Effects of Elevated Temperature on Pisum sativum Nodule Development: II—Phytohormonal Responses
Abstract
1. Introduction
2. Results
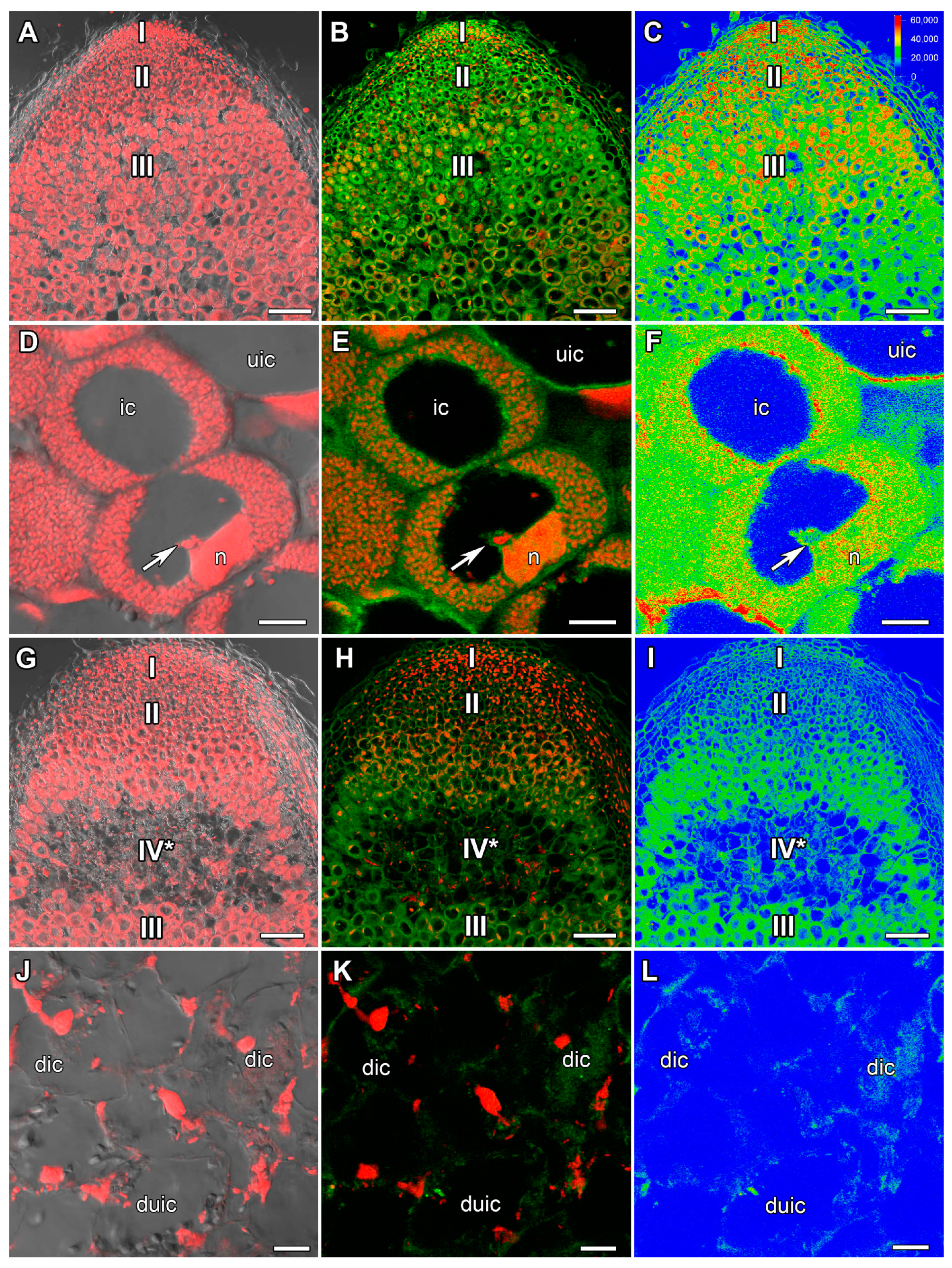
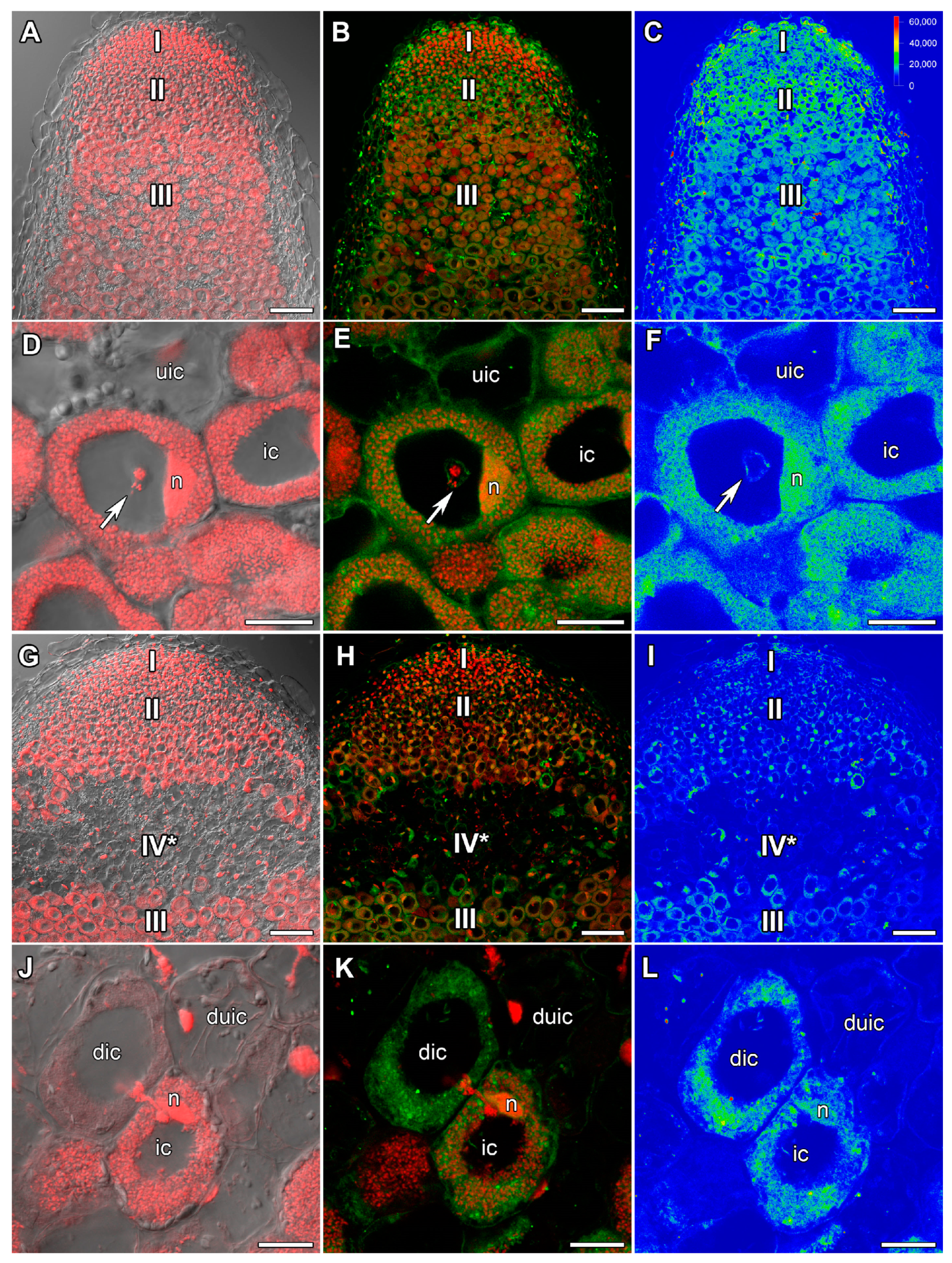
2.1. ABA Immunolocalization
2.2. ACC Immunolocalization
2.3. GA3 Immunolocalization
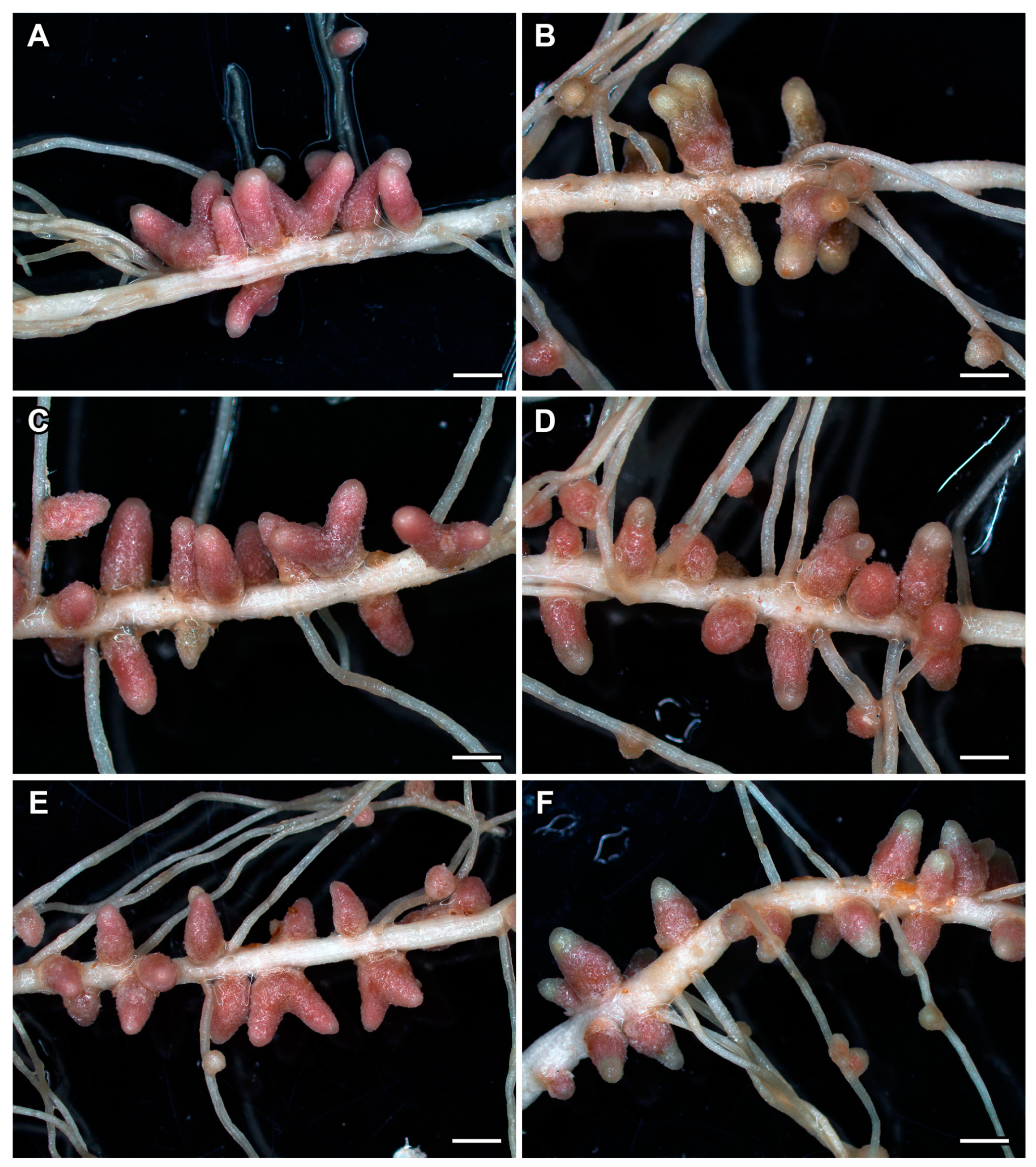
2.4. Nodule Phenotype in Pea Plants of the Line SGE Exposed to Elevated Temperature in Combination with Pharmacological Treatment
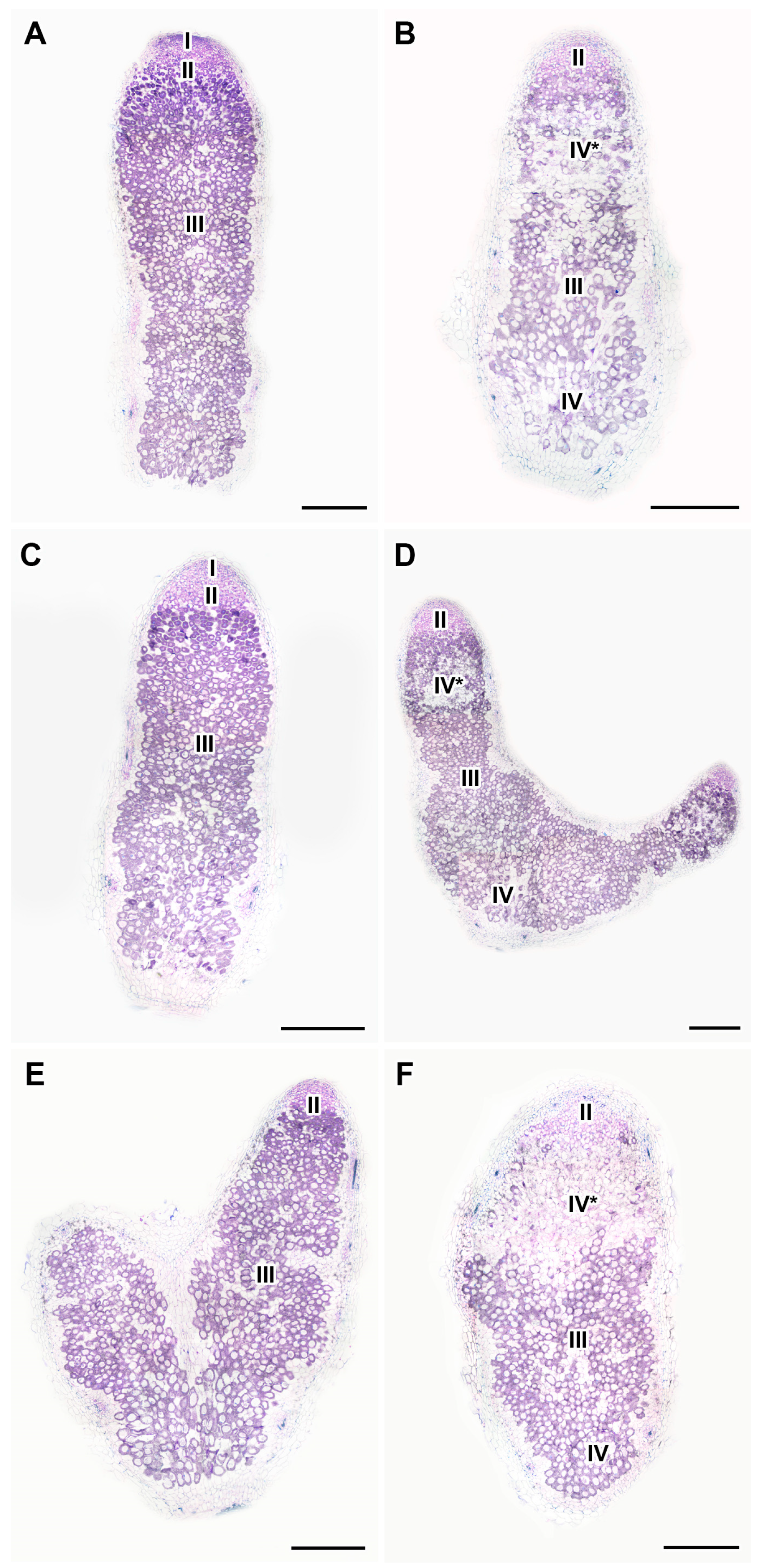
2.5. Histological Organization of Nodules in Pea Plants of the Line SGE Exposed to Elevated Temperature in Combination with Pharmacological Treatment
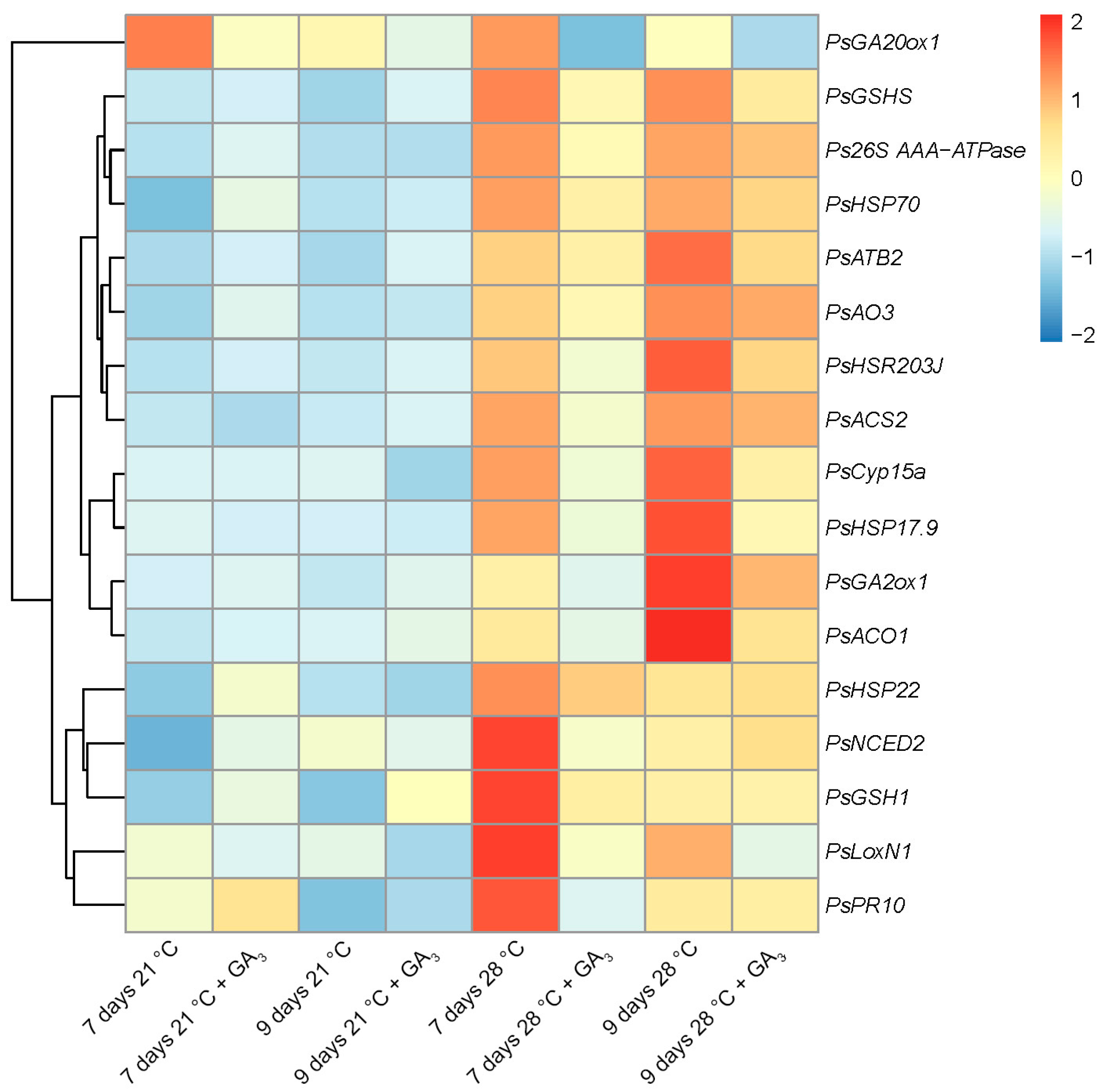
2.6. Expression Analysis of the Genes Associated with Senescence, Heat Stress, and Defense Responses in Nodules of Pea Plants of the Line SGE Exposed to Elevated Temperature in Combination with GA3 Treatment
3. Discussion
4. Materials and Methods
4.1. Plant Material, Bacterial Strain and Plant Growth Conditions
4.2. Growing of Plants at Elevated Temperature
4.3. Cultivation of Plants of Pea Line SGE under Elevated Temperature Conditions in Combination with Pharmacological Treatment
4.4. Nodule Phenotypes
4.5. Light Microscopy
4.6. Immunolocalization of Phytohormones
4.7. Expression Analysis of Genes Associated with Senescence, Heat Stress, and Defense Responses
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Christophe, S.; Jean-Christophe, A.; Annabelle, L.; Alain, O.; Marion, P.; Anne-Sophie, V. Plant N fluxes and modulation by nitrogen, heat and water stresses: A review based on comparison of legumes and non legume plants. In Abiotic Stress in Plants–Mechanisms Adaptations; InTech: London, UK, 2011; pp. 79–118. [Google Scholar] [CrossRef]
- Schlenker, W.; Roberts, M.J. Nonlinear temperature effects indicate severe damages to U.S. crop yields under climate change. Proc. Natl. Acad. Sci. USA 2009, 106, 15594–15598. [Google Scholar] [CrossRef]
- Teixeira, E.I.; Fischer, G.; Van Velthuizen, H.; Walter, C.; Ewert, F. Global hot-spots of heat stress on agricultural crops due to climate change. Agric. For. Meteorol. 2013, 170, 206–215. [Google Scholar] [CrossRef]
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M.R. Heat tolerance in plants: An overview. Environ. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Andrews, M.; Hodge, S. Climate change, a challenge for cool season grain legume crop production. In Climate Change and Management of Cool Season Grain Legume Crops; Yadav, S., Redden, R., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 1–9. [Google Scholar] [CrossRef]
- Devi, J.; Sagar, V.; Mishra, G.P.; Jha, P.K.; Gupta, N.; Dubey, R.K.; Singh, P.M.; Behera, T.K.; Prasad, P. Heat stress tolerance in peas (Pisum sativum L.): Current status and way forward. Front. Plant Sci. 2023, 13, 1108276. [Google Scholar] [CrossRef] [PubMed]
- Stoddard, F.L.; Balko, C.; Erskine, W.; Khan, H.; Link, W.; Sarker, A. Screening techniques and sources of resistance to abiotic stresses in cool-season food legumes. Euphytica 2006, 147, 167–186. [Google Scholar] [CrossRef]
- Sita, K.; Sehgal, A.; HanumanthaRao, B.; Nair, R.M.; Vara Prasad, P.; Kumar, S.; Gaur, P.M.; Farooq, M.; Siddique, K.H.; Varshney, R.K. Food legumes and rising temperatures: Effects, adaptive functional mechanisms specific to reproductive growth stage and strategies to improve heat tolerance. Front. Plant Sci. 2017, 8, 1658. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.; Mir, R.R.; Shafi, S.; Sen Gupta, D.; Djalovic, I.; Miladinovic, J.; Kumar, R.; Kumar, S.; Kumar, R. Genomics associated interventions for heat stress tolerance in cool season adapted grain legumes. Int. J. Mol. Sci. 2021, 23, 399. [Google Scholar] [CrossRef] [PubMed]
- Gladish, D.K.; Rost, T.L. The effects of temperature on primary root growth dynamics and lateral root distribution in garden pea (Pisum sativum L., cv.“Alaska”). Environ. Exp. Bot. 1993, 33, 243–258. [Google Scholar] [CrossRef]
- Fletcher, H.; Maurer, A.; Ormrod, D.; Stanfield, B. Response of peas to environment: I. Planting date and location. Can. J. Plant Sci. 1966, 46, 77–85. [Google Scholar] [CrossRef]
- Guilioni, L.; Wery, J.; Tardieu, F. Heat stress-induced abortion of buds and flowers in pea: Is sensitivity linked to organ age or to relations between reproductive organs? Ann. Bot. 1997, 80, 159–168. [Google Scholar] [CrossRef]
- Sadras, V.; Lake, L.; Leonforte, A.; McMurray, L.; Paull, J. Screening field pea for adaptation to water and heat stress: Associations between yield, crop growth rate and seed abortion. Field Crops Res. 2013, 150, 63–73. [Google Scholar] [CrossRef]
- Parihar, A.K.; Hazra, K.K.; Lamichaney, A.; Singh, A.K.; Dixit, G.P. Delineating the role of plant stature towards heat stress tolerance in field pea (Pisum sativum L.). Heliyon 2023, 9, e14539. [Google Scholar] [CrossRef]
- Lambert, R.G.; Linck, A. Effects of high temperature on yield of peas. Plant Physiol. 1958, 33, 347. [Google Scholar] [CrossRef] [PubMed]
- Nonnecke, I.; Adedipe, N.; Ormrod, D. Temperature and humidity effects on the growth and yield of pea cultivars. Can. J. Plant Sci. 1971, 51, 479–484. [Google Scholar] [CrossRef]
- Jiang, Y.; Lahlali, R.; Karunakaran, C.; Kumar, S.; Davis, A.R.; Bueckert, R.A. Seed set, pollen morphology and pollen surface composition response to heat stress in field pea. Plant Cell Environ. 2015, 38, 2387–2397. [Google Scholar] [CrossRef] [PubMed]
- Guilioni, L.; Wéry, J.; Lecoeur, J. High temperature and water deficit may reduce seed number in field pea purely by decreasing plant growth rate. Funct. Plant Biol. 2003, 30, 1151–1164. [Google Scholar] [CrossRef] [PubMed]
- Frings, J.F.J. The Rhizobium-Pea Symbiosis as Affected by High Temperatures. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 1976. [Google Scholar]
- Larmure, A.; Munier-Jolain, N.G. High temperatures during the seed-filling period decrease seed nitrogen amount in pea (Pisum sativum L.): Evidence for a sink limitation. Front. Plant Sci. 2019, 10, 1608. [Google Scholar] [CrossRef]
- Lamichaney, A.; Parihar, A.K.; Hazra, K.K.; Dixit, G.P.; Katiyar, P.K.; Singh, D.; Singh, A.K.; Kumar, N.; Singh, N.P. Untangling the influence of heat stress on crop phenology, seed set, seed weight, and germination in field pea (Pisum sativum L.). Front. Plant Sci. 2021, 12, 635868. [Google Scholar] [CrossRef] [PubMed]
- Susmita, C.; Aghora, T.; Mohan, N.; Bhatt, R. Breeding for improvement of high temperature tolerance in garden pea (Pisum sativum L.) for off season cultivation. J. Hortic. Sci. 2020, 15, 62–66. [Google Scholar] [CrossRef]
- Newcomb, W. A correlated light and electron microscopic study of symbiotic growth and differentiation in Pisum sativum root nodules. Can. J. Bot. 1976, 54, 2163–2186. [Google Scholar] [CrossRef]
- Guinel, F.C. Getting around the legume nodule: I. The structure of the peripheral zone in four nodule types. Botany 2009, 87, 1117–1138. [Google Scholar] [CrossRef]
- Mathesius, U. Hormonal interactions in the regulation of the nitrogen-fixing legume-Rhizobium symbiosis. In Advances in Botanical Research; Frendo, P., Frugier, F., Masson-Boivin, C., Eds.; Academic Press: Cambridge, MA, USA, 2020; Volume 94, pp. 41–66. [Google Scholar]
- Yang, J.; Lan, L.; Jin, Y.; Yu, N.; Wang, D.; Wang, E. Mechanisms underlying legume–rhizobium symbioses. J. Integr. Plant Biol. 2022, 64, 244–267. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; LaRue, T.A. Ethylene as a possible mediator of light- and nitrate-induced inhibition of nodulation of Pisum sativum L. cv Sparkle. Plant Physiol. 1992, 100, 1334–1338. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; LaRue, T.A. Exogenous ethylene inhibits nodulation of Pisum sativum L. cv Sparkle. Plant Physiol. 1992, 100, 1759–1763. [Google Scholar] [CrossRef] [PubMed]
- McAdam, E.L.; Reid, J.B.; Foo, E. Gibberellins promote nodule organogenesis but inhibit the infection stages of nodulation. J. Exp. Bot. 2018, 69, 2117–2130. [Google Scholar] [CrossRef]
- Ferguson, B.J.; Foo, E.; Ross, J.J.; Reid, J.B. Relationship between gibberellin, ethylene and nodulation in Pisum sativum. New Phytol. 2011, 189, 829–842. [Google Scholar] [CrossRef]
- Dolgikh, E.A.; Kusakin, P.G.; Kitaeva, A.B.; Tsyganova, A.V.; Kirienko, A.N.; Leppyanen, I.V.; Dolgikh, A.V.; Ilina, E.L.; Demchenko, K.N.; Tikhonovich, I.A.; et al. Mutational analysis indicates that abnormalities in rhizobial infection and subsequent plant cell and bacteroid differentiation in pea (Pisum sativum) nodules coincide with abnormal cytokinin responses and localization. Ann. Bot. 2020, 125, 905–923. [Google Scholar] [CrossRef] [PubMed]
- Syōno, K.; Newcomb, W.; Torrey, J.G. Cytokinin production in relation to the development of pea root nodules. Can. J. Bot. 1976, 54, 2155–2162. [Google Scholar] [CrossRef]
- Libbenga, K.R.; van Iren, F.; Bogers, R.J.; Schraag-Lamers, M.F. The role of hormones and gradients in the initiation of cortex proliferation and nodule formation in Pisum sativum L. Planta 1973, 114, 29–39. [Google Scholar] [CrossRef]
- Syõno, K.; Torrey, J.G. Identification of cytokinins of root nodules of the garden pea, Pisum sativum L. Plant Physiol. 1976, 57, 602–606. [Google Scholar] [CrossRef]
- Jones, J.M.C.; Clairmont, L.; Macdonald, E.S.; Weiner, C.A.; Emery, R.J.N.; Guinel, F.C. E151 (sym15), a pleiotropic mutant of pea (Pisum sativum L.), displays low nodule number, enhanced mycorrhizae, delayed lateral root emergence, and high root cytokinin levels. J. Exp. Bot. 2015, 66, 4047–4059. [Google Scholar] [CrossRef]
- Kantsurova, E.S.; Ivanova, A.N.; Kozyulina, P.Y.; Dolgikh, E.A. Exogenously applied cytokinin altered the bacterial release and subsequent stages of nodule development in pea ipd3/cyclops mutant. Plants 2023, 12, 657. [Google Scholar] [CrossRef]
- Foo, E.; Davies, N.W. Strigolactones promote nodulation in pea. Planta 2011, 234, 1073. [Google Scholar] [CrossRef]
- McAdam, E.L.; Hugill, C.; Fort, S.; Samain, E.; Cottaz, S.; Davies, N.W.; Reid, J.B.; Foo, E. Determining the site of action of strigolactones during nodulation. Plant Physiol. 2017, 175, 529–542. [Google Scholar] [CrossRef] [PubMed]
- Van Dingenen, J.; De Keyser, A.; Desmet, S.; Clarysse, A.; Beullens, S.; Michiels, J.; Planque, M.; Goormachtig, S. Strigolactones repress nodule development and senescence in pea. Plant J. 2023, 116, 7–22. [Google Scholar] [CrossRef]
- Serova, T.A.; Kusakin, P.G.; Kitaeva, A.B.; Seliverstova, E.V.; Gorshkov, A.P.; Romanyuk, D.A.; Zhukov, V.A.; Tsyganova, A.V.; Tsyganov, V.E. Effects of elevated temperature on Pisum sativum nodule development: I—Detailed characteristic of unusual apical senescence. Int. J. Mol. Sci. 2023, accepted. [Google Scholar]
- Gorshkov, A.P.; Tsyganova, A.V.; Vorobiev, M.G.; Tsyganov, V.E. The fungicide tetramethylthiuram disulfide negatively affects plant cell walls, infection thread walls, and symbiosomes in pea (Pisum sativum L.) symbiotic nodules. Plants 2020, 9, 1488. [Google Scholar] [CrossRef] [PubMed]
- Tsyganova, A.V.; Seliverstova, E.V.; Tsyganov, V.E. Influence of mutation in pea (Pisum sativum L.) cdt (cadmium tolerance) gene on histological and ultrastructural nodule organization. Ekol. Genet. 2019, 17, 71–80. [Google Scholar] [CrossRef]
- Gorshkov, A.P.; Kusakin, P.G.; Borisov, Y.G.; Tsyganova, A.V.; Tsyganov, V.E. Effect of triazole fungicides Titul Duo and Vintage on the development of pea (Pisum sativum L.) symbiotic nodules. Int. J. Mol. Sci. 2023, 24, 8646. [Google Scholar] [CrossRef] [PubMed]
- Van de Velde, W.; Guerra, J.C.P.; Keyser, A.D.; De Rycke, R.; Rombauts, S.; Maunoury, N.; Mergaert, P.; Kondorosi, E.; Holsters, M.; Goormachtig, S. Aging in legume symbiosis. A molecular view on nodule senescence in Medicago truncatula. Plant Physiol. 2006, 141, 711–720. [Google Scholar] [CrossRef]
- Puppo, A.; Groten, K.; Bastian, F.; Carzaniga, R.; Soussi, M.; Lucas, M.M.; De Felipe, M.R.; Harrison, J.; Vanacker, H.; Foyer, C.H. Legume nodule senescence: Roles for redox and hormone signalling in the orchestration of the natural aging process. New Phytol. 2005, 165, 683–701. [Google Scholar] [CrossRef]
- Zhou, S.; Zhang, C.; Huang, Y.; Chen, H.; Yuan, S.; Zhou, X. Characteristics and research progress of legume nodule senescence. Plants 2021, 10, 1103. [Google Scholar] [CrossRef]
- Kazmierczak, T.; Yang, L.; Boncompagni, E.; Meilhoc, E.; Frugier, F.; Frendo, P.; Bruand, C.; Gruber, V.; Brouquisse, R. Legume nodule senescence: A coordinated death mechanism between bacteria and plant cells. In Advances in Botanical Research; Frendo, P., Frugier, F., Masson-Boivin, C., Eds.; Academic Press: Cambridge, MA, USA, 2020; Volume 94, pp. 181–212. [Google Scholar]
- Serova, T.A.; Tikhonovich, I.A.; Tsyganov, V.E. Analysis of nodule senescence in pea (Pisum sativum L.) using laser microdissection, real-time PCR, and ACC immunolocalization. J. Plant Physiol. 2017, 212, 29–44. [Google Scholar] [CrossRef] [PubMed]
- Serova, T.A.; Tsyganova, A.V.; Tikhonovich, I.A.; Tsyganov, V.E. Gibberellins inhibit nodule senescence and stimulate nodule meristem bifurcation in pea (Pisum sativum L.). Front. Plant Sci. 2019, 10, 285. [Google Scholar] [CrossRef]
- Suzuki, N.; Rivero, R.M.; Shulaev, V.; Blumwald, E.; Mittler, R. Abiotic and biotic stress combinations. New Phytol. 2014, 203, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, M.; Canales, E.; Borroto, C.J.; Carmona, E.; Lopez, J.; Pujol, M.; Borrás-Hidalgo, O. Identification of genes induced upon water-deficit stress in a drought-tolerant rice cultivar. J. Plant Physiol. 2006, 163, 577–584. [Google Scholar] [CrossRef]
- Awasthi, R.; Kaushal, N.; Vadez, V.; Turner, N.C.; Berger, J.; Siddique, K.H.; Nayyar, H. Individual and combined effects of transient drought and heat stress on carbon assimilation and seed filling in chickpea. Funct. Plant Biol. 2014, 41, 1148–1167. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.K.; Andersen, S.B.; Ottosen, C.O.; Rosenqvist, E. Wheat cultivars selected for high Fv/Fm under heat stress maintain high photosynthesis, total chlorophyll, stomatal conductance, transpiration and dry matter. Physiol. Plant. 2015, 153, 284–298. [Google Scholar] [CrossRef]
- Yang, H.; Gu, X.; Ding, M.; Lu, W.; Lu, D. Heat stress during grain filling affects activities of enzymes involved in grain protein and starch synthesis in waxy maize. Sci. Rep. 2018, 8, 15665. [Google Scholar] [CrossRef]
- Awasthi, R.; Bhandari, K.; Nayyar, H. Temperature stress and redox homeostasis in agricultural crops. Front. Environ. Sci. 2015, 3, 11. [Google Scholar] [CrossRef]
- Barnabás, B.; Jäger, K.; Fehér, A. The effect of drought and heat stress on reproductive processes in cereals. Plant Cell Environ. 2008, 31, 11–38. [Google Scholar] [CrossRef]
- Aranjuelo, I.; Aldasoro, J.; Arrese-Igor, C.; Erice, G.; Sanz-Sáez, Á. How does high temperature affect legume nodule symbiotic activity? In Legume Nitrogen Fixation in a Changing Environment: Achievements and Challenges; Sulieman, S., Tran, L.-S.P., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 67–87. [Google Scholar] [CrossRef]
- Serova, T.A.; Tsyganova, A.V.; Tsyganov, V.E. Early nodule senescence is activated in symbiotic mutants of pea (Pisum sativum L.) forming ineffective nodules blocked at different nodule developmental stages. Protoplasma 2018, 255, 1443–1459. [Google Scholar] [CrossRef]
- Charbonneau, G.A.; Newcomb, W. Growth regulators in developing effective root nodules of the garden pea (Pisum sativum L.). Biochemie und Physiologie der Pflanzen 1985, 180, 667–681. [Google Scholar] [CrossRef]
- Karmarkar, V.M. Transcriptional Regulation of Nodule Development and Senescence in Medicago truncatula. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2014. [Google Scholar]
- Guinel, F.C. Ethylene, a hormone at the center-stage of nodulation. Front. Plant Sci. 2015, 6, 1121. [Google Scholar] [CrossRef] [PubMed]
- Fukudome, M.; Watanabe, E.; Osuki, K.-I.; Imaizumi, R.; Aoki, T.; Becana, M.; Uchiumi, T. Stably transformed Lotus japonicus plants overexpressing phytoglobin LjGlb1-1 show decreased nitric oxide levels in roots and nodules as well as delayed nodule senescence. Plant Cell Physiol. 2019, 60, 816–825. [Google Scholar] [CrossRef] [PubMed]
- da Silva, H.A.P.; Caetano, V.S.; Pessoa, D.D.V.; Pacheco, R.S.; Simoes-Araujo, J.L. Molecular and biochemical changes of aging-induced nodules senescence in common bean. Symbiosis 2019, 79, 33–48. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, H.; Purves, R.W.; Bueckert, R.; Tar’an, B.; Warkentin, T.D. Comparative analysis of heat-stress-induced abscisic acid and heat shock protein responses among pea varieties. Crop Sci. 2023, 63, 139–150. [Google Scholar] [CrossRef]
- Wilmowicz, E.; Kućko, A.; Golińska, P.; Burchardt, S.; Przywieczerski, T.; Świdziński, M.; Brzozowska, P.; Kapuścińska, D. Abscisic acid and ethylene in the control of nodule-specific response on drought in yellow lupine. Environ. Exp. Bot. 2020, 169, 103900. [Google Scholar] [CrossRef]
- Colebrook, E.H.; Thomas, S.G.; Phillips, A.L.; Hedden, P. The role of gibberellin signalling in plant responses to abiotic stress. J. Exp. Biol. 2014, 217, 67–75. [Google Scholar] [CrossRef]
- Achard, P.; Gong, F.; Cheminant, S.; Alioua, M.; Hedden, P.; Genschik, P. The cold-inducible CBF1 factor–dependent signaling pathway modulates the accumulation of the growth-repressing DELLA proteins via its effect on gibberellin metabolism. Plant Cell 2008, 20, 2117–2129. [Google Scholar] [CrossRef]
- Zhou, M.; Chen, H.; Wei, D.; Ma, H.; Lin, J. Arabidopsis CBF3 and DELLAs positively regulate each other in response to low temperature. Sci. Rep. 2017, 7, 39819. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-I.; Li, P.-F.; Yang, C.-H. NAC-like gene GIBBERELLIN SUPPRESSING FACTOR regulates the gibberellin metabolic pathway in response to cold and drought stresses in Arabidopsis. Sci. Rep. 2019, 9, 19226. [Google Scholar] [CrossRef]
- Toh, S.; Imamura, A.; Watanabe, A.; Nakabayashi, K.; Okamoto, M.; Jikumaru, Y.; Hanada, A.; Aso, Y.; Ishiyama, K.; Tamura, N.; et al. High temperature-induced abscisic acid biosynthesis and its role in the inhibition of gibberellin action in Arabidopsis seeds. Plant Physiol. 2008, 146, 1368–1385. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Gull, S.; Ali, M.M.; Yousef, A.F.; Ercisli, S.; Kalaji, H.M.; Telesiński, A.; Auriga, A.; Wróbel, J.; Radwan, N.S.; et al. Heat stress mitigation in tomato (Solanum lycopersicum L.) through foliar application of gibberellic acid. Sci. Rep. 2022, 12, 11324. [Google Scholar] [CrossRef] [PubMed]
- Hamayun, M.; Khan, S.A.; Khan, A.L.; Shin, J.-H.; Ahmad, B.; Shin, D.-H.; Lee, I.-J. Exogenous gibberellic acid reprograms soybean to higher growth and salt stress tolerance. J. Agric. Food Chem. 2010, 58, 7226–7232. [Google Scholar] [CrossRef] [PubMed]
- Kosterin, O.E.; Rozov, S.M. Mapping of the new mutation blb and the problem of integrity of linkage group I. Pisum Genet. 1993, 25, 27–31. [Google Scholar]
- Ivanova, K.A.; Tsyganova, A.V.; Brewin, N.J.; Tikhonovich, I.A.; Tsyganov, V.E. Induction of host defences by Rhizobium during ineffective nodulation of pea (Pisum sativum L.) carrying symbiotically defective mutations sym40 (PsEFD), sym33 (PsIPD3/PsCYCLOPS) and sym42. Protoplasma 2015, 252, 1505–1517. [Google Scholar] [CrossRef]
- Glenn, A.R.; Poole, P.S.; Hudman, J.F. Succinate uptake by free-living and bacteroid forms of Rhizobium leguminosarum. Microbiology 1980, 119, 267–271. [Google Scholar] [CrossRef][Green Version]
- Peng, Y.B.; Zou, C.; Wang, D.H.; Gong, H.Q.; Xu, Z.H.; Bai, S.N. Preferential localization of abscisic acid in primordial and nursing cells of reproductive organs of Arabidopsis and cucumber. New Phytol. 2006, 170, 459–466. [Google Scholar] [CrossRef]
- Die, J.V.; Román, B.; Nadal, S.; Dita, M.Á.; González-Verdejo, C.I. Expression analysis of Pisum sativum putative defence genes during Orobanche crenata infection. Crop Pasture Sci. 2009, 60, 490–498. [Google Scholar] [CrossRef]
- Weston, D.E.; Elliott, R.C.; Lester, D.R.; Rameau, C.; Reid, J.B.; Murfet, I.C.; Ross, J.J. The pea DELLA proteins LA and CRY are important regulators of gibberellin synthesis and root growth. Plant Physiol. 2008, 147, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, K.A.; Chernova, E.N.; Kulaeva, O.A.; Tsyganova, A.V.; Kusakin, P.G.; Russkikh, I.V.; Tikhonovich, I.A.; Tsyganov, V.E. The regulation of pea (Pisum sativum L.) symbiotic nodule infection and defense responses by glutathione, homoglutathione, and their ratio. Front. Plant Sci. 2022, 13, 843565. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsova, E.; Seddas-Dozolme, P.M.A.; Arnould, C.; Tollot, M.; van Tuinen, D.; Borisov, A.; Gianinazzi, S.; Gianinazzi-Pearson, V. Symbiosis-related pea genes modulate fungal and plant gene expression during the arbuscule stage of mycorrhiza with Glomus intraradices. Mycorrhiza 2010, 20, 427–443. [Google Scholar] [CrossRef] [PubMed]
- Kolde, R. pheatmap: Pretty Heatmaps, R Package Version 1.0.12. 2019.




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kitaeva, A.B.; Serova, T.A.; Kusakin, P.G.; Tsyganov, V.E. Effects of Elevated Temperature on Pisum sativum Nodule Development: II—Phytohormonal Responses. Int. J. Mol. Sci. 2023, 24, 17062. https://doi.org/10.3390/ijms242317062
Kitaeva AB, Serova TA, Kusakin PG, Tsyganov VE. Effects of Elevated Temperature on Pisum sativum Nodule Development: II—Phytohormonal Responses. International Journal of Molecular Sciences. 2023; 24(23):17062. https://doi.org/10.3390/ijms242317062
Chicago/Turabian StyleKitaeva, Anna B., Tatiana A. Serova, Pyotr G. Kusakin, and Viktor E. Tsyganov. 2023. "Effects of Elevated Temperature on Pisum sativum Nodule Development: II—Phytohormonal Responses" International Journal of Molecular Sciences 24, no. 23: 17062. https://doi.org/10.3390/ijms242317062
APA StyleKitaeva, A. B., Serova, T. A., Kusakin, P. G., & Tsyganov, V. E. (2023). Effects of Elevated Temperature on Pisum sativum Nodule Development: II—Phytohormonal Responses. International Journal of Molecular Sciences, 24(23), 17062. https://doi.org/10.3390/ijms242317062






