Mechanisms That Protect Mammalian Sperm from the Spontaneous Acrosome Reaction
Abstract
1. Introduction
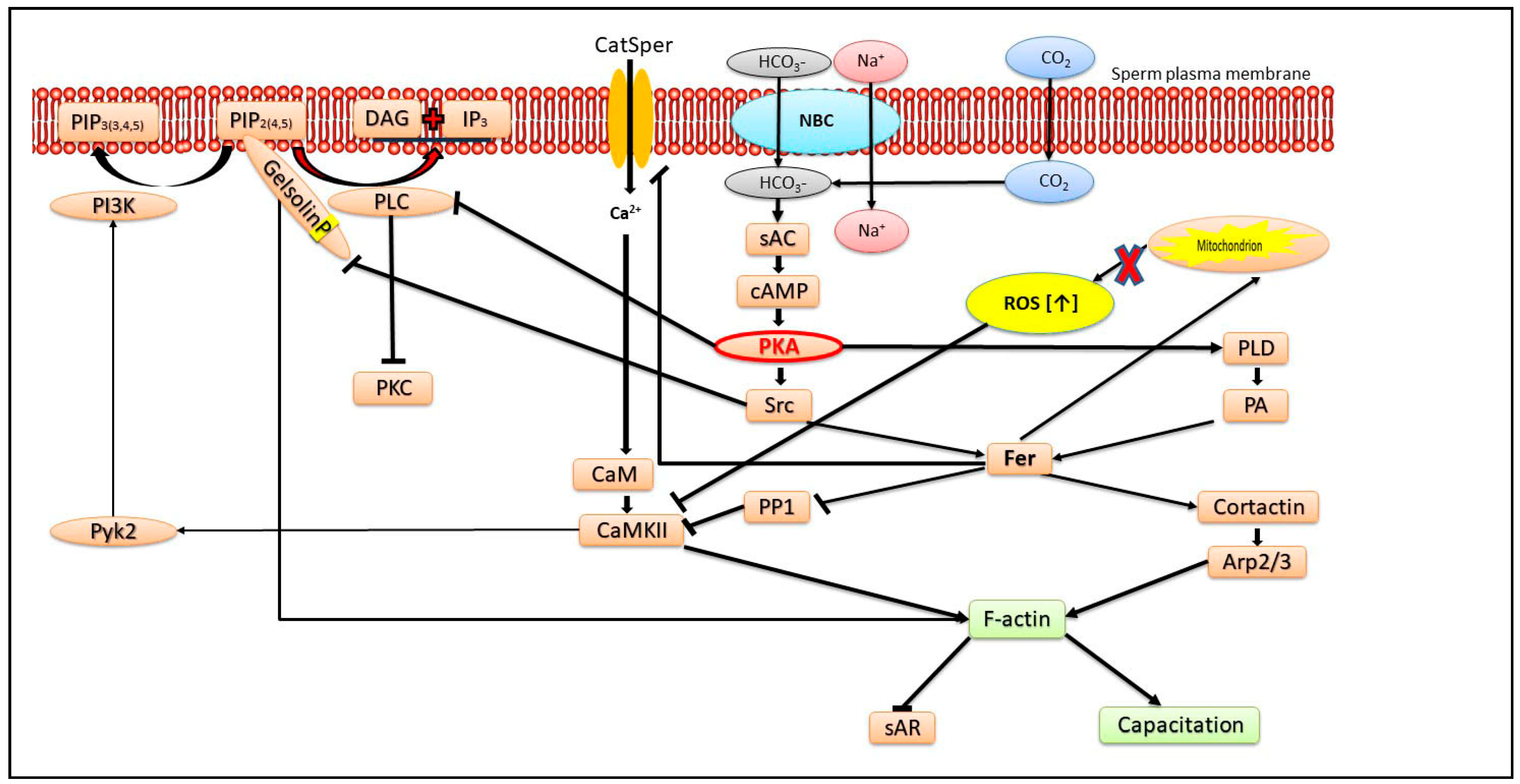
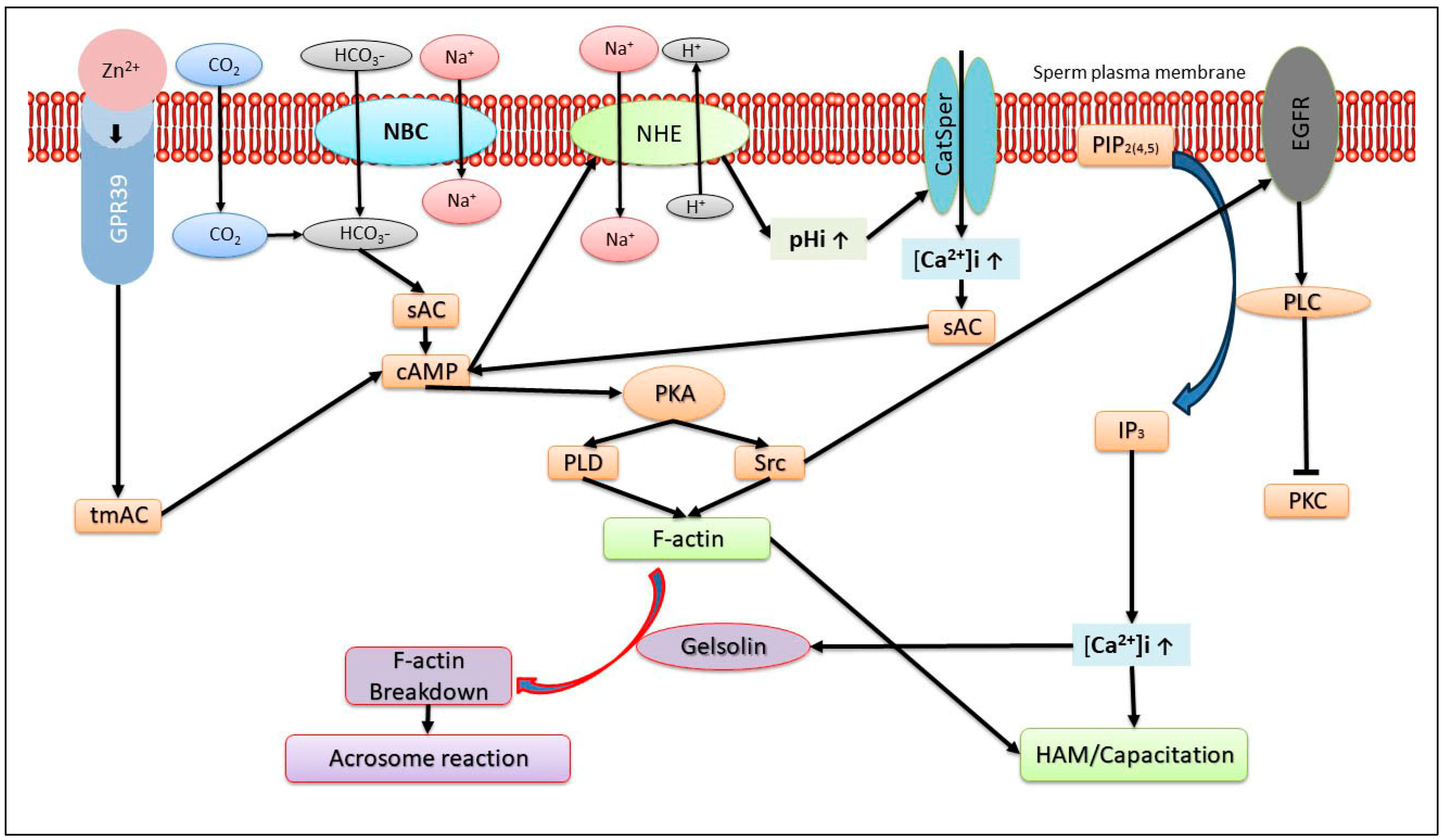
2. Role of Actin Polymerization
3. Ca2+ Transport Mechanisms Regulates the AR
4. Role of Reactive Oxygen Species (ROS) and Mitochondrial Activity in the sAR
5. Role of Energy Metabolism in the sAR
6. Role of Zn2+ in the sAR
7. Role of Protein Acetylation in the sAR
8. Additional Factors Regulating sAR
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Florman, H.M.; Jungnickel, M.K.; Sutton, K.A. Regulating the acrosome reaction. Int. J. Dev. Biol. 2008, 52, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Jungnickel, M.K.; Sutton, K.A.; Wang, Y.; Florman, H.M. Phosphoinositide-dependent pathways in mouse sperm are regulated by egg ZP3 and drive the acrosome reaction. Dev. Biol. 2007, 304, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Florman, H.M.; Storey, B.T. Mouse gamete interactions: The zona pellucida is the site of the acrosome reaction leading to fertilization in vitro. Dev. Biol. 1982, 91, 121–130. [Google Scholar] [CrossRef] [PubMed]
- La Spina, F.A.; Puga Molina, L.C.; Romarowski, A.; Vitale, A.M.; Falzone, T.L.; Krapf, D.; Hirohashi, N.; Buffone, M.G. Mouse sperm begin to undergo acrosomal exocytosis in the upper isthmus of the oviduct. Dev. Biol. 2016, 411, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, T.; Ikawa, M.; Yamada, S.; Parvinen, M.; Baba, T.; Nishimune, Y.; Okabe, M. Real-time observation of acrosomal dispersal from mouse sperm using GFP as a marker protein. FEBS Lett. 1999, 449, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Baibakov, B.; Gauthier, L.; Talbot, P.; Rankin, T.L.; Dean, J. Sperm binding to the zona pellucida is not sufficient to induce acrosome exocytosis. Development 2007, 134, 933–943. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Fujiwara, E.; Kakiuchi, Y.; Okabe, M.; Satouh, Y.; Baba, S.A.; Chiba, K.; Hirohashi, N. Most fertilizing mouse spermatozoa begin their acrosome reaction before contact with the zona pellucida during in vitro fertilization. Proc. Natl. Acad. Sci. USA 2011, 108, 4892–4896. [Google Scholar] [CrossRef]
- Amari, S.; Yonezawa, N.; Mitsui, S.; Katsumata, T.; Hamano, S.; Kuwayama, M.; Hashimoto, Y.; Suzuki, A.; Takeda, Y.; Nakano, M. Essential role of the nonreducing terminal alpha-mannosyl residues of the N-linked carbohydrate chain of bovine zona pellucida glycoproteins in sperm-egg binding. Mol. Reprod. Dev. 2001, 59, 221–226. [Google Scholar] [CrossRef]
- Hirohashi, N.; Yanagimachi, R. Sperm acrosome reaction: Its site and role in fertilization. Biol. Reprod. 2018, 99, 127–133. [Google Scholar] [CrossRef]
- Visconti, P.E.; Krapf, D.; de la Vega-Beltran, J.L.; Acevedo, J.J.; Darszon, A. Ion channels, phosphorylation and mammalian sperm capacitation. Asian J. Androl. 2011, 13, 395–405. [Google Scholar] [CrossRef]
- Brener, E.; Rubinstein, S.; Cohen, G.; Shternall, K.; Rivlin, J.; Breitbart, H. Remodeling of the actin cytoskeleton during mammalian sperm capacitation and acrosome reaction. Biol. Reprod. 2003, 68, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Shabtay, O.; Breitbart, H. CaMKII prevents spontaneous acrosomal exocytosis in sperm through induction of actin polymerization. Dev. Biol. 2016, 415, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Fenichel, P.; Donzeau, M.; Farahifar, D.; Basteris, B.; Ayraud, N.; Hsi, B.L. Dynamics of human sperm acrosome reaction: Relation with in vitro fertilization. Fertil. Steril. 1991, 55, 994–999. [Google Scholar] [CrossRef]
- Bozhedomov, V.A.; Lipatova, N.A.; Rokhlikov, I.M.; Alexeev, R.A.; Ushakova, I.V.; Sukhikh, G.T. Male fertility and varicocoele: Role of immune factors. Andrology 2014, 2, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Samavat, J.; Natali, I.; Degl’Innocenti, S.; Filimberti, E.; Cantini, G.; Di Franco, A.; Danza, G.; Seghieri, G.; Lucchese, M.; Baldi, E.; et al. Acrosome reaction is impaired in spermatozoa of obese men: A preliminary study. Fertil. Steril. 2014, 102, 1274–1281.e2. [Google Scholar] [CrossRef] [PubMed]
- Bunay, J.; Gallardo, L.M.; Torres-Fuentes, J.L.; Aguirre-Arias, M.V.; Orellana, R.; Sepulveda, N.; Moreno, R.D. A decrease of docosahexaenoic acid in testes of mice fed a high-fat diet is associated with impaired sperm acrosome reaction and fertility. Asian J. Androl. 2021, 23, 306–313. [Google Scholar] [PubMed]
- Mu, Y.; Yan, W.-J.; Yin, T.-L.; Zhang, Y.; Li, J.; Yang, J. Diet-induced obesity impairs spermatogenesis: A potential role for autophagy. Sci. Rep. 2017, 7, 43475. [Google Scholar] [CrossRef] [PubMed]
- Francou, M.M.; Girela, J.L.; De Juan, A.; Ten, J.; Bernabeu, R.; De Juan, J. Human sperm motility, capacitation and acrosome reaction are impaired by 2-arachidonoylglycerol endocannabinoid. Histol. Histopathol. 2017, 32, 1351–1358. [Google Scholar]
- Miller, M.R.; Mannowetz, N.; Iavarone, A.T.; Safavi, R.; Gracheva, E.O.; Smith, J.F.; Hill, R.Z.; Bautista, D.M.; Kirichok, Y.; Lishko, P.V. Unconventional endocannabinoid signaling governs sperm activation via the sex hormone progesterone. Science 2016, 352, 555–559. [Google Scholar] [CrossRef]
- Xuan, X.J.; Xu, C.; Zhao, Y.R.; Wu, K.L.; Chen, T.; Zhang, H.B.; Li, X.; Su, S.Z.; Ma, G.; Tang, R.; et al. Application of spontaneous acrosome reaction of sperm in prediction of outcome of in-vitro fertilization and embryo transfer. Zhonghua Yi Xue Za Zhi 2016, 96, 1285–1288. [Google Scholar]
- Ushiyama, A.; Tajima, A.; Ishikawa, N.; Asano, A. Modification of membrane cholesterol and desmosterol in chicken spermatozoa improves post-thaw survival and prevents impairment of sperm function after cryopreservation. Reprod. Fertil. Dev. 2018, 30, 591–599. [Google Scholar] [CrossRef]
- Kholkute, S.D.; Meherji, P.; Puri, C.P. Capacitation and the acrosome reaction in sperm from men with various semen profiles monitored by a chlortetracycline fluorescence assay. Int. J. Androl. 1992, 15, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Topfer-Petersen, E.; Volcker, C.; Heissler, E.; Schill, W.B. Absence of acrosome reaction in polyzoospermia. Andrologia 1987, 19, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Schill, W.B.; Topfer-Petersen, E.; Heissler, E. The sperm acrosome: Functional and clinical aspects. Hum. Reprod. 1988, 3, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Herrera, J.; Fierro, R.; Zayas, H.; Conejo, J.; Jimenez, I.; Garcia, A.; Betancourt, M. Acrosome reaction in fertile and subfertile boar sperm. Arch. Androl. 2002, 48, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Guidobaldi, H.A.; Hirohashi, N.; Cubilla, M.; Buffone, M.G.; Giojalas, L.C. An intact acrosome is required for the chemotactic response to progesterone in mouse spermatozoa. Mol. Reprod. Dev. 2017, 84, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Suarez, S.S.; Katz, D.F.; Overstreet, J.W. Movement characteristics and acrosomal status of rabbit spermatozoa recovered at the site and time of fertilization. Biol. Reprod. 1983, 29, 1277–1287. [Google Scholar] [CrossRef]
- Cohen, G.; Rubinstein, S.; Gur, Y.; Breitbart, H. Crosstalk between protein kinase A and C regulates phospholipase D and F-actin formation during sperm capacitation. Dev. Biol. 2004, 267, 230–241. [Google Scholar] [CrossRef]
- Visconti, P.E.; Bailey, J.L.; Moore, G.D.; Pan, D.; Olds-Clarke, P.; Kopf, G.S. Capacitation of mouse spermatozoa. I. Correlation between the capacitation state and protein tyrosine phosphorylation. Development 1995, 121, 1129–1137. [Google Scholar] [CrossRef]
- Visconti, P.E.; Moore, G.D.; Bailey, J.L.; Laclerc, P.; Connors, S.A.; Pan, D.; Olds-Clarke, P.; Kopf, G.S. Capacitation in mouse spermatozoa. II. Protein tyrosine phosphorylation and capacitation are regulated by a cAMP-dependent pathway. Development 1995, 121, 1139–1150. [Google Scholar] [CrossRef]
- Tsirulnikov, E.; Huta, Y.; Breitbart, H. PKA and PI3K activities during capacitation protect sperm from undergoing spontaneous acrosome reaction. Theriogenology 2019, 128, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.A.; Kopf, G.S.; Storey, B.T. Effects of phorbol esters and a diacylglycerol on the mouse sperm acrosome reaction induced by the zona pellucida. Biol. Reprod. 1987, 36, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Albrizio, M.; Lacalandra, G.M.; Cinone, M. The role of bicarbonate in the modulation of capacitation, spontaneous acrosome reaction and motility of equine fresh and frozen spermatozoa. Theriogenology 2022, 187, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Visconti, P.E.; Stewart-Savage, J.; Blasco, A.; Battaglia, L.; Miranda, P.; Kopf, G.S.; Tezon, J.G. Roles of bicarbonate, cAMP, and protein tyrosine phosphorylation on capacitation and the spontaneous acrosome reaction of hamster sperm. Biol. Reprod. 1999, 61, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Green, S.; Fishel, S. Morphology comparison of individually selected hyperactivated and non-hyperactivated human spermatozoa. Hum. Reprod. 1999, 14, 123–130. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Angeles-Floriano, T.; Roa-Espitia, A.L.; Baltierrez-Hoyos, R.; Cordero-Martinez, J.; Elizondo, G.; Hernandez-Gonzalez, E.O. Absence of aryl hydrocarbon receptor alters CDC42 expression and prevents actin polymerization during capacitation. Mol. Reprod. Dev. 2016, 83, 1015–1026. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, M.; Etkovitz, N.; Breitbart, H. Role and regulation of sperm gelsolin prior to fertilization. J. Biol. Chem. 2010, 285, 39702–39709. [Google Scholar] [CrossRef] [PubMed]
- Etkovitz, N.; Rubinstein, S.; Daniel, L.; Breitbart, H. Role of PI3-kinase and PI4-kinase in actin polymerization during bovine sperm capacitation. Biol. Reprod. 2007, 77, 263–273. [Google Scholar] [CrossRef]
- Rotfeld, H.; Hillman, P.; Ickowicz, D.; Breitbart, H. PKA and CaMKII mediate PI3K activation in bovine sperm by inhibition of the PKC/PP1 cascade. Reproduction 2014, 147, 347–356. [Google Scholar] [CrossRef]
- Belenky, M.; Breitbart, H. Role and regulation of glycogen synthase kinase-3 beta in bovine spermatozoa. Mol. Reprod. Dev. 2017, 84, 8–18. [Google Scholar] [CrossRef]
- Grinshtain, E.; Shpungin, S.; Baum, M.; Nir, U.; Breitbart, H. The Fer tyrosine kinase protects sperm from spontaneous acrosome reaction. Dev. Biol. 2022, 487, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshana, C.; Setiawan, R.; Tajima, A.; Asano, A. Src family kinases-mediated negative regulation of sperm acrosome reaction in chickens (Gallus gallus domesticus). PLoS ONE 2020, 15, e0241181. [Google Scholar] [CrossRef] [PubMed]
- Caran, N.; Johnson, L.D.; Jenkins, K.J.; Tombes, R.M. Cytosolic targeting domains of gamma and delta calmodulin-dependent protein kinase II. J. Biol. Chem. 2001, 276, 42514–42519. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, F.; Zitranski, N.; Borth, H.; Buech, T.; Gudermann, T.; Boekhoff, I. CaMKIIalpha interacts with multi-PDZ domain protein MUPP1 in spermatozoa and prevents spontaneous acrosomal exocytosis. J. Cell Sci. 2009, 122 Pt 24, 4547–4557. [Google Scholar] [CrossRef] [PubMed]
- Huta, Y.; Nitzan, Y.; Breitbart, H. Ezrin protects bovine spermatozoa from spontaneous acrosome reaction. Theriogenology 2020, 151, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Stock, C.E.; Fraser, L.R. Divalent cations, capacitation and the acrosome reaction in human spermatozoa. J. Reprod. Fertil. 1989, 87, 463–478. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Luconi, M.; Krausz, C.; Forti, G.; Baldi, E. Extracellular calcium negatively modulates tyrosine phosphorylation and tyrosine kinase activity during capacitation of human spermatozoa. Biol. Reprod. 1996, 55, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Dahan, T.; Breitbart, H. Involvement of metabolic pathway in the sperm spontaneous acrosome reaction. Theriogenology 2022, 192, 38–44. [Google Scholar] [CrossRef]
- Walensky, L.D.; Snyder, S.H. Inositol 1,4,5-trisphosphate receptors selectively localized to the acrosomes of mammalian sperm. J. Cell Biol. 1995, 130, 857–869. [Google Scholar] [CrossRef]
- Itzhakov, D.; Nitzan, Y.; Breitbart, H. Protein kinase A inhibition induces EPAC-dependent acrosomal exocytosis in human sperm. Asian J. Androl. 2019, 21, 337–344. [Google Scholar]
- Endo, D.; Kon, S.; Sato, T.; Toyama, F.; Katsura, Y.; Nakauchi, Y.; Takayama-Watanabe, E.; Watanabe, A. NMDA-type glutamate receptors mediate the acrosome reaction and motility initiation in newt sperm. Mol. Reprod. Dev. 2019, 86, 1106–1115. [Google Scholar] [CrossRef]
- Lv, M.G.; Chen, W.Q.; Weng, S.Q.; Chen, H.Y.; Cheng, Y.M.; Luo, T. Rosmarinic acid compromises human sperm functions by an intracellular Ca2+ concentration-related mechanism. Reprod. Toxicol. 2018, 81, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.-H.; Navarro, B.; Xia, X.-M.; Clapham, D.E.; Lingle, C.J. Simultaneous knockout of Slo3 and CatSper1 abolishes all alkalization- and voltage-activated current in mouse spermatozoa. J. Gen. Physiol. 2013, 142, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Navarro, B.; Kirichok, Y.; Clapham, D.E. KSper, a pH-sensitive K+ current that controls sperm membrane potential. Proc. Natl. Acad. Sci. USA 2007, 104, 7688–7692. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.H.; Yen, Y.K.; Ling, T.Y.; Cheng, K.T.; Shu, J.A.; Au, H.K.; Huang, Y.H. Capacitation suppression by mouse seminal vesicle autoantigen involves a decrease in plasma membrane Ca2+-ATPase (PMCA)-mediated intracellular calcium. J. Cell. Biochem. 2010, 111, 1188–1198. [Google Scholar] [CrossRef] [PubMed]
- Mata-Martinez, E.; Darszon, A.; Trevino, C.L. pH-dependent Ca2+ oscillations prevent untimely acrosome reaction in human sperm. Biochem. Biophys. Res. Commun. 2018, 497, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Cardenas, C.; Servin-Vences, M.R.; Jose, O.; Trevino, C.L.; Hernandez-Cruz, A.; Darszon, A. Acrosome reaction and Ca2+ imaging in single human spermatozoa: New regulatory roles of [Ca2+]i. Biol. Reprod. 2014, 91, 67. [Google Scholar] [CrossRef] [PubMed]
- Koh, T.W.; Bellen, H.J. Synaptotagmin I, a Ca2+ sensor for neurotransmitter release. Trends Neurosci. 2003, 26, 413–422. [Google Scholar] [CrossRef]
- Brose, N. For better or for worse: Complexins regulate SNARE function and vesicle fusion. Traffic 2008, 9, 1403–1413. [Google Scholar] [CrossRef]
- Brukman, N.G.; Nunez, S.Y.; Puga Molina, L.D.C.; Buffone, M.G.; Darszon, A.; Cuasnicu, P.S.; Da Ros, V.G. Tyrosine phosphorylation signaling regulates Ca(2+) entry by affecting intracellular pH during human sperm capacitation. J. Cell. Physiol. 2019, 234, 5276–5288. [Google Scholar] [CrossRef]
- McMahon, H.T.; Missler, M.; Li, C.; Sudhof, T.C. Complexins: Cytosolic proteins that regulate SNAP receptor function. Cell 1995, 83, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Sudhof, T.C.; Rothman, J.E. Membrane fusion: Grappling with SNARE and SM proteins. Science 2009, 323, 474–477. [Google Scholar] [CrossRef] [PubMed]
- Weber, T.; Zemelman, B.V.; McNew, J.A.; Westermann, B.; Gmachl, M.; Parlati, F.; Sollner, T.H.; Rothman, J.E. SNAREpins: Minimal machinery for membrane fusion. Cell 1998, 92, 759–772. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, T.; Ikawa, M.; Yamada, S.; Toshimori, K.; Okabe, M. Alkalinization of acrosome measured by GFP as a pH indicator and its relation to sperm capacitation. Dev. Biol. 2001, 237, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Lishko, P.V.; Kirichok, Y. The role of Hv1 and CatSper channels in sperm activation. J. Physiol. 2010, 588, 4667–4672. [Google Scholar] [CrossRef] [PubMed]
- Barati, E.; Nikzad, H.; Karimian, M. Oxidative stress and male infertility: Current knowledge of pathophysiology and role of antioxidant therapy in disease management. Cell. Mol. Life Sci. 2020, 77, 93–113. [Google Scholar] [CrossRef] [PubMed]
- O’Flaherty, C.; de Lamirande, E.; Gagnon, C. Reactive oxygen species modulate independent protein phosphorylation pathways during human sperm capacitation. Free Radic. Biol. Med. 2006, 40, 1045–1055. [Google Scholar] [CrossRef]
- Bui, A.D.; Sharma, R.; Henkel, R.; Agarwal, A. Reactive oxygen species impact on sperm DNA and its role in male infertility. Andrologia 2018, 50, e13012. [Google Scholar] [CrossRef]
- Alvarez, J.G.; Touchtone, J.C.; Blasco, L.; Storey, B.T. Spontaneous lipid peroxidation and production of hydrogen peroxide and superoxide in human spermatozoa: Superoxide dismutase as a major enzyme protectant against oxygen toxicity. J. Androl. 1987, 8, 338–348. [Google Scholar] [CrossRef]
- Jeulin, C.; Soufir, J.C.; Weber, P.; Laval-Martin, D.; Calvayrac, R. Catalase activity in human spermatozoa and seminal plasma. Gamete Res. 1989, 24, 185–196. [Google Scholar] [CrossRef]
- Nir, U.; Grinshtain, E.; Breitbart, H. Fer and FerT: A New Regulatory Link between Sperm and Cancer Cells. Int. J. Mol. Sci. 2023, 24, 5256. [Google Scholar] [CrossRef] [PubMed]
- de Lamirande, E.; Gagnon, C. A positive role for the superoxide anion in triggering hyperactivation and capacitation of human spermatozoa. Int. J. Androl. 1993, 16, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Rivlin, J.; Mendel, J.; Rubinstein, S.; Etkovitz, N.; Breitbart, H. Role of hydrogen peroxide in sperm capacitation and acrosome reaction. Biol. Reprod. 2004, 70, 518–522. [Google Scholar] [CrossRef] [PubMed]
- de Lamirande, E.; O’Flaherty, C. Sperm activation: Role of reactive oxygen species and kinases. Biochim. Biophys. Acta 2008, 1784, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Faggi, M.; Vanzetti, A.; Teijeiro, J.M. Effect of glucose and reactive oxygen species on boar sperm induced-acrosome exocytosis. Res. Vet. Sci. 2023, 164, 105013. [Google Scholar] [CrossRef] [PubMed]
- Yaffe, E.; Hikri, E.; Elkis, Y.; Cohen, O.; Segal, A.; Makovski, A.; Varvak, A.; Shpungin, S.; Nir, U. Oncogenic properties of a spermatogenic meiotic variant of fer kinase expressed in somatic cells. Cancer Res. 2014, 74, 6474–6485. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Wen, Z.Z.; Wu, B.; Zhu, H.X.; Zhang, A.Z.; Li, J.Y.; Gao, J.G. Deletion of Aldh4a1 Leads to Impaired Sperm Maturation in Mice. Mol. Biol. 2022, 56, 585–594. [Google Scholar] [CrossRef]
- Choi, Y.J.; Uhm, S.J.; Song, S.J.; Song, H.; Park, J.K.; Kim, T.; Park, C.; Kim, J.H. Cytochrome c upregulation during capacitation and spontaneous acrosome reaction determines the fate of pig sperm cells: Linking proteome analysis. J. Reprod. Dev. 2008, 54, 68–83. [Google Scholar] [CrossRef]
- delBarco-Trillo, J.; Mateo, R.; Roldan, E.R. Differences in the fatty-acid composition of rodent spermatozoa are associated to levels of sperm competition. Biol. Open 2015, 4, 466–473. [Google Scholar] [CrossRef]
- Li, H.L.; Liu, D.P.; Liang, C.C. Paraoxonase gene polymorphisms, oxidative stress, and diseases. J. Mol. Med. 2003, 81, 766–779. [Google Scholar] [CrossRef]
- Efrat, M.; Stein, A.; Pinkas, H.; Breitbart, H.; Unger, R.; Birk, R. Paraoxonase 1 (PON1) attenuates sperm hyperactivity and spontaneous acrosome reaction. Andrology 2019, 7, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Volk, M.; Jaklic, H.; Zorn, B.; Peterlin, B. Association between male infertility and genetic variability at the PON1/2 and GSTM1/T1 gene loci. Reprod. Biomed. Online 2011, 23, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Plum, L.M.; Rink, L.; Haase, H. The essential toxin: Impact of zinc on human health. Int. J. Environ. Res. Public Health 2010, 7, 1342–1365. [Google Scholar] [CrossRef] [PubMed]
- Sauer, A.; Hagmeyer, S.; Grabrucker, A. Zinc Deficiency. In Nutritional Deficiency; IntechOpen: London, UK, 2016; pp. 23–46. [Google Scholar]
- Wani, A.; Parveen, N.; Ansari, M.O.; Ahmad, F.; Jameel, S.; Shadab, G.G.H.A. Zinc: An element of extensive medical importance. Curr. Med. Res. Pract. 2017, 7, 90–98. [Google Scholar] [CrossRef]
- Albert, A. Selective Toxicity: The Physico-Chemical Basis of Therapy; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Vijayalakshmi, K.; Sivaraj, D. Enhanced antibacterial activity of Cr doped ZnO nanorods synthesized using microwave processing. RSC Adv. R. Soc. Chem. 2015, 5, 68461–68469. [Google Scholar] [CrossRef]
- Colagar, A.H.; Marzony, E.T.; Chaichi, M.J. Zinc levels in seminal plasma are associated with sperm quality in fertile and infertile men. Nutr. Res. 2009, 29, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Brito, M.; Figueroa, J.; Vera, J.C.; Cortés, P.; Hott, R.; Burzio, L.O. Phosphoproteins are structural components of bull sperm outer dense fiber. Gamete Res. 1986, 15, 327–336. [Google Scholar] [CrossRef]
- O’Flaherty, C.; Matsushita-Fournier, D. Reactive oxygen species and protein modifications in spermatozoa. Biol. Reprod. 2017, 97, 577–585. [Google Scholar] [CrossRef]
- Lee, S.R. Critical Role of Zinc as Either an Antioxidant or a Prooxidant in Cellular Systems. Oxidative Med. Cell. Longev. 2018, 2018, 9156285. [Google Scholar] [CrossRef]
- Kerns, K.; Sharif, M.; Zigo, M.; Xu, W.; Hamilton, L.E.; Sutovsky, M.; Ellersieck, M.; Drobnis, E.Z.; Bovin, N.; Oko, R.; et al. Sperm Cohort-Specific Zinc Signature Acquisition and Capacitation-Induced Zinc Flux Regulate Sperm-Oviduct and Sperm-Zona Pellucida Interactions. Int. J. Mol. Sci. 2020, 21, 2121. [Google Scholar] [CrossRef]
- Clapper, D.L.; Davis, J.A.; Lamothe, P.J.; Patton, C.; Epel, D. Involvement of zinc in the regulation of pHi, motility, and acrosome reactions in sea urchin sperm. J. Cell Biol. 1985, 100, 1817–1824. [Google Scholar] [CrossRef] [PubMed]
- Allouche-Fitoussi, D.; Bakhshi, D.; Breitbart, H. Signaling pathways involved in human sperm hyperactivated motility stimulated by Zn2. Mol. Reprod. Dev. 2018, 85, 543–556. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.; Forster, H.; Boersma, A.; Seiler, A.; Wehnes, H.; Sinowatz, F.; Neumuller, C.; Deutsch, M.J.; Walch, A.; Hrabe de Angelis, M.; et al. Mitochondrial glutathione peroxidase 4 disruption causes male infertility. FASEB J. 2009, 23, 3233–3242. [Google Scholar] [CrossRef] [PubMed]
- Michailov, Y.; Ickowicz, D.; Breitbart, H. Zn2+-stimulation of sperm capacitation and of the acrosome reaction is mediated by EGFR activation. Dev. Biol. 2014, 396, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Etkovitz, N.; Tirosh, Y.; Chazan, R.; Jaldety, Y.; Daniel, L.; Rubinstein, S.; Breitbart, H. Bovine sperm acrosome reaction induced by G-protein-coupled receptor agonists is mediated by epidermal growth factor receptor transactivation. Dev. Biol. 2009, 334, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Windler, W.; Bönigk, W.; Körschen, H.G.; Grahn, E.; Strünker, T.; Seifert, R.; Kaupp, U.B. The solute carrier SLC9C1 is a Na+/H+-exchanger gated by an S4-type voltage-sensor and cyclic-nucleotide binding. Nat. Commun. 2018, 9, 2809. [Google Scholar] [CrossRef]
- Ho, H.C.; Suarez, S.S. An inositol 1,4,5-trisphosphate receptor-gated intracellular Ca2+ store is involved in regulating sperm hyperactivated motility. Biol. Reprod. 2001, 65, 1606–1615. [Google Scholar] [CrossRef]
- Bowker, Z.; Goldstein, S.; Breitbart, H. Protein acetylation protects sperm from spontaneous acrosome reaction. Theriogenology 2022, 191, 231–238. [Google Scholar] [CrossRef]
- Anderson, K.A.; Hirschey, M.D. Mitochondrial protein acetylation regulates metabolism. Essays Biochem. 2012, 52, 23–35. [Google Scholar]
- Sun, G.; Jiang, M.; Zhou, T.; Guo, Y.; Cui, Y.; Guo, X.; Sha, J. Insights into the lysine acetylproteome of human sperm. J. Proteom. 2014, 109, 199–211. [Google Scholar] [CrossRef]
- Yu, H.; Diao, H.; Wang, C.; Lin, Y.; Yu, F.; Lu, H.; Xu, W.; Li, Z.; Shi, H.; Zhao, S.; et al. Acetylproteomic analysis reveals functional implications of lysine acetylation in human spermatozoa (sperm). Mol. Cell. Proteom. 2015, 14, 1009–1023. [Google Scholar] [CrossRef]
- Piperno, G.; Fuller, M.T. Monoclonal antibodies specific for an acetylated form of alpha-tubulin recognize the antigen in cilia and flagella from a variety of organisms. J. Cell Biol. 1985, 101, 2085–2094. [Google Scholar] [CrossRef]
- Bhagwat, S.; Dalvi, V.; Chandrasekhar, D.; Matthew, T.; Acharya, K.; Gajbhiye, R.; Kulkarni, V.; Sonawane, S.; Ghosalkar, M.; Parte, P. Acetylated alpha-tubulin is reduced in individuals with poor sperm motility. Fertil. Steril. 2014, 101, 95–104.e3. [Google Scholar] [CrossRef] [PubMed]
- Ritagliati, C.; Luque, G.M.; Stival, C.; Baro Graf, C.; Buffone, M.G.; Krapf, D. Lysine acetylation modulates mouse sperm capacitation. Sci. Rep. 2018, 8, 13334. [Google Scholar] [CrossRef] [PubMed]
- Branham, M.T.; Bustos, M.A.; De Blas, G.A.; Rehmann, H.; Zarelli, V.E.; Trevino, C.L.; Darszon, A.; Mayorga, L.S.; Tomes, C.N. Epac activates the small G proteins Rap1 and Rab3A to achieve exocytosis. J. Biol. Chem. 2009, 284, 24825–24839. [Google Scholar] [CrossRef] [PubMed]
- Branham, M.T.; Mayorga, L.S.; Tomes, C.N. Calcium-induced acrosomal exocytosis requires cAMP acting through a protein kinase A-independent, Epac-mediated pathway. J. Biol. Chem. 2006, 281, 8656–8666. [Google Scholar] [CrossRef] [PubMed]
- Azoulay, Y.; Malik, Z.; Breitbart, H. Sperm interaction with bacteria induces the spontaneous acrosome reaction. Theriogenology 2023, 203, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.S.; Webb, S.; Lettice, L.; Tardif, S.; Kilanowski, F.; Tyrrell, C.; Macpherson, H.; Semple, F.; Tennant, P.; Baker, T.; et al. Partial deletion of chromosome 8 beta-defensin cluster confers sperm dysfunction and infertility in male mice. PLoS Genet. 2013, 9, e1003826. [Google Scholar] [CrossRef] [PubMed]
- Dorin, J.R. Novel phenotype of mouse spermatozoa following deletion of nine beta-defensin genes. Asian J. Androl. 2015, 17, 716–719. [Google Scholar] [CrossRef]
- Sebkova, N.; Ded, L.; Vesela, K.; Dvorakova-Hortova, K. Progress of sperm IZUMO1 relocation during spontaneous acrosome reaction. Reproduction 2014, 147, 231–240. [Google Scholar] [CrossRef]
- Inoue, N.; Ikawa, M.; Nakanishi, T.; Matsumoto, M.; Nomura, M.; Seya, T.; Okabe, M. Disruption of mouse CD46 causes an accelerated spontaneous acrosome reaction in sperm. Mol. Cell. Biol. 2003, 23, 2614–2622. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.M.; Clift, L.E.; Andrlikova, P.; Jursova, M.; Flanagan, B.F.; Cummerson, J.A.; Stopka, P.; Dvorakova-Hortova, K. Rapid sperm acrosome reaction in the absence of acrosomal CD46 expression in promiscuous field mice (Apodemus). Reproduction 2007, 134, 739–747. [Google Scholar] [CrossRef] [PubMed]
- Clift, L.E.; Andrlikova, P.; Frolikova, M.; Stopka, P.; Bryja, J.; Flanagan, B.F.; Johnson, P.M.; Dvorakova-Hortova, K. Absence of spermatozoal CD46 protein expression and associated rapid acrosome reaction rate in striped field mice (Apodemus agrarius). Reprod. Biol. Endocrinol. 2009, 7, 29. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Liu, D.; Zhu, H.; Sun, X.; Xiao, Y.; Lin, Z.; Zhang, A.; Ye, C.; Gao, J. Deficiency for Lcn8 causes epididymal sperm maturation defects in mice. Biochem. Biophys. Res. Commun. 2021, 548, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Jaldety, Y.; Glick, Y.; Orr-Urtreger, A.; Ickowicz, D.; Gerber, D.; Breitbart, H. Sperm epidermal growth factor receptor (EGFR) mediates alpha7 acetylcholine receptor (AChR) activation to promote fertilization. J. Biol. Chem. 2012, 287, 22328–22340. [Google Scholar] [CrossRef]
- Makino, Y.; Hiradate, Y.; Umezu, K.; Hara, K.; Tanemura, K. Expression and Possible Role of Nicotinic Acetylcholine Receptor epsilon Subunit (AChRe) in Mouse Sperm. Biology 2021, 10, 46. [Google Scholar] [CrossRef] [PubMed]
- Oyeyipo, I.P.; Maartens, P.J.; du Plessis, S.S. In vitro effects of nicotine on human spermatozoa. Andrologia 2014, 46, 887–892. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Breitbart, H.; Grinshtein, E. Mechanisms That Protect Mammalian Sperm from the Spontaneous Acrosome Reaction. Int. J. Mol. Sci. 2023, 24, 17005. https://doi.org/10.3390/ijms242317005
Breitbart H, Grinshtein E. Mechanisms That Protect Mammalian Sperm from the Spontaneous Acrosome Reaction. International Journal of Molecular Sciences. 2023; 24(23):17005. https://doi.org/10.3390/ijms242317005
Chicago/Turabian StyleBreitbart, Haim, and Elina Grinshtein. 2023. "Mechanisms That Protect Mammalian Sperm from the Spontaneous Acrosome Reaction" International Journal of Molecular Sciences 24, no. 23: 17005. https://doi.org/10.3390/ijms242317005
APA StyleBreitbart, H., & Grinshtein, E. (2023). Mechanisms That Protect Mammalian Sperm from the Spontaneous Acrosome Reaction. International Journal of Molecular Sciences, 24(23), 17005. https://doi.org/10.3390/ijms242317005







