Xenograft Tumor Growth" International Journal of Molecular Sciences 24, no. 23: 17060. https://doi.org/10.3390/ijms242317060
APA StyleWise, J. T. F., & Kondo, K. (2023). Increased Lipogenesis Is Important for Hexavalent Chromium-Transformed Lung Cells and Xenograft Tumor Growth. International Journal of Molecular Sciences, 24(23), 17060. https://doi.org/10.3390/ijms242317060





