Abstract
Pyogenic granuloma (PG) is a benign vascular lesion found predominantly in the oral cavity. Characterized by rapid growth and propensity to bleed, PG presents diagnostic challenges due to its similarity and alarming proliferation. This narrative review synthesizes current knowledge on the epidemiology, etiopathogenesis, clinical manifestations, and management of oral PG, with emphasis on recent advances in diagnostic and therapeutic approaches. The epidemiology of the injury is meticulously analyzed, revealing a higher incidence in women and a wide range of ages of onset. It delves into the etiopathogenesis, highlighting the uncertainty surrounding the exact causal factors, although historical attributions suggest an infectious origin. It exhaustively analyzes the clinical and histopathological aspects of oral PG, offering information on its various presentations and the importance of an accurate diagnosis to guide effective treatment. It details treatment strategies, emphasizing the personalized approach based on individual patient characteristics. This comprehensive review consolidates current knowledge on oral PG, highlighting the need for further research to clarify its pathogenesis and optimize treatment protocols.
1. Introduction
Pyogenic granuloma (PG) is a benign connective tissue proliferation that is predominantly characterized by granulation tissue hyperplasia, and it occurs frequently in the skin or mucous membranes [,,,]. It is considered one of the most common lesions responsible for soft tissue enlargements, due to its rapid and alarming growth rate [,,,].
Pyogenic granuloma (PG) was first described in 1897 by Poncet and Dor, who reported four patients with “vascular tumors” on the fingers which they named “Botrichomycosis hominis” []. The term “pyogenic granuloma” was introduced in 1904 by Hartzell. However, the name is considered inappropriate as it is neither related to pus formation, nor is it histologically a true granuloma [,,]. Due to the controversy regarding its true pathological nature, this lesion has been given several names such as granuloma pediculatum benignum, benign vascular tumor, septic granuloma, hemangiomatous granuloma, vascular epulis, fibroangioma, polypoid capillary hemangioma, eruption capillary hemangioma, non-lobular capillary hemangioma, and Crocker and Hartzell’s disease []. In pregnant women, GP is identified as pregnancy granuloma, pyogenic granuloma of pregnancy, or granuloma gravidarum []. In the dermatological literature, Cawson et al. (1998) have described this disease as “granuloma telangiectacticum” due to the presence of numerous blood vessels observed in histological sections. Some pyogenic granulomas (also known as lobular capillary hemangiomas) are categorized as vascular tumors, according to the classification of the International Society for the Study of Vascular Anomalies (ISSVA, 2022).
Given that PG is a common lesion in the oral cavity, this article was aimed at reviewing its prevalence, etiopathogenesis, clinical picture, radiographical and histopathological features, as well as its differential diagnosis and treatment.
2. Epidemiology
Oral PG manifests within the range of 4.5 to 93 years, and it shows a preeminent incidence in the second and fifth decades, with the female gender often being more affected than the male gender [,]. On the other hand, some researchers have suggested that this disease manifests mostly in males younger than 18 years and in females aged 18–39 years, with equal gender distribution in older patients []. With regard to its prevalence, in a retrospective study conducted at the Oral and Maxillofacial Pathology Department, Faculty of Dentistry, Shiraz University of Medical Sciences in Iran, analysis was performed on 1000 biopsies of gingival lesions, out of which 92.4% of the cases were found to be non-neoplastic lesions. The most common reports were related to reactive lesions (71.8%), with the highest PG-associated prevalence of 24.6% [,]. In their descriptive study of 427 patients, PG accounted for 50.35% of all reactive oral cavity lesions reported at Dharwad Dental Institute in Karnataka, India. Shammin et al. [] analyzed 244 cases of gingival lesions at the Department of Oral Pathology, Government Dental College, Calicut, India, and reported that non-neoplastic lesions comprised 75.5% of the cases, out of which PG accounted for 52.7%. A descriptive study of 923 Iranian pregnant women revealed that only 2 of them (0.22%) presented with PG []. On the other hand, a 4.2% PG prevalence has been reported in this group of patients in a medical center in Kerman/Iran []. These discrepancies in prevalence could be attributed to the differences in diagnostic methods applied. The diagnosis was based only on the clinical picture of the lesion, while Nejad et al. [] implemented confirmation through histopathological studies.
3. Etiopathogenesis
Some factors are implicated in the etiopathogenesis of PG. However, the exact cause is unknown. Historically, some researchers consider it to be a pathology attributable to an infectious agent, hence the term “pyogenic” [,]. Kerr in 1951 was of the view that the factors that influence the progression of PG were bebotryomycosis, staphylococci, foreign particles, and the accumulation of infection in the endothelium of blood vessels []. In a study by Bhaskar and Jacoway, Gram-positive and Gram-negative bacilli were identified in GP. However, these microorganisms could be members of the oral microbiota, since they were more frequent in ulcerated lesions than in non-ulcerated lesions []. On the other hand, in 2001, Lee and Lynde pioneered the establishment of a statistically significant association between Bartonella seropositivity and PG, which has important therapeutic implications []. This association suggested the possibility of non-surgical treatment with an antibiotic such as erythromycin. In addition, they suggested that such treatment may also decrease recurrence and satellitosis of PG.
Several researchers define the PG as a “reactive” or “reparative” process. Regezi et al. consider PG a reactive or repairing process in which a certain stimulus generates an exuberant proliferation of connective tissue [,]. The etiological factors considered as stimuli that trigger this reactive process are trauma, dental calculus, dental biofilm, chronic irritation, pre-existing vascular lesions, chronic irritation due to exfoliation of primary teeth, injury of a primary tooth, eruption of permanent teeth, defective restorations in the area of the lesion, occlusal interference, food impaction, periodontitis, and trauma from toothbrushing [,,]. These factors are summarized in Figure 1.
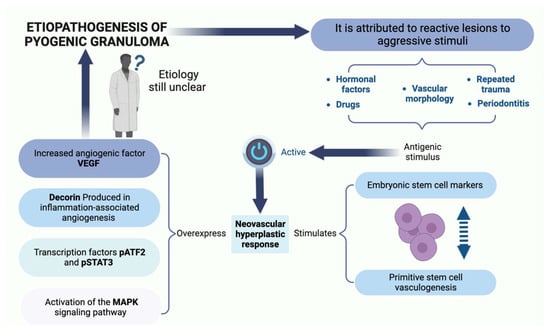
Figure 1.
The etiopathogenesis and risk factors of PG observed. Figure created with BioRender, © biorender.com.
Pyogenic granuloma (PG) may manifest after a hypersensitivity reaction associated with the use of drugs such as calcineurin inhibitors (cyclosporine and tacrolimus), carbamazepine, phenytoin, nifedipine, levothyroxine and ramucirumab [,,,,,,,]. Additionally, PG is associated with retinoid, antineoplastic and antiretroviral agents []. The mechanism involved in the presence of PG following hematopoietic cell transplant (HCT) is unknown. Cheney and Lund described five cases of pediatric patients who developed oral PG after HCT, under treatment with the calcineurin inhibitors cyclosporine A or tacrolimus []. It has been suggested that a side effect of cyclosporine, which is less common in tacrolimus, is the proliferation of fibroblasts, thereby generating increased collagen synthesis in the oral mucosa, resulting in triggering of a critical gingival hyperplasia that leads to PG. Moreover, there is an increase in the level of connective tissue growth factor [,]. Cyclosporine decreases the production of collagenase and matrix metalloproteinases, and also upregulates the expressions of the inhibitors of these metalloproteinases, thereby playing a crucial role in tissue growth. These hyperplastic effects on the gums may be implicated in the development of PG associated with calcineurin inhibitors. Cheney et al. described a case series in which they included five pediatric/adolescent patients who developed oral PG after HCT for acute lymphoblastic leukemia, Fanconi anemia, nodular sclerosis Hodgkin’s lymphoma, or junctional epidermolysis bullosa []. It was suggested that calcineurin inhibitors, which are used for graft versus host disease, play a crucial role due to irritation and chronic inflammatory changes in the oral cavity, leading to tissue proliferation, and eventually to PG formation. Regarding carbamazepine, the release of angiogenic factors stimulated by the inflammatory process and the impairment of liver functions contribute to the development of PG []. Levothyroxine has been considered a possible etiological factor in PG through its pro-angiogenic and proliferative effects []. Piraccini et al. reported that a side effect in psoriasis or acne patients treated with retinoids (systemic isotretinoin, systemic etretinate, systemic acitretin, topical retinoic acid, and topical tazarotene) is the appearance of periungual PGs. Retinoids decrease the attachments amongst keratinocytes, exert angiogenic properties, and inhibit the activities of collagenases and gelatinases in vitro [,]. Antiretrovirals, particularly indinavir and lamivudine, are associated with the presence of PG in the nail folds, and the time of appearance varies from 2 months to 1 year from the initiation of therapy [,]. It has been suggested that protease inhibitors may have a retinoid-like effect by virtue of homologies between the amino acid sequences of cellular retinoic acid binding protein 1 (CRABP1) and the catalytic site of HIV-1 protease. Thus, the specific retinoid receptor may be occupied and activated by the antiretroviral agent, thereby increasing the activity of vitamin A and its analogues []. Multiple PGs have been described in metastatic carcinoma patients treated with antineoplastic drugs (epidermal growth factor receptor inhibitors, capecitabine, cyclosporin, docetaxel, and mitoxantrone) []. Aragaki et al. described two clinical cases with the presence of oral PG during administration of ramucirumab for gastric cancer. Ramucirumab, an entirely human IgG1 monoclonal antibody, favors the appearance of this pathology (PG) by generating systemic deterioration of the angiogenic balance and local deterioration of the oral environment [].
Hormonal changes, especially in estrogen and progesterone during puberty and pregnancy, may promote the development of PG or pregnancy granuloma. Increased levels of these hormones during puberty deteriorate already established gingival inflammation by increasing the dilation and proliferation of blood vessels and releasing vasoactive mediators from damaged mast cells [,]. In pregnancy in particular, these hormonal changes have been associated with vascular, microbiological, cellular and immunological modifications which generate a favorable environment for the initiation and development of PG [,,,]. The hormones estrogen, progesterone and chorionic gonadotropin induce certain alterations in the microcirculatory system, including swelling of endothelial cells, increased adhesion of platelets and leukocytes to vessel walls, formation of microthrombi, disruption of perivascular mast cells, increased vascular permeability, and vascular proliferation [,] (Figure 2). The oral microbiota may present changes characterized by an increase in the proportion of anaerobic and aerobic bacteria such as Bacteroides melaninogenicus, Prevotella intermedia, and Porphyromonas gingivalis. Thus, high levels of Fusobacterium nucleatum and Aggregatibacter actinomycetemcomitans have also been observed, particularly in the second and third trimesters of pregnancy [,,,,]. The cellular changes comprise a decrease in the keratinization of the gingival epithelium, an increase in epithelial glycogen, proliferation of fibroblasts, and a blockage in collagen degradation, leading to changes in the epithelial barrier that result in an increased response against irritating factors, especially dental biofilm []. Progesterone may act as an immunosuppressant in the periodontal tissues of pregnant women, thereby preventing the appearance of an acute inflammatory response to an irritant stimulus. However, it allows an increase in chronic tissue reactions, which results in an exaggerated appearance of inflammation clinically []. Additionally, there is a decrease in the antimicrobial activity of peripheral neutrophils which constitute essential components of the innate immune defenses of periodontal tissues. All these changes result in the exacerbation of the prevalence and/or severity of some pathologies in the oral cavity during pregnancy, particularly in the tissues, especially from the second month onwards, because it is exactly at this point that elevations in plasma estrogen and progesterone levels occur [,].
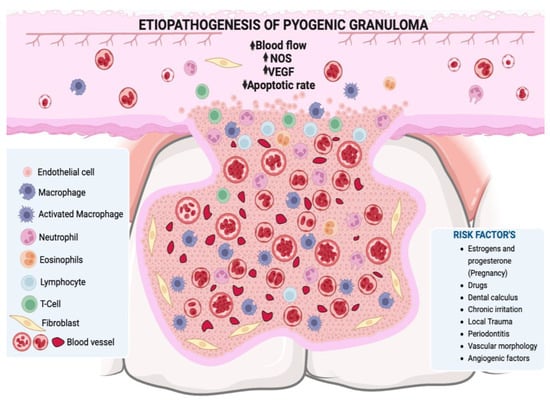
Figure 2.
A lobular mass of hyperplastic granulation tissue is observed, formed by abundant vascular spaces, young fibroblasts, infiltration of acute and/or chronic inflammatory cells with macrophages, neutrophils, lymphocytes, and polymorphonuclear cells. NOS: nitric oxide synthase, VEGF: vascular endothelium-derived grow. Figure created with BioRender, © biorender.com.
The imbalance between angiogenesis enhancers and inhibitors is one of the hypotheses for the etiopathogenesis of PG. It highlights the important role of certain factors such as basic fibroblast growth factor (bFGF), vascular endothelial growth factor (VEGF), tyrosine kinase with immunoglobulin-like, EGF-like domains-2 (Tie-2), angiopoietin-1 (Ang-1), angiopoietin-2 (Ang-2), ephrin-B2 and Eph-B4 in the processes involved in adult inflammatory neovascularization [,]. On the other hand, Shetty et al. reported that PG is triggered by the presence of local and/or systemic factors that generate the release of various endogenous substances (tumor cell angiogenic factors and vascular morphogenic factors), leading to alterations in the vascular system of the affected area []. Decorin is an integral component of new capillaries in in vivo angiogenesis, especially angiogenesis associated with severe inflammation. Nelimarkka et al. have demonstrated that decorin is present in the endothelial cells of capillary neovessels in PGs and in the granulation tissue of healing dermal wounds [].
PG is also known as intravascular lobular capillary hemangioma (ILCH), as it is considered a benign vascular tumor that is usually located within the blood vessels of the head, neck and upper extremities [,,]. In PG, excessive growth of vascular tissues is observed towards the interior of the vessel in addition to the invasion of surrounding tissues. In the histopathological analysis, it is observed that this type of hemangioma presents groups of capillaries enclosed by flattened endothelial cells within the upper layers of mucosa, as well as dense groups of vessels lined with fibrovascular endothelium with robust walls located in the deeper layers (Figure 2) []. Congenital endothelial hyperplasia is considered to be a contributing factor in its pathogenesis [], and recent studies have also highlighted the participation of intussusceptive angiogenesis, also known as angiogenesis by division []. Histologically, ILCH exhibits a distinctive lobular architecture marked by a nonstratified endothelium that forms capillaries within an edematous fibromyxoid stroma, these lobes are separated by venular structures. Characteristic features, such as capillary-sized vessels and inter-endothelial contacts, distinguish ILCH from other vascular tumors [,].
PG is considered within the group of reactive oral lesions that develop in response to chronic irritation or trauma and where the inflammatory response of the tissue increases []. The chronic inflammatory immune response to chronic irritation is characterized by simultaneous tissue remodeling and repair. Remodeling begins with the migration of inflammatory cells regulating the immune system, the proliferation of vascular endothelial cells, fibroblasts and the synthesis of the extracellular matrix. Cytokines, growth factors and angiogenic factors play a definitive role in the repair process []. Mast cells and macrophages have been reported to contribute to the pathogenesis of reactive oral lesions through their effects on endothelial cells and fibroblasts. They synthesize increased amounts of basic Fibroblast Growth Factor (bFGF) to be released into the extracellular matrix and also promote neovascularization through pro-angiogenic cytokines primarily Vascular Endothelial Growth Factor (VEGF). Mast cell mediator, namely tryptase, promotes fibroblast activation, collagen deposition, and fibrosis [,,,]. Mast cells release mediators that increase vascular permeability and vasodilation, facilitating the migration of inflammatory cells []. The outcome of mutual mast cell–fibroblast interactions promotes granulation tissue formation, a hallmark of PG [], which encouraged us to evaluate the mast cells numbers in both histopathological subtypes. Several authors [], have found a positive correlation between the number of micro vessels and mast cells in a group of so-called reactive lesions, including LCH, fibrous hyperplasia, inflammatory fibrous hyperplasia, and peripheral giant cell lesions, while others found a negative correlation []. The major basic protein of eosinophils has proangiogenic effects and may participate in inflammation and tissue remodeling. Furthermore, in addition to VEGF, eosinophils have been shown to produce other proangiogenic factors such as IL-8 and nerve growth factor [,]. The contribution of eosinophils to the pathogenesis of oral reactive lesions was previously studied, but eosinophils were not found to be involved in the fibrotic process or in the variation of the microscopic characteristics of these lesions []. Mast cell mediator, namely tryptase, promotes fibroblast activation, collagen deposition, and fibrosis [,,,]. During the initial stage of the lesion, inflammatory cell infiltration, vascular proliferation, and appearance of myofibroblasts and type III collagen can be observed in response to the injury and repair. When the lesions mature, the connective tissue consists of densely packed type I collagen with no inflammation and the myofibroblasts disappear, suggestive of healing [].
A study carried out by Vasanthi et al. in 2022 where they evaluated the severity of inflammation of reactive lesions, observed a great inflammatory activity in PG, followed by inflammatory fibrous hyperplasia and irritation fibroma []. This was attributed to the presence of elevated levels of estrogen and progesterone in the periodontal tissues. Estrogen leads to increased proliferation of gingival fibroblasts and gingival inflammation [].
VEGF, commonly upregulated in cancer to promote angiogenesis and sustain tumor growth, is also elevated in benign conditions such as PG, although with clear differences. In PG, VEGF-driven vascular proliferation is localized and benign, lacking the invasive and metastatic potential seen in cancer []. The regulatory mechanisms behind VEGF expression in PG are not as well understood as in cancer, where genetic mutations often drive its persistent elevation [,]. Treatment responses also diverge; PG usually resolves with local management and VEGF levels normalize, while cancer may require systemic anti-VEGF therapies to control tumor progression []. Therefore, while VEGF is a common factor in both PG and cancerous growths, its role and the clinical management of its effects are markedly different, reflecting the benign versus malignant nature of these conditions.
Pyogenic granuloma (PG) is considered a neovascular hyperplastic response in which inducible nitric oxide synthase expression, increased VEGF expression, and low apoptotic rate expression of Bax/Bcl-2 proteins have been reported []. In this regard, Blackwell et al. [] demonstrated the expressions of embryonic stem cell markers, i.e., OCT4, SOX2, pSTAT3 and NANOG, suggesting that the endothelium of PG shows a primitive phenotype. Chen et al. (2008) found overexpression of phosphorylated (p)-activating transcription factor-2 (p-ATF2) and phosphorylated (p)-signal transducer and activator of transcription-3 (p-STAT3) in cutaneous angiosarcoma and PG. Additionally, activation of the MAPK/ERK pathway demonstrated by immunohistochemical evidence for phospho-ERK1/2 positivity, has been identified in oral PG endothelial cells (Figure 3). However, further studies are needed to elucidate the mechanism involved [].
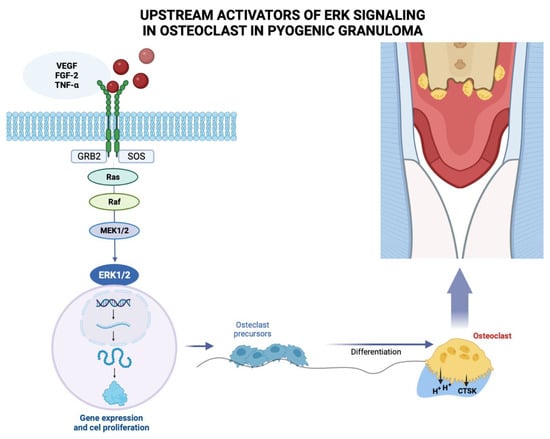
Figure 3.
Growth factors such as vascular endothelium-derived growth factor (VEGF), fibroblast growth factor-2 (FGF-2), tumor necrosis factor α (TNF-α) among other molecules, bind to the extracellular domain of a receptor tyrosine kinase that acts with docking sites for GRB2 adapter proteins that also binds to a guanine nucleotide exchange factor SOS, for a protein called Ras, which activates another signaling protein called Raf, acting on the MEK substrate 1/2, which phosphorylates and activates ERK 1/2, which enters the nucleus where it activates specific transcription factors and genes involved in the proliferation of osteoclast precursors that differentiate into mature osteoclasts that will resorb the bone crest in the pyogenic granuloma area. Figure created with BioRender, © biorender.com.
Investigation of the genetic and genomic alterations associated with PG could provide valuable information on its etiology and pathogenesis. Whole-genome sequencing and comparative genomic analyses could uncover specific genetic mutations or alterations [].
Recently, HRAS mutations were identified in cutaneous PG with lobular capillary arrangements. Subsequently, KRAS and BRAF mutations were also detected in sporadic cutaneous PG arguing against its classification as a reactive hyperplasia [,]. These genes are key components in the mitogen-activated protein kinases (MAPK)/extracellular signal-regulated kinase (ERK) signaling pathway [].
In a study carried out by Pereira et al., in which they evaluated hotspot mutations in the HRAS, KRAS, NRAS, BRAF, GNA11, and GNA14 genes to determine if oral PG shares the same genetic alterations reported in cutaneous PG, representing the same disease in a different topography, they concluded that MAPK/ERK pathway activation in oral PG is not driven by mutations in the HRAS, KRAS, NRAS, BRAF, GNA11 and GNA14 genes as is PG in skin [].
4. Clinical Features
Pyogenic granuloma (PG) occurs most often in the skin or in the oral cavity, but rarely in the gastrointestinal tract, trachea, urinary bladder, and central nervous system [,]. In the oral cavity, the gingiva accounts for 75% of the sites of predilection of this pathology. However, PG may occur in other areas such as the lips, tongue, buccal mucosa, hard plate and peri-implant mucosa, and it affects the maxilla more than the mandible, the anterior region more than the posterior region, with the buccal surfaces more affected than the lingual surfaces [,,]. In the literature, the floor of the mouth is not considered as a site of occurrence of PG. This is perhaps due to the fact that in addition to the absence of sufficient amounts of connective tissue in the mucosa of the floor of the mouth, the tongue protects this region from traumatic injury [].
Oral PG is a pathology that manifests as a raised, smooth or exophytic growth on a sessile or pedunculated broad base with a smooth and lobulated surface covered with red hemorrhagic and erythematous compressible papules which appear lobulated and warty, complete with ulcerations and covered by a yellow brackish membrane [,,,]. The surface of the pathology is frequently ulcerated in areas subjected to trauma, and due to its pronounced vascularity, occasional bleeding may occur, especially during mastication. The clinical course of PG is generally slow, asymptomatic and painless [,]. The growth of PG is slow, and it takes from weeks to months to reach optimal size [,]. As shown in Table 1, the size of PG may vary in diameter from a few millimeters to several centimeters [,,]. The color of this pathology depends on its age: younger PGs tend to be reddish due to the large number of blood vessels, while older ones appear pink in color. The consistency of the oral PG depends on the age of the lesion: as the lesion matures, collagen fibers increase in number, and the lesion becomes firm [,].
The signs and/or symptoms referred to by the PG patients in anamnesis are bleeding [,,,,,,,,], difficulty in chewing [,,,,,,,], and pain and tenderness [].

Table 1.
Reviewed articles.
Table 1.
Reviewed articles.
| Year | Author | Country | Age (Years) | Gender | Location | Consistency | Comorbidity | Radiographic | Lesion Size | Diagnosis | Treatment |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Features | |||||||||||
| 2001 | Akyol et al., 2001 [] | Turkey | 4 m | B | Tongue | Soft | No | NA | 1 × 0.8 × 0.8 cm | H | Surgical excision |
| 2002 | Aguilo L. 2002 [] | Spain | 19 m | B | Gingiva | Soft | No | Fracture of the crown of 61 | NA | H | Surgical excision |
| 2006 | Parisi E et al., 2006 [] | USA | 33 | F | Gingiva | NA | NA | NA | 0.5 × 0.5 cm | H | Intralesional corticosteroids |
| 2006 | Patil et al., 2006 [] | India | 50 | F | Lower Lip | NA | No | NA | 1 × 0.5 cm | H | Surgical excision |
| 2006 | Shenoy S. 2006 [] | India | 8 | G | Gingiva | NA | NA | loss of alveolar crestal bone | 2.0 × 1.0 × 1.0 cm | H | Surgical excision |
| 2008 | Amirchaghmaghi et al., 2008 [] | Iran | 16 | M | Hard palate | Firm | No | NA | 0.7 cm | H | Surgical excision |
| 2009 | Goncalves 2009 [] | Brazil | 12 | F | Upper lip | NA | NA | NA | 1.0 cm | H | Surgical excision |
| 2009 | Olmedo D et al., 2009 [] | Argentina | 75 | F | Peri-implant mucosa | NA | No | No abnormalities | 1.0 × 1.0 × 0.6 cm | H | Surgical excision |
| Argentina | 64 | F | Peri-implant mucosa | Firm | No | Bone loss | 0.6 × 0.5 × 0.4 cm | H | Surgical excision | ||
| 2010 | Gondivkar et al., 2010 [] | India | 25 | F | Gingiva | Soft | Pregnancy | Alveolar bone loss | 3 × 7 cm | H | Surgical excision |
| 2010 | Rizwanulla et al., 2010 [] | Nepal | 13 | F | Gingiva | Firm | No | NA | 2.0 × 1.0 × 1.0 cm | H | Surgical excision |
| 2011 | Behl et al., 2011 [] | India | 60 | F | Gingiva | Firm | No | Vertical bone loss | 3.2 × 3.4 cm | H | Surgical excision |
| 2011 | Mubben et al., 2011 [] | India | 63 | F | Gingiva | Soft | No | NA | 5.0 × 3.5 cm | H | Surgical excision |
| 2011 | Penseriya et al. 2011 [] | India | 30 | M | Gingiva | NA | No | Interdental bone loss | 2.0 × 1.5 cm | H | Surgical excision |
| 2011 | Shivaswamy et al., 2011 [] | India | 19 | M | Gingiva and palate | Soft | NA | Horizontal bone loss | 4.0 × 5.0 mm | H | Surgical excision |
| 2012 | Chandrashekar 2012 [] | India | 28 | F | Gingiva | Soft | No | NA | NA | H | Surgical excision |
| 2012 | Panjwani et al., 2012 [] | India | 69 | M | Tongue | Firm | No | NA | 2.0 × 3.0 cm | H | Surgical excision |
| 2012 | Ravi et al., 2012 [] | India | 33 | M | Lower lip | Firm | NA | NA | 3.0 × 2.0 cm | H | Surgical excision |
| 2012 | Piscoya et al., 2012 [] | Brazil | 44 | M | Lower lip | NA | NA | NA | NA | H | Surgical excision |
| 2012 | Verma et al., 2012 [] | India | 30 | F | Gingiva | NA | No | Alveolar bone loss | 1.5 × 1.0 cm | H | Surgical excision |
| 2013 | Adusumilli et al., 2013 [] | India | 24 | F | Gingiva | Firm | NA | NA | 2 × 3.5 cm | H | Surgical excision |
| 27 | F | Gingiva | Firm | NA | NA | 2 × 3.5 cm | Surgical excision | ||||
| 27 | F | Gingiva | Firm | NA | NA | 2 × 3.5 cm | H | Surgical excision | |||
| 32 | F | Gingiva | Firm | NA | NA | 1.5 × 2.5 cm | H | Surgical excision | |||
| 23 | F | Gingiva | Soft | NA | NA | 1.5 × 1.75 cm | H | Surgical excision | |||
| 26 | F | Gingiva | Soft | NA | NA | 2.0 × 2.5 cm | H | Surgical excision | |||
| 28 | F | Gingiva | Soft | NA | NA | 0.5 × 0.8 cm | H | Surgical excision | |||
| 2013 | Deshmukh et al., 2013 [] | India | 9 | M | Gingiva and mucogingival junction | Soft | No | Horizontal bone loss | NA | H | Surgical excision |
| 2013 | Gomes et al., 2013 [] | India | 22 | M | Gingiva | NA | NA | No bone loss | 2.1 × 4.4 cm | H | Surgical excision |
| 2013 | Kamala 2013 [] | India | 30 | F | Upper lip | Firm | No | NA | 0.8 cm | H | Surgical excision |
| 2013 | Mahabob et al., 2013 [] | India | 22 | F | Palate | Firm | Pregnancy | NA | 2 × 2 cm | H | Surgical excision |
| 2013 | Moraes, et al., 2013 [] | Brazil | 65 | M | Gingiva | NA | Diabetes and high blood pressure | No abnormalities | NA | H | Surgical excision |
| 2013 | Sangamesh et al., 2013 [] | India | 40 | F | Buccal mucosa | Firm | NA | NA | 1.5 × 1.5 cm | H | Surgical excision |
| 2014 | Asha et al., 2014 [] | India | 54 | M | Lower lip | Firm | No | NA | 3.0 × 3.0 cm | H | Surgical excision |
| 2014 | Fekrazad et al., 2014 [] | Iran | 24 | F | Gingiva | Soft | NA | No abnormalities | 1.4 × 0.8 mm | H | Laser excision |
| 2014 | Ghalayani et al., 2014 [] | Iran | 45 | M | Tongue | NA | Epilepsy beginning | NA | 4.0 × 3.0 × 1.0 cm | H | Surgical excision |
| 2014 | Kejriwal et al., 2014 [] | India | 59 | M | Gingiva | Firm | No | No abnormalities | 1.5 × 2.0 × 1.0 cm | H | Surgical excision |
| 2014 | Mastammanavar et al., 2014 [] | India | 44 | F | Gingiva | Soft | NA | NA | 1.5 × 3.0 cm | H | Surgical excision |
| 2014 | Sun et al., 2014 [] | China | 22 | F | Gingiva | NA | Pregnancy | NA | 3 cm | C | No |
| 2015 | Asnaashari et al., 2015 [] | Iran | 6 | M | Gingiva | Soft | No | No abnormalities | 1.1 × 1.3 cm | H | Laser excision |
| 2015 | Bugshan et al., 2015 [] | USA | 51 | F | Gingiva and palatal | NA | NA | NA | 0.9 × 0.6 cm buccal and 0.8 × 0.7 cm palatal. | H | Intralesional injections |
| 2015 | De Carvalho et al., 2015 [] | Brazil | 11 | B | Upper Lip | NA | No | NA | 4.5 cm | H | Surgical |
| 2015 | Ganesan A. 2015 [] | India | 49 | F | Gingiva | Firm | No | No bone loss | 4.0 × 3.0 cm | H | Surgical excision |
| 2015 | Sachdeva 2015 [] | India | 45 | F | Buccal mucosa | Firm | No | NA | 2 × 1 cm | H | Surgical excision |
| 2015 | Tripathi et al., 2015 [] | India | 55 | M | Gingiva | NA | No | Alveolar bone loss | 3 × 3 cm | H | Surgical excision |
| 2016 | Agarwal N et al., 2016 [] | India | 8 d | B | Gingiva | Soft | No | NA | 0.5 × 0.8 cm | H | Surgical excision |
| 2016 | Al-Mohaya et al., 2016 [] | Saudí arabia | 51 | F | Gingiva | Firm | Uncontrolled type II diabetes mellitus | NA | 2.0 × 1.5 cm | H | Laser excision |
| 2016 | Marla et al., 2016 [] | Nepal | 40 | F | Gingiva | NA | NA | NA | 1.0–2.0 cm | H | Surgical excision |
| Nepal | 40 | F | Buccal mucosa | NA | NA | NA | 1.0–2.0 cm | H | Surgical excision | ||
| Nepal | 9 | M | Buccal mucosa | NA | NA | NA | <1.0 cm | H | Surgical excision | ||
| Nepal | 23 | F | Gingiva | NA | NA | NA | 1.0–2.0 cm | H | Surgical excision | ||
| 2016 | Cheney et al., 2016 [] | USA | 16 | M | Tongue, Buccal mucosa, | Soft | Acute lymphoblastic leukemia | N/A | N/A | Surgical excision | |
| USA | 14 | G | Tongue | N/A | Fanconi anemia | No | 0.5 × 0.2cm 1.0 × 0.3 cm 0.3 × 0.2 | H | Surgical excision | ||
| USA | 11 | G | Tongue | N/A | Fanconi anemia | No | 2.0 × 2.5 cm | H | Surgical excision | ||
| USA | 15 | Buccal mucosa | Stage IIIB nodular sclerosing Hodgkin lymphoma. | No | 1 cm 2.0 × 4.0 cm | H | CO2 laser | ||||
| USA | 6 | B | Tongue | N/A | Junctional epidermolysis bullosa | No | 1.0 × 0.5 cm | H | Surgical excision | ||
| 2017 | Rosa et al., 2017 [] | México | 34 | F | Gingiva | Firm | No | Absence of interproximal contact | 1.5 × 0.9 cm | H | Surgical excision |
| 2018 | Tenore et al., 2018 [] | Italy | 28 | F | Retromolar region | Soft | No | No abnormalities | NA | H | CO2 laser |
| 58 | M | Gingiva | Soft | No | No abnormalities | H | CO2 laser | ||||
| 19 | M | Retromolar region | Soft | No | No abnormalities | NA | H | CO2 laser | |||
| 21 | M | Retromolar region | Soft | No | No abnormalities | NA | H | CO2 laser | |||
| 2018 | Canivell et al., 2018 [] | Spain | 32 | F | Lower lip | NA | Pregnancy | NA | 0.5 × 1.0 cm | H | Surgical excision |
| 2018 | Parajuli et al., 2018 [] | Nepal | 26 | F | Tongue | Soft | No | NA | 2.5 × 2.0 cm | H | Surgical excision |
| Nepal | 15 | G | Upper lip | NA | NA | NA | 0.5 × 0.5 cm | C | Surgical excision | ||
| 2019 | Poudel et al., 2019 [] | Nepal | 49 | M | Upper lip | Firm | NA | NA | 0.6 × 0.8 cm | H | Surgical excision |
| 2020 | Gutierrez 2020 [] | Perú | 51 | F | Alveolar ridge | NA | Hypothyroidism erythrodermic psoriasis | Alveolar bone loss | 2.5 × 2.5 cm | H | Surgical excision |
| 2020 | Banjar et al., 2020 [] | Saudí arabia | 15 | B | Lower lip | Soft | No | NA | 1.2 × 0.8 × 0.6 cm | H | Surgical excision |
| 2021 | Aragaki et al., 2021 [] | Japan | 55 | M | Tongue | Soft | Gastric Cancer | No abnormalities | 0.6cm | H | Surgical excision |
| 67 | M | Upper lip | Soft | Gastric Cancer | No abnormalities | 0.5 mm | H | Surgical excision | |||
| 2021 | Pisano et al., 2021 [] | Italy | 11 | G | Lower lip | Soft | No | N/A | 1.5 cm | H | Diode Laser |
| 2023 | Lomelí et al., 2023 [] | México | 32 | F | Hard palate | Soft | No | Alveolar bone loss | 25 × 12 mm | H | Surgical excision |
| México | 42 | F | Gingiva | Soft | No | Alveolar bone loss | 16 × 10 mm | H | Surgical excision | ||
| 38 | F | Gingiva | 20 × 15 mm |
Note: (m): months. (d): Day. (F): Female. (M): Male. (B): Boy. (G): Girl. (NA): data not available. (H): Histopathological. (C): Clinical.
5. Radiographic Features
Oral PG generally does not present radiographic findings: some authors make a presumptive diagnosis with the clinical features. Thus, most of the clinical cases described in the literature do not present radiographic analysis and/or description. However, some authors have reported bone loss or erosion of the alveolar ridge associated with the area where the PG is located [,,,,,,,,,] (Table 1).
The molecular mechanisms leading to bone loss or erosion associated with oral PG are unclear. However, PG is a benign inflammatory lesion that expresses significantly more VEGFs and basic fibroblast growth factors than healthy gingiva and periodontitis []. Growth factors such as fibroblast growth factor-2, growth arrest-specific gene 6, and TNF-α, among other molecules, stimulate mature osteoclast function and survival through activation of extracellular signal-regulated kinase (ERK), resulting in degradation or resorption of organic and inorganic bone components []. The ERK signaling pathway has been associated with the regulation of osteoclasts with respect to survival, proliferation, apoptosis, formation, polarity, podosome disassembly, and differentiation [].
This signaling cascade constitutes the core of three serially phosphorylated protein kinases. Activation of Raf isoforms through the Ras–Raf interaction stimulates MAPKK, MEK1 and MEK2, and then activates ERK1 and ERK2 via dual phosphorylation at the conserved Thr–Glu–Tyr (TEY) motif, leading to phosphorylation of various downstream substrates [,,]. Among the latter are c-Fos, NFATc1, MITF, TFE3, Hedgehog-Gli, Egr2, RSK2 and MMp-9, which ultimately activate the ERK signaling pathway []. Pereira et al. [] reported MAPK/ERK pathway activation, as demonstrated by the immunohistochemical positivity of phospho-ERK1/2 in oral PG endothelial cells. Future research should focus on elucidating the molecular mechanisms that trigger PG-associated bone loss or erosion, particularly the role of the MAPK/ERK pathway in this process (Figure 3).
6. Microscopic Features
Most PGs are constituted by a lobular mass of hyperplastic granulation tissue representing a non-neoplastic proliferation formed by abundant vascular spaces, young fibroblasts, and infiltration of acute and/or chronic inflammatory cells with lymphocytes, plasma cells, histiocytes, and polymorphonuclear cells in a collagen matrix. This entity is partially or totally covered by parakeratotic or non-keratinized stratified squamous epithelium. Ulcerated lesions exhibit an extensive fibrin layer in the epithelium [,].
7. Differential Diagnosis of Oral PG
The features used for differential diagnosis of oral PG include red or reddish-blue growths such as gingival hyperplasia, peripheral giant cell granuloma, hemangiomas, conventional granulation tissue, peripheral ossifying fibroma, peripheral odontogenic fibroma, metastatic cancer, Kaposi’s sarcoma, bacillary angiomatosis, angiosarcoma, and non-Hodgkin’s lymphoma [,,,,].
8. Treatment of Oral PG
The treatment or the management of oral PG depends on the particular characteristics presented by each patient. However, the treatment of choice is conventional surgical excision. Other minimally invasive treatment modalities have been suggested, including laser, corticosteroid injections, cryosurgery and sclerotherapy [,,] (Table 1).
Surgical excision consists of the complete removal of the lesion and the extension of the cut to the periosteum, including a 2 mm margin to the adjacent soft tissues. If the PG is located near adjacent teeth, it is important that after removal of the lesion, debridement is performed both supra- and sub-gingivally to the biofilm and/or dental calculus. Additionally, it is important to remove all irritating agents (foreign materials, sources of trauma, overhang crowns, etc.) that are present in the area of the lesion. These suggested measures, both in the surgical technique and in the removal of irritants, are aimed at avoiding recurrence of PG [,,,].
Chandrashekar implemented a minimally invasive approach as a treatment strategy for oral PG. This protocol consists of performing scaling and root planning in the area where the lesion is located. In addition, it is crucial to maintain complete oral hygiene by brushing twice a day and using a 0.12% chlorhexidine rinse twice a day. It is necessary to monitor the evolution of the lesion every week. If the lesion persists, scaling and root planning should be implemented every week for four consecutive weeks in order to continue with the non-invasive approach. At the same time, it is recommended that patients should maintain adequate brushing and flossing twice a day. This minimally invasive treatment may be considered when the PG is small in size, painless, and without bleeding [].
Recently, a laser-assisted removal treatment was used for oral PG [,,]. Asnaashari et al. [] implemented the Er:YAG Laser therapy using the Diode Laser Gallium–Aluminum–Arsenide (GA–LA–AS). Moreover, Al-Mohaya et al. [] used the 940 nm diode laser in an uncontrolled diabetic patient without observing recurrences and/or postoperative complications. Additionally, Tenore et al. [] presented four cases of PG in patients with a history of excision of oral neoplastic lesions and rehabilitation by a free revascularized flap of the iliac crest; excisional biopsies were performed by carbon dioxide (CO2) laser. These cases presented, suggest that limitations in oral functions and maintenance of oral hygiene measures subsequent to reconstruction surgery with revascularized free flap probably played a role in the development of gingival reactive hyperplastic lesions with the presence of triggering factors such as local trauma, chronic infection or inadequate prosthesis. The advantages of applying the laser are that by sealing the blood vessels and nerve bundles, there is no bleeding during surgery, thereby ensuring better visualization of the surgery site, sterile conditions, cutting precision, reduced number of instruments used, and a suture-less procedure with minimal postoperative pain [,]. In addition, the laser instantly disinfects the surgical wound, resulting in lower possibility of postoperative infection, minimal inflammation, and better healing of the surgical wound. In oral cavity lesions, the use of a laser reduces intraoperative and postoperative complications, when compared to surgical excision [].
A proposed therapeutic alternative is corticosteroid injection into the oral PG, an option which was identified in two studies in this review (Table 1). Parisi et al. [] were pioneers in implementing, for the first time, the use of a series of corticosteroid injections into lesions for the management of multiple intraoral PG nodules as a new conservative treatment option for this pathology. However, Bugshan et al. [] suggested a series of injections in five different sites of the lesion with 0.1 mL of 10 mg/mL triamcinolone acetonide, without exceeding a total of 0.5 mL, in addition to local application of 0.05% clobetasol propionate for 2 weeks. The latter protocol is appropriate and effective, particularly in patients with high PG recurrences due to poor surgical excisions. The exact mechanism of action of corticosteroid therapy is still unknown. However, these drugs may improve the response of the lesion in the vascular bed to vasoconstrictor agents. A corticosteroid such as dexamethasone, stops vascular proliferation by downregulating proangiogenic factors such as VEGF-A, MMP1, and IL-6 [].
Cryotherapy is a simple, easy-to-execute, inexpensive, and safe treatment that has become one of the therapeutic options used for PG patients. Its implementation is simpler than that of surgical excision, and it is cheaper than laser. It allows resolution of the pathology without leaving significant scarring. Endothelial cells may be more vulnerable to cryotherapy than collagen fibers [].
Sodium tetradecyl sulfate sclerotherapy is among the alternative therapies that have been implemented. This technique offers a better alternative to excision due to its simplicity and the absence of scarring, although multiple treatment sessions are required. The therapeutic effects of this treatment may be mediated through a mechanism involving the specific and non-specific actions of sodium tetradecyl sulfate, which specifically causes damage to endothelial cells and obliterates the lumen of vessels. Additionally, in stromal tissues, it may cause non-specific necrotic changes. The adverse effects of treatment with sodium tetradecyl sulfate include allergic reactions, skin necrosis, and hyperpigmentation. Sodium tetradecyl sulfate injection is usually painless. Therefore, extravasation may develop asymptomatically. To avoid skin necrosis, slow and careful injection with some pressure is necessary [].
During pregnancy, choosing the treatment modality for oral PG is challenging, and it depends on the severity of the symptoms. Gondivkar et al. [] and Canivell et al. [] have proposed surgical excision of the granuloma during pregnancy. On the other hand, Su et al. [] suggest that for patients without hemorrhagic or painless lesions, oral hygiene instructions, clinical monitoring of development of PG, follow-up, and oral self-care at home should be implemented. Regardless of the treatment implemented for PG in pregnant women, we suggest, particularly in this group of patients, a strict control of oral hygiene, the continuous removal of biofilm and/or dental calculus, the use of soft toothbrushes, as well as flossing in order to avoid recurrence.
The formation of a reactive lesion (for example, PG) around dental implants is a complication that has received attention. These lesions may lead to marginal bone loss and, consequently, implant failure. Therefore, several treatments for implant-associated PG have been considered, with the treatment of choice being conservative surgical excision with total removal of the base of the lesion and bone curettage []. Additionally, it is recommended that the implant surface should be polished so as to eliminate any irritating factor that may be causing or favoring the appearance of PG []. Photodynamic therapy is a simple, non-invasive adjunctive treatment for peri-implant diseases. It allows for control of disease progression through de-contamination of infected surfaces. However, further studies are needed to establish its efficacy in cases of reactive lesions around dental implants [,].
Regarding postoperative management for arresting recurrence of PG after treatment, some authors have suggested implementation of oral hygiene measures, among which are the use of a soft toothbrush, keeping the lesion area clean, use of mouth rinses, and antibiotic therapy [,,,]. The suggested rinses entail the use of 0.2% chlorhexidine [,,] and 0.12% chlorhexidine []; use of gluconate twice daily, and a saline rinse []. The antibiotic of choice frequently used for adequate intervention is amoxicillin at a dose of 500 mg every 8 h for 5 days [,,]. In pediatric patients, good oral hygiene is recommended for keeping the lesion area clean, and neither analgesics nor antibiotics are prescribed [,].
The recurrence rate of PG is 15%, and this is associated with incomplete lesion removal and failure to eliminate etiological factors [,]. Frumkin et al. [] proposed a conservative protocol for preventing recurrence. This entails removal of irritants with debridement under local anesthesia, along with ancillary measures such as chlorhexidine rinse and oral hygiene instructions. In addition, the protocol suggests a follow-up schedule that involves visits every 2 weeks during the first 2 months, followed by maintenance visits once every 2 months. However, recurrence is uncommon in extra-gingival locations after surgical excision [].
9. Future Perspective
Future research on PG is aimed at making significant advances by delving into the molecular genesis of the lesion, seeking novel and minimally invasive therapeutic approaches, and understanding the complexities behind its tendency to recur. A concerted effort to analyze the hormonal interaction at work, together with the search for reliable biomarkers for diagnosis and prognosis, will shed new light on the clinical trajectory of PG. Adopting cutting-edge diagnostic technologies, gathering extensive epidemiological knowledge, and conducting rigorous clinical trials will be critical to refining treatment regimens. Additionally, the focus on refining patient management strategies and developing comprehensive, standardized treatment protocols is expected to improve. Notably for the quality of life and clinical outcomes of people fighting this enigmatic injury.
10. Conclusions
Pyogenic granuloma (PG) manifests as a benign proliferation of connective tissue, primarily characterized by granulation tissue hyperplasia. This phenomenon often occurs on the skin or mucous membranes and is considered a reactive process triggered by various factors such as trauma, dental calculi, chronic irritation, the use of certain drugs, and is linked to elevated levels of estrogen and progesterone. In its pathogenesis, intense inflammatory activity stands out, facilitated by the imbalance between enhancers and inhibitors of angiogenesis, as well as interactions between mast cells and fibroblasts. Exploring the entire genome and comparative genomic analyses could reveal specific mutations or genetic alterations associated with the underlying molecular mechanisms of PG. This approach could uncover potential innovative and less invasive therapeutic pathways.
Funding
This research did not receive external funding.
Conflicts of Interest
The author declares no conflict of interest.
References
- Sarwal, P.; Lapumnuaypol, K. Pyogenic Granuloma. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA.
- Gomes, S.R.; Shakir, Q.J.; Thaker, P.V.; Tavadia, J.K. Pyogenic granuloma of the gingiva: A misnomer?—A case report and review of literature. J. Indian Soc. Periodontol. 2013, 17, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Kamal, R.; Dahiya, P.; Puri, A. Oral pyogenic granuloma: Various concepts of etiopathogenesis. J. Oral Maxillofac. Pathol. 2012, 16, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Chandra, S.; Gupta, S.; Srivastava, S. Heterogeneous conceptualization of etiopathogenesis: Oral pyogenic granuloma. Natl. J. Maxillofac. Surg. 2019, 10, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Ghalayani, P.; Hajisadeghi, S.; Babadi, F. Extragingival pyogenic granuloma associated with medication: Report of an unusual case. Dent. Res. J. 2014, 11, 400–404. [Google Scholar]
- Parisi, E.; Glick, P.H.; Glick, M. Recurrent intraoral pyogenic granuloma with satellitosis treated with corticosteroids. Oral. Dis. 2006, 12, 70–72. [Google Scholar] [CrossRef] [PubMed]
- Al-Noaman, A.S. Pyogenic granuloma: Clinicopathological and treatment scenario. J. Indian Soc. Periodontol. 2020, 24, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Poncet, A.; Dor, L. Botyromycose humaine. Rev. Chir. 1897, 18, 996. [Google Scholar]
- Greenberger, S.; Boscolo, E.; Adini, I.; Mulliken, J.B.; Bischoff, J. Corticosteroid suppression of VEGF-A in infantile hemangio-ma-derived stem cells. N. Engl. J. Med. 2010, 362, 1005–1013. [Google Scholar] [CrossRef]
- Jafarzadeh, H.; Sanatkhani, M.; Mohtasham, N. Oral pyogenic granuloma: A review. J. Oral. Sci. 2006, 48, 167–175. [Google Scholar] [CrossRef]
- Wiener, R.C.; Wiener-Pla, R. Literacy, pregnancy and potential oral health changes: The Internet and readability levels. Matern. Child Health J. 2014, 18, 657–662. [Google Scholar] [CrossRef]
- Montazer Lotf-Elahi, M.S.; Farzinnia, G.; Jaafari-Ashkavandi, Z. Clinicopathological study of 1000 biopsied gingival lesions among dental outpatients: A 22-year retrospective study. BMC Oral Health 2022, 22, 154. [Google Scholar] [CrossRef] [PubMed]
- Krishnapillai, R.; Punnoose, K.; Angadi, P.V.; Koneru, A. Oral pyogenic granuloma—A review of 215 cases in a South Indian Teaching Hospital, Karnataka, over a period of 20 years. Oral Maxillofac. Surg. 2012, 16, 305–309. [Google Scholar] [CrossRef]
- Shamim, T.; Varghese, V.I.; Shameena, P.M.; Sudha, S. A retrospective analysis of gingival biopsied lesions in South Indian population: 2001–2006. Med. Oral. Patol. Oral Cir. Bucal 2008, 13, E414–E418. [Google Scholar]
- Nejad, E.S.; BigomTaheri, J.; Azimi, S. Frequency of gingival pregnancy tumor in Iran (confirmed by biopsy). J. Int. Oral Health 2014, 6, 72–76. [Google Scholar] [PubMed]
- Chamani, G.; Navvabi, N.; Abdollahzadeh, S.H. The frequency of pregnancy tumor in pregnant mothers. Dent. J. Shiraz Med. Sci. Univ. 2009, 10, 79–82. [Google Scholar]
- Lee, J.; Lynde, C. Pyogenic granuloma: Pyogenic again? Association between pyogenic granuloma and Bartonella. J. Cutan. Med. Surg. 2001, 5, 467–470. [Google Scholar] [CrossRef]
- Kerr, D.A. Granuloma pyogenicum. Oral Surg. Oral Med. Oral Pathol. 1951, 4, 158–176. [Google Scholar] [CrossRef]
- Bhaskar, S.N.; Jacoway, J.R. Pyogenic granuloma—Clinical features, incidence, histology, and result of treatment: Report of 242 cases. J. Oral Surg. 1966, 24, 391–398. [Google Scholar]
- Piraccini, B.M.; Bellavista, S.; Misciali, C.; Tosti, A.; de Berker, D.; Richert, B. Periungual and subungual pyogenic granuloma. Br. J. Dermatol. 2010, 163, 941–953. [Google Scholar] [CrossRef]
- Cheney-Peters, D.; Lund, T.C. Oral Pyogenic Granuloma After Bone Marrow Transplant in the Pediatric/Adolescent Population: Report of 5 Cases. J. Pediatr. Hematol. Oncol. 2016, 38, 570–573. [Google Scholar] [CrossRef]
- Al-Mohaya, M.; Treister, N.; Al-Khadra, O.; Lehmann, L.; Padwa, B.; Woo, S.B. Calcineurin inhibitor-associated oral inflammatory polyps after transplantation. J. Oral Pathol. Med. 2007, 36, 570–574. [Google Scholar] [CrossRef] [PubMed]
- Palmero, M.L.; Pope, E. Eruptive pyogenic granulomas developing after drug hypersensitivity reaction. J. Am. Acad. Dermatol. 2009, 60, 855–857. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez Patiño-Paul, A.A. Un inusual caso de granuloma piogeno oral multiple,¿ asociado a tratamiento con levotiroxina? Rev. Estomatol. Hered. 2020, 30, 294–301. [Google Scholar] [CrossRef]
- Tosti, A.; Piraccini, B.M.; D’Antuono, A.; Marzaduri, S.; Bettoli, V. Paronychia associated with antiretroviral therapy. Br. J. Dermatol. 1999, 140, 1165–1168. [Google Scholar] [CrossRef] [PubMed]
- Leung, A.K.C.; Barankin, B.; Hon, K.L. Pyogenic Granuloma. Clin. Mother. Child. Health 2014, 11, e106. [Google Scholar] [CrossRef]
- Aragaki, T.; Tomomatsu, N.; Michi, Y.; Hosaka, H.; Fukai, Y.; Iijima, M.; Yoda, T. Ramucirumab-related Oral Pyogenic Granuloma: A Report of Two Cases. Intern. Med. 2021, 60, 2601–2605. [Google Scholar] [CrossRef] [PubMed]
- Uzel, M.I.; Kantarci, A.; Hong, H.H.; Uygur, C.; Sheff, M.C.; Firatli, E.; Trackman, P.C. Connective tissue growth factor in drug-induced gingival overgrowth. J. Periodontol. 2001, 72, 921–931. [Google Scholar] [CrossRef]
- Baran, R. Etretinate and the nails (study of 130 cases) possible mechanisms of some side-effects. Clin. Exp. Dermatol. 1986, 11, 148–152. [Google Scholar] [CrossRef]
- Williams, L.H.; Fleckman, P. Painless periungual pyogenic granulomata associated with reverse transcriptase inhibitor therapy in a patient with human immunodeficiency virus infection. Br. J. Dermatol. 2007, 156, 163–164. [Google Scholar] [CrossRef]
- Silva de Araujo Figueiredo, C.; Gonçalves Carvalho Rosalem, C.; Costa Cantanhede, A.L.; Abreu Fonseca Thomaz, É.B.; Fontoura Nogueira da Cruz, M.C. Systemic alterations and their oral manifestations in pregnant women. J. Obstet. Gynaecol. Res. 2017, 43, 16–22. [Google Scholar] [CrossRef]
- Figuero-Ruiz, E.; Prieto Prieto, I.; Bascones-Martínez, A. Cambios hormonales asociados al embarazo. Afectación gingivo-periodontal. Av. Periodoncia Implantol. Oral 2006, 18, 101–113. [Google Scholar]
- Cardoso, J.A.; Spanemberg, J.C.; Cherubini, K.; Figueiredo, M.A.; Salum, F.G. Oral granuloma gravidarum: A retrospective study of 41 cases in Southern Brazil. J. Appl. Oral. Sci. 2013, 21, 215–218. [Google Scholar] [CrossRef]
- Jang, H.; Patoine, A.; Wu, T.T.; Castillo, D.A.; Xiao, J. Oral microflora and pregnancy: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 16870. [Google Scholar] [CrossRef] [PubMed]
- Borgo, P.V.; Rodrigues, V.A.; Feitosa, A.C.; Xavier, K.C.; Avila-Campos, M.J. Association between periodontal condition and sub-gingival microbiota in women during pregnancy: A longitudinal study. J. Appl. Oral Sci. 2014, 22, 528–533. [Google Scholar] [CrossRef]
- Ojanotko-Harri, A.O.; Harri, M.P.; Hurttia, H.M.; Sewon, L.A. Altered tissue metabolism of progesterone in pregnancy gingivitis and granuloma. J. Clin. Periodontol. 1991, 18, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Yuan, K.; Jin, Y.T.; Lin, M.T. Expression of Tie-2, angiopoietin-1, angiopoietin-2, ephrinB2 and EphB4 in pyogenic granuloma of human gingiva implicates their roles in inflammatory angiogenesis. J. Periodontol. Res. 2000, 35, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Yuan, K.; Jin, Y.T.; Lin, M.T. The detection and comparison of angiogenesis-associated factors in pyogenic granuloma by im-munohistochemistry. J. Periodontol. 2000, 71, 701–709. [Google Scholar] [CrossRef]
- Shetty, S.J.; Hallikeri, K.; Anehosur, V.; Desai, A. An aggressive pyogenic granuloma masquerading as a vascular neoplasm. J. Indian. Soc. Periodontol. 2020, 24, 276–279. [Google Scholar] [CrossRef]
- Nelimarkka, L.; Salminen, H.; Kuopio, T.; Nikkari, S.; Ekfors, T.; Laine, J.; Pelliniemi, L.; Järveläinen, H. Decorin is produced by capillary endothelial cells in inflammation-associated angiogenesis. Am. J. Pathol. 2001, 158, 345–353. [Google Scholar] [CrossRef]
- Cooper, P.H.; McAllister, H.A.; Helwig, E.B. Intravenous pyogenic granuloma. A study of 18 cases. Am. J. Surg. Pathol. 1979, 3, 221–228. [Google Scholar] [CrossRef]
- Fakoory, J.M.W.; Smith, A.L.; Depeiza, N. Intravascular lobular capillary hemangioma of the temporal artery. J. Vasc. Surg. Cases Innov. Tech. 2022, 8, 237–239. [Google Scholar] [CrossRef] [PubMed]
- Jing, W.; Wen, X.; Zhang, M.; Zhou, C.; Huang, J.; He, Y. Ultrasonographic features of an intravascular lobular capillary hemangioma originating in the basilic vein: Case report and literature review. J. Clin. Ultrasound. 2021, 49, 741–745. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.H.; Kuo, H.W.; Chiu, Y.K.; Huang, P.H. Intravascular pyogenic granuloma arising in an acquired arteriovenous malformation: Report of a case and review of the literature. Dermatol. Surg. 2004, 30, 1050–1053. [Google Scholar] [CrossRef] [PubMed]
- Mahapatra, Q.S.; Sahai, K.; Malik, A.; Mani, N.S. Intravascular papillary endothelial hyperplasia: An unusual histopathological entity. Indian Dermatol. Online J. 2015, 6, 277–279. [Google Scholar] [PubMed]
- Díaz-Flores, L.; Gutiérrez, R.; González-Gómez, M.; García, M.P.; Carrasco, J.L.; Díaz-Flores, L., Jr.; Madrid, J.F.; Álvarez-Argüelles, H. Participation of intussusceptive angiogenesis in the morphogenesis of lobular capillary hemangioma. Sci. Rep. 2020, 10, 4987. [Google Scholar] [CrossRef] [PubMed]
- Sepulveda, A.; Buchanan, E.P. Vascular tumors. Semin. Plast. Surg. 2014, 28, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Blaya, E.; Vento, V.; Kuntz, S.; Bruyns, L.; Ohana, M.; Weingertner, N.; Lejay, A.; Chakfé, N. Intravenous lobular capillary haemangioma (pyogenic granuloma) of the superior vena cava: Case report and literature review. EJVES Vasc. Forum 2021, 50, 32–36. [Google Scholar] [CrossRef]
- Naderi, N.J.; Eshghyar, N.; Esfehanian, H. Reactive lesions of the oral cavity: A retrospective study on 2068 cases. Dent. Res. J. 2012, 9, 251–255. [Google Scholar]
- Wynn, T.A. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J. Clin. Investig. 2007, 117, 524–529. [Google Scholar] [CrossRef]
- Kapoor, P.; Deshmukh, R.S. VEGF: A critical driver for angiogenesis and subsequent tumor growth: An IHC study. J. Oral Maxillofac. Pathol. 2012, 16, 330–337. [Google Scholar] [CrossRef]
- Aghbali, A.; Akbarzadeh, A.; Kouhsoltani, M. The role of macrophages and eosinophils in reactive lesions of the oral cavity. J. Oral Maxillofac. Pathol. 2018, 22, 147. [Google Scholar] [CrossRef] [PubMed]
- Sheethal, H.S.; Kn, H.; Smitha, T.; Chauhan, K. Role of mast cells in inflammatory and reactive pathologies of pulp, periapical area and periodontium. J. Oral Maxillofac. Pathol. 2018, 22, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Shekar, S.; Angadi, P.V. Quantification of mast cells in reactive oral lesions—A clue to the morphologic diversity. Indian. J. Health Sci. Biomed. Res. 2019, 12, 123–126. [Google Scholar]
- Komi, D.; Khomtchouk, K.; Maria, P. A review of the contribution of mast cells in wound healing: Involved molecular and cellular mechanisms. Clin. Rev. Allergy Immunol. 2020, 58, 298–312. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.; Xavier, F.; Freitas, M.; Nunes, F.; Gurgel, C.; Cangussu, M. Involvement of mast cells and microvessels density in reactive lesions of oral cavity: A comparative immunohistochemical study. Pathol. Res. Pract. 2016, 212, 761–766. [Google Scholar] [CrossRef] [PubMed]
- Kouhsoltani, M.; Khiavi, M.M.; Tahamtan, S. Evaluation of mast cell counts and microvessel density in reactive lesions of the oral cavity. J. Dent. Res. Dent. Clin. Dent. Prospects. 2016, 10, 241–246. [Google Scholar] [CrossRef]
- Puxeddu, I.; Berkman, N.; Nissim Ben Efraim, A.H.; Davies, D.E.; Ribatti, D.; Gleich, G.J.; Levi-Schaffer, F. The role of eosinophil major basic protein in angiogenesis. Allergy 2009, 64, 368–374. [Google Scholar] [CrossRef]
- Nissim Ben Efraim, A.H.; Levi-Schaffer, F. Roles of eosinophils in the modulation of angiogenesis. Chem. Immunol. Allergy 2014, 99, 138–154. [Google Scholar] [CrossRef]
- Nakamura, F.; Fifita, S.F.; Kuyama, K. A study of oral irritation fibroma with special reference to clinicopathological and immunohistochemical features of stromal spindle cells. Int. J. Oral Med. Sci. 2005, 4, 83–91. [Google Scholar] [CrossRef][Green Version]
- Vasanthi, V.; Divya, B.; Ramadoss, R.; Deena, P.; Annasamy, R.K.; Rajkumar, K. Quantification of inflammatory, angiogenic and fibrous components of reactive oral lesions with an insight into pathogenesis. J. Oral Maxillofac. Pathol. 2022, 26, 600. [Google Scholar]
- Hunasgi, S.; Koneru, A.; Vanishree, M.; Manvikar, V. Assessment of reactive gingival lesions of oral cavity: A histopathological study. J. Oral Maxillofac. Pathol. 2017, 21, 180. [Google Scholar] [CrossRef] [PubMed]
- Vara, J.T.; Gurudu, V.S.; Ananthaneni, A.; Bagalad, B.S.; Kuberappa, P.H.; Ponnapalli, H.P. Correlation of Vascular and Inflammatory Index in Oral Pyogenic Granuloma and Periapical Granuloma—An Insight into Pathogenesis. J. Clin. Diagn. Res. 2017, 11, ZC25–ZC28. [Google Scholar] [CrossRef] [PubMed]
- Abdulla, M.H.; Shaik, A.S.; Vaali-Mohammed, M.A.; Al Khayal, K.A.; Traiki, T.B.; Zubaidi, A.M.; Al-Johani, T.; Shakoor, Z.; Al-Obeed, O.A. Expression of VEGF, EGF and HGF in early- and late-stage colorectal cancer. Mol. Clin. Oncol. 2021, 15, 251. [Google Scholar] [CrossRef] [PubMed]
- Blackwell, M.G.; Itinteang, T.; Chibnall, A.M.; Davis, P.F.; Tan, S.T. Expression of embryonic stem cell markers in pyogenic granu-loma. J. Cutan. Pathol. 2016, 43, 1096–1101. [Google Scholar] [CrossRef]
- Pereira, T.D.S.F.; de Amorim, L.S.D.; Pereira, N.B.; Vitório, J.G.; Duarte-Andrade, F.F.; Guimarães, L.M.; Diniz, M.G.; Gomes, C.C.; Gomez, R.S. Oral pyogenic granulomas show MAPK/ERK signaling pathway activation, which occurs independently of BRAF, KRAS, HRAS, NRAS, GNA11, and GNA14 mutations. J. Oral Pathol. Med. 2019, 48, 906–910. [Google Scholar] [CrossRef]
- Gapizov, A.; Sidhu, H.K.; Affaf, M.; Safdar, S.; Irfan, R.; Ekhator, C.; Saddique, M.N.; Devi, M.; Cabana, E.O.S.; Kamran, M.; et al. Unraveling Intravascular Lobular Capillary Hemangioma: A Comprehensive Scoping Review. Cureus 2023, 15, e45142. [Google Scholar] [CrossRef]
- Lim, Y.H.; Douglas, S.R.; Ko, C.J.; Antaya, R.J.; McNiff, J.M.; Zhou, J.; Choate, K.A.; Narayan, D. Somatic activating RAS mutations cause vascular tumors including pyogenic granuloma. J. Investig. Dermatol. 2015, 135, 1698–1700. [Google Scholar] [CrossRef]
- Groesser, L.; Peterhof, E.; Evert, M.; Landthaler, M.; Berneburg, M.; Hafner, C. BRAF and RAS mutations in sporadic and secondary pyogenic granuloma. J. Investig. Dermatol. 2016, 136, 481–486. [Google Scholar] [CrossRef]
- Lim, Y.H.; Bacchiocchi, A.; Qiu, J.; Straub, R.; Bruckner, A.; Bercovitch, L.; Narayan, D.; McNiff, J.; Ko, C.; Robinson-Bostom, L.; et al. GNA14 somatic mutation causes congenital and sporadic vascular tumors by MAPK activation. Am. J. Hum. Genet. 2016, 99, 443–450. [Google Scholar] [CrossRef]
- Yao, T.; Nagai, E.; Utsunomiya, T.; Tsuneyoshi, M. An intestinal counterpart of pyogenic granuloma of the skin: A newly proposed entity. Am. J. Surg. Pathol. 1995, 19, 1054–1060. [Google Scholar] [CrossRef]
- Akyol, M.U.; Yalçiner, E.G.; Doğan, A.I. Pyogenic granuloma (lobular capillary hemangioma) of the tongue. Int. J. Pediatr. Otorhinolaryngol. 2001, 58, 239–241. [Google Scholar] [CrossRef] [PubMed]
- Rosa, G.; Cartagena, C.; Andrea, L.; La Torre, C. Oral pyogenic granuloma diagnosis and treatment: A series of cases. Rev. Odontol. Mex. 2017, 21, 253–261. Available online: http://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S1870-199X2017000400253&lng=es&tlng=en (accessed on 4 September 2023). [CrossRef]
- Parajuli, R.; Maharjan, S. Unusual presentation of oral pyogenic granulomas: A review of two cases. Clin. Case Rep. 2018, 6, 690–693. [Google Scholar] [CrossRef] [PubMed]
- Panjwani, S.; Issrani, R.; Keluskar, V.; Naik, Z. An unusual presentation of pyogenic granuloma. Indian J. Dent. 2012, 3, 178–181. [Google Scholar] [CrossRef]
- Mubeen, K.; Vijayalakshmi, K.R.; Abhishek, R.P. Oral pyogenic granuloma with mandible involvement: An unusual presentation. J. Dent. Oral Hyg. 2011, 3, 6–9. [Google Scholar]
- Mastammanavar, D.; Hunasgi, S.; Koneru, A.; Vanishree, M.R.; Surekha, R.; Vardendra, M. Aggressive Pyogenic Granuloma: A Case Report. Int. J. Oral Maxillofac. Pathol. 2014, 5, 29–32. [Google Scholar]
- Gonçales, E.S.; Damante, J.H.; Fischer Rubira, C.M.; Taveira, L.A. Pyogenic granuloma on the upper lip: An unusual location. J. Appl. Oral Sci. 2010, 18, 538–541. [Google Scholar] [CrossRef]
- Fekrazad, R.; Nokhbatolfoghahaei, H.; Khoei, F.; Kalhori, K.A. Pyogenic Granuloma: Surgical Treatment with Er:YAG Laser. J. Lasers Med. Sci. 2014, 5, 199–205. [Google Scholar]
- Ganesan, A.; Kumar, N.G.; Azariah, E.; Asokan, G.S. Oral pyogenic granuloma: A case report and a comprehensive review. SRM J. Res. Dent. Sci. 2015, 6, 257–260. Available online: https://www.srmjrds.in/text.asp?2015/6/4/257/170284 (accessed on 17 July 2023). [CrossRef]
- Aguilo, L. Pyogenic granuloma subsequent to injury of a primary tooth—A case report. Int. J. Paediatr. Dent. 2002, 12, 438–441. [Google Scholar] [CrossRef]
- Panseriya, B.J.; Hungund, S. Pyogenic granuloma associated with periodontal abscess and bone loss—A rare case report. Contemp. Clin. Dent. 2011, 2, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh JKulkarni, V.K.; Katti, G.; Deshpande, S. Pyogenic Granuloma, An Unusual Presentation in Pediatric Patient—A Case Report. Indian J. Dent. Sci. 2013, 5, 90–93. [Google Scholar]
- Asha, V.; Dhanya, M.; Patil, B.A.; Revanna, G. An unusual presentation of pyogenic granuloma of the lower lip. Contemp. Clin. Dent. 2014, 5, 524–526. [Google Scholar] [CrossRef] [PubMed]
- Behl, A.B.; Bali, V.; Bali, R. Pyogenic Granuloma—A case report and review of literature. Int. J. Stomatol. Occlusion Med. 2011, 4, 166–170. [Google Scholar] [CrossRef]
- Patil, K.; Mahima, V.G.; Lahari, K. Extragingival pyogenic granuloma. Indian J. Dent. Res. 2006, 17, 199–202, Erratum in Indian J. Dent. Res. 2007, 18, 45. [Google Scholar] [CrossRef] [PubMed]
- Shenoy, S.S.; Dinkar, A.D. Pyogenic granuloma associated with bone loss in an eight year old child: A case report. J. Indian. Soc. Pedod. Prev. Dent. 2006, 24, 201–203. [Google Scholar] [CrossRef]
- Amirchaghmaghi, M.; Falaki, F.; Mohtasham, N.; Mozafari, P.M. Extragingival pyogenic granuloma: A case report. Cases J. 2008, 1, 371. [Google Scholar] [CrossRef]
- Olmedo, D.G.; Paparella, M.L.; Brandizzi, D.; Cabrini, R.L. Reactive lesions of peri-implant mucosa associated with titanium dental implants: A report of 2 cases. Int. J. Oral Maxillofac. Surg. 2010, 39, 503–507. [Google Scholar] [CrossRef]
- Gondivkar, S.M.; Gadbail, A.; Chole, R. Oral pregnancy tumor. Contemp. Clin. Dent. 2010, 1, 190–192. [Google Scholar] [CrossRef]
- Rizwanulla, M.D.; Koirala, B.; Sharma, S.; Adhikari, L.; Pradhan, A. Pyogenic Granuloma: A Case Report. Health Renaiss. 2011, 8, 196–198. [Google Scholar] [CrossRef]
- Shivaswamy, S.; Siddiqui, N.; Jain, S.A.; Koshy, A.; Tambwekar, S.; Shankar, A. A rare case of generalized pyogenic granuloma: A case report. Quintessence Int. 2011, 42, 493–499. [Google Scholar] [PubMed]
- Chandrashekar, B. Minimally invasive approach to eliminate pyogenic granuloma: A case report. Case Rep. Dent. 2012, 2012, 909780. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ravi, V.; Jacob, M.; Sivakumar, A.; Saravanan, S.; Priya, K. Pyogenic granuloma of labial mucosa: A misnomer in an anomolous site. J. Pharm. Bioallied Sci. 2012, 4 (Suppl. 2), S194–S196. [Google Scholar] [CrossRef] [PubMed]
- de Vasconcellos Piscoya MD, B.; de Alencar Ximenes, R.A.; Silva, G.M.; Jamelli, S.R.; Coutinho, S.B. Periodontitis-associated risk factors in pregnant women. Clinics 2012, 67, 27–33. [Google Scholar] [CrossRef]
- Verma, P.K.; Srivastava, R.; Baranwal, H.C.; Chaturvedi, T.P.; Gautam, A.; Singh, A. Pyogenic granuloma—Hyperplastic lesion of the gingiva: Case reports. Open Dent. J. 2012, 6, 153–156, Erratum in Open Dent. J. 2012, 6, 188. [Google Scholar] [CrossRef][Green Version]
- Adusumilli, S.; Yalamanchili, P.S.; Manthena, S. Pyogenic granuloma near the midline of the oral cavity: A series of case reports. J. Indian Soc. Periodontol. 2014, 18, 236–239. [Google Scholar] [CrossRef]
- Mahabob, N.; Kumar, S.; Raja, S. Palatal pyogenic granulomaa. J. Pharm. Bioallied Sci. 2013, 5 (Suppl. 2), S179–S181. [Google Scholar] [CrossRef]
- Moraes, S.H.; Moraes, G.F.; Durski, J.; Viero, F.L.; Da Silva Meira, D.D.; Caron, M.E. Granuloma Piogênico: Relato de Caso Clínico. Rev. Gestão Saúde 2013, 9, 12–19. [Google Scholar]
- Sangamesh, N.C.; Poornima, B.; Vidya, K.C.; Sakri, S.B. Extragingival pyogenic granuloma: A rare case report. J. Sci. Soc. 2013, 40, 49–51. [Google Scholar] [CrossRef]
- Kejriwal, S.; Bhandary, R.; Thomas, B. Oral pyogenic granuloma: A case report. Nitte Univ. J. Health Sci. 2014, 4, 123–125. [Google Scholar] [CrossRef]
- Sun, W.L.; Lei, L.; Zhong, L.; Yu, S.; Zhou, J.W. Multiple gingival pregnancy tumors with rapid growth. J. Dent. Sci. 2013, 9, 289–293. [Google Scholar] [CrossRef]
- Asnaashari, M.; Mehdipour, M.; MoradiAbbasabadi, F.; Azari-Marhabi, S. Expedited removal of pyogenic granuloma by diode laser in a pediatric patient. J. Lasers Med. Sci. 2015, 6, 40–44. [Google Scholar]
- Bugshan, A.; Patel, H.; Garber, K.; Meiller, T.F. Alternative Therapeutic Approach in the Treatment of Oral Pyogenic Granuloma. Case Rep. Oncol. 2015, 8, 493–497. [Google Scholar] [CrossRef]
- de Carvalho, F.K.; Pinheiro, T.N.; Arid, J.; de Queiroz, A.M.; de Rossi, A.; Nelson-Filho, P. Trauma-Induced Giant Pyogenic Granuloma in the Upper Lip. J. Dent. Child 2015, 82, 168–170. [Google Scholar]
- Sachdeva, S.K. Extragingival Pyogenic Granuloma: An Unusual Clinical Presentation. J. Dent. 2015, 16 (Suppl. 3), 282–285. [Google Scholar]
- Tripathi, A.K.; Upadhaya, V.; Kumar, V.; Saimbi, C.S. Hyperactive lesions of gingiva associated with severe alveolar bone loss: A rare finding. Contemp. Clin. Dent. 2015, 6, 223–225. [Google Scholar] [CrossRef]
- Agarwal, N.; Kumar, D.; Vaish, A.; Anand, A. A Rare Case of Pyogenic Granuloma with a Natal Tooth. J. Clin. Diagn. Res. 2016, 10, ZD28–ZD29. [Google Scholar] [CrossRef]
- Marla, V.; Shrestha, A.; Goel, K.; Shrestha, S. The Histopathological Spectrum of Pyogenic Granuloma: A Case Series. Case Rep. Dent. 2016, 2016, 1323798. [Google Scholar] [CrossRef]
- Tenore, G.; Mohsen, A.; Pompa, G.; Brauner, E.; Cassoni, A.; Valentini, V.; Polimeni, A.; Romeo, U. Gingival Reactive Lesions in Orally Rehabilitated Patients by Free Revascularized Flap. Case Rep. Dent. 2018, 2018, 2474706. [Google Scholar] [CrossRef]
- Canivell-Zabaleta, M.; Martin-Lozano, G.; Olmos-Juarez, E.; Fontillon-Alberdi, M.; Infante-Cossio, P. Extragingival Pregnancy Pyogenic Granuloma on the Lip. J. Craniofac. Surg. 2018, 29, e49–e50. [Google Scholar] [CrossRef]
- Poudel, P.; Chaurasia, N.; Marla, V.; Srii, R. Pyogenic granuloma of the upper lip: A common lesion in an uncommon location. J. Taibah Univ. Med. Sci. 2018, 14, 95–98. [Google Scholar] [CrossRef]
- Banjar, A.; Abdrabuh, A.; Al-Habshi, M.; Parambil, M.; Bastos, P.; Abed, H. Labial pyogenic granuloma related to trauma: A case report and mini-review. Dent. Traumatol. 2020, 36, 446–451. [Google Scholar] [CrossRef]
- Pisano, M.; Sammartino, P.; Di Vittorio, L.; Iandolo, A.; Caggiano, M.; Roghi, M.; Bizzoca, M.E.; Lo Muzio, L. Use of Diode Laser for Surgical Removal of Pyogenic Granuloma of the Lower Lip in a Pediatric Patient: A Case Report. Am. J. Case Rep. 2021, 22, e929690. [Google Scholar] [CrossRef]
- Lomelí Martínez, S.M.; Bocanegra Morando, D.; Mercado González, A.E.; Gómez Sandoval, J.R. Unusual clinical presentation of oral pyogenic granuloma with severe alveolar bone loss: A case report and review of literature. World J. Clin. Cases. 2023, 11, 3907–3914. [Google Scholar] [CrossRef]
- Ma, Y.; Nicolet, J. Specificity models in MAPK cascade signaling. FEBS Open Biol. 2023, 13, 1177–1192. [Google Scholar] [CrossRef]
- Rodríguez-Carballo, E.; Gámez, B.; Ventura, F. p38 MAPK Signaling in Osteoblast Differentiation. Front. Cell Dev. Biol. 2016, 4, 40. [Google Scholar] [CrossRef]
- Al-Mohaya, M.A.; Al-Malik, A.M. Excision of oral pyogenic granuloma in a diabetic patient with 940 nm diode laser. Saudi Med. J. 2016, 37, 1395–1400. [Google Scholar] [CrossRef]
- Mirshams, M.; Daneshpazhooh, M.; Mirshekari, A.; Taheri, A.; Mansoori, P.; Hekmat, S. Cryotherapy in the treatment of pyogenic granuloma. J. Eur. Acad. Dermatol. Venereol. 2006, 20, 788–790. [Google Scholar] [CrossRef]
- Moon, S.E.; Hwang, E.J.; Cho, K.H. Treatment of pyogenic granuloma by sodium tetradecyl sulfate sclerotherapy. Arch. Dermatol. 2005, 141, 644–646. [Google Scholar] [CrossRef]
- Román-Quesada, N.; González-Navarro, B.; Izquierdo-Gómez, K.; Jané-Salas, E.; Marí-Roig, A.; Estrugo-Devesa, A.; López-López, J. An analysis of the prevalence of peripheral giant cell granuloma and pyogenic granuloma in relation to a dental implant. BMC Oral Health 2021, 21, 204. [Google Scholar] [CrossRef]
- Baesso, R.C.P.; de Lima Jacy Monteiro Barki, M.C.; de Souza Azevedo, R.; da Costa Fontes, K.B.F.; Pereira, D.L.; Tucci, R.; Pires, F.R.; Picciani, B.L.S. Peripheral giant cell granuloma associated with a dental implant. BMC Oral Health 2019, 19, 283. [Google Scholar] [CrossRef] [PubMed]
- Frumkin, N.; Nashef, R.; Shapira, L.; Wilensky, A. Nonsurgical treatment of recurrent gingival pyogenic granuloma: A case report. Quintessence Int. 2015, 46, 539–544. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).