Abstract
The purinergic system has a dual role: the maintenance of energy balance and signaling within cells. Adenosine and adenosine triphosphate (ATP) are essential for maintaining these functions. Sarcopenia is characterized by alterations in the control of energy and signaling in favor of catabolic pathways. This review details the association between the purinergic system and muscle and adipose tissue homeostasis, discussing recent findings in the involvement of purinergic receptors in muscle wasting and advances in the use of the purinergic system as a novel therapeutic target in the management of sarcopenia.
1. Management of Energy Distribution in the Body
Energy homeostasis is defined as the regulation of energy utilization for essential physiological processes and the production of compounds. It revolves around maintaining a balance between energy intake and expenditure to sustain bodily functions and weight [1]. Energy expenditure refers to the amount of energy an individual uses to maintain essential bodily functions such as breathing, circulation, and digestion [2].
The energy generated by the metabolism is primarily derived from glucose. Glucose is converted to ATP as immediate energy needed for many essential processes in organisms and cells [3,4]. Following hydrolysis of its phosphate groups, ATP releases its storage energy and provides energy for cells [5,6]. The efficiency of ATP formation is only about 43%, and the remaining 57% is lost as heat in the body’s metabolism [7].
The relationship between body composition and energy metabolism has been the subject of research for many years. Keesey and Hirvonen demonstrated that energy requirements increase proportionally with bodyweight in rats [8]. Even at rest, there is a baseline energy consumption associated with organs with high metabolic rates [9]. The rate of energy consumption for each organ depends on its individual metabolism and size, and this dynamic process can vary with growth, the onset of diseases, and nutritional status. It is known that under resting conditions, skeletal muscle, heart, liver, brain, kidneys, and adipose tissue exhibit the highest basal metabolic rates [9].
During an illness, the basal metabolic rate can vary dramatically depending on the type, severity, and stage of the disease [10]. In untreated inflammatory conditions, the immune system is the primary energy-consuming organ, accounting for 10–15% of the total energy expenditure [11]. Chronic, low-grade systemic inflammation, characterized by increased pro-inflammatory cytokines, such as obesity, cachexia, or exercise, also results in significant energy expenditure. In exercise, interleukin 6 (IL-6) myokine acts as an energy sensor, triggering lipolysis and ATP generation as muscle glycogen is consumed during contraction [12,13]. On the other hand, IL-6 produced by macrophages in obesity and aging causes an increase in lipolysis and high levels of free fatty acids [11].
Caloric restriction is the most commonly employed method for weight loss. While it can yield initial results, prolonged caloric restriction often leads to diminished effectiveness in achieving weight loss. During caloric restriction, the first organs to reduce their metabolism are skeletal muscle and adipose tissue, whereas the remaining organs and tissues tend to be largely preserved [3].
2. The Double Edge of the Purinergic System
In 1929, Albert Szent-Gyorgyi and Alan Drury proved that purines and pyrimidines are involved in extracellular signaling [4]. However, it was not until 1972 when Geoffrey Burnstock showed that ATP was a transmitter in non-adrenergic and non-cholinergic inhibitory nerves that purinergic signaling was proposed [14]. Adenosine and ATP play a dual role within the purinergic system. They mediate both energy storage and release through ATP and their phosphate hydrolysis to ADP and AMP, thereby meeting cellular energy demands and facilitating nucleotide assembly. Additionally, they serve as signaling molecules [15].
The purinergic system mediates cell signaling through the activation of selective receptors (named purinergic receptors) and secondary pathways for the control of physiological actions (such as cell proliferation/differentiation) [16]. Purinergic receptors are divided into two groups (based on agonist selectivity), namely P1 adenosine receptors and P2 nucleotide receptors. Two subfamilies of P2 receptors (ionotropic P2X receptors and metabotropic P2Y receptors) [16,17] and four different P1 receptor sub-types (A1R, A2AR, A2BR, and A3R) have been characterized [18]. P1 receptors are activated at different adenosine concentrations, where A1R and A2AR are high-affinity receptors (<1 µM adenosine) and A2BR and A3R are low-affinity receptors (<10 µM adenosine) [19].
The sequential hydrolysis of extracellular ATP to adenosine is catalyzed by ectonucleotidases (CD39, CD73). Once adenosine is produced, it is released via cell membrane equilibrative (ENT) and concentrative (CNT) nucleoside transporters. Adenosine release via ENT keeps intracellular and extracellular adenosine levels in balance, while CNT favors intracellular adenosine levels [20]. Extracellular adenosine can either be converted to inosine by adenosine deaminase [21] or it can activate adenosine receptors. Moreover, intracellular adenosine can sequentially be converted to AMP, ADP, and ATP via phosphorylation or into uric acid as a final metabolite [17].
All adenosine receptors belong to the GPCR family of seven transmembrane receptors linked to calcium mobilization, either promoting (A2AR/A2BR) or inhibiting (A1R/A3R) the generation of cyclic AMP (cAMP) [22]. A1R and A3R are negatively coupled to adenylate cyclase, while A2AR and A2BR are positively coupled to adenylate cyclase [23]. The inhibition of cAMP due to A1R and A3R stimulation has been shown to promote the contraction of smooth muscle cells via MAPK and ERK1/2 activation [24]. Increased cAMP levels lead to the activation of protein kinase A (PKA) and the exchange protein directly activated by cAMP (EPAC) [25]. PKA and EPAC may perform individual, combination, or opposite effects [25,26]. Studies in cancer and neuronal models have shown that PKA activation promotes proliferation, while EPAC activation promotes differentiation [26]. Furthermore, EPAC is able to promote osteoclast formation, and PKA counteracts this effect [27,28]. In contrast, in vitro smooth muscle models have shown that both PKA and EPAC promote muscle proliferation [29]. Therefore, the role of these cAMP receptors is tissue dependent. The activation of the cAMP response element binding protein (CREB) is triggered by the phosphorylation of PKA in the nucleus. CREB phosphorylation has been related to the activation of pro-anabolic genes [30] and the reduction in pro-atrophic genes in muscle [31].
ATP is released to the extracellular space via connexins (e.g., connexin-43) and pannexin channels (Panx-1, Panx-2, Panx-3) [15,17,23]. In addition, some experiments show that connexins are not clearly expressed in adult muscles. Consequently, the process of releasing ATP to the extracellular space might be carried out by pannexin1 hemichannels [32]. The physiological effects of elevated extracellular ATP are mediated by P2X and P2Y receptors. P2X receptors are ligand-gated ion channels, while P2Y receptors are members of the G protein-coupled receptor (GPCR) family. There are seven sub-types of P2X receptor (1–7) and eight sub-types of P2Y receptors (P2Y1, P2Y2, P2Y4, P2Y6, P2Y11–14), all of which are selective for ATP, ADP, UTP, and UDP [4,17,33].
Moreover, the purinergic system exerts its role to fulfill cellular energy demands via modulation of the AMP-activated protein kinase (AMPK)-mediated mechanism. AMPK is a ubiquitously distributed serine/threonine protein kinase that regulates cellular energy homeostasis, acting as a central energy sensor and maintaining energy stores by fine-tuning anabolic and catabolic pathways through the activation of pathways that generate ATP (e.g., glucose transport and glycolysis) and the deactivation of energy-consuming anabolic pathways (e.g., inhibition of fatty acid synthesis) [34]. AMPK is activated via the phosphorylation of tyrosine residue 172 with the increase in the intracellular AMP–ATP ratio [35]. Moreover, creatine kinase (CK) mediates an energetic role in the purinergic system. ATP generated via mitochondrial oxidative phosphorylation is used to catalyze the conversion of creatine (Cr) to phosphocreatine (PCr) [36]. Then, PCr is released into the cytosol and activates CK to regenerate the ATP consumed during muscle expenditure [36]. Elevated levels of PCr in the cytosol are related to inactive AMPK due to high ATP concentrations [37]. Therefore, AMPK and CK act as mediators of energy control in the purinergic system [38]. Alterations in the energy balance between anabolism and catabolism generate a poor metabolic rate, which leads to wasting conditions, such as aging [39].
3. Sarcopenia
Sarcopenia is a generalized and progressive loss of skeletal muscle mass and function, concomitant with an increased risk of adverse outcomes such as falls, metabolic dysfunction, disability, poor quality of life, and death [40,41]. After the age of 30, an individual loses between 3 and 8% of muscular mass every decade, with this rate increasing after the age of 60 [42]. This decrease in muscle mass produces a decline in strength and muscular function, with qualitative changes in muscular tissue due to a reduction in motor units affecting both nervous and muscular fibers, especially fiber type II, and, therefore, altering the contractile activity [43,44].
Sarcopenia can be categorized as primary or secondary sarcopenia. Primary sarcopenia is associated with the aging process, seen in the elderly, and secondary sarcopenia is associated with one or more of the following causes that could promote the loss of muscle mass: sedentary lifestyle, immobilization, malnutrition, diabetes, obesity, cancer, and other acute or chronic inflammatory diseases (e.g., rheumatoid sarcopenia) [45,46]. The European Working Group on Sarcopenia in Older People (EWGSOP) described the following criteria for the diagnosis of sarcopenia: low skeletal muscle mass (diagnosed via DXA or anthropometry) and either low muscle performance (walking speed, muscle power) or low muscle strength (e.g., handgrip) [47,48].
In addition, when considering the duration, sarcopenia can be described as acute sarcopenia when lasting less than 6 months and chronic sarcopenia when the duration is longer than 6 months, and it is associated with other chronic conditions, including aging [49] (Figure 1).
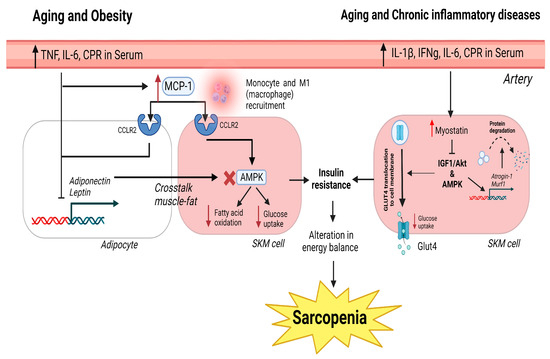
Figure 1.
Canonical pathways in obesity, aging, and chronic inflammatory diseases that generate sarcopenia. In obesity and aging, the increase in pro-inflammatory cytokines and CRP in the serum generates high levels of MCP-1, which binds to the CCLR2 receptor in muscle and adipose cells. In adipose tissue, this causes the inhibition of adiponectin and leptin expression. The inhibition causes a negative cross-talk between muscle and the inhibition of AMPK, fatty acid oxidation, and glucose uptake. In chronic inflammatory disorders and aging, the presence of pro-inflammatory molecules in the serum produces an increase in myostatin. Myostatin inhibits IGF1/Akt via AMPK activation. This causes a reduction in glucose uptake and an increase in atrogene expression underlying protein degradation and muscle atrophy. The inhibition of glucose uptake in obesity, aging, and chronic inflammatory disorders generates insulin resistance and muscle loss due to the failure to restore muscle cell energy demands. Red cross indicates blockade of the expression of AMPK. Red arrows inform of up or down regulation of which is indicated next to it.
Although sarcopenia has a severe impact on the quality of life of elderly people and society, the pathophysiological mechanisms underlying this disease have not been elucidated due to the intricacy of the network of interactions and causes (chronic inflammation, muscle protein turnover alterations, neuromuscular junction dysfunction, and hormone levels, among others) [50]. Muscle loss in both primary and secondary sarcopenia appears to be driven by different mechanisms, involving changes in biochemical molecules in different signaling pathways [51,52]. Sarcopenia in the elderly is primarily caused by anabolic resistance induced by myostatin [51], while secondary sarcopenia appears to be activated by catabolic processes [52]. In this regard, the severity of secondary sarcopenia observed in chronic debilitating conditions and inflammatory diseases varies with the intensity of systemic inflammation [53].
Low-grade chronic inflammation, produced by slight elevations in circulating pro-inflammatory mediators (such as C-reactive protein (CRP), tumor necrosis factor (TNF), and IL-6), is among the causes of inducing sarcopenia. Catabolic inflammatory processes are involved in the development of sarcopenia, especially at an advanced age, even in healthy individuals [54]. This low-grade chronic inflammation presents immune cell senescence, alterations in T cell function and the extracellular matrix, foci of chronic infection, and an increased fat mass [55]. Recent data from sarcopenic elderly individuals suggest that circulating TNF and IL-6 are significantly elevated, and these circulating levels are correlated with and increased risk of muscle strength loss [56,57]. Under normal circumstances, CRP, TNF, and IL-6 maintain the balance between catabolism and synthetic metabolism in skeletal muscles, but higher levels of inflammatory markers are associated with physical decline, resulting in increased catabolism, with an inhibition of protein synthesis, and damage in muscle integrity and function, resulting in sarcopenia [58,59]. On the other hand, IL-6 has been proven to act as a sensor of muscle damage, promoting the activation and migration of T cells via gap junction proteins, such as connexins and pannexins [60]. Anti-inflammatory cytokines (IL-4, IL-10, and IL-15) are able to antagonize pro-inflammatory cytokine activity to reduce muscle atrophy and retard sarcopenia [61]. It has been observed that IL-4 improves glucose metabolism in muscle cells and acts as a myoblast recruitment factor, promoting myogenesis and muscle regeneration [62,63] (Figure 1).
Frailty is a multi-system syndrome associated with lower resilience against stressors and an increased risk of adverse health outcomes [64,65]. Both low muscle strength or function and weight loss are phenotypic characteristics of the frailty syndrome [66], reflecting that frailty and sarcopenia are linked, although they are distinct, as not all frailty in patients is related to skeletal muscle mass or function [64]. Both conditions share the same pathophysiology and clinical outcomes, with sarcopenia being considered a component of frailty, but not vice versa [67]. Therefore, diagnostic criteria are essential for the recognition of each condition in clinical practice. According to Fried et al., individuals can be categorized as non-frail (0 Fried criteria present), pre-frail or intermediate (1–2 criteria), or frail (≥3 criteria) [64], with these criteria observing the following factors: low gait speed and low grip strength, weight loss, self-reported low physical activity, and exhaustion [66]. Sarcopenia is then considered the physical component of frailty. As described above, it is clear that the degree and chronicity of inflammation are responsible for the effect on muscle mass, strength, and quality. Relatively mild inflammation levels that occur in normal aging or obesity may not be sufficient to observe the effects on muscle mass or strength lost but affect metabolic quality and, therefore, contribute to sarcopenia development [68]. In a more severe systemic inflammation scenario, as seen in frailty, pro-inflammatory cytokines contribute to muscle mass and strength loss [69]. Exogenous TNF administration to mice induced anorexia and muscle loss, the upregulation of leptin, activation of NFκB (nuclear factor kappa B), atrophy and activation of the ubiquitin–proteasome pathway, and suppression of the AKT serine–threonine protein kinase and mammalian target of rapamycin (Akt-mTOR) pathway [70]. Moreover, neutrophils and NET (neutrophil extracellular traps) generation are impaired with age [71]. Higher neutrophil counts have been associated with frailty and low levels of physical activity, and higher white cell count in healthy individuals can predict frailty over the years [72]. The neutrophil chemotactic ability is reduced with age, inducing an inefficient migration, higher tissue damage, and secondary systemic inflammation, suggesting that neutrophils play a key role in sarcopenia and frailty [67,73].
Rheumatoid arthritis (RA) is the most common autoimmune disease, with 1% of the world population affected [74]. Apart from articular manifestations, RA has several systemic comorbidities, including rheumatoid cachexia (RC), a condition that impacts function and quality of life and affects approximately 11% to 26% of RA patients worldwide [51,75]. It is characterized by a reduced skeletal muscle mass with either stable or increased fat mass, degradation of balance and disruption of muscle protein synthesis, decreased muscle mass, strength, and function, together with total energy expenditure, insulin resistance, increased basal metabolic rate, and inflammation [51]. The mechanism involved is not fully understood but includes cytokine-driven hypermetabolism produced by TNF, IL-1β, and IL-6 (associated with resting energy expenditure and sarcopenia in RA patients), as well as the limitation of physical activity and insulin resistance [51,76]. Novel approaches indicate that insulin resistance is produced in RA patients due to the increase in energy expenditure via high levels of IL-6 in the serum [77].
Employing an antigen-induced arthritis animal model, we have previously demonstrated that rabbits developed a rheumatoid cachexia-like secondary sarcopenia with increased muscle protein breakdown and a compensatory anabolic response [51]. Rheumatoid rabbits showed weight loss, decreased muscle size, and an upregulation of atrogenes in muscles, along with a decrease in myostatin expression and a reduction in the signal transducer and activator of transcription 3 (p-STAT-3) levels. This response suggests that the inflamed muscle could contribute to secondary sarcopenia through an autocrine mechanism of atrophy triggered by pro-inflammatory mediators [51]. In an adjuvant-induced arthritis model, the authors have observed a 20% decrease in skeletal muscle and muscle weight [78]. In a collagen-induced arthritis (CIA) rat model, Hartog et al. have described reduced weight and spontaneous locomotion. Additionally, a 31% reduction in the gastrocnemius relative weight has also been demonstrated 21 days after the induction of arthritis [78,79]. Numerous studies have monitored alterations in body composition parameters and, consequently, the development of rheumatoid cachexia (RC). This was carried out through the implementation of specific drug treatments or a combination of therapeutic approaches. Disease-modifying anti-rheumatic drugs (DMARDs) play a crucial role in managing disease activity by impeding inflammatory signaling pathways, such as those involving TNF and IL-6. Methotrexate (MTX) monotherapy is a first line DMARD agent for RA. It is unknown whether MTX monotherapy is beneficial for RC, but some authors have demonstrated that MTX in combination with different drugs may protect against the development of RC [75]. The use of Janus kinase (JAK) inhibitors has also been explored. The IL-6/JAK/STAT pathway is key for muscle fiber development and regeneration. Multiple studies have demonstrated that the JAK/STAT pathway controls the myogenic development of adult satellite cells [80]. Furthermore, the JAK/STAT pathway induced the expression of atrogenes Murf1 and Atrogin1 in muscle alterations derived from RA [81]. In an antigen-induced arthritis rabbit model of RA, the inhibition of the JAK/STAT pathway with tofacitinib prevents the expression of the atrogenes and myogenic alterations in muscles [81].
Sarcopenia is sometimes accompanied by changes in adipose tissue. The modulation of myokine and adipokine levels contributes to the cross-talk between muscle and adipose tissue [82]. Secretions of these cytokines regulate anabolic and catabolic responses in muscles. These cytokines are altered with high adiposity and age-related muscle wasting [82]. Myostatin and irisin are the main myokines involved in the muscle–fat cross-talk [83,84]. Myostatin is a human growth factor that produces a downregulation of protein synthesis in muscles via Smad2/3 and inhibits insulin-like growth factor-1 (IGF-1)/Akt via (forkhead box transcription factors) FOXO; moreover, it inhibits glucose transporter protein type-4 (GLUT4) and AMPK [85,86]. This pathway produces a reduction in glucose uptake and leads to muscle atrophy. With aging, myostatin expression increases with a strong correlation to decreased strength [87]. Clinical studies have shown that patients with obesity have an increased presence of myostatin in the serum [88]. Therefore, there is a relationship between fat and myostatin expression that has not been elucidated (Figure 1).
In contrast, irisin correlates with increased strength and muscle maintenance via Akt/mTor [89]. The activation of this pathway has been demonstrated in a C2C12 model, in which irisin treatment was observed to contribute to the development of muscle hypertrophy [90,91]. In addition, a decrease in irisin expression was found in patients with severe obesity [92]. Therefore, there is a relationship that has not been elucidated between fat and the expression of both myostatin and irisin. On the other hand, among the adipokines involved in the muscle–fat relationship are leptin and adiponectin. Leptin is known to be a pro-inflammatory adipokine. It is closely related to the amount of body fat and acts on muscle via the modulation of AMPK levels [93]. Old obese rats show resistance to leptin. On the other hand, the caloric restriction of these models increases responsiveness to leptin (especially in aging) [94]. In humans, serum leptin levels were positively correlated with body mass index (BMI) and negatively correlated with skeletal muscle index (SMI) [95]. This indicates that leptin is a good marker to indicate the risk of sarcopenic obesity [95].
Adiponectin is a key regulator synthesized by adipose tissue involved during glucose and fatty acid metabolism. Adiponectin promotes the ability of insulin to stimulate glucose uptake through increased GLUT4 translocation to the plasma membrane. C2C12 myoblast cells transfected with adiponectin showed reduced lipid accumulation [96]. The role of adiponectin in sarcopenia is unclear, but clinical studies have shown that the level of adiponectin is significantly lower in sarcopenic patients [97]. However, a parallel clinical study observed that strength was negatively correlated with adiponectin expression [98].
The principal function of monocyte chemoattractant protein-1 (MCP-1/CCL2) is to regulate monocyte/macrophage migration and infiltration [99]. Increased adipose tissue has been shown to correlate with increased MCP-1, which promotes macrophage migration into the adipose tissue and the synthesis of other cytokines, such as IL-6 and TNF [100,101,102]. Cytokine expression has been studied in patients with cachexia, and only MCP-1 was found to be increased, indicating a major role in muscle loss [103]. This was confirmed in a clinical study, where sarcopenic patients showed increased serum MCP-1 expression [104]. Therefore, MCP-1 favors a pro-inflammatory state in adipose and muscle tissues, leading to the development of sarcopenia (Figure 1).
4. P2 Receptors in Muscle and Fat in Sarcopenia
The presence of P2 purinergic receptors has been proven in muscles using immunochemistry. P2Y11 and P2X1 are more expressed in the cytosol of muscle fibers. In addition, P2X1 is expressed in the plasma membrane, as well as P2Y4. In contrast, other receptors, such as P2Y1, P2Y2, P2Y12, and P2X4, are not expressed in muscle cells [105]. In sarcolemma, receptors P2X1 and P2Y4 are visible. P2X1 is found inside vesicles in sarcolemma and P2Y4 does not have these vesicles. The P2Y11 receptor is expressed in type I fibers, while it is less expressed in type 2 fibers and almost absent in sarcolemma [105].
P2 receptors are correlated with muscle blood flow. Experiments with the continuous infusion of ATP have been shown to stimulate muscle blood flow, allowing us to prove that P2 receptors are involved during physical exercise in muscles. This was proven using a P2Y1R blocker. It has been shown that upon blocking this receptor, the potentiation (contraction) in the EDL muscle was prevented, whereas it was not prevented in the soleus. This information suggests that ATP is able to activate P2Y1 receptors in fast muscles [32]. On the other hand, the use of P2 receptor inhibitors led to a decrease in muscle blood flow [106]. In addition, the use of ATP increases the abundance of NA+–K+ pumps in muscles. This means that P2 receptors are involved in muscle excitation during intense exercise [107].
Additionally, chronic inflammatory diseases that generate muscle dystrophies produce an increase in the amount of ATP in muscle tissue [107]. This ATP activates the P2X7 receptor, which has been correlated with muscle fiber atrophy [108]. Experiments in a co-culture with osteoclast and muscle cells showed that the mechanical stimulation of osteoclast releases ATP in the medium. This ATP initiates a cross-talk with muscles, inducing the P2–PI3K–Akt-mTOR pathway in muscles [109]. On the other hand, knockout models of P2X7 showed a reduction in the expression of CCL2 and IL6 in WAT, but no alterations were observed in BAT thermogenesis [110]. In adipocytes, it has been observed that the expression of P2X7 is related to an inhibition of SIRT3/5 genes involved in browning, favoring the adipogenesis process [111]. Therefore, it is possible that the expression of P2X7 generates muscle atrophy, increases adipogenesis, and reduces BAT, contributing to the development of obese sarcopenia in chronic inflammation.
The action of P2Y receptors is controversial. P2Y1 and P2Y2 receptors improve muscle regeneration, which is blocked when these receptors are inhibited [112]. However, in skeletal muscle fibroblasts, P2Y2 receptors promote fibrosis and muscle atrophy via Akt/ERK/PKC activation [113]. Therefore, the role of P2Y receptors in the development of sarcopenia remains unknown.
5. Adenosine Receptors in Muscles and Fat and Their Role in Sarcopenia
The expression and distribution of adenosine receptors in skeletal muscle may change depending on the species, and within the species, it may depend on the muscle state, type of muscle fiber, and location within the fiber. In vitro, in the C2C12 myoblastic line, A1R was first characterized, correlating its expression with the cellular enhancement of glucose uptake [114]. A subsequent study analyzed the gene expression of all receptors in C2C12, in which A2BR was the most highly expressed. A1R, A2AR, and A3R were expressed to a lesser extent compared to A2BR [115]. In mouse tibialis, the expression of A1R, A2AR, and A2BR was observed via a polymerase chain reaction (PCR) [115,116]. Moreover, in rats, the expression of these receptors was also confirmed via Western blotting [117]. In human skeletal muscle, the expression of A2AR and A2BR in the plasma membrane and cytosol and the absence of A1R were verified via immunohistochemistry. A2AR is equally distributed in type 1 and 2 fibers and is slightly more expressed in the cytoplasm than in the membrane of type 1 fibers. In contrast, A2BR has major expression in the cytoplasm of type 2 and the plasma membrane of type 1 fibers. Therefore, according to the expression of the receptors in the cell membrane in comparison with cytosol, A2AR is responsible for glucose transport across the cell membrane in type 2 fibers and A2BR in type 1 fibers [118]. When blocking adenosine receptors or using adenosine deaminase to remove extracellular adenosine, there is a great decrease in glucose transport in skeletal muscle fibers, which confirms their role in glucose metabolism [119].
The activation of PKA and AMPK depends on the expression of adenosine A2A and A2B receptors [120,121]. The upregulation of A2A and A2B promotes the formation of cyclic AMP from ATP by adenylate cyclase [122]. This cAMP activates both PKA and exchange proteins directly activated by cyclic AMP (EPAC) proteins. Next, cAMP is reduced to AMP by phosphodiesterase [123]. This increases the intracellular AMP concentration and thus the AMP/ATP ratio, similar to events during muscle contraction [124]. This ratio activates AMPK via phosphorylation and allows for the restoration of ATP levels from AMP [125]. The activation of both PKA and AMPK has been observed to promote CREB expression [121,126,127]. CREB promotes the expression of genes involved in mitochondrial biogenesis (PGC-1 and TFAM) and muscle regeneration, preventing alterations in muscles [128,129] (Figure 2).
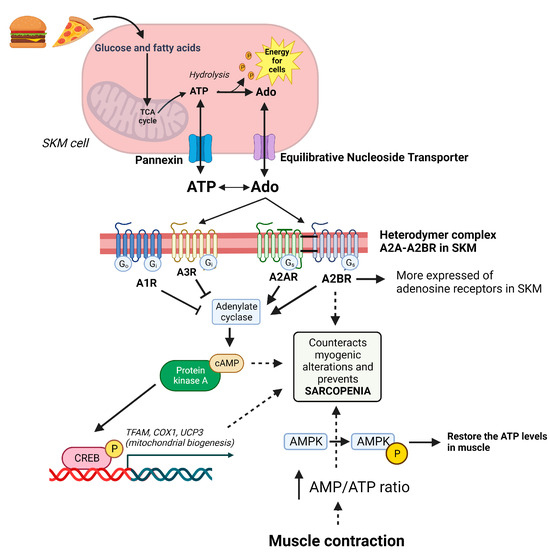
Figure 2.
Purinergic system in the management of muscle homeostasis. Fatty acids and glucose are used to produce cell energy via ATP and are recovered via adenosine. ATP and adenosine maintain a balance in the cytosol and extracellular space via pannexin and an equilibrative nucleoside transporter, respectively. In muscles, the A2B receptor (the most expressed of the adenosine receptors in skeletal muscle (SKM)) forms a heterodymer with the A2A receptor to exert its function. These receptors activate cAMP/PKA/CREB, which increases the mitochondrial biogenesis to maintain energy balance in the muscle cell. On the other hand, muscle contraction uses available ATP. This produces an increase in the AMP/ATP ratio and activates AMPK, which is able to restore ATP levels in the cell. Therefore, A2BR, cAMP, and AMPK are essential molecules in the control of muscle homeostasis and future pharmacological targets in the treatment of sarcopenia.
Adenosine receptors differ in expression within adipose tissue depending on whether they are located in white (WAT) or brown (BAT) adipose tissue. Vassaux et. al. analyzed the mRNA expression of adenosine receptors in the white adipocytes and pre-adipocytes extracted from rat epididymal white fat pad. A2R expression was characterized in pre-adipocytes but not adipocytes, and A1R was only in adipocytes [130,131]. However, in studies on mesenchymal cell differentiation to adipocytes, it has been observed that both A1R and A2AR are more expressed with the passage of days of adipocyte differentiation [132]. On the other hand, in murine BAT, the expression of the four adenosine receptors was demonstrated [115]. There is some controversy because some studies suggest that A2AR is the most abundant adenosine receptor in human and murine BAT [133,134]. However, subsequent studies have shown a predominant role of A2BR in murine BAT [115]. This may be due to the heterodimerization of A2AR–A2BR, in which the expression of both is required for activation [115]. Methotrexate treatment in RA increases the adenosine available to adenosine receptors, decreases joint inflammation, and induces the browning of adipose tissue with a high expression of genes that induce thermogenesis in RA [135].
In a first study, treatment with a cAMP analog (db-cAMP) was associated with an extended average lifespan and the maintenance of muscle mass, thus preventing the occurrence of sarcopenic events in mice [136]. The production of cAMP in skeletal muscle is primarily dependent on A2BR [115,137]. An A2BR−/− (skeletal muscle-specific knockout) murine model led to a loss of muscle mass and strength and increased senescence markers and mitochondrial alteration [115]. In addition, in skeletal muscle explants from patients, a higher expression of A2BR was correlated with a lower expression of the senescence marker p21 [115]. In primary myocytes isolated from these same patients and treated with an A2BR agonist, an increase in differentiation and proliferation markers was observed, as well as an improvement in the expression of mitochondrial oxidative phosphorylation markers [115].
Because the development of sarcopenia is accompanied by an increase in fat in what is known as obese sarcopenia, it is important to establish the role of adenosine receptors in both WAT and BAT [138,139]. Adenosine is secreted from adipocytes, activating the A1R, which is involved in multiple functions. In isolated rat adipocytes, increased A1R expression has been correlated with increased lipolysis [140]. On the other hand, increased A2BR expression appears to inhibit lipogenesis and adipogenesis [141]. In human and murine brown adipocytes, adenosine activates its receptors at nanomolar levels. The inhibition of A2AR, both pharmacologically and in a murine A2Aknockout model, has shown that A2A is an essential contributor to the process of thermogenesis [133]. Subsequently, it was proven in the A2BR murine knockout model that the activation of this receptor with an agonist increased the thermogenesis process and decreased the induction of obesity via diet [115]. The aforementioned evidence seems to lead to the conclusion that both A2AR and A2BR (as heterodymers) make an essential contribution to preventing the onset of the sarcopenic process and the development of obesity correlated with muscle loss [115].
6. Therapeutic Possibilities: Adenosine in Tissue Regeneration
The main role of adenosine is to maintain cellular homeostasis and it is of special interest as a target in the treatment of many diseases and disorders [6]. Clinical treatment with adenosine is not very effective due to its short lifetime and receptor non-specificity [142]. However, several approaches have been developed for the therapeutic use of the purinergic system, including the oral or intravenous administration of ATP, the use of AR agonists, the use of adenosine analogs or drugs that modulate cellular levels of adenosine and increase its selectivity toward an AR, and the use of a cAMP analog [143].
In relation to muscle disorders, some therapeutic strategies correlated with the purinergic system were investigated.
6.1. A2B Signaling via AMPK/cAMP and Mitochondrial ADP Sensibility Counteracts Aging Sarcopenia
AMPK activation has been shown to inhibit the progression of aging via FoxO, mTOR, CREB, and sirtuin (SIRT) 1 signaling pathways [144]. AMPK activation has been correlated with increased levels of cAMP via Ca2+/calmodulin-dependent protein kinase II (CaMKII) [145,146]. Because the increase in cAMP in muscles primarily depends on A2BR, an A2BR agonist could be a good therapeutic agent to counteract muscle loss in aging [115]. In addition, adenosine has been shown to prevent age-related loss of muscle contraction [147,148]. In a parallel study, CaMKII has been shown to decrease muscular fatigue by reducing calcium release during intense exercise [149]. CaMKII also promotes ATP signaling via P2R and pannexin, contributing to the migration of dendritic cells during muscular damage [150]. Furthermore, the commitment of myoblasts to the myogenic lineage relies on an increase in intracellular free calcium levels. Potential cell membrane pathways implicated in these calcium increases include P2 receptors and connexin and/or pannexin hemichannels, recognized for their ability to allow for the passage of calcium [151].
A downregulation of A2BR in aging was observed. Studies on the A2BR receptor agonist BAY 60-6583 in rats have demonstrated an improvement in muscle contraction [147]. The increase in cAMP as a molecule to counteract sarcopenia has not only been proven by A2BR stimulation but also by using a cAMP analog (db-cAMP) [136]. Muscle cAMP levels decreased in 24-month-old mice compared to 6-month-old mice, concomitant with a loss of motor activity that was recovered with db-cAMP treatment [136]. On the other hand, AMPK activation depends on the intracellular AMP/ATP ratio, and an increase in AMP levels using a pharmacological modulator is a potential strategy to address sarcopenia in aging [152]. Alternatively, ADP sensitivity has been shown to be reduced in old mouse gastrocnemius, with an increased production of reactive oxygen species (ROS) [153]. This increase in ROS leads to an age-associated increase in H2O2 release [154]. Insulin use has been shown to contribute to increased ADP sensitivity in mitochondria [155]. As such, insulin may be a good stimulating agent of mitochondrial biogenesis via ADP in aging.
Recently, it was reported that the pharmacological increase in extracellular adenosine by dipyridamole in the myoblastic line C3C12 leads to an increase in A2B adenosine receptor expression [127]. Subsequently, this leads to cAMP–PKA–CREB increase and AMPK activation [127]. Furthermore, the pharmacological stimulation of cAMP and AMPK by dipyridamole is able to prevent alterations in muscle myogenesis in vitro [127]. Therefore, a new therapeutic via the treatment of sarcopenia is introduced with the use of drugs that modulate adenosine levels in muscles (e.g., dipyridamole) [127]. On the other hand, the use of tenofovir in the C2C12 line inhibits ATP release in the extracellular space [127]. This produces a decrease in extracellular adenosine levels, with a reduced expression in the adenosine A2B receptor [127]. The decrease in the adenosine 2B receptor promotes alterations in muscle myogenesis with the inhibition of PKA/AMPK pathways [127].
6.2. cAMP Treatment to Prevent Muscle Atrophy
Muscle atrophy is characterized by alterations in protein metabolism, leading to a loss of muscle function [156]. AMP deaminase 3 controls the content of intracellular adenine nucleotides. AMP deaminase 3 has been shown to be increased in a murine skeletal muscle atrophy model [157]. The overexpression of AMP deaminase 3 is related toa decrease in ATP and an increase in inosine monophosphate (IMP) levels [157]. Furthermore, the upregulation of AMP deaminase 3 produces an inhibition of AMPK phosphorylation and decreases in the mitochondrial protein synthesis rate [157]. In this case, the stimulation of AMP production by an analog may be a good therapeutic approach to avoid muscle wasting in atrophic muscles. In addition, AMPK activity is decreased in the extensor digitorum longus of atrophic rats [158]. Therefore, implementing a cAMP analog, as mentioned previously, could increase the intracellular AMP/ATP ratio, thereby preventing AMPK inactivation [152].
6.3. ATP as a Therapy for Cancer-Associated Cachexia
Cancer-associated cachexia occurs in half of all cancer patients [159]. The level of muscle loss varies with the progression and type of tumor; therefore, maintaining muscle mass is essential to improve the quality of life and treatment efficacy [160]. Patients with gastric cancer have a lower content of ATP, ADP, AMP, and adenosine [161]. This decrease in purines and pyrimidines is not due to a lack of nutrients. This was proven in the muscle of a cancer cachexia model in pair-fed rodents, in which tumor resection increased ATP levels [162,163]. Intravenous ATP has already been safely tested in lung cancer patients. Both in phase I and phase II studies, ATP has shown promising results in muscle maintenance and nutritional status [164]. In murine models, it has been observed that intraperitoneal ATP inhibits weight loss in animals with advanced tumor growth independent of its antineoplastic action [165]. On the other hand, prolonged oral use of ATP (0 to 5000 mg/day) has been shown to lead to a decrease in ATP plasma levels in biodistribution studies, which decreases its therapeutic potential [166].
6.4. Adenosine Modulators as a Treatment for Duchenne Muscular Dystrophy
Duchenne muscular dystrophy (DMD) is the most common, severe, and widely studied type of dystrophy in humans [167]. In patients with DMD, ATP and total adenosine are severely reduced in muscles (±50%). This decrease could be due to mitochondrial dysfunctions and an increased degradation of adenosine that is secreted by urine [168]. Treatments were carried out to prevent the loss of adenosine levels by adenylosuccinic acid (which increases cellular adenosine levels) or by inhibiting purine breakdown with allopurinol, leading to improved muscle strength and reduced lipid deposition [169].
7. Conclusions
In conclusion, the purinergic system is largely involved in the control of sarcopenia and muscle homeostasis. Adenosine A2A–A2B receptors play a fundamental role in muscle maintenance. Therapeutically, the activation of these receptors could prevent myogenic alterations and muscle loss in sarcopenia associated with aging and other pathologies. Nevertheless, more research is necessary to find new therapeutic strategies for secondary sarcopenia, including rheumatoid cachexia and other disorders.
8. Patents
A.M., M.M.-B., M.F., R.L., and G.H.-B. have filed a patent on the use of dipyridamole as a novel therapy for muscular myogenesis disorders and inflammatory arthritis.
Author Contributions
A.M. conceptualized, wrote, and revised the manuscript. M.M.-B. and M.F. were primarily responsible for writing, editing, and revising the manuscript. G.H.-B. and R.L. wrote, edited, and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from Instituto de Salud Carlos III through the “Miguel Servet” program (CP15/00053, CPII20/00017 co-funded by Union Europea, and a research grant from the Spanish Instituto de Salud Carlos III (PI19/00744, PI22/00347).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
A.M. has filed a patent for the use of adenosine A2AR agonists to prevent prosthesis loosening (pending) and a separate patent for the use of A2AR agonists and agents that increase adenosine levels to promote bone formation/regeneration. A.M., M.M.-B., M.F., R.L., and G.H.-B. have filed a patent for the use of dipyridamole as a novel therapy for muscular myogenesis disorders and inflammatory arthritis. R.L. and G.H.-B. have filed a patent for the use of 6-shogaol in osteoarthritis.
References
- Galgani, J.; Ravussin, E. Energy Metabolism, Fuel Selection and Body Weight Regulation. Int. J. Obes. 2008, 32, S109–S119. [Google Scholar] [CrossRef] [PubMed]
- Gellman, M.D.; Turner, J.R. (Eds.) Encyclopedia of Behavioral Medicine; Springer: New York, NY, USA, 2013; ISBN 978-1-4419-1004-2. [Google Scholar]
- Gallagher, D.; Kelley, D.E.; Thornton, J.; Boxt, L.; Pi-Sunyer, X.; Lipkin, E.; Nyenwe, E.; Janumala, I.; Heshka, S. Changes in Skeletal Muscle and Organ Size after a Weight-Loss Intervention in Overweight and Obese Type 2 Diabetic Patients. Am. J. Clin. Nutr. 2017, 105, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Cheek, D.J.; McHugh, J.M.; Blood-Siegfried, J.; McFetridge, J.F.; Turner, B.S. A Historical Perspective on the Discovery of Adenyl Purines. Biol. Res. For. Nurs. 2000, 1, 265–275. [Google Scholar] [CrossRef]
- Nesci, S.; Trombetti, F.; Ventrella, V.; Pagliarani, A. Opposite Rotation Directions in the Synthesis and Hydrolysis of ATP by the ATP Synthase: Hints from a Subunit Asymmetry. J. Membr. Biol. 2015, 248, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Samsel, M.; Dzierzbicka, K. Therapeutic Potential of Adenosine Analogues and Conjugates. Pharmacol. Rep. 2011, 63, 601–617. [Google Scholar] [CrossRef] [PubMed]
- Neufer, P.D. The Bioenergetics of Exercise. Cold Spring Harb. Perspect. Med. 2018, 8, a029678. [Google Scholar] [CrossRef] [PubMed]
- Keesey, R.E.; Hirvonen, M.D. Body Weight Set-Points: Determination and Adjustment. J. Nutr. 1997, 127, 1875S–1883S. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ying, Z.; Bosy-Westphal, A.; Zhang, J.; Schautz, B.; Later, W.; Heymsfield, S.B.; Müller, M.J. Specific Metabolic Rates of Major Organs and Tissues across Adulthood: Evaluation by Mechanistic Model of Resting Energy Expenditure. Am. J. Clin. Nutr. 2010, 92, 1369–1377. [Google Scholar] [CrossRef]
- Elia, M. Insights into Energy Requirements in Disease. Public Health Nutr. 2005, 8, 1037–1052. [Google Scholar] [CrossRef]
- Straub, R.H. The Brain and Immune System Prompt Energy Shortage in Chronic Inflammation and Ageing. Nat. Rev. Rheumatol. 2017, 13, 743–751. [Google Scholar] [CrossRef]
- Leal, L.G.; Lopes, M.A.; Batista, M.L. Physical Exercise-Induced Myokines and Muscle-Adipose Tissue Crosstalk: A Review of Current Knowledge and the Implications for Health and Metabolic Diseases. Front. Physiol. 2018, 9, 1307. [Google Scholar] [CrossRef]
- Laurens, C.; Bergouignan, A.; Moro, C. Exercise-Released Myokines in the Control of Energy Metabolism. Front. Physiol. 2020, 11, 91. [Google Scholar] [CrossRef]
- Burnstock, G.; Dumsday, B.; Smythe, A. Atropine Resistant Excitation of the Urinary Bladder: The Possibility of Transmission via Nerves Releasing a Purine Nucleotide. Br. J. Pharmacol. 1972, 44, 451–461. [Google Scholar] [CrossRef]
- Spinozzi, E.; Baldassarri, C.; Acquaticci, L.; Del Bello, F.; Grifantini, M.; Cappellacci, L.; Riccardo, P. Adenosine Receptors as Promising Targets for the Management of Ocular Diseases. Med. Chem. Res. 2021, 30, 353–370. [Google Scholar] [CrossRef]
- Antonioli, L.; Blandizzi, C.; Pacher, P.; Haskó, G. The Purinergic System as a Pharmacological Target for the Treatment of Immune-Mediated Inflammatory Diseases. Pharmacol. Rev. 2019, 71, 345–382. [Google Scholar] [CrossRef]
- Burnstock, G. Purine and Purinergic Receptors. Brain Neurosci. Adv. 2018, 2, 239821281881749. [Google Scholar] [CrossRef]
- Huang, Z.; Xie, N.; Illes, P.; Di Virgilio, F.; Ulrich, H.; Semyanov, A.; Verkhratsky, A.; Sperlagh, B.; Yu, S.-G.; Huang, C.; et al. From Purines to Purinergic Signalling: Molecular Functions and Human Diseases. Signal Transduct. Target. Ther. 2021, 6, 162. [Google Scholar] [CrossRef] [PubMed]
- Fredholm, B.B. Adenosine, an Endogenous Distress Signal, Modulates Tissue Damage and Repair. Cell Death Differ. 2007, 14, 1315–1323. [Google Scholar] [CrossRef] [PubMed]
- Puchałowicz, K.; Baranowska-Bosiacka, I.; Dziedziejko, V.; Chlubek, D. Purinergic Signaling and the Functioning of the Nervous System Cells. Cell. Mol. Biol. Lett. 2015, 20, 867–918. [Google Scholar] [CrossRef] [PubMed]
- Arin, R.M.; Gorostidi, A.; Navarro-Imaz, H.; Rueda, Y.; Fresnedo, O.; Ochoa, B. Adenosine: Direct and Indirect Actions on Gastric Acid Secretion. Front. Physiol. 2017, 8, 737. [Google Scholar] [CrossRef]
- Chiarella, A.M.; Ryu, Y.K.; Manji, G.A.; Rustgi, A.K. Extracellular ATP and Adenosine in Cancer Pathogenesis and Treatment. Trends Cancer 2021, 7, 731–750. [Google Scholar] [CrossRef] [PubMed]
- Fredholm, B.B.; Arslan, G.; Halldner, L.; Kull, B.; Schulte, G.; Ådén, U.; Svenningsson, P. Adenosine Receptor Signaling in Vitro and in Vivo: Adenosine Receptor Signaling In Vitro and In Vivo. Drug Dev. Res. 2001, 52, 274–282. [Google Scholar] [CrossRef]
- Ansari, H.R.; Teng, B.; Nadeem, A.; Roush, K.P.; Martin, K.H.; Schnermann, J.; Mustafa, S.J. A 1 Adenosine Receptor-Mediated PKC and P42/P44 MAPK Signaling in Mouse Coronary Artery Smooth Muscle Cells. Am. J. Physiol.-Heart Circ. Physiol. 2009, 297, H1032–H1039. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Ji, Z.; Tsalkova, T.; Mei, F. Epac and PKA: A Tale of Two Intracellular cAMP Receptors. Acta Biochim. Biophys. Sin. 2008, 40, 651–662. [Google Scholar] [CrossRef]
- Bacallao, K.; Monje, P.V. Opposing Roles of Pka and Epac in the cAMP-Dependent Regulation of Schwann Cell Proliferation and Differentiation. PLoS ONE 2013, 8, e82354. [Google Scholar] [CrossRef]
- Mediero, A.; Perez-Aso, M.; Cronstein, B.N. Activation of EPAC1/2 Is Essential for Osteoclast Formation by Modulating NFκB Nuclear Translocation and Actin Cytoskeleton Rearrangements. FASEB J. 2014, 28, 4901–4913. [Google Scholar] [CrossRef]
- Mediero, A.; Perez-Aso, M.; Cronstein, B.N. Activation of Adenosine A 2A Receptor Reduces Osteoclast Formation via PKA- and ERK1/2-Mediated Suppression of NFκB Nuclear Translocation: A 2A R Inhibits Osteoclast Differentiation via cAMP. Br. J. Pharmacol. 2013, 169, 1372–1388. [Google Scholar] [CrossRef]
- Hewer, R.C.; Sala-Newby, G.B.; Wu, Y.-J.; Newby, A.C.; Bond, M. PKA and Epac Synergistically Inhibit Smooth Muscle Cell Proliferation. J. Mol. Cell. Cardiol. 2011, 50, 87–98. [Google Scholar] [CrossRef]
- Zhang, H.; Kong, Q.; Wang, J.; Jiang, Y.; Hua, H. Complex Roles of cAMP–PKA–CREB Signaling in Cancer. Exp. Hematol. Oncol. 2020, 9, 32. [Google Scholar] [CrossRef]
- Silveira, W.A.; Gonçalves, D.A.; Machado, J.; Lautherbach, N.; Lustrino, D.; Paula-Gomes, S.; Pereira, M.G.; Miyabara, E.H.; Sandri, M.; Kettelhut, I.C.; et al. cAMP-dependent Protein Kinase Inhibits FoxO Activity and Regulates Skeletal Muscle Plasticity in Mice. FASEB J. 2020, 34, 12946–12962. [Google Scholar] [CrossRef]
- Riquelme, M.A.; Cea, L.A.; Vega, J.L.; Boric, M.P.; Monyer, H.; Bennett, M.V.L.; Frank, M.; Willecke, K.; Sáez, J.C. The ATP Required for Potentiation of Skeletal Muscle Contraction Is Released via Pannexin Hemichannels. Neuropharmacology 2013, 75, 594–603. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, K.A.; Delicado, E.G.; Gachet, C.; Kennedy, C.; Kügelgen, I.; Li, B.; Miras-Portugal, M.T.; Novak, I.; Schöneberg, T.; Perez-Sen, R.; et al. Update of P2Y Receptor Pharmacology: IUPHAR Review 27. Br. J. Pharmacol. 2020, 177, 2413–2433. [Google Scholar] [CrossRef]
- Kjøbsted, R.; Hingst, J.R.; Fentz, J.; Foretz, M.; Sanz, M.; Pehmøller, C.; Shum, M.; Marette, A.; Mounier, R.; Treebak, J.T.; et al. AMPK in Skeletal Muscle Function and Metabolism. FASEB J. 2018, 32, 1741–1777. [Google Scholar] [CrossRef]
- Kim, J.; Yang, G.; Kim, Y.; Kim, J.; Ha, J. AMPK Activators: Mechanisms of Action and Physiological Activities. Exp. Mol. Med. 2016, 48, e224. [Google Scholar] [CrossRef]
- Baird, M.F.; Graham, S.M.; Baker, J.S.; Bickerstaff, G.F. Creatine-Kinase- and Exercise-Related Muscle Damage Implications for Muscle Performance and Recovery. J. Nutr. Metab. 2012, 2012, 960363. [Google Scholar] [CrossRef]
- Wright, N.A.; Wilcox, S.H.; Thomson, D.M. Month-Long AICAR Treatment Reverses Age-Related mTOR Pathway Hyperactivity. FASEB J. 2019, 33, 539.9. [Google Scholar] [CrossRef]
- Ponticos, M. Dual Regulation of the AMP-Activated Protein Kinase Provides a Novel Mechanism for the Control of Creatine Kinase in Skeletal Muscle. EMBO J. 1998, 17, 1688–1699. [Google Scholar] [CrossRef]
- Moldakozhayev, A.; Gladyshev, V.N. Metabolism, Homeostasis, and Aging. Trends Endocrinol. Metab. 2023, 34, 158–169. [Google Scholar] [CrossRef]
- Critchley, M. The Neurology of Old Age. Lancet 1931, 217, 1119–1127. [Google Scholar] [CrossRef]
- Rosenberg, I.H. Symposium: Sarcopenia: Diagnosis and Mechanisms. J. Nutr. 1997, 127, 990S–991S. [Google Scholar] [CrossRef] [PubMed]
- Janssen, I.; Shepard, D.S.; Katzmarzyk, P.T.; Roubenoff, R. The Healthcare Costs of Sarcopenia in the United States. J. Am. Geriatr. Soc. 2004, 52, 80–85. [Google Scholar] [CrossRef]
- Rolland, Y.; Czerwinski, S.; van Kan, G.A.; Morley, J.E.; Cesari, M.; Onder, G.; Woo, J.; Baumgartner, R.; Pillard, F.; Boirie, Y.; et al. Sarcopenia: Its Assessment, Etiology, Pathogenesis, Consequences and Future Perspectives. J. Nutr. Health Aging 2008, 12, 433–450. [Google Scholar] [CrossRef] [PubMed]
- Masanés Torán, F.; Navarro López, M.; Sacanella Meseguer, E.; López Soto, A. ¿Qué es la sarcopenia? Semin. Fund. Española Reumatol. 2010, 11, 14–23. [Google Scholar] [CrossRef]
- Muscaritoli, M.; Anker, S.D.; Argilés, J.; Aversa, Z.; Bauer, J.M.; Biolo, G.; Boirie, Y.; Bosaeus, I.; Cederholm, T.; Costelli, P.; et al. Consensus Definition of Sarcopenia, Cachexia and Pre-Cachexia: Joint Document Elaborated by Special Interest Groups (SIG) “Cachexia-Anorexia in Chronic Wasting Diseases” and “Nutrition in Geriatrics”. Clin. Nutr. 2010, 29, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Cannataro, R.; Carbone, L.; Petro, J.L.; Cione, E.; Vargas, S.; Angulo, H.; Forero, D.A.; Odriozola-Martínez, A.; Kreider, R.B.; Bonilla, D.A. Sarcopenia: Etiology, Nutritional Approaches, and miRNAs. Int. J. Mol. Sci. 2021, 22, 9724. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.-P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European Consensus on Definition and Diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Fielding, R.A.; Vellas, B.; Evans, W.J.; Bhasin, S.; Morley, J.E.; Newman, A.B.; Abellan van Kan, G.; Andrieu, S.; Bauer, J.; Breuille, D.; et al. Sarcopenia: An Undiagnosed Condition in Older Adults. Current Consensus Definition: Prevalence, Etiology, and Consequences. International Working Group on Sarcopenia. J. Am. Med. Dir. Assoc. 2011, 12, 249–256. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European Consensus on Definition and Diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Sayer, A.A. Sarcopenia. Lancet 2019, 393, 2636–2646. [Google Scholar] [CrossRef]
- Little, R.D.; Prieto-Potin, I.; Pérez-Baos, S.; Villalvilla, A.; Gratal, P.; Cicuttini, F.; Largo, R.; Herrero-Beaumont, G. Compensatory Anabolic Signaling in the Sarcopenia of Experimental Chronic Arthritis. Sci. Rep. 2017, 7, 6311. [Google Scholar] [CrossRef]
- Narici, M.V.; Maffulli, N. Sarcopenia: Characteristics, Mechanisms and Functional Significance. Br. Med. Bull. 2010, 95, 139–159. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Baos, S.; Prieto-Potin, I.; Román-Blas, J.A.; Sánchez-Pernaute, O.; Largo, R.; Herrero-Beaumont, G. Mediators and Patterns of Muscle Loss in Chronic Systemic Inflammation. Front. Physiol. 2018, 9, 409. [Google Scholar] [CrossRef] [PubMed]
- Beyer, I.; Mets, T.; Bautmans, I. Chronic Low-Grade Inflammation and Age-Related Sarcopenia. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Zanni, F. Marked Increase with Age of Type 1 Cytokines within Memory and Effector/Cytotoxic CD8+ T Cells in Humans: A Contribution to Understand the Relationship between Inflammation and Immunosenescence. Exp. Gerontol. 2003, 38, 981–987. [Google Scholar] [CrossRef] [PubMed]
- Bian, A.-L.; Hu, H.-Y.; Rong, Y.-D.; Wang, J.; Wang, J.-X.; Zhou, X.-Z. A Study on Relationship between Elderly Sarcopenia and Inflammatory Factors IL-6 and TNF-α. Eur. J. Med. Res. 2017, 22, 25. [Google Scholar] [CrossRef] [PubMed]
- Schaap, L.A.; Pluijm, S.M.F.; Deeg, D.J.H.; Visser, M. Inflammatory Markers and Loss of Muscle Mass (Sarcopenia) and Strength. Am. J. Med. 2006, 119, 526.e9–526.e17. [Google Scholar] [CrossRef]
- Sharma, B.; Dabur, R. Role of Pro-Inflammatory Cytokines in Regulation of Skeletal Muscle Metabolism: A Systematic Review. Curr. Med. Chem. 2020, 27, 2161–2188. [Google Scholar] [CrossRef]
- Aleman, H.; Esparza, J.; Ramirez, F.A.; Astiazaran, H.; Payette, H. Longitudinal Evidence on the Association between Interleukin-6 and C-Reactive Protein with the Loss of Total Appendicular Skeletal Muscle in Free-Living Older Men and Women. Age Ageing 2011, 40, 469–475. [Google Scholar] [CrossRef]
- Corvalán, L.A.; Araya, R.; Brañes, M.C.; Sáez, P.J.; Kalergis, A.M.; Tobar, J.A.; Theis, M.; Willecke, K.; Sáez, J.C. Injury of Skeletal Muscle and Specific Cytokines Induce the Expression of Gap Junction Channels in Mouse Dendritic Cells. J. Cell. Physiol. 2007, 211, 649–660. [Google Scholar] [CrossRef]
- Hofmann, S.R.; Rösen-Wolff, A.; Tsokos, G.C.; Hedrich, C.M. Biological Properties and Regulation of IL-10 Related Cytokines and Their Contribution to Autoimmune Disease and Tissue Injury. Clin. Immunol. 2012, 143, 116–127. [Google Scholar] [CrossRef]
- Heredia, J.E.; Mukundan, L.; Chen, F.M.; Mueller, A.A.; Deo, R.C.; Locksley, R.M.; Rando, T.A.; Chawla, A. Type 2 Innate Signals Stimulate Fibro/Adipogenic Progenitors to Facilitate Muscle Regeneration. Cell 2013, 153, 376–388. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-H.; Tsai, J.-N.; Chen, T.-L.; Ho, K.-T.; Cheng, H.-Y.; Hsiao, C.-W.; Shiau, M.-Y. Interleukin-4 Promotes Myogenesis and Boosts Myocyte Insulin Efficacy. Mediat. Inflamm. 2019, 2019, 4182015. [Google Scholar] [CrossRef] [PubMed]
- Cooper, C.; Dere, W.; Evans, W.; Kanis, J.A.; Rizzoli, R.; Sayer, A.A.; Sieber, C.C.; Kaufman, J.-M.; Abellan van Kan, G.; Boonen, S.; et al. Frailty and Sarcopenia: Definitions and Outcome Parameters. Osteoporos. Int. 2012, 23, 1839–1848. [Google Scholar] [CrossRef] [PubMed]
- Morley, J.E.; Vellas, B.; Abellan van Kan, G.; Anker, S.D.; Bauer, J.M.; Bernabei, R.; Cesari, M.; Chumlea, W.C.; Doehner, W.; Evans, J.; et al. Frailty Consensus: A Call to Action. J. Am. Med. Dir. Assoc. 2013, 14, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in Older Adults: Evidence for a Phenotype. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2001, 56, M146–M157. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.; Jackson, T.; Sapey, E.; Lord, J.M. Frailty and Sarcopenia: The Potential Role of an Aged Immune System. Ageing Res. Rev. 2017, 36, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Murton, A.J.; Maddocks, M.; Stephens, F.B.; Marimuthu, K.; England, R.; Wilcock, A. Consequences of Late-Stage Non–Small-Cell Lung Cancer Cachexia on Muscle Metabolic Processes. Clin. Lung Cancer 2017, 18, e1–e11. [Google Scholar] [CrossRef]
- Greiwe, J.S.; Cheng, B.; Rubin, D.C.; Yarasheski, K.E.; Semenkovich, C.F. Resistance Exercise Decreases Skeletal Muscle Tumor Necrosis Factor α in Frail Elderly Humans. FASEB J. 2001, 15, 475–482. [Google Scholar] [CrossRef]
- Pijet, B.; Pijet, M.; Litwiniuk, A.; Gajewska, M.; Pająk, B.; Orzechowski, A. TNF-α and IFN-s-Dependent Muscle Decay Is Linked to NF- κ B- and STAT-1 α-Stimulated Atrogin1 and MuRF1 Genes in C2C12 Myotubes. Mediat. Inflamm. 2013, 2013, 171437. [Google Scholar] [CrossRef]
- Hazeldine, J.; Lord, J.M. Innate Immunesenescence: Underlying Mechanisms and Clinical Relevance. Biogerontology 2015, 16, 187–201. [Google Scholar] [CrossRef]
- Fernández-Garrido, J.; Navarro-Martínez, R.; Buigues-González, C.; Martínez-Martínez, M.; Ruiz-Ros, V.; Cauli, O. The Value of Neutrophil and Lymphocyte Count in Frail Older Women. Exp. Gerontol. 2014, 54, 35–41. [Google Scholar] [CrossRef]
- Hazeldine, J.; Harris, P.; Chapple, I.L.; Grant, M.; Greenwood, H.; Livesey, A.; Sapey, E.; Lord, J.M. Impaired Neutrophil Extracellular Trap Formation: A Novel Defect in the Innate Immune System of Aged Individuals. Aging Cell 2014, 13, 690–698. [Google Scholar] [CrossRef] [PubMed]
- Glass, D.; Roubenoff, R. Recent Advances in the Biology and Therapy of Muscle Wasting: Glass & Roubenoff. Ann. N. Y. Acad. Sci. 2010, 1211, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Efthymiou, E.; Grammatikopoulou, M.G.; Gkiouras, K.; Efthymiou, G.; Zafiriou, E.; Goulis, D.G.; Sakkas, L.I.; Bogdanos, D.P. Time to Deal with Rheumatoid Cachexia: Prevalence, Diagnostic Criteria, Treatment Effects and Evidence for Management. Mediterr. J. Rheumatol. 2022, 33, 271. [Google Scholar] [CrossRef]
- Visser, M.; Pahor, M.; Taaffe, D.R.; Goodpaster, B.H.; Simonsick, E.M.; Newman, A.B.; Nevitt, M.; Harris, T.B. Relationship of Interleukin-6 and Tumor Necrosis Factor- With Muscle Mass and Muscle Strength in Elderly Men and Women: The Health ABC Study. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2002, 57, M326–M332. [Google Scholar] [CrossRef] [PubMed]
- Straub, R.H. Insulin Resistance, Selfish Brain, and Selfish Immune System: An Evolutionarily Positively Selected Program Used in Chronic Inflammatory Diseases. Arthritis Res. Ther. 2014, 16, S4. [Google Scholar] [CrossRef] [PubMed]
- Filippin, L.I.; Teixeira, V.N.; Viacava, P.R.; Lora, P.S.; Xavier, L.L.; Xavier, R.M. Temporal Development of Muscle Atrophy in Murine Model of Arthritis Is Related to Disease Severity. J. Cachexia Sarcopenia Muscle 2013, 4, 231–238. [Google Scholar] [CrossRef]
- Hartog, A.; Hulsman, J.; Garssen, J. Locomotion and Muscle Mass Measures in a Murine Model of Collagen-Induced Arthritis. BMC Musculoskelet. Disord. 2009, 10, 59. [Google Scholar] [CrossRef]
- Toth, K.G.; McKay, B.R.; De Lisio, M.; Little, J.P.; Tarnopolsky, M.A.; Parise, G. IL-6 Induced STAT3 Signalling Is Associated with the Proliferation of Human Muscle Satellite Cells Following Acute Muscle Damage. PLoS ONE 2011, 6, e17392. [Google Scholar] [CrossRef]
- Bermejo-Álvarez, I.; Pérez-Baos, S.; Gratal, P.; Medina, J.P.; Largo, R.; Herrero-Beaumont, G.; Mediero, A. Effects of Tofacitinib on Muscle Remodeling in Experimental Rheumatoid Sarcopenia. Int. J. Mol. Sci. 2023, 24, 13181. [Google Scholar] [CrossRef]
- Paris, M.T.; Bell, K.E.; Mourtzakis, M. Myokines and Adipokines in Sarcopenia: Understanding Cross-Talk between Skeletal Muscle and Adipose Tissue and the Role of Exercise. Curr. Opin. Pharmacol. 2020, 52, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Kirk, B.; Feehan, J.; Lombardi, G.; Duque, G. Muscle, Bone, and Fat Crosstalk: The Biological Role of Myokines, Osteokines, and Adipokines. Curr. Osteoporos. Rep. 2020, 18, 388–400. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-M.; Zhao, Y.-P.; Zhao, Y.; Deng, S.-L.; Yu, K. Regulation of Myostatin on the Growth and Development of Skeletal Muscle. Front. Cell Dev. Biol. 2021, 9, 785712. [Google Scholar] [CrossRef]
- Trendelenburg, A.U.; Meyer, A.; Rohner, D.; Boyle, J.; Hatakeyama, S.; Glass, D.J. Myostatin Reduces Akt/TORC1/p70S6K Signaling, Inhibiting Myoblast Differentiation and Myotube Size. Am. J. Physiol.-Cell Physiol. 2009, 296, C1258–C1270. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Wang, X.; Wei, Z.; Yang, M.; Zhou, X.; Lei, J.; Bai, C.; Su, G.; Liu, X.; Yang, L.; et al. Myostatin Deficiency Enhances Antioxidant Capacity of Bovine Muscle via the SMAD-AMPK-G6PD Pathway. Oxidative Med. Cell. Longev. 2022, 2022, 3497644. [Google Scholar] [CrossRef] [PubMed]
- Mafi, F.; Biglari, S.; Ghardashi Afousi, A.; Gaeini, A.A. Improvement in Skeletal Muscle Strength and Plasma Levels of Follistatin and Myostatin Induced by an 8-Week Resistance Training and Epicatechin Supplementation in Sarcopenic Older Adults. J. Aging Phys. Act. 2019, 27, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Ryan, A.S.; Li, G. Skeletal Muscle Myostatin Gene Expression and Sarcopenia in Overweight and Obese Middle-aged and Older Adults. JCSM Clin. Rep. 2021, 6, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Ding, H.; Deng, Y.; Liu, H.; Xiong, X.; Yang, Y. Irisin: A New Code Uncover the Relationship of Skeletal Muscle and Cardiovascular Health During Exercise. Front. Physiol. 2021, 12, 620608. [Google Scholar] [CrossRef]
- Ye, X.; Shen, Y.; Ni, C.; Ye, J.; Xin, Y.; Zhang, W.; Ren, Y. Irisin Reverses Insulin Resistance in C2C12 Cells via the P38-MAPK-PGC-1α Pathway. Peptides 2019, 119, 170120. [Google Scholar] [CrossRef]
- Zhi, S.; Yang, L.; Yang, G.; Qin, C.; Yan, X.; Niu, M.; Zhang, W.; Liu, M.; Zhao, M.; Nie, G. Irisin Regulates Hepatic Glucose Metabolism via AMPK and PI3K/Akt Activation. Aquac. Nutr. 2022, 2022, 1946960. [Google Scholar] [CrossRef]
- Mai, S.; Grugni, G.; Mele, C.; Vietti, R.; Vigna, L.; Sartorio, A.; Aimaretti, G.; Scacchi, M.; Marzullo, P. Irisin Levels in Genetic and Essential Obesity: Clues for a Potential Dual Role. Sci. Rep. 2020, 10, 1020. [Google Scholar] [CrossRef] [PubMed]
- Minokoshi, Y.; Kim, Y.-B.; Peroni, O.D.; Fryer, L.G.D.; Müller, C.; Carling, D.; Kahn, B.B. Leptin Stimulates Fatty-Acid Oxidation by Activating AMP-Activated Protein Kinase. Nature 2002, 415, 339–343. [Google Scholar] [CrossRef]
- D’souza, A.M.; Asadi, A.; Johnson, J.D.; Covey, S.D.; Kieffer, T.J. Leptin Deficiency in Rats Results in Hyperinsulinemia and Impaired Glucose Homeostasis. Endocrinology 2014, 155, 1268–1279. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yang, Z.-Y.; Chen, W.-L. Examining the Association Between Serum Leptin and Sarcopenic Obesity. J. Inflamm. Res. 2021, 14, 3481–3487. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Long, Q.; Lei, T.; Chen, X.; Long, H.; Feng, B.; Peng, Y.; Wu, Y.; Yang, Z. Lipid Accumulation Mediated by Adiponectin in C2C12 Myogenesis. BMB Rep. 2009, 42, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yu, K.; Shyh-Chang, N.; Li, G.; Jiang, L.; Yu, S.; Xu, L.; Liu, R.; Guo, Z.; Xie, H.; et al. Circulating Factors Associated with Sarcopenia during Ageing and after Intensive Lifestyle Intervention. J. Cachexia Sarcopenia Muscle 2019, 10, 586–600. [Google Scholar] [CrossRef]
- Komici, K.; Dello Iacono, A.; De Luca, A.; Perrotta, F.; Bencivenga, L.; Rengo, G.; Rocca, A.; Guerra, G. Adiponectin and Sarcopenia: A Systematic Review With Meta-Analysis. Front. Endocrinol. 2021, 12, 576619. [Google Scholar] [CrossRef]
- Singh, S.; Anshita, D.; Ravichandiran, V. MCP-1: Function, Regulation, and Involvement in Disease. Int. Immunopharmacol. 2021, 101, 107598. [Google Scholar] [CrossRef]
- Rajasekaran, M.; Sul, O.-J.; Choi, E.-K.; Kim, J.-E.; Suh, J.-H.; Choi, H.-S. MCP-1 Deficiency Enhances Browning of Adipose Tissue via Increased M2 Polarization. J. Endocrinol. 2019, 242, 91–101. [Google Scholar] [CrossRef]
- Sartipy, P.; Loskutoff, D.J. Monocyte Chemoattractant Protein 1 in Obesity and Insulin Resistance. Proc. Natl. Acad. Sci. USA 2003, 100, 7265–7270. [Google Scholar] [CrossRef]
- Hulsmans, M.; Geeraert, B.; Arnould, T.; Tsatsanis, C.; Holvoet, P. PPAR Agonist-Induced Reduction of Mcp1 in Atherosclerotic Plaques of Obese, Insulin-Resistant Mice Depends on Adiponectin-Induced Irak3 Expression. PLoS ONE 2013, 8, e62253. [Google Scholar] [CrossRef] [PubMed]
- Talbert, E.E.; Lewis, H.L.; Farren, M.R.; Ramsey, M.L.; Chakedis, J.M.; Rajasekera, P.; Haverick, E.; Sarna, A.; Bloomston, M.; Pawlik, T.M.; et al. Circulating Monocyte Chemoattractant Protein-1 (MCP-1) Is Associated with Cachexia in Treatment-Naïve Pancreatic Cancer Patients: A Biomarker Analysis in Pancreatic Adenocarcinoma-Induced Cachexia. J. Cachexia Sarcopenia Muscle 2018, 9, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Afandy, N.O.; Lock, H.S.; Tay, L.; Yeo, A.; Yew, S.; Leung, B.P.; Lim, W.S. Association of Monocyte Chemotactic Protein-1 and Dickkopf-1 with Body Composition and Physical Performance in Community-Dwelling Older Adults in Singapore. J. Frailty Sarcopenia Falls 2021, 06, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Bornø, A.; Ploug, T.; Bune, L.T.; Rosenmeier, J.B.; Thaning, P. Purinergic Receptors Expressed in Human Skeletal Muscle Fibres. Purinergic Signal. 2012, 8, 255–264. [Google Scholar] [CrossRef]
- Mortensen, S.P.; McAllister, R.M.; Yang, H.T.; Hellsten, Y.; Laughlin, M.H. The Effect of Purinergic P2 Receptor Blockade on Skeletal Muscle Exercise Hyperemia in Miniature Swine. Eur. J. Appl. Physiol. 2014, 114, 2147–2155. [Google Scholar] [CrossRef] [PubMed]
- Zaripova, K.А.; Belova, S.P.; Shenkman, B.S.; Nemirovskaya, T.L. The Role of P2Y Receptors in the Regulation of Atrophic Processes in Rat Skeletal Muscles under Unloading. J. Evol. Biochem. Phys. 2022, 58, 1708–1719. [Google Scholar] [CrossRef]
- Zabłocki, K.; Górecki, D.C. The Role of P2X7 Purinoceptors in the Pathogenesis and Treatment of Muscular Dystrophies. Int. J. Mol. Sci. 2023, 24, 9434. [Google Scholar] [CrossRef] [PubMed]
- Morales-Jiménez, C.; Balanta-Melo, J.; Arias-Calderón, M.; Hernández, N.; Gómez-Valenzuela, F.; Escobar, A.; Jaimovich, E.; Buvinic, S. Mechanical Disturbance of Osteoclasts Induces ATP Release That Leads to Protein Synthesis in Skeletal Muscle through an Akt-mTOR Signaling Pathway. Int. J. Mol. Sci. 2022, 23, 9444. [Google Scholar] [CrossRef]
- Tian, T.; Heine, M.; Evangelakos, I.; Jaeckstein, M.Y.; Schaltenberg, N.; Stähler, T.; Koch-Nolte, F.; Kumari, M.; Heeren, J. The P2X7 Ion Channel Is Dispensable for Energy and Metabolic Homeostasis of White and Brown Adipose Tissues. Purinergic Signal. 2020, 16, 529–542. [Google Scholar] [CrossRef]
- Chiang, C.-H.; Cheng, C.-Y.; Lien, Y.-T.; Huang, K.-C.; Lin, W.-W. P2X7 Activation Enhances Lipid Accumulation During Adipocytes Differentiation Through Suppressing the Expression of Sirtuin-3, Sirtuin-5, and Browning Genes. Front. Pharmacol. 2022, 13, 852858. [Google Scholar] [CrossRef]
- Wang, M.-J.; Yang, B.-R.; Jing, X.-Y.; Wang, Y.-Z.; Kang, L.; Ren, K.; Kang, L. P2Y1R and P2Y2R: Potential Molecular Triggers in Muscle Regeneration. Purinergic Signal. 2023, 19, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Chen, H.; Gu, Y.; Sun, P.; Sun, J.; Yu, H.; Zheng, H.; Chen, D. P2Y2 Promotes Fibroblasts Activation and Skeletal Muscle Fibrosis through AKT, ERK, and PKC. BMC Musculoskelet. Disord. 2021, 22, 680. [Google Scholar] [CrossRef] [PubMed]
- Liu, I.-M.; Lai, T.-Y.; Tsai, C.-C.; Cheng, J.-T. Characterization of Adenosine A1 Receptor in Cultured Myoblast C2C12 Cells of Mice. Auton. Neurosci. 2001, 87, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Gnad, T.; Navarro, G.; Lahesmaa, M.; Reverte-Salisa, L.; Copperi, F.; Cordomi, A.; Naumann, J.; Hochhäuser, A.; Haufs-Brusberg, S.; Wenzel, D.; et al. Adenosine/A2B Receptor Signaling Ameliorates the Effects of Aging and Counteracts Obesity. Cell Metab. 2020, 32, 56–70.e7. [Google Scholar] [CrossRef] [PubMed]
- Warren, G.L.; Hulderman, T.; Liston, A.; Simeonova, P.P. Toll-like and Adenosine Receptor Expression in Injured Skeletal Muscle. Muscle Nerve 2011, 44, 85–92. [Google Scholar] [CrossRef]
- Sacramento, J.F.; Martins, F.O.; Rodrigues, T.; Matafome, P.; Ribeiro, M.J.; Olea, E.; Conde, S.V. A2 Adenosine Receptors Mediate Whole-Body Insulin Sensitivity in a Prediabetes Animal Model: Primary Effects on Skeletal Muscle. Front. Endocrinol. 2020, 11, 262. [Google Scholar] [CrossRef]
- Lynge, J.; Hellsten, Y. Distribution of Adenosine A1, A2A and A2B Receptors in Human Skeletal Muscle: Adenosine Receptors in Human Skeletal Muscle. Acta Physiol. Scand. 2000, 169, 283–290. [Google Scholar] [CrossRef]
- Han, D.H.; Hansen, P.A.; Nolte, L.A.; Holloszy, J.O. Removal of Adenosine Decreases the Responsiveness of Muscle Glucose Transport to Insulin and Contractions. Diabetes 1998, 47, 1671–1675. [Google Scholar] [CrossRef]
- Berdeaux, R.; Stewart, R. cAMP Signaling in Skeletal Muscle Adaptation: Hypertrophy, Metabolism, and Regeneration. Am. J. Physiol.-Endocrinol. Metab. 2012, 303, E1–E17. [Google Scholar] [CrossRef]
- Thomson, D.M.; Herway, S.T.; Fillmore, N.; Kim, H.; Brown, J.D.; Barrow, J.R.; Winder, W.W. AMP-Activated Protein Kinase Phosphorylates Transcription Factors of the CREB Family. J. Appl. Physiol. 2008, 104, 429–438. [Google Scholar] [CrossRef]
- Eisenstein, A.; Chitalia, S.V.; Ravid, K. Bone Marrow and Adipose Tissue Adenosine Receptors Effect on Osteogenesis and Adipogenesis. Int. J. Mol. Sci. 2020, 21, 7470. [Google Scholar] [CrossRef]
- Wang, Z.; Shi, F. Phosphodiesterase 4 and Compartmentalization of Cyclic AMP Signaling. Chin. Sci. Bull. 2007, 52, 34–46. [Google Scholar] [CrossRef]
- Thomson, D. The Role of AMPK in the Regulation of Skeletal Muscle Size, Hypertrophy, and Regeneration. Int. J. Mol. Sci. 2018, 19, 3125. [Google Scholar] [CrossRef]
- Hardie, D.G. AMP-Activated Protein Kinase—An Energy Sensor That Regulates All Aspects of Cell Function. Genes Dev. 2011, 25, 1895–1908. [Google Scholar] [CrossRef]
- Chen, A.E.; Ginty, D.D.; Fan, C.-M. Protein Kinase A Signalling via CREB Controls Myogenesis Induced by Wnt Proteins. Nature 2005, 433, 317–322. [Google Scholar] [CrossRef]
- Marco-Bonilla, M.; Herencia, R.; Fresnadillo, M.; Huete-Toral, F.; Carracedo, G.; Largo, R.; Herrero-Beaumont, G.; Mediero, A. Dipyridamole activates adenosine A2B receptor and AMPK/cAMP signaling and promotes myogenic differentiation of myoblastic C2C12 cells. Front. Pharmacol. 2023, 14, 1247664. [Google Scholar] [CrossRef] [PubMed]
- Stewart, R.; Flechner, L.; Montminy, M.; Berdeaux, R. CREB Is Activated by Muscle Injury and Promotes Muscle Regeneration. PLoS ONE 2011, 6, e24714. [Google Scholar] [CrossRef]
- Dasgupta, D.; Mahadev Bhat, S.; Price, A.L.; Delmotte, P.; Sieck, G.C. Molecular Mechanisms Underlying TNFα-Induced Mitochondrial Biogenesis in Human Airway Smooth Muscle. Int. J. Mol. Sci. 2023, 24, 5788. [Google Scholar] [CrossRef] [PubMed]
- Vassaux, G.; Gaillard, D.; Mari, B.; Ailhaud, G.; Negrel, R. Differential Expression of Adenosine A1 and Adenosine A2 Receptors in Preadipocytes and Adipocytes. Biochem. Biophys. Res. Commun. 1993, 193, 1123–1130. [Google Scholar] [CrossRef]
- Børglum, J.D.; Vassaux, G.; Richelsen, B.; Gaillard, D.; Darimont, C.; Ailhaud, G.; Négrel, R. Changes in Adenosine Al- and A2-Receptor Expression during Adipose Cell Differentiation. Mol. Cell. Endocrinol. 1996, 117, 17–25. [Google Scholar] [CrossRef]
- Gharibi, B.; Abraham, A.A.; Ham, J.; Evans, B.A. Adenosine Receptor Subtype Expression and Activation Influence the Differentiation of Mesenchymal Stem Cells to Osteoblasts and Adipocytes. J. Bone Miner. Res. 2011, 26, 2112–2124. [Google Scholar] [CrossRef] [PubMed]
- Gnad, T.; Scheibler, S.; von Kügelgen, I.; Scheele, C.; Kilić, A.; Glöde, A.; Hoffmann, L.S.; Reverte-Salisa, L.; Horn, P.; Mutlu, S.; et al. Adenosine Activates Brown Adipose Tissue and Recruits Beige Adipocytes via A2A Receptors. Nature 2014, 516, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Lahesmaa, M.; Oikonen, V.; Helin, S.; Luoto, P.; U Din, M.; Pfeifer, A.; Nuutila, P.; Virtanen, K.A. Regulation of Human Brown Adipose Tissue by Adenosine and A2A Receptors—Studies with [15O]H2O and [11C]TMSX PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Verma, N.; Perie, L.; Corciulo, C.; Leucht, P.; Ramkhelawon, B.; Cronstein, B.N.; Mueller, E. Browning of Adipose Tissue and Increased Thermogenesis Induced by Methotrexate. FASEB BioAdvances 2021, 3, 877–887. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, L.; Liang, Y.; Zhang, C.; Xu, Z.; Zhang, L.; Fuji, R.; Mu, W.; Li, L.; Jiang, J.; et al. Cyclic AMP Mimics the Anti-Ageing Effects of Calorie Restriction by Up-Regulating Sirtuin. Sci. Rep. 2015, 5, 12012. [Google Scholar] [CrossRef] [PubMed]
- Carroll, S.H.; Wigner, N.A.; Kulkarni, N.; Johnston-Cox, H.; Gerstenfeld, L.C.; Ravid, K. A2B Adenosine Receptor Promotes Mesenchymal Stem Cell Differentiation to Osteoblasts and Bone Formation in Vivo. J. Biol. Chem. 2012, 287, 15718–15727. [Google Scholar] [CrossRef]
- Zamboni, M.; Rubele, S.; Rossi, A.P. Sarcopenia and Obesity. Curr. Opin. Clin. Nutr. Metab. Care 2019, 22, 13–19. [Google Scholar] [CrossRef]
- Roh, E.; Choi, K.M. Health Consequences of Sarcopenic Obesity: A Narrative Review. Front. Endocrinol. 2020, 11, 332. [Google Scholar] [CrossRef]
- Szkudelski, T.; Szkudelska, K.; Nogowski, L. Effects of Adenosine A1 Receptor Antagonism on Lipogenesis and Lipolysis in Isolated Rat Adipocytes. Physiol. Res. 2009, 58, 863–871. [Google Scholar] [CrossRef]
- Gharibi, B.; Abraham, A.A.; Ham, J.; Evans, B.A.J. Contrasting Effects of A1 and A2b Adenosine Receptors on Adipogenesis. Int. J. Obes. 2012, 36, 397–406. [Google Scholar] [CrossRef]
- Poulsen, S.-A.; Quinn, R.J. Adenosine Receptors: New Opportunities for Future Drugs. Bioorg. Med. Chem. 1998, 6, 619–641. [Google Scholar] [CrossRef]
- Sachdeva, S.; Gupta, M. Adenosine and Its Receptors as Therapeutic Targets: An Overview. Saudi Pharm. J. 2013, 21, 245–253. [Google Scholar] [CrossRef]
- Salminen, A.; Kaarniranta, K.; Kauppinen, A. Age-Related Changes in AMPK Activation: Role for AMPK Phosphatases and Inhibitory Phosphorylation by Upstream Signaling Pathways. Ageing Res. Rev. 2016, 28, 15–26. [Google Scholar] [CrossRef]
- Laudette, M.; Sainte-Marie, Y.; Cousin, G.; Bergonnier, D.; Belhabib, I.; Brun, S.; Formoso, K.; Laib, L.; Tortosa, F.; Bergoglio, C.; et al. Cyclic AMP-Binding Protein Epac1 Acts as a Metabolic Sensor to Promote Cardiomyocyte Lipotoxicity. Cell Death Dis. 2021, 12, 824. [Google Scholar] [CrossRef]
- Raney, M.A.; Turcotte, L.P. Evidence for the Involvement of CaMKII and AMPK in Ca2+-Dependent Signaling Pathways Regulating FA Uptake and Oxidation in Contracting Rodent Muscle. J. Appl. Physiol. 2008, 104, 1366–1373. [Google Scholar] [CrossRef] [PubMed]
- Weller, J.; Pose, M.; Protzel, C.; Mader, F.; Porath, K.; Köhling, R.; Hakenberg, O.W.; Kirschstein, T. Age-Related Decrease of Adenosine-Mediated Relaxation in Rat Detrusor Is a Result of A2B Receptor Downregulation: Adenosine Relaxation in Aging Detrusor. Int. J. Urol. 2015, 22, 322–329. [Google Scholar] [CrossRef]
- Aoyagi, Y.; Shephard, R.J. Aging and Muscle Function. Sports Med. 1992, 14, 376–396. [Google Scholar] [CrossRef] [PubMed]
- Flück, M.; Sanchez, C.; Jacquemond, V.; Berthier, C.; Giraud, M.-N.; Jacko, D.; Bersiner, K.; Gehlert, S.; Baan, G.; Jaspers, R.T. Enhanced Capacity for CaMKII Signaling Mitigates Calcium Release Related Contractile Fatigue with High Intensity Exercise. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2024, 1871, 119610. [Google Scholar] [CrossRef] [PubMed]
- Sáez, P.J.; Vargas, P.; Shoji, K.F.; Harcha, P.A.; Lennon-Duménil, A.-M.; Sáez, J.C. ATP Promotes the Fast Migration of Dendritic Cells through the Activity of Pannexin 1 Channels and P2X7 Receptors. Sci. Signal. 2017, 10, eaah7107. [Google Scholar] [CrossRef] [PubMed]
- Riquelme, M.A.; Cea, L.A.; Vega, J.L.; Puebla, C.; Vargas, A.A.; Shoji, K.F.; Subiabre, M.; Sáez, J.C. Pannexin Channels Mediate the Acquisition of Myogenic Commitment in C2C12 Reserve Cells Promoted by P2 Receptor Activation. Front. Cell Dev. Biol. 2015, 3, 25. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G. Minireview: The AMP-Activated Protein Kinase Cascade: The Key Sensor of Cellular Energy Status. Endocrinology 2003, 144, 5179–5183. [Google Scholar] [CrossRef]
- Pharaoh, G.; Brown, J.; Ranjit, R.; Ungvari, Z.; Van Remmen, H. Reduced Adenosine Diphosphate Sensitivity in Skeletal Muscle Mitochondria Increases Reactive Oxygen Species Production in Mouse Models of Aging and Oxidative Stress but Not Denervation. JCSM Rapid Commun. 2021, 4, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Holloway, G.P.; Holwerda, A.M.; Miotto, P.M.; Dirks, M.L.; Verdijk, L.B.; van Loon, L.J.C. Age-Associated Impairments in Mitochondrial ADP Sensitivity Contribute to Redox Stress in Senescent Human Skeletal Muscle. Cell Rep. 2018, 22, 2837–2848. [Google Scholar] [CrossRef] [PubMed]
- Brunetta, H.S.; Petrick, H.L.; Vachon, B.; Nunes, E.A.; Holloway, G.P. Insulin Rapidly Increases Skeletal Muscle Mitochondrial ADP Sensitivity in the Absence of a High Lipid Environment. Biochem. J. 2021, 478, 2539–2553. [Google Scholar] [CrossRef] [PubMed]
- Dumitru, A.; Radu, B.M.; Radu, M.; Cretoiu, S.M. Muscle Changes During Atrophy. In Muscle Atrophy; Xiao, J., Ed.; Advances in Experimental Medicine and Biology; Springer: Singapore, 2018; Volume 1088, pp. 73–92. ISBN 9789811314346. [Google Scholar]
- Miller, S.G.; Hafen, P.S.; Law, A.S.; Springer, C.B.; Logsdon, D.L.; O’Connell, T.M.; Witczak, C.A.; Brault, J.J. AMP Deamination Is Sufficient to Replicate an Atrophy-like Metabolic Phenotype in Skeletal Muscle. Metabolism 2021, 123, 154864. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Zhu, M.J.; Ma, C.; Du, M. Rat Hindlimb Unloading Down-Regulates Insulin like Growth Factor-1 Signaling and AMP-Activated Protein Kinase, and Leads to Severe Atrophy of the Soleus Muscle. Appl. Physiol. Nutr. Metab. 2007, 32, 1115–1123. [Google Scholar] [CrossRef] [PubMed]
- Argilés, J.M.; Busquets, S.; Stemmler, B.; López-Soriano, F.J. Cancer Cachexia: Understanding the Molecular Basis. Nat. Rev. Cancer 2014, 14, 754–762. [Google Scholar] [CrossRef] [PubMed]
- Donohoe, C.L.; Ryan, A.M.; Reynolds, J.V. Cancer Cachexia: Mechanisms and Clinical Implications. Gastroenterol. Res. Pract. 2011, 2011, 601434. [Google Scholar] [CrossRef]
- Symreng, T.; Larsson, J.; Schildt, B.O.; Wetterfors, J. Nutritional Assessment Reflects Muscle Energy Metabolism in Gastric Carcinoma. Ann. Surg. 1983, 198, 146–150. [Google Scholar] [CrossRef]
- Schneeberger, A.L.; Thompson, R.T.; Driedger, A.A.; Finley, R.J.; Inaiici, R.I. Effect of Cancer on the in Vivo Energy State of Rat Liver and Skeletal Muscle. Cancer Res. 1989, 49, 1160–1164. [Google Scholar]
- Hochwald, S.N.; Harrison, L.E.; Port, J.L.; Blumberg, D.; Brennan, M.F.; Burt, M. Depletion of High Energy Phosphate Compounds in the Tumor-Bearing State and Reversal after Tumor Resection. Surgery 1996, 120, 534–541. [Google Scholar] [CrossRef]
- Haskell, C.M.; Wong, M.; Williams, A.; Lee, L.-Y. Phase I Trial of Extracellular Adenosine 5′-Triphosphate in Patients with Advanced Cancer. Med. Pediatr. Oncol. 1996, 27, 165–173. [Google Scholar] [CrossRef]
- Agteresch, H.J.; Leij-Halfwerk, S.; Hordijk-Luijk, C.H.; Wilson, J.H.P.; Dagnelie, P.C. Effects of ATP Infusion on Glucose Turnover and Gluconeogenesis in Patients with Advanced Non-Small-Cell Lung Cancer. Clin. Sci. 2000, 98, 689–695. [Google Scholar] [CrossRef]
- Coolen, E.J.C.M.; Arts, I.C.W.; Bekers, O.; Vervaet, C.; Bast, A.; Dagnelie, P.C. Oral Bioavailability of ATP after Prolonged Administration. Br. J. Nutr. 2011, 105, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Bonsett, C.A.; Rudman, A. The Dystrophin Connection—ATP? Med. Hypotheses 1992, 38, 139–154. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.G.; Hafen, P.S.; Brault, J.J. Increased Adenine Nucleotide Degradation in Skeletal Muscle Atrophy. Int. J. Mol. Sci. 2019, 21, 88. [Google Scholar] [CrossRef]
- Thomson, W.H.S.; Smith, I. X-Linked Recessive (Duchenne) Muscular Dystrophy (DM D) and Purine Metabolism: Effects of Oral Allopurinol and Adenylate. Metabolism 1978, 27, 151–163. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).