Comparison of the Therapeutic Effects of Adipose- and Bone Marrow-Derived Mesenchymal Stem Cells on Renal Fibrosis
Abstract
:1. Introduction
2. Results
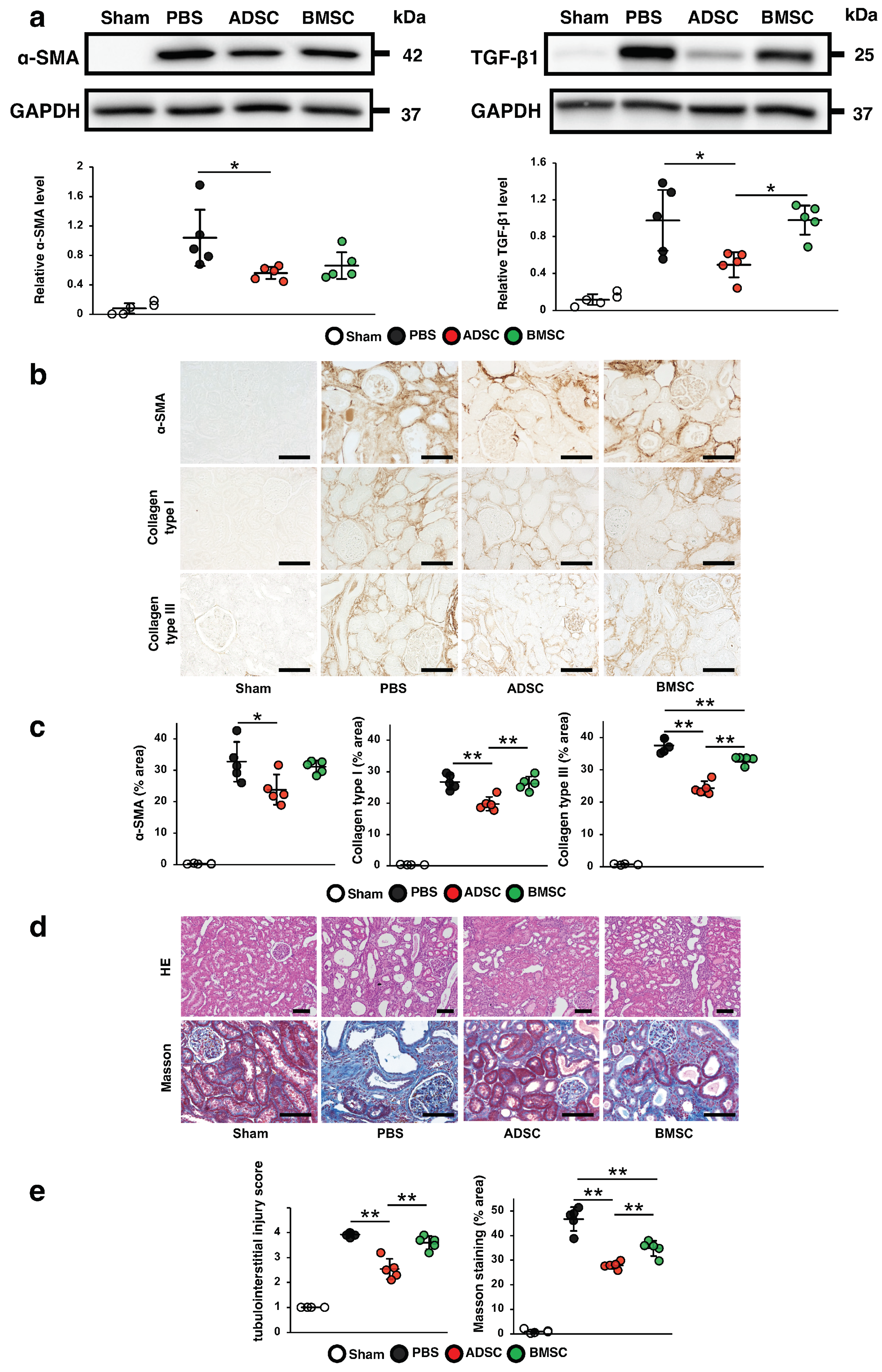
2.1. Comparison of the Therapeutic Effects of ADSCs and BMSCs Cultured in Serum-Containing Medium on IRI-Induced Renal Fibrosis in Rats
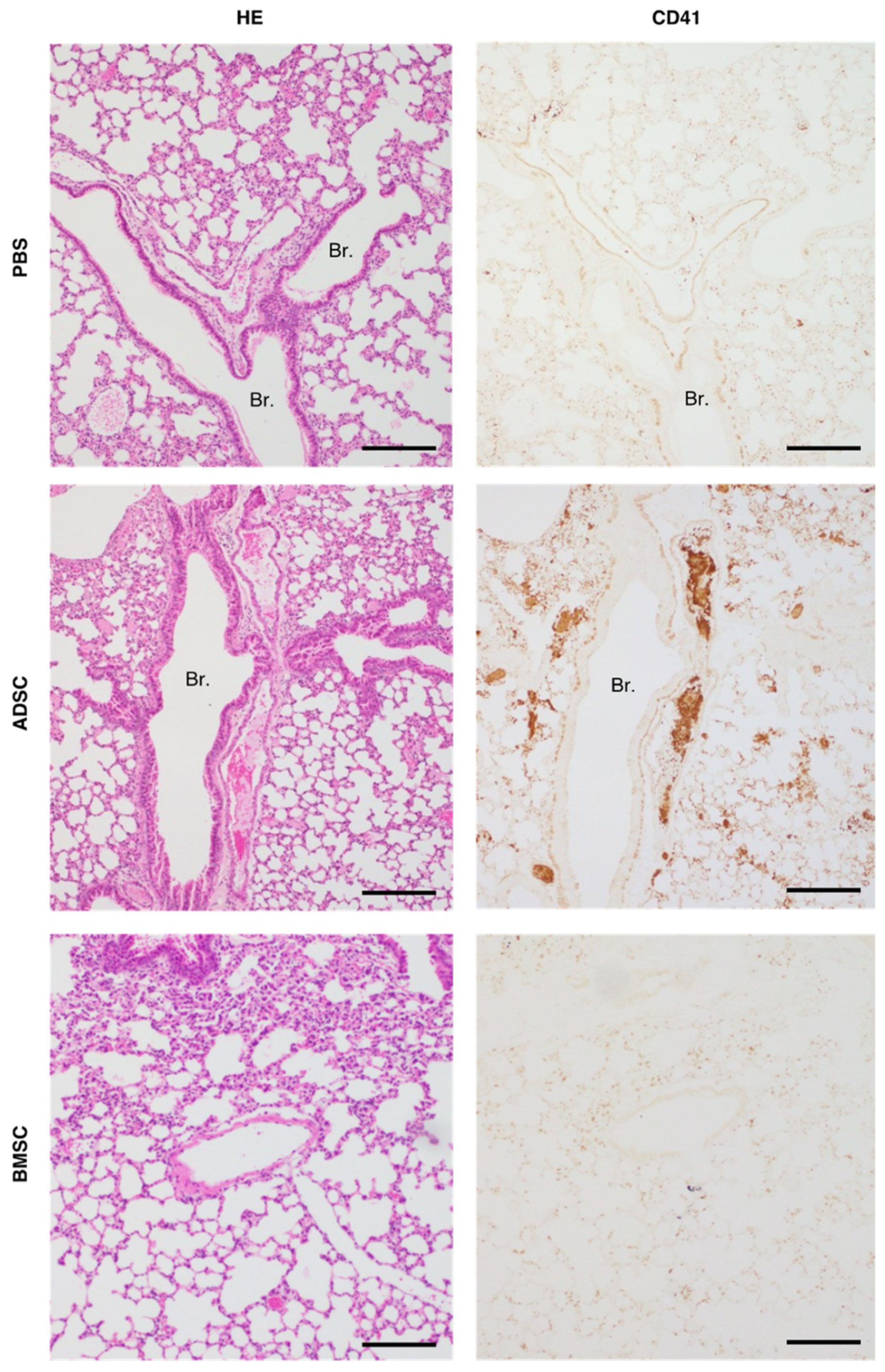
2.2. Comparison of Tail Vein Infusion of ADSCs and BMSCs on the Incidence of Death in Mice
2.3. Comparison of the Procoagulation Factors and Humoral Factors Involved in the Therapeutic Effects of ADSCs and BMSCs
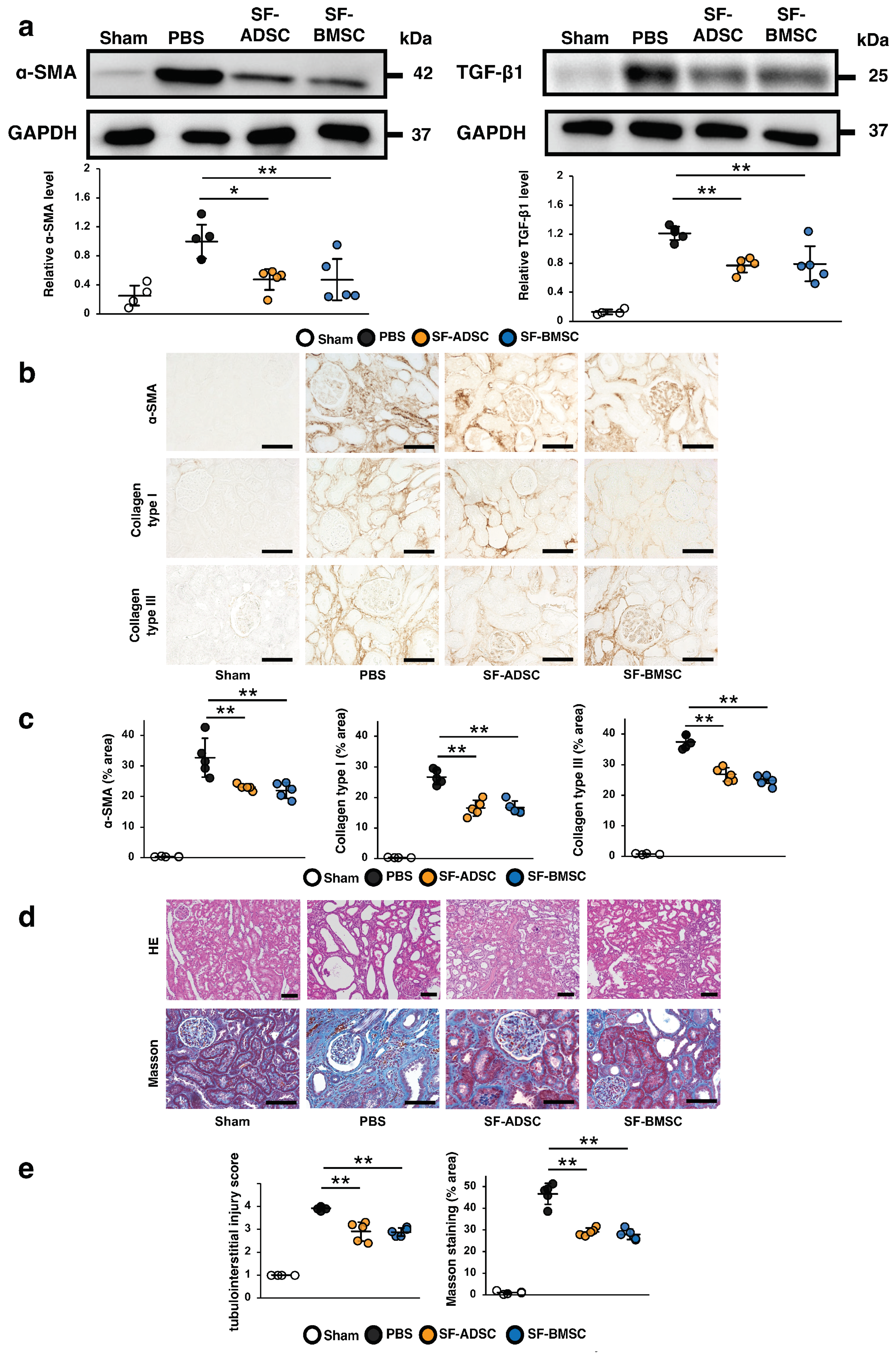
2.4. Comparison of the Therapeutic Effects of ADSCs and BMSCs Cultured in Serum-Free Medium on Renal Fibrosis in IRI Rats
2.5. Comparison of Tail Vein Infusion of SF-ADSCs and SF-BMSCs on the Incidence of Death in Mice
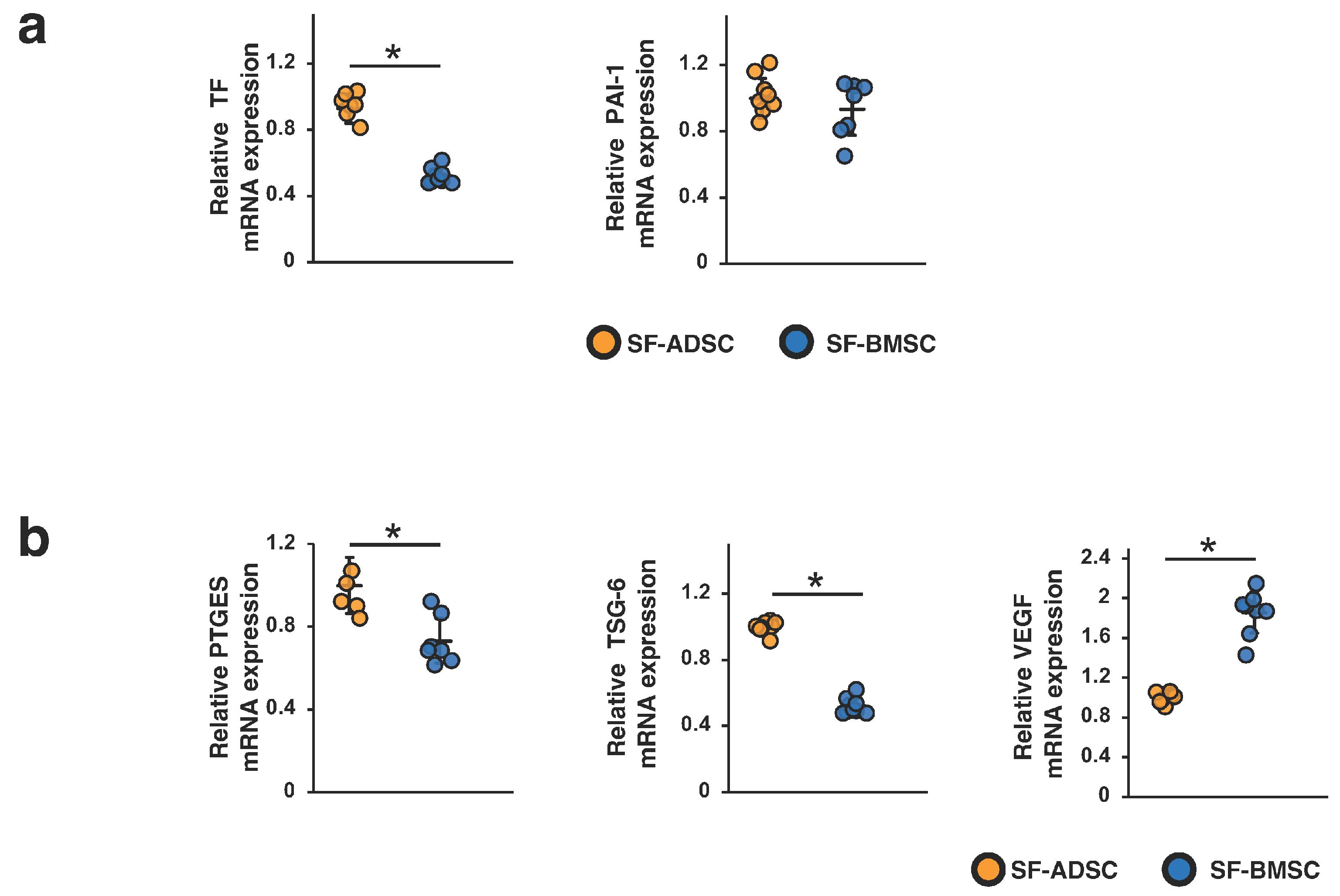
2.6. Comparison of the Procoagulation Factors and Humoral Factors Involved in the Therapeutic Effects of SF-ADSCs and SF-BMSCs
3. Discussion
4. Materials and Methods
4.1. MSC Preparation
4.2. Characterization of MSCs
4.3. Animals
4.4. Experimental Animal Models
4.5. Histological Analysis
4.6. Immunohistochemical Analysis
4.7. Western Blotting
4.8. Quantitative Real-Time Reverse Transcription PCR
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liyanage, T.; Ninomiya, T.; Jha, V.; Neal, B.; Patrice, H.M.; Okpechi, I.; Zhao, M.H.; Lv, J.; Garg, A.X.; Knight, J.; et al. Worldwide access to treatment for end-stage kidney disease: A systematic review. Lancet 2015, 385, 1975–1982. [Google Scholar] [CrossRef] [PubMed]
- Brenner, B.M.; Cooper, M.E.; Zeeuw, D.D.; Keane, W.F.; Mitch, W.E.; Parving, H.H.; Remuzzi, G.; Snapinn, S.M.; Zhang, Z.; Shahinfar, S.; et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N. Engl. J. Med. 2001, 345, 861–869. [Google Scholar] [CrossRef]
- Heerspink, H.J.L.; Stefánsson, B.V.; Correa-Rotter, R.; Chertow, G.M.; Greene, T.; Hou, F.-F.; Mann, J.F.E.; McMurray, J.J.V.; Lindberg, M.; Rossing, P.; et al. Dapagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2020, 383, 1436–1446. [Google Scholar] [CrossRef] [PubMed]
- The EMPA-KIDNEY Collaborative Group. Empagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2023, 388, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Hickson, L.T.J.; Abedalqader, T.; Ben-Bernard, G.; Mondy, J.M.; Bian, X.; Conley, S.M.; Zhu, X.; Herrmann, S.M.; Kukla, A.; Lorenz, E.C.; et al. A systematic review and meta-analysis of cell-based interventions in experimental diabetic kidney disease. Stem Cells Transl. Med. 2021, 10, 1304–1319. [Google Scholar] [CrossRef] [PubMed]
- Morigi, M.; Imberti, B.; Zoja, C.; Corna, D.; Tomasoni, S.; Abbate, M.; Rottoli, D.; Angioletti, S.; Benigni, A.; Perico, N.; et al. Mesenchymal stem cells are renotropic, helping to repair the kidney and improve function in acute renal failure. J. Am. Soc. Nephrol. 2004, 15, 1794–1804. [Google Scholar] [CrossRef]
- Imberti, B.; Morigi, M.; Tomasoni, S.; Rota, C.; Corna, D.; Longaretti, L.; Rottoli, D.; Valsecchi, F.; Benigni, A.; Wang, J.; et al. Insulin-like growth factor-1 sustains stem cell-mediated renal repair. J. Am. Soc. Nephrol. 2007, 18, 2921–2928. [Google Scholar] [CrossRef]
- Tögel, F.; Cohen, A.; Zhang, P.; Yang, Y.; Hu, Z.; Westenfelder, C. Autologous and allogeneic marrow stromal cells are safe and effective for the treatment of acute kidney injury. Stem Cells Dev. 2009, 18, 475–485. [Google Scholar] [CrossRef]
- Semedo, P.; Donizetti-Oliveira, C.; Burgos-Silva, M.; Cenedeze, M.A.; Avancini Costa Malheiros, D.M.; Pacheco-Silva, A.; Camara, N.O.S. Bone marrow mononuclear cells attenuate fibrosis development after severe acute kidney injury. Lab. Investig. 2010, 90, 685–695. [Google Scholar] [CrossRef]
- Alfarano, C.; Roubeix, C.; Chaaya, R.; Ceccaldi, C.; Calise, D.; Mias, C.; Cussac, D.; Bascands, J.L.; Parini, A. Intraparenchymal Injection of bone marrow mesenchymal stem cells reduces kidney fibrosis after ischemia-reperfusion in cyclosporine-immunosuppressed rats. Cell Transplant. 2012, 21, 2009–2019. [Google Scholar] [CrossRef]
- Zhu, F.; Shin, O.L.S.C.L.; Pei, G.; Hu, Z.; Yang, J.; Zhu, H.; Wang, M.; Mou, J.; Sun, J.; Wang, Y.; et al. Adipose-derived mesenchymal stem cells employed exosomes to attenuate AKI-CKD transition through tubular epithelial cell dependent Sox9 activation. Oncotarget 2017, 8, 70707–70726. [Google Scholar] [CrossRef] [PubMed]
- Ishiy, C.S.R.A.; Ormanji, M.S.; Maquigussa, E.; Ribeiro, R.S.; Da Silva Novaes, A.; Boim, M.A. Comparison of the Effects of Mesenchymal Stem Cells with Their Extracellular Vesicles on the Treatment of Kidney Damage Induced by Chronic Renal Artery Stenosis. Stem Cells Int. 2020, 2020, 8814574. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Nakashima, A.; Doi, S.; Ueno, T.; Okubo, T.; Kawano, K.; Kanawa, M.; Kato, Y.; Higashi, Y.; Masaki, T. Serum-free medium enhances the immunosuppressive and antifibrotic abilities of mesenchymal stem cells utilized in experimental renal fibrosis. Stem Cells Transl. Med. 2018, 7, 893–905. [Google Scholar] [CrossRef]
- Moll, G.; Ankrum, J.A.; Kamhieh-Milz, J.; Bieback, K.; Ringdén, O.; Volk, H.D.; Geissler, S.; Reinke, P. Intravascular Mesenchymal Stromal/Stem Cell Therapy Product Diversification: Time for New Clinical Guidelines. Trends Mol. Med. 2019, 25, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Tatsumi, K.; Ohashi, K.; Matsubara, Y.; Kohori, A.; Ohno, T.; Kakidachi, H.; Horii, A.; Kanegae, K.; Utoh, R.; Iwata, T.; et al. Biochemical and Biophysical Research Communications Tissue factor triggers procoagulation in transplanted mesenchymal stem cells leading to thromboembolism. Biochem. Biophys. Res. Commun. 2013, 431, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Moll, G.; Rasmusson-Duprez, I.; Von Bahr, L.; Connolly-Andersen, A.; Elgue, G.; Funke, L.; Hamad, O.; Lönnies, H.; Magnusson, P.; Sanchez, J.; et al. Are therapeutic human mesenchymal stromal cells compatible with human blood? Stem Cells 2012, 30, 1565–1574. [Google Scholar] [CrossRef]
- George, M.J.; Prabhakara, K.; Toledano-Furman, N.E.; Wang, Y.W.; Gill, B.S.; Wade, C.E.; Olson, S.D.; Cox, C.S. Clinical Cellular Therapeutics Accelerate Clot Formation. Stem Cells Transl. Med. 2018, 7, 731–739. [Google Scholar] [CrossRef]
- Frischmuth, T.; Hindberg, K.; Aukrust, P.; Ueland, T.; Brækkan, S.K.; Hansen, J.B.; Morelli, V.M. Elevated plasma levels of plasminogen activator inhibitor-1 are associated with risk of future incident venous thromboembolism. J. Thromb. Haemost. 2022, 20, 1618–1626. [Google Scholar] [CrossRef]
- Kanai, R.; Nakashima, A.; Doi, S.; Kimura, T.; Yoshida, K.; Maeda, S.; Ishiuchi, N.; Yamada, Y.; Ike, T.; Doi, T.; et al. Interferon-γ enhances the therapeutic effect of mesenchymal stem cells on experimental renal fibrosis. Sci. Rep. 2021, 11, 850. [Google Scholar] [CrossRef]
- Kang, D.H.; Hughes, J.; Mazzali, M.; Schreiner, G.F.; Johnson, R.J. Impaired angiogenesis in the remnant kidney model: II. Vascular endothelial growth factor administration reduces renal fibrosis and stabilizes renal function. J. Am. Soc. Nephrol. 2001, 12, 1448–1457. [Google Scholar] [CrossRef]
- Ishiuchi, N.; Nakashima, A.; Doi, S.; Yoshida, K.; Maeda, S.; Kanai, R.; Yamada, Y.; Ike, T.; Doi, T.; Kato, Y.; et al. Hypoxia-preconditioned mesenchymal stem cells prevent renal fibrosis and inflammation in ischemia-reperfusion rats. Stem Cell Res. Ther. 2020, 11, 130. [Google Scholar] [CrossRef]
- Zhao, L.; Han, F.; Wang, J.; Chen, J. Current understanding of the administration of mesenchymal stem cells in acute kidney injury to chronic kidney disease transition: A review with a focus on preclinical models. Stem Cell Res. Ther. 2019, 10, 385. [Google Scholar] [CrossRef]
- Perico, N.; Casiraghi, F.; Remuzzi, G. Clinical Translation of Mesenchymal Stromal Cell Therapies in Nephrology. J. Am. Soc. Nephrol. 2018, 29, 362–375. [Google Scholar] [CrossRef]
- Selim, R.E.; Ahmed, H.H.; Abd-Allah, S.H.; Sabry, G.M.; Hassan, R.E.; Khalil, W.K.B.; Abouhashem, N.S. Mesenchymal Stem Cells: A Promising Therapeutic Tool for Acute Kidney Injury. Appl. Biochem. Biotechnol. 2019, 189, 284–304. [Google Scholar] [CrossRef]
- Zare, H.; Jamshidi, S.; Dehghan, M.M.; Saheli, M.; Piryaei, A. Bone marrow or adipose tissue mesenchymal stem cells: Comparison of the therapeutic potentials in mice model of acute liver failure. J. Cell. Biochem. 2018, 119, 5834–5842. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Lin, J.; Zhao, K.; Jin, K.; He, Q.; Hu, Y.; Feng, G.; Cai, Y.; Xia, C.; Liu, H.; et al. Single-Cell Profiles and Clinically Useful Properties of Human Mesenchymal Stem Cells of Adipose and Bone Marrow Origin. Am. J. Sports Med. 2019, 47, 1722–1733. [Google Scholar] [CrossRef] [PubMed]
- Ikegame, Y.; Yamashita, K.; Hayashi, S.I.; Mizuno, H.; Tawada, M.; You, F.; Yamada, K.; Tanaka, Y.; Egashira, Y.; Nakashima, S.; et al. Comparison of mesenchymal stem cells from adipose tissue and bone marrow for ischemic stroke therapy. Cytotherapy 2011, 13, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, Y.; Sato, Y.; Kitase, Y.; Suzuki, T.; Kondo, T.; Mikrogeorgiou, A.; Horinouchi, A.; Maruyama, S.; Shimoyama, Y.; Tsuji, M.; et al. Intravenous administration of bone marrow-derived mesenchymal stem cell, but not adipose tissue-derived stem cell, ameliorated the neonatal hypoxic-ischemic brain injury by changing cerebral inflammatory state in rat. Front. Neurol. 2018, 9, 757. [Google Scholar] [CrossRef]
- Nammian, P.; Asadi-Yousefabad, S.L.; Daneshi, S.; Sheikhha, M.H.; Tabei, S.M.B.; Razban, V. Comparative analysis of mouse bone marrow and adipose tissue mesenchymal stem cells for critical limb ischemia cell therapy. Stem Cell Res. Ther. 2021, 12, 58. [Google Scholar] [CrossRef]
- Liao, L.; Shi, B.; Chang, H.; Su, X.; Zhang, L.; Bi, C.; Shuai, Y.; Du, X.; Deng, Z.; Jin, Y. Heparin improves BMSC cell therapy: Anticoagulant treatment by heparin improves the safety and therapeutic effect of bone marrow-derived mesenchymal stem cell cytotherapy. Theranostics 2017, 7, 106–116. [Google Scholar] [CrossRef]
- Jung, J.W.; Kwon, M.; Choi, J.C.; Shin, J.W.; Park, I.W.; Choi, B.W.; Kim, J.Y. Familial occurrence of pulmonary embolism after intravenous, adipose tissue-derived stem cell therapy. Yonsei Med. J. 2013, 54, 1293–1296. [Google Scholar] [CrossRef] [PubMed]
- Moll, G.; Ignatowicz, L.; Catar, R.; Luecht, C.; Sadeghi, B.; Hamad, O.; Jungebluth, P.; Dragun, D.; Schmidtchen, A.; Ringdén, O. Different Procoagulant Activity of Therapeutic Mesenchymal Stromal Cells Derived from Bone Marrow and Placental Decidua. Stem Cells Dev. 2015, 24, 2269–2279. [Google Scholar] [CrossRef]
- Christy, B.A.; Herzig, M.C.; Montgomery, R.K.; Delavan, C.; Bynum, J.A.; Reddoch, K.M.; Cap, A.P.; Houston, F.S. Procoagulant activity of human mesenchymal stem cells. J. Trauma Acute Care Surg. 2017, 83, S164–S169. [Google Scholar] [CrossRef] [PubMed]
- Doi, T.; Doi, S.; Nakashima, A.; Ueno, T.; Yokoyama, Y.; Kohno, N.; Masaki, T. Mizoribine ameliorates renal injury and hypertension along with the attenuation of renal caspase-1 expression in aldosterone-salt-treated rats. PLoS ONE 2014, 9, e93513. [Google Scholar] [CrossRef] [PubMed]


| Number of Injected Cells | Total Mouse Number | Number of Surviving Mice | Survival Rate |
|---|---|---|---|
| 1.0 × 105 ADSCs/mouse | 10 | 6 | 60% |
| 1.0 × 105 BMSCs/mouse | 8 | 8 | 100% |
| 0 (PBS, the same volume) | 6 | 6 | 100% |
| Number of Injected Cells | Total Mouse Number | Number of Surviving Mice | Survival Rate |
|---|---|---|---|
| 1.0 × 105 SF-ADSCs/mouse | 10 | 10 | 100% |
| 2.0 × 105 SF-ADSCs/mouse | 6 | 6 | 100% |
| 3.0 × 105 SF-ADSCs/mouse | 10 | 10 | 100% |
| 4.0 × 105 SF-ADSCs/mouse | 10 | 1 | 10% |
| 4.0 × 105 SF-BMSCs/mouse | 10 | 9 | 90% |
| 0 (PBS, the same volume) | 6 | 6 | 100% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshida, M.; Nakashima, A.; Ishiuchi, N.; Miyasako, K.; Morimoto, K.; Tanaka, Y.; Sasaki, K.; Maeda, S.; Masaki, T. Comparison of the Therapeutic Effects of Adipose- and Bone Marrow-Derived Mesenchymal Stem Cells on Renal Fibrosis. Int. J. Mol. Sci. 2023, 24, 16920. https://doi.org/10.3390/ijms242316920
Yoshida M, Nakashima A, Ishiuchi N, Miyasako K, Morimoto K, Tanaka Y, Sasaki K, Maeda S, Masaki T. Comparison of the Therapeutic Effects of Adipose- and Bone Marrow-Derived Mesenchymal Stem Cells on Renal Fibrosis. International Journal of Molecular Sciences. 2023; 24(23):16920. https://doi.org/10.3390/ijms242316920
Chicago/Turabian StyleYoshida, Maria, Ayumu Nakashima, Naoki Ishiuchi, Kisho Miyasako, Keisuke Morimoto, Yoshiki Tanaka, Kensuke Sasaki, Satoshi Maeda, and Takao Masaki. 2023. "Comparison of the Therapeutic Effects of Adipose- and Bone Marrow-Derived Mesenchymal Stem Cells on Renal Fibrosis" International Journal of Molecular Sciences 24, no. 23: 16920. https://doi.org/10.3390/ijms242316920
APA StyleYoshida, M., Nakashima, A., Ishiuchi, N., Miyasako, K., Morimoto, K., Tanaka, Y., Sasaki, K., Maeda, S., & Masaki, T. (2023). Comparison of the Therapeutic Effects of Adipose- and Bone Marrow-Derived Mesenchymal Stem Cells on Renal Fibrosis. International Journal of Molecular Sciences, 24(23), 16920. https://doi.org/10.3390/ijms242316920





