The Reduction of Carbonyl Compounds with Dicyclopentylzinc: A New Example of Asymmetric Amplifying Autocatalysis
Abstract
:1. Introduction
2. Results
2.1. Discovery of the Reducing Properties of Dicyclopentylzinc
2.2. Reactions of Dicyclopentylzinc with Aldehydes
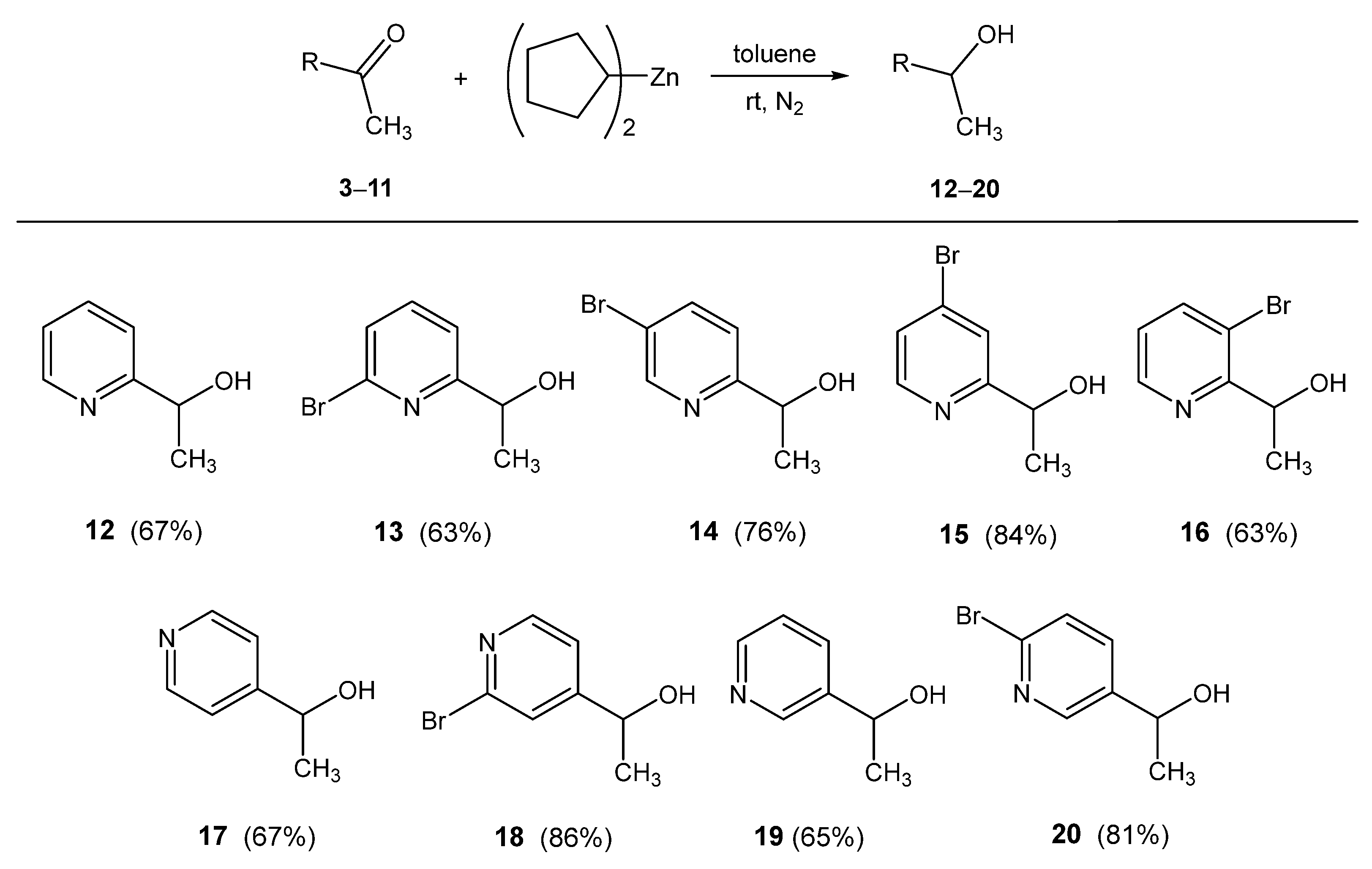
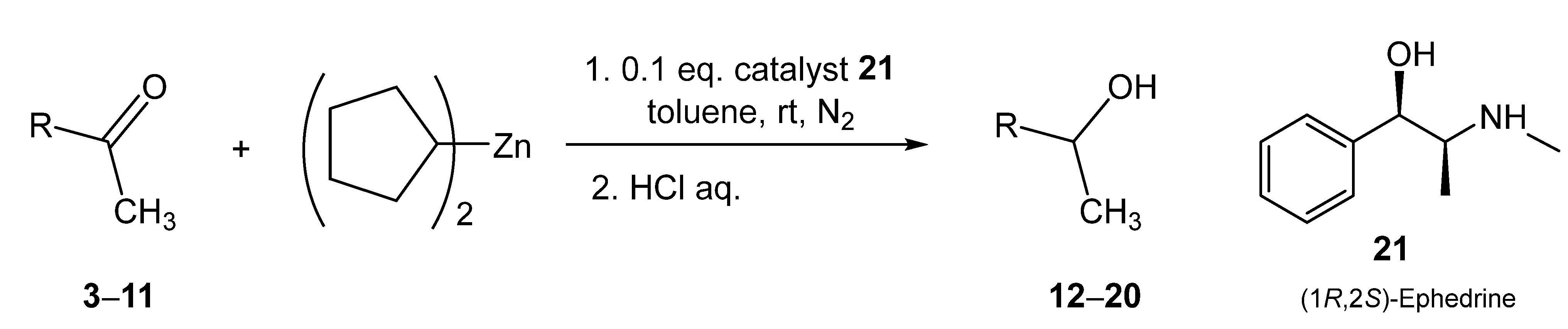
2.3. Reactions of Dicyclopentylzinc with Ketones
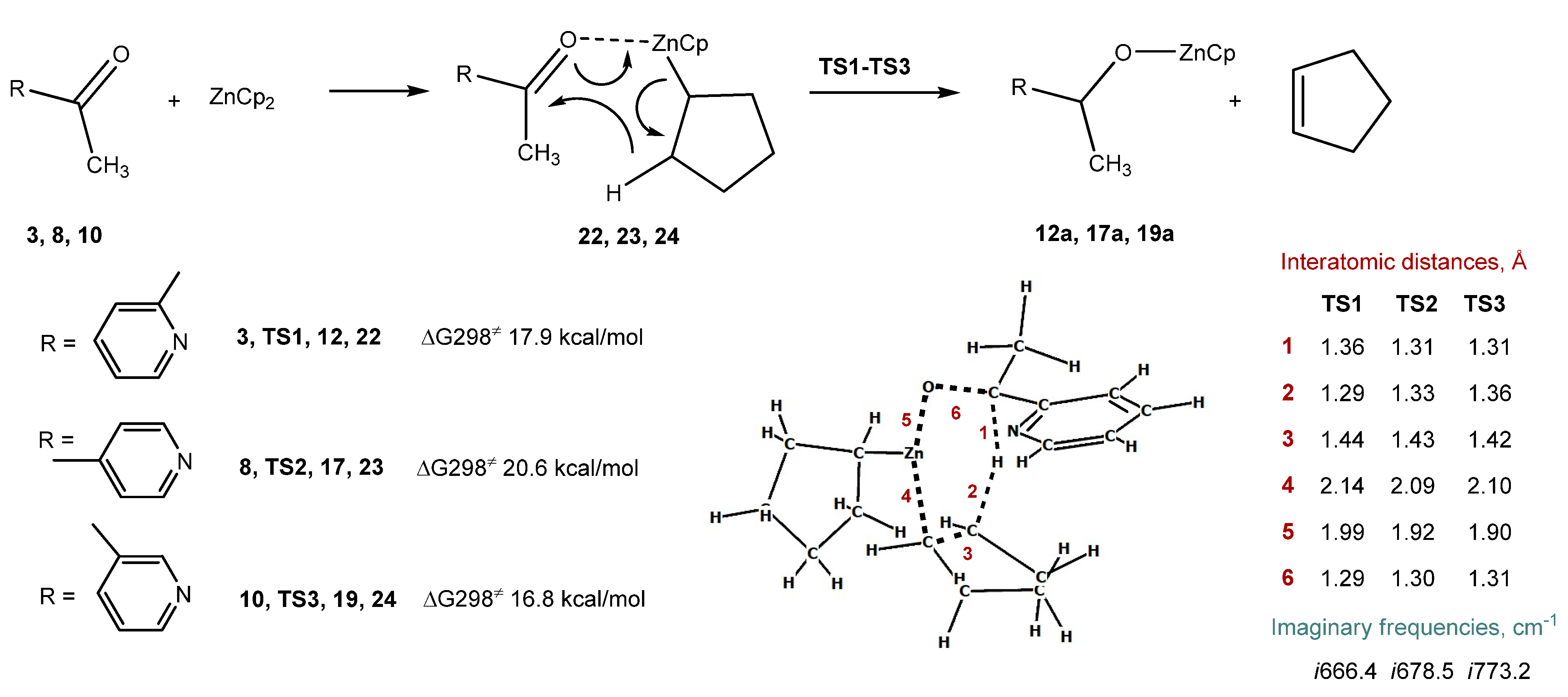
2.4. Computational Studies of the Reduction of Pyridinic Ketones with Dicyclopentylzinc
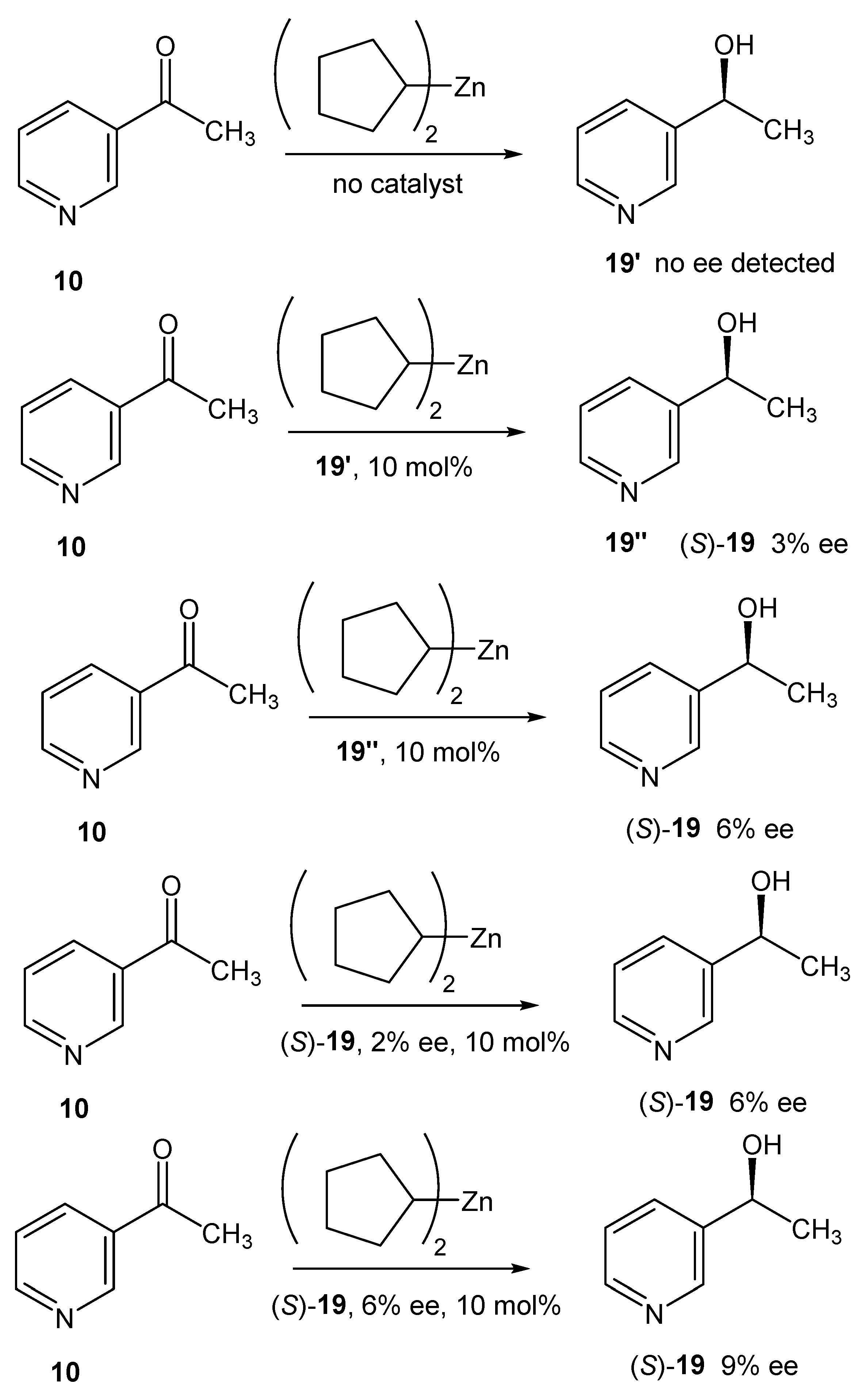
2.5. Asymmetric Amplifying Autocatalysis in the Reaction of Dicyclopentylzinc with 3-Acetylpyridine
2.6. Computational Search of the Possible Mechanism of Autoamplification
3. Discussion
4. Materials and Methods
4.1. Experimental Details
4.2. Computational Details
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Soai, K.; Shibata, T.; Morioka, H.; Choji, K. Asymmetric autocatalysis and amplification of enantiomeric excess of a chiral molecule. Nature 1995, 378, 767–768. [Google Scholar] [CrossRef]
- Soai, K.; Shibata, T.; Sato, I. Discovery and development of asymmetric autocatalysis. Bull. Chem. Soc. Jpn. 2004, 77, 1063–1073. [Google Scholar] [CrossRef]
- Mislow, K. Absolute asymmetric synthesis: A commentary. Collect. Czech. Chem. Commun. 2003, 68, 849–864. [Google Scholar] [CrossRef]
- Soai, K.; Sato, I.; Shibata, T.; Komiya, S.; Hayashi, M.; Matsueda, Y.; Imamura, H.; Hayase, T.; Morioka, H.; Tabira, H.; et al. Asymmetric synthesis of pyrimidyl alkanol without adding chiral substances by the addition of diisopropylzinc to pyrimidine-5-carbaldehyde in conjunction with asymmetric autocatalysis. Tetrahedron Asymmetry 2003, 14, 185–188. [Google Scholar] [CrossRef]
- Gridnev, I.D.; Serafimov, J.M.; Quiney, H.; Brown, J.M. Reflections on spontaneous asymmetric synthesis by amplifying autocatalysis. Org. Biomol. Chem. 2003, 1, 3811–3819. [Google Scholar] [CrossRef]
- Singleton, D.A.; Vo, L.K. A few molecules can control the enantiomeric outcome. Evidence supporting absolute asymmetric synthesis using the Soai asymmetric autocatalysis. Org. Lett. 2003, 23, 4337–4339. [Google Scholar] [CrossRef]
- Tanji, S.; Kodaka, Y.; Ohno, A.; Shibata, T.; Sato, I.; Soai, K. Asymmetric autocatalysis of 5-carbamoyl-3-pyridyl alkanols with amplification of enantiomeric excess. Tetrahedron Asymmetry 2000, 11, 4249–4253. [Google Scholar] [CrossRef]
- Athavale, S.V.; Simon, A.; Houk, K.N.; Denmark, S.E. Structural contributions to autocatalysis and asymmetric amplification in the Soai reaction. J. Am. Chem. Soc. 2020, 142, 18387–18406. [Google Scholar] [CrossRef]
- Lu, N.; Dai, Z.; Zhang, Q.; Zhang, C.; Kwong, C.Y.R.; Xia, C. Metal Complex Containing Three Different. Ligands. Patent No. US20200358010A1, 12 November 2020. [Google Scholar]
- Chikugi, Y.; Komiya, M.; Furuta, T.; Iwamoto, K.; Takahashi, Y.; Nonoyama, A. Preparation of New Benzoxazolone. Compounds. Patent No. JP2017001991, 5 January 1991. [Google Scholar]
- Campbell, J.B.; Firor, J.W. Synthesis of 5-substituted aminopyrrolo[3,4-b]quinolinones by a diorganozinc-mediated tandem alkylation/cyclization. Synth. Commun. 2012, 42, 1613–1621. [Google Scholar] [CrossRef]
- Hyodo, I.; Tobisu, M.; Chatani, N. Regioselective C–H bond functionalizations of acridines using organozinc reagents. Chem. Commun. 2012, 48, 308–310. [Google Scholar] [CrossRef]
- Jadhav, A.S.; Anand, R.V. 1,6-Conjugate addition of zinc alkyls to para-quinone methides in a continuous-flow microreactor. Org. Biomol. Chem. 2017, 15, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Angelini, L.; Sanz, L.M.; Leonori, D. Divergent nickel-catalysed ring-opening–functionalization of cyclobutanone oximes with organozincs. Synlett 2020, 31, 37–40. [Google Scholar]
- Schuppe, A.W.; Huang, D.; Chen, Y.; Newhouse, T.R. Total synthesis of (−)-Xylogranatopyridine B via a palladium-catalyzed oxidative stannylation of enones. J. Am. Chem. Soc. 2018, 140, 2062–2066. [Google Scholar] [CrossRef] [PubMed]
- Hatano, M.; Mizuno, T.; Ishihara, K. Catalytic enantioselective synthesis of sterically demanding alcohols using di(2-alkyl)zinc prepared by the refined Charette’s method. Chem. Commun. 2010, 46, 5443–5445. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, K.; Hatano, M. Preparation of Optically Active Alcohols. Patent No. WO2011062280, 26 May 2011. [Google Scholar]
- Soai, K.; Niwa, S.; Kobayashi, T. Asymmetric synthesis of pyridylethanols and amino alcohols by enantioselective reduction with lithium borohydride modified with N,N′-dibenzoylcystine. J. Chem. Soc. Chem. Commun. 1987, 11, 801–802. [Google Scholar] [CrossRef]
- Tuulmets, A.; Sassian, M. Reactions of partially solvated Grignard reagents with a ketone. J. Organomet. Chem. 1999, 586, 145–149. [Google Scholar] [CrossRef]
- Bartolo, N.D.; Read, J.A.; Valentín, E.M.; Woerpel, K.A. Reactions of allylmagnesium reagents with carbonyl compounds and compounds with C=N double bonds: Their diastereoselectivities generally cannot be analyzed using the Felkin–Anh and chelation-control models. Chem. Rev. 2020, 120, 1513–1619. [Google Scholar] [CrossRef]
- Soai, K.; Niwa, S.; Hori, H. Asymmetric self-catalytic reaction. Self-production of chiral 1-(3-pyridyl)alkanols as chiral self-catalysts in the enantioselective addition of dialkylzinc reagents to pyridine-3-carbaldehyde. J. Chem. Soc. Chem. Commun. 1990, 14, 982–983. [Google Scholar] [CrossRef]
- Gridnev, I.D.; Vorobiev, A.K. Quantification of sophisticated equilibria in the reaction pool and amplifying catalytic cycle of the Soai reaction. ACS Catal. 2012, 2, 2137–2149. [Google Scholar] [CrossRef]
- Thiele, K.H.; Wilcke, S.; Ehrhardt, M. Uber dicycloalkyl-verbindungen des zinks. J. Organomet. Chem. 1968, 14, 13–19. [Google Scholar] [CrossRef]
- Integrated Spectral Data Base System of Organic Compounds, National Institute of Advanced Industrial Science and Technology (Japan). Available online: http://sdbs.db.aist.go.jp (accessed on 25 November 2023).
- Clarker, M.L.; Díaz-Valenzuela, M.B.; Slawin, A.M.Z. Hydrogenation of aldehydes, esters, imines, and ketones catalyzed by a ruthenium complex of a chiral tridentate ligand. Organometallics 2007, 26, 16–19. [Google Scholar] [CrossRef]
- Jones, G.; Mouat, D.J.; Tonkinson, D.J. Triazolopyridines. Part 6. Ring opening reactions of triazolopyridines. J. Chem. Soc. Perkin Trans. 1985, 1, 2719–2723. [Google Scholar] [CrossRef]
- Cho, S.-D.; Park, Y.-D.; Kim, J.-J.; Falck, J.R.; Yoon, Y.-J. Facile reduction of carboxylic acids, esters, acid chlorides, amides and nitriles to alcohols or amines using NaBH4/BF3⋅Et2O. Bull. Korean Chem. Soc. 2004, 25, 407–409. [Google Scholar] [CrossRef]
- Basavaiah, D.; Sharada, D.S.; Veerendhar, A. Organo-base mediated Cannizzaro reaction. Tetrahedron Lett. 2006, 47, 5771–5774. [Google Scholar] [CrossRef]
- Knorr, R.; Lattke, E. 2-Methyl-1-phenyl-1-propenyllithium. Ein katalytisch ummetallierbares Vinyllithiumderivat. Chem. Ber. 1981, 114, 2116–2131. [Google Scholar] [CrossRef]
- Trecourt, F.; Breton, G.; Bonnet, V.; Mongin, F.; Marsais, F.; Queguiner, G. New syntheses of substituted pyridines via bromine-magnesium exchange. Tetrahedron 2000, 56, 1349–1360. [Google Scholar] [CrossRef]
- Bolm, C.; Ewald, M.; Felder, M.; Schlingloff, G. Enantioselective synthesis of optically active pyridine derivatives and C2-symmetric 2,2′-bipyridines. Chem. Ber. 1992, 125, 1169–1190. [Google Scholar] [CrossRef]
- Lebedev, Y.; Polishchuk, I.; Maity, B.; Guerreiro, M.D.V.; Cavallo, L.; Rueping, M. Asymmetric hydroboration of heteroaryl ketones by aluminumcatalysis. J. Am. Chem. Soc. 2019, 141, 19415−19423. [Google Scholar] [CrossRef] [PubMed]
- Babaoglu, K.; Brizgys, G.; Cha, J.; Chen, X.; Guo, H.; Halcomb, R.L.; Han, X.; Huang, R.; Liu, H.; McFadden, R.; et al. Benzothiazol-6-ylacetic acid derivatives as anti-HIV agents and their preparation and use for treating an HIV infection. Patent No. WO2013159064, 24 October 2013. [Google Scholar]
- Subota, A.I.; Lutsenko, A.O.; Vashchenko, B.V.; Volochnyuk, D.M.; Levchenko, V.; Dmytriv, Y.V.; Rusanov, E.B.; Gorlova, A.O.; Ryabukhin, S.V.; Grygorenko, O.O. Scalable and straightforward synthesis of all isomeric (cyclo)alkylpiperidines. Eur. J. Org. Chem. 2019, 22, 3636–3648. [Google Scholar] [CrossRef]
- Gottarelli, G.; Samori, B. Circular dichroism of isomeric pyridylethanols. J. Chem. Soc. Perkin Trans. 2 1974, 12, 1462–1465. [Google Scholar] [CrossRef]
- Hatano, M.; Suzuki, S.; Ishihara, K. Highly efficient alkylation to ketones and aldimines with Grignard reagents catalyzed by Zinc(II) chloride. J. Am. Chem. Soc. 2006, 128, 9998–9999. [Google Scholar] [CrossRef]
- Cook, B.N.; Disalvo, D.; Fandrick, D.R.; Harcken, C.; Kuzmich, D.; Lee, T.W.H.; Liu, P.; Lord, J.; Mao, C.; Neu, J.; et al. Azaindazole compounds as ccr1 receptor antagonists. Patent No. WO2010036632A1, 1 April 2010. [Google Scholar]
- Lunniss, C.J.; Palmer, J.T.; Pitt, G.R.W.; Axford, L.C.; Davies, D. Antibacterial compounds. Patent No. WO2013138860, 26 September 2013. [Google Scholar]
- Harriman, G.; Kolz, C.; Roth, B.; Song, Y.; Trivedi, B. Functionalized heterocycles as chemokine receptor modulators. Patent No. WO00/42045, 20 July 2000. [Google Scholar]
- Chai, J.-D.; Head-Gordon, M. Long-range corrected hybrid density functionals with damped atom–atom dispersion corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A Gen. Phys. 1988, 38, 3098–3100. [Google Scholar] [CrossRef] [PubMed]
- Ditchfield, R.; Hehre, W.J.; Pople, J.A. Self-consistent molecular-orbital methods. IX. An extended Gaussian-type basis for molecular-orbital studies of organic molecules. J. Chem. Phys. 1971, 54, 724–728. [Google Scholar] [CrossRef]
- Hehre, W.J.; Ditchfield, R.; Pople, J.A. Self-consistent molecular orbital methods. XII. Further extensions of Gaussian-type basis sets for use in molecular orbital studies of organic molecules. J. Chem. Phys. 1972, 56, 2257–2261. [Google Scholar] [CrossRef]
- Hariharan, P.C.; Pople, J.A. The influence of polarization functions on molecular orbital hydrogenation energies. Theor. Chim. Acta 1973, 28, 213–222. [Google Scholar] [CrossRef]
- Francl, M.M.; Pietro, W.J.; Hehre, W.; Binkley, J.S.; Gordon, M.S.; DeFrees, D.J.; Pople, J.A. Self-consistent molecular orbital methods. XXIII. A polarization-type basis set for second-row elements. J. Chem. Phys. 1982, 77, 3654–3665. [Google Scholar] [CrossRef]
- Gordon, M.S.; Binkley, J.S.; Pople, J.A.; Pietro, W.J.; Hehre, W.J. Self-consistent molecular-orbital methods. 22. Small split-valence basis sets for second-row elements. J. Am. Chem. Soc. 1982, 104, 2797–2803. [Google Scholar] [CrossRef]
- Rassolov, V.A.; Pople, J.A.; Ratner, M.A.; Windus, T.L. 6–31G* basis set for atoms K through Zn. J. Chem. Phys. 1998, 109, 1223–1229. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal solvation model based on solute electron density and on continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef]



| Entry | Starting Compound | Reaction Conditions | Product | Yield, % a |
|---|---|---|---|---|
| 1 | 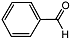 | 1 eq. ZnCp2, rt, 30 min, hexane | 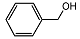 | 99 |
| 2 | 0.5 eq. ZnCp2, rt, 30 min, hexane | 90 | ||
| 3 | 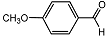 | 1 eq. ZnCp2, rt, 18 h, hexane | 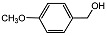 | 80 |
| 4 | 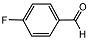 | 1 eq. ZnCp2, rt, 1 h, hexane |  | 90 |
| 5 |  | 1 eq. ZnCp2, rt,1 h, hexane | 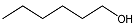 | 58 |
| 6 | 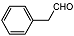 | 1 eq. ZnCp2, rt, 3.5 h, hexane | 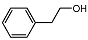 | 70 |
| 7 | 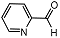 | 1 eq. ZnCp2, rt, 0.5 h, hexane |  | 93 |
| 8 | 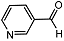 | 1 eq. ZnCp2, rt, 0.5 h, hexane | 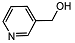 | 84 |
| 9 | 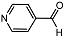 | 1 eq. ZnCp2, rt, 0.5 h, hexane |  | 99 |
| Entry | Starting Compound | Reaction Conditions | Product | Yield, % a | ee, % (HPLC) |
|---|---|---|---|---|---|
| 1 | 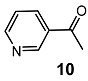 | 5 eq. ZnCp2, rt, 5 h, toluene | 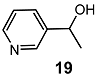 | 65 | – |
| 2 | 3 eq. ZnCp2, 0.1 eq. 21, rt, 24 h | 55 | 92 (S) | ||
| 3 | 3 eq. ZnCp2, 0.1 eq. 21, rt, 5 h | 57 | >99 (S) | ||
| 4 | 3 eq. ZnCp2, 0.1 eq. (S)-19, b rt, 5 h | 45 c,d | 95 (S) | ||
| 5 | 5 eq. ZnCp2, 0.1 eq. (S)-19, b rt, 5 h | 42 c,d | 96 (S) | ||
| 6 | 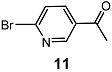 | 3 eq. ZnCp2, rt, 24 h, toluene | 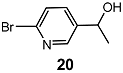 | 81 | – |
| 7 | 3 eq. ZnCp2, 0.1 eq. 21, rt, 24 h | 64 | 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saigitbatalova, E.S.; Latypova, L.Z.; Zagidullin, A.A.; Kurbangalieva, A.R.; Gridnev, I.D. The Reduction of Carbonyl Compounds with Dicyclopentylzinc: A New Example of Asymmetric Amplifying Autocatalysis. Int. J. Mol. Sci. 2023, 24, 17048. https://doi.org/10.3390/ijms242317048
Saigitbatalova ES, Latypova LZ, Zagidullin AA, Kurbangalieva AR, Gridnev ID. The Reduction of Carbonyl Compounds with Dicyclopentylzinc: A New Example of Asymmetric Amplifying Autocatalysis. International Journal of Molecular Sciences. 2023; 24(23):17048. https://doi.org/10.3390/ijms242317048
Chicago/Turabian StyleSaigitbatalova, Elena Sh., Liliya Z. Latypova, Almaz A. Zagidullin, Almira R. Kurbangalieva, and Ilya D. Gridnev. 2023. "The Reduction of Carbonyl Compounds with Dicyclopentylzinc: A New Example of Asymmetric Amplifying Autocatalysis" International Journal of Molecular Sciences 24, no. 23: 17048. https://doi.org/10.3390/ijms242317048
APA StyleSaigitbatalova, E. S., Latypova, L. Z., Zagidullin, A. A., Kurbangalieva, A. R., & Gridnev, I. D. (2023). The Reduction of Carbonyl Compounds with Dicyclopentylzinc: A New Example of Asymmetric Amplifying Autocatalysis. International Journal of Molecular Sciences, 24(23), 17048. https://doi.org/10.3390/ijms242317048







