Homer1 Protects against Retinal Ganglion Cell Pyroptosis by Inhibiting Endoplasmic Reticulum Stress-Associated TXNIP/NLRP3 Inflammasome Activation after Middle Cerebral Artery Occlusion-Induced Retinal Ischemia
Abstract
:1. Introduction
2. Results
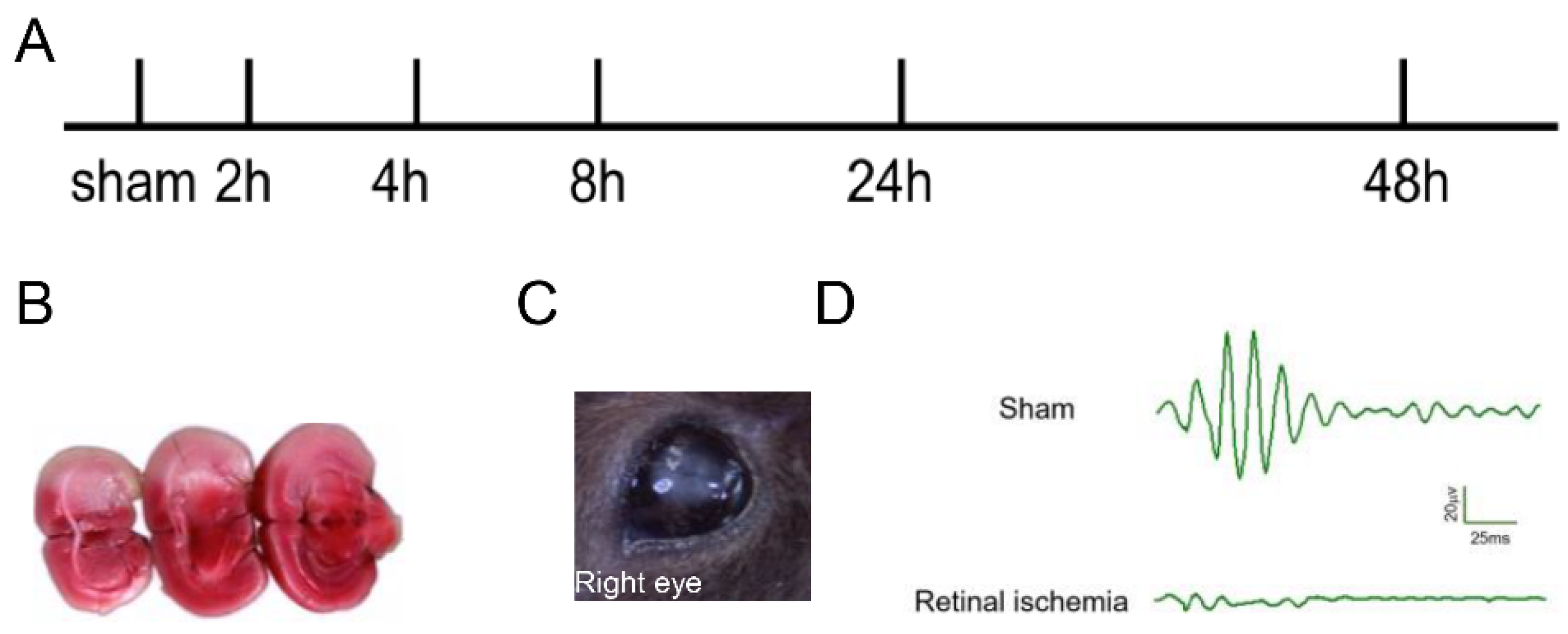
2.1. Generation of MCAO-Induced Retinal Ischemia Model
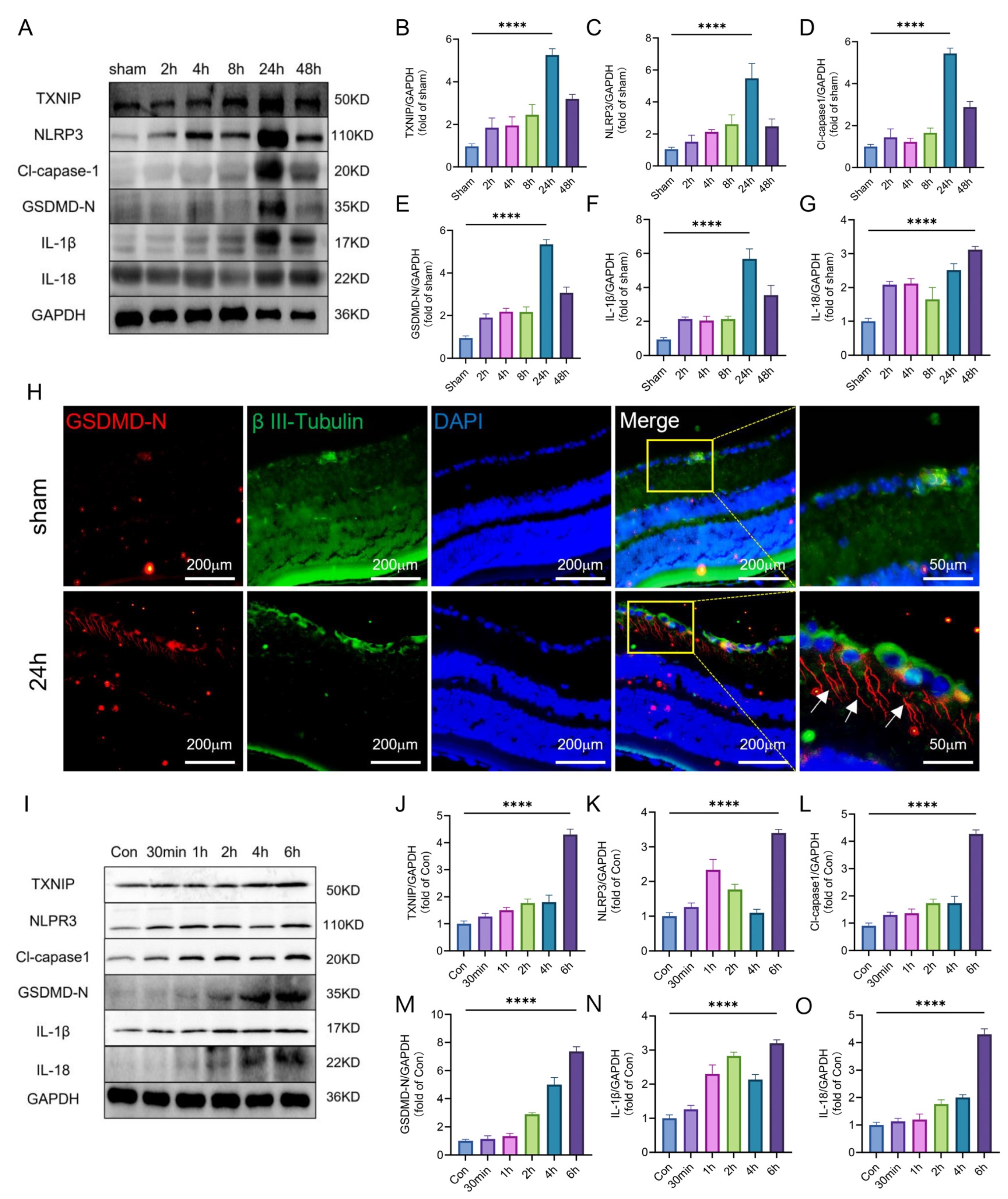
2.2. TXNIP/NLRP3-Mediated Pyroptosis Was Activated after Retinal Ischemia
2.3. Occurrence of Pyroptosis Coincided with the Change of Homer1 Expression in the Retina after Retinal Ischemia
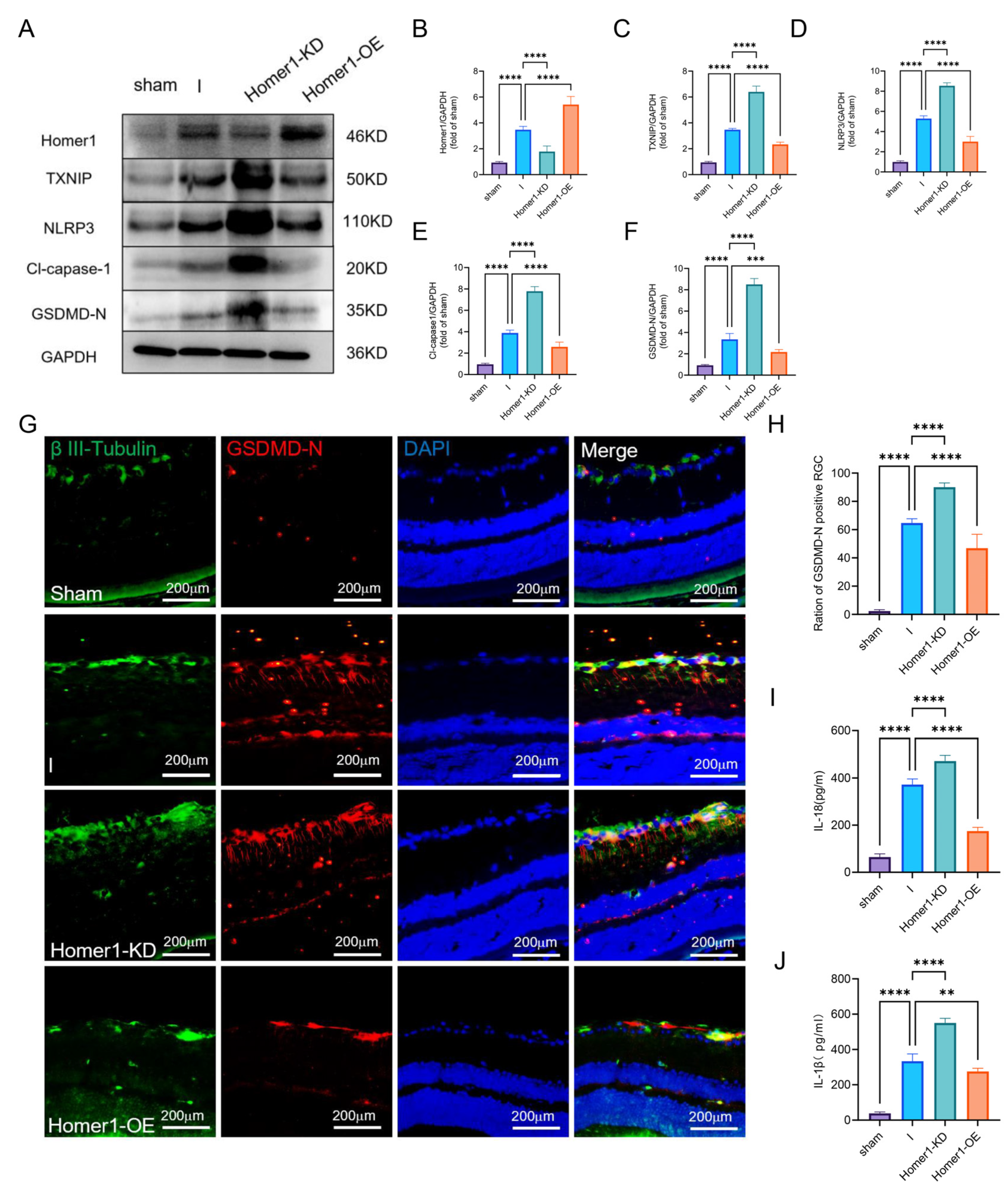
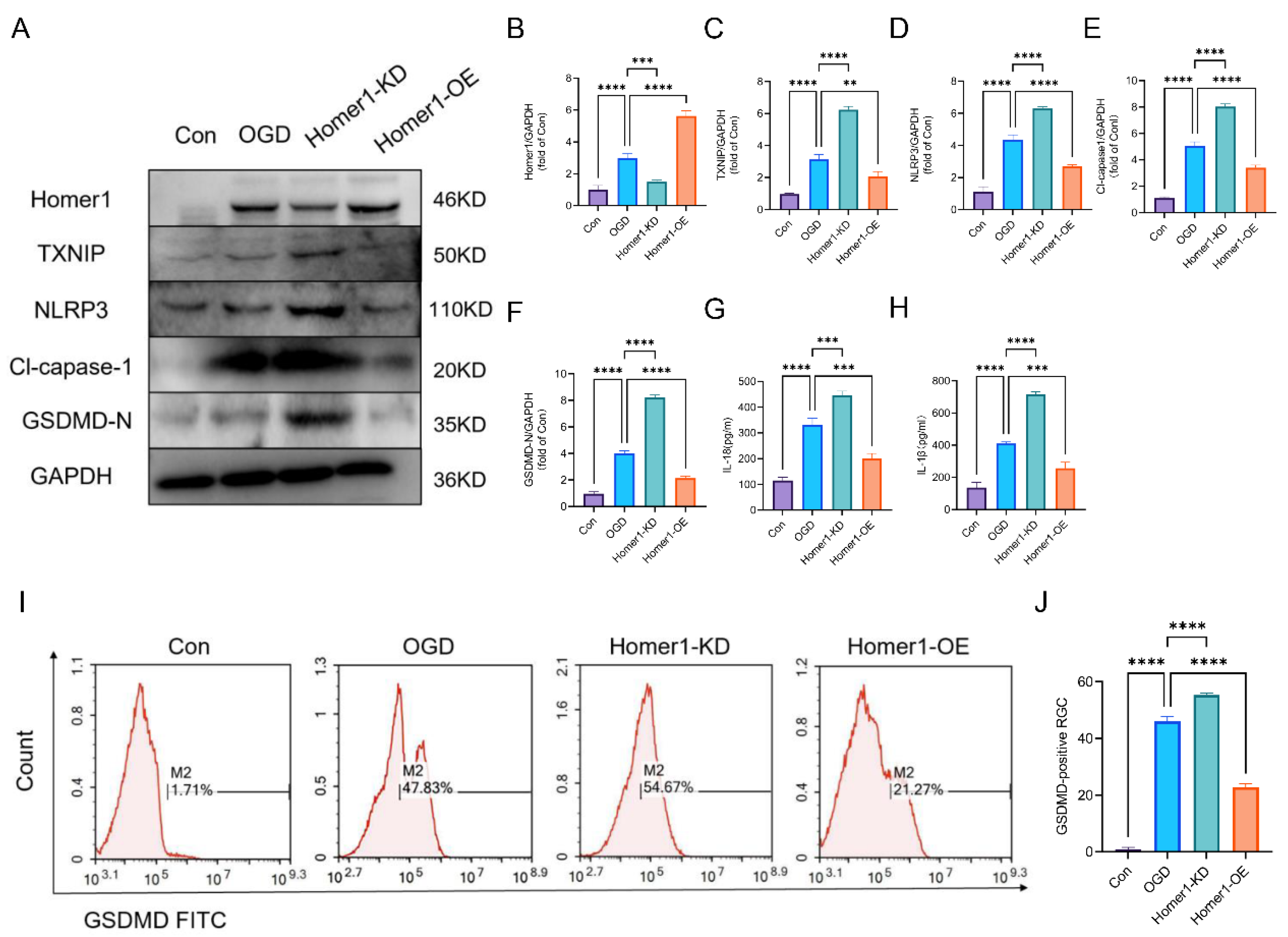
2.4. Overexpression of Homer1 Alleviated RGC Pyroptosis after Retinal Ischemia
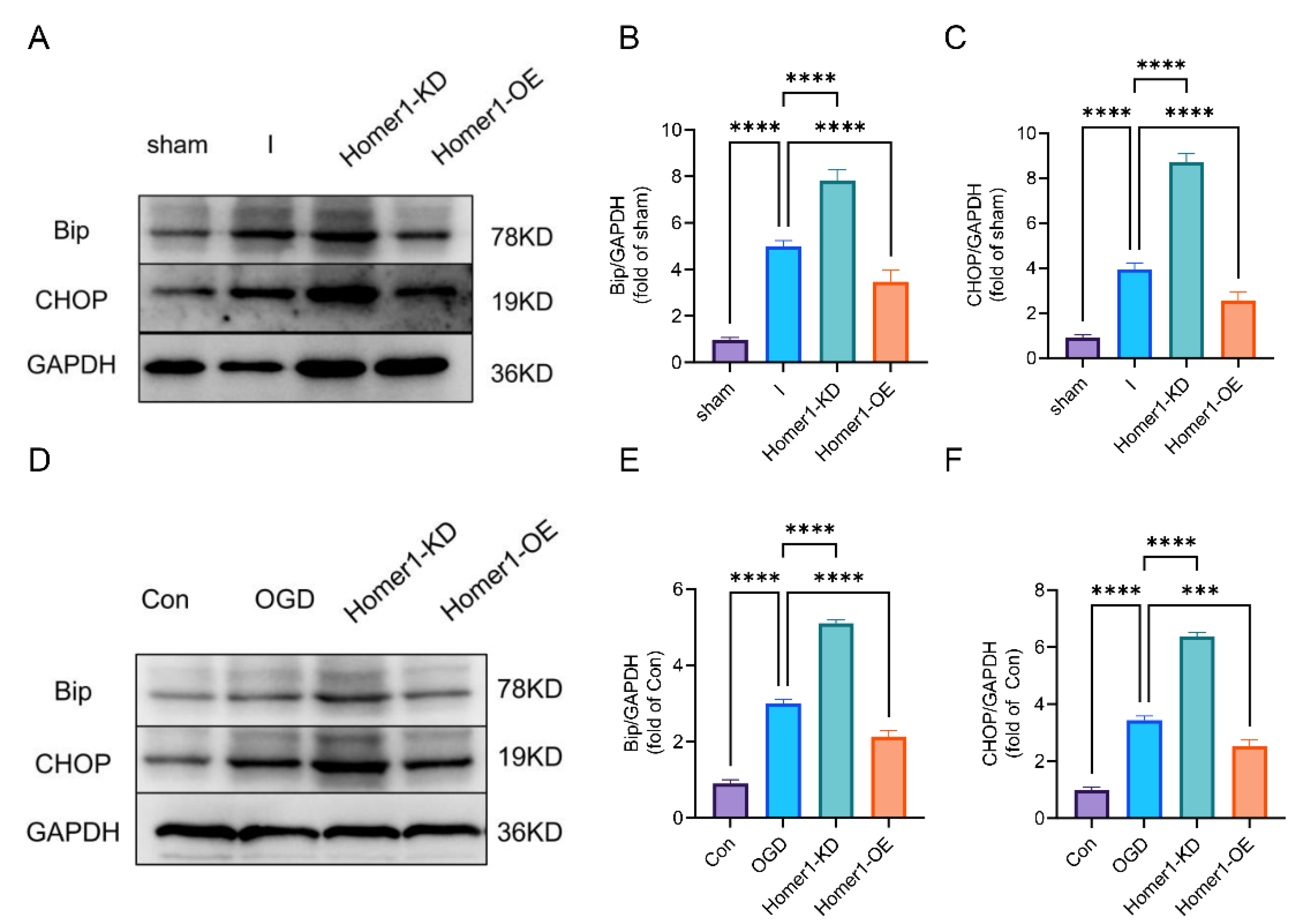
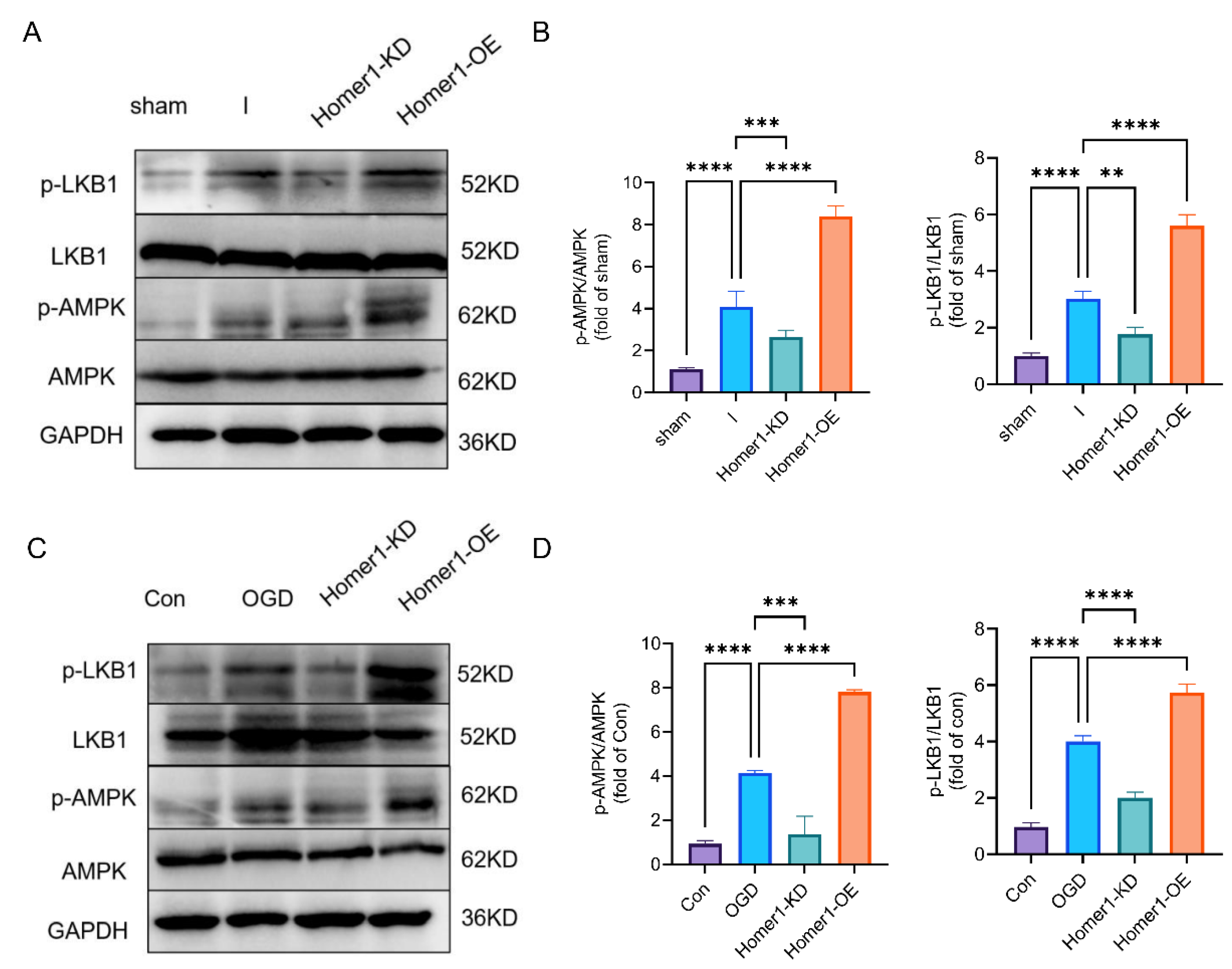
2.5. Overexpression of Homer1 Inhibited ER Stress by Regulating AMPK Activity after Retinal Ischemia
2.6. Overexpression of Homer1 Preserved Visual Function after Retinal Ischemia
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Lentivirus and Adeno-Associated Virus
4.3. Experimental Design
4.4. Induction of Permanent MCAO-Induced Retinal Ischemia
4.5. Isolation of Primary Cultured Retinal Ganglion Cells (RGCs)
4.6. Oxygen and Glucose Deprivation (OGD)
4.7. 2, 3, 5-Triphenyl Tetrazolium Chloride Stain (TTC)
4.8. Immunofluorescence Staining of Retinal Sections
4.9. Western Blot
4.10. qPCR
4.11. Flash Visually Evoked Potential Recordings (FVEPs)
4.12. HE
4.13. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Zhang, R.; Wu, Y.; Xie, F.; Zhong, Y.; Wang, Y.; Xu, M.; Feng, J.; Charish, J.; Monnier, P.P.; Qin, X. RGMa mediates reactive astrogliosis and glial scar formation through TGFβ1/Smad2/3 signaling after stroke. Cell Death Differ. 2018, 25, 1503–1516. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Wu, X.; Zhou, Y.; Shao, B.; Niu, X.; Zhang, W.; Lin, Y. Maternal high-fat diet programs cerebrovascular remodeling in adult rat offspring. J. Cereb. Blood Flow Metab. 2018, 38, 1954–1967. [Google Scholar] [CrossRef] [PubMed]
- Sifat, A.E.; Vaidya, B.; Villalba, H.; Albekairi, T.H.; Abbruscato, T.J. Neurovascular unit transport responses to ischemia and common coexisting conditions: Smoking and diabetes. Am. J. Physiol. Cell Physiol. 2019, 316, C2–C15. [Google Scholar] [CrossRef] [PubMed]
- Kingsbury, C.; Heyck, M.; Bonsack, B.; Lee, J.Y.; Borlongan, C.V. Stroke gets in your eyes: Stroke-induced retinal ischemia and the potential of stem cell therapy. Neural Regen. Res. 2020, 15, 1014–1018. [Google Scholar] [CrossRef] [PubMed]
- Pollock, A.; Hazelton, C.; Henderson, C.A.; Angilley, J.; Dhillon, B.; Langhorne, P.; Livingstone, K.; Munro, F.A.; Orr, H.; Rowe, F.J.; et al. Interventions for age-related visual problems in patients with stroke. Cochrane Database Syst. Rev. 2012, 3, Cd008390. [Google Scholar] [CrossRef] [PubMed]
- Egan, R.A.; Lutsep, H.L. Prevalence of Retinal Emboli and Acute Retinal Artery Occlusion in Acute Ischemic Stroke. J. Stroke Cerebrovasc. Dis. 2020, 29, 104446. [Google Scholar] [CrossRef] [PubMed]
- Steele, E.C., Jr.; Guo, Q.; Namura, S. Filamentous middle cerebral artery occlusion causes ischemic damage to the retina in mice. Stroke 2008, 39, 2099–2104. [Google Scholar] [CrossRef] [PubMed]
- Renner, M.; Stute, G.; Alzureiqi, M.; Reinhard, J.; Wiemann, S.; Schmid, H.; Faissner, A.; Dick, H.B.; Joachim, S.C. Optic Nerve Degeneration after Retinal Ischemia/Reperfusion in a Rodent Model. Front. Cell. Neurosci. 2017, 11, 254. [Google Scholar] [CrossRef]
- Jóhannesson, G.; Qvarlander, S.; Wåhlin, A.; Ambarki, K.; Hallberg, P.; Eklund, A.; Lindén, C. Intraocular Pressure Decrease Does Not Affect Blood Flow Rate of Ophthalmic Artery in Ocular Hypertension. Investig. Ophthalmol. Vis. Sci. 2020, 61, 17. [Google Scholar] [CrossRef]
- Sehi, M.; Goharian, I.; Konduru, R.; Tan, O.; Srinivas, S.; Sadda, S.R.; Francis, B.A.; Huang, D.; Greenfield, D.S. Retinal blood flow in glaucomatous eyes with single-hemifield damage. Ophthalmology 2014, 121, 750–758. [Google Scholar] [CrossRef]
- Venugopalan, P.; Wang, Y.; Nguyen, T.; Huang, A.; Muller, K.J.; Goldberg, J.L. Transplanted neurons integrate into adult retinas and respond to light. Nat. Commun. 2016, 7, 10472. [Google Scholar] [CrossRef]
- Wang, Y.; Rajala, A.; Cao, B.; Ranjo-Bishop, M.; Agbaga, M.P.; Mao, C.; Rajala, R.V. Cell-Specific Promoters Enable Lipid-Based Nanoparticles to Deliver Genes to Specific Cells of the Retina In Vivo. Theranostics 2016, 6, 1514–1527. [Google Scholar] [CrossRef]
- Pita-Thomas, W.; Mahar, M.; Joshi, A.; Gan, D.; Cavalli, V. HDAC5 promotes optic nerve regeneration by activating the mTOR pathway. Exp. Neurol. 2019, 317, 271–283. [Google Scholar] [CrossRef]
- Jia, Y.; Cui, R.; Wang, C.; Feng, Y.; Li, Z.; Tong, Y.; Qu, K.; Liu, C.; Zhang, J. Metformin protects against intestinal ischemia-reperfusion injury and cell pyroptosis via TXNIP-NLRP3-GSDMD pathway. Redox Biol. 2020, 32, 101534. [Google Scholar] [CrossRef]
- Zhou, R.; Yazdi, A.S.; Menu, P.; Tschopp, J. A role for mitochondria in NLRP3 inflammasome activation. Nature 2011, 469, 221–225. [Google Scholar] [CrossRef]
- Han, Y.; Xu, X.; Tang, C.; Gao, P.; Chen, X.; Xiong, X.; Yang, M.; Yang, S.; Zhu, X.; Yuan, S.; et al. Reactive oxygen species promote tubular injury in diabetic nephropathy: The role of the mitochondrial ros-txnip-nlrp3 biological axis. Redox Biol. 2018, 16, 32–46. [Google Scholar] [CrossRef]
- Tanti, J.F.; Ceppo, F.; Jager, J.; Berthou, F. Implication of inflammatory signaling pathways in obesity-induced insulin resistance. Front. Endocrinol. 2012, 3, 181. [Google Scholar] [CrossRef]
- Li, L.; Ismael, S.; Nasoohi, S.; Sakata, K.; Liao, F.F.; McDonald, M.P.; Ishrat, T. Thioredoxin-Interacting Protein (TXNIP) Associated NLRP3 Inflammasome Activation in Human Alzheimer’s Disease Brain. J. Alzheimers Dis. 2019, 68, 255–265. [Google Scholar] [CrossRef]
- Sprenkle, N.T.; Sims, S.G.; Sánchez, C.L.; Meares, G.P. Endoplasmic reticulum stress and inflammation in the central nervous system. Mol. Neurodegener. 2017, 12, 42. [Google Scholar] [CrossRef]
- Lerner, A.G.; Upton, J.P.; Praveen, P.V.; Ghosh, R.; Nakagawa, Y.; Igbaria, A.; Shen, S.; Nguyen, V.; Backes, B.J.; Heiman, M.; et al. IRE1α induces thioredoxin-interacting protein to activate the NLRP3 inflammasome and promote programmed cell death under irremediable ER stress. Cell Metab. 2012, 16, 250–264. [Google Scholar] [CrossRef]
- Wang, H.Y.; MacDonald, M.L.; Borgmann-Winter, K.E.; Banerjee, A.; Sleiman, P.; Tom, A.; Khan, A.; Lee, K.C.; Roussos, P.; Siegel, S.J.; et al. mGluR5 hypofunction is integral to glutamatergic dysregulation in schizophrenia. Mol. Psychiatry 2020, 25, 750–760. [Google Scholar] [CrossRef]
- Fei, X.; Dou, Y.N.; Wang, L.; Wu, X.; Huan, Y.; Wu, S.; He, X.; Lv, W.; Wei, J.; Fei, Z. Homer1 promotes the conversion of A1 astrocytes to A2 astrocytes and improves the recovery of transgenic mice after intracerebral hemorrhage. J. Neuroinflammation 2022, 19, 67. [Google Scholar] [CrossRef]
- Murotomi, K.; Takagi, N.; Muroyama, A.; Kaji, N.; Takeo, S.; Tanonaka, K. Transient focal cerebral ischemia differentially decreases Homer1a and 1b/c contents in the postsynaptic density. Neurosci. Lett. 2012, 515, 92–96. [Google Scholar] [CrossRef]
- Tchantchou, F.; Puche, A.A.; Leiste, U.; Fourney, W.; Blanpied, T.A.; Fiskum, G. Rat Model of Brain Injury to Occupants of Vehicles Targeted by Land Mines: Mitigation by Elastomeric Frame Designs. J. Neurotrauma 2018, 35, 1192–1203. [Google Scholar] [CrossRef]
- Fu, Y.; Shen, J.; Li, Y.; Liu, F.; Ning, B.; Zheng, Y.; Jiang, X. Inhibition of the PERK/TXNIP/NLRP3 Axis by Baicalin Reduces NLRP3 Inflammasome-Mediated Pyroptosis in Macrophages Infected with Mycobacterium tuberculosis. Mediat. Inflamm. 2021, 2021, 1805147. [Google Scholar] [CrossRef]
- Wang, Y.; Ruan, W.; Mi, J.; Xu, J.; Wang, H.; Cao, Z.; Saavedra, J.M.; Zhang, L.; Lin, H.; Pang, T. Balasubramide derivative 3C modulates microglia activation via CaMKKβ-dependent AMPK/PGC-1α pathway in neuroinflammatory conditions. Brain Behav. Immun. 2018, 67, 101–117. [Google Scholar] [CrossRef]
- Neumann, D. Is TAK1 a Direct Upstream Kinase of AMPK? Int. J. Mol. Sci. 2018, 19, 2412. [Google Scholar] [CrossRef]
- Heyck, M.; Bonsack, B.; Zhang, H.; Sadanandan, N.; Cozene, B.; Kingsbury, C.; Lee, J.Y.; Borlongan, C.V. The brain and eye: Treating cerebral and retinal ischemia through mitochondrial transfer. Exp. Biol. Med. 2019, 244, 1485–1492. [Google Scholar] [CrossRef]
- Kovacs, S.B.; Miao, E.A. Gasdermins: Effectors of Pyroptosis. Trends Cell Biol. 2017, 27, 673–684. [Google Scholar] [CrossRef]
- Mao, R.; Zong, N.; Hu, Y.; Chen, Y.; Xu, Y. Neuronal Death Mechanisms and Therapeutic Strategy in Ischemic Stroke. Neurosci. Bull. 2022, 38, 1229–1247. [Google Scholar] [CrossRef]
- Hsu, S.K.; Li, C.Y.; Lin, I.L.; Syue, W.J.; Chen, Y.F.; Cheng, K.C.; Teng, Y.N.; Lin, Y.H.; Yen, C.H.; Chiu, C.C. Inflammation-related pyroptosis, a novel programmed cell death pathway, and its crosstalk with immune therapy in cancer treatment. Theranostics 2021, 11, 8813–8835. [Google Scholar] [CrossRef]
- Cao, Z.; Fang, Y.; Lu, Y.; Tan, D.; Du, C.; Li, Y.; Ma, Q.; Yu, J.; Chen, M.; Zhou, C.; et al. Melatonin alleviates cadmium-induced liver injury by inhibiting the TXNIP-NLRP3 inflammasome. J. Pineal Res. 2017, 62, e12389. [Google Scholar] [CrossRef]
- Jiang, L.; Fei, D.; Gong, R.; Yang, W.; Yu, W.; Pan, S.; Zhao, M.; Zhao, M. CORM-2 inhibits TXNIP/NLRP3 inflammasome pathway in LPS-induced acute lung injury. Inflamm. Res. 2016, 65, 905–915. [Google Scholar] [CrossRef]
- Zhu, Y.; Yu, J.; Gong, J.; Shen, J.; Ye, D.; Cheng, D.; Xie, Z.; Zeng, J.; Xu, K.; Shen, J.; et al. PTP1B inhibitor alleviates deleterious microglial activation and neuronal injury after ischemic stroke by modulating the ER stress-autophagy axis via PERK signaling in microglia. Aging 2021, 13, 3405–3427. [Google Scholar] [CrossRef]
- Ahsan, A.; Zheng, Y.R.; Wu, X.L.; Tang, W.D.; Liu, M.R.; Ma, S.J.; Jiang, L.; Hu, W.W.; Zhang, X.N.; Chen, Z. Urolithin A-activated autophagy but not mitophagy protects against ischemic neuronal injury by inhibiting ER stress in vitro and in vivo. CNS Neurosci. Ther. 2019, 25, 976–986. [Google Scholar] [CrossRef]
- Rodenhizer, D.; Gaude, E.; Cojocari, D.; Mahadevan, R.; Frezza, C.; Wouters, B.G.; McGuigan, A.P. A three-dimensional engineered tumour for spatial snapshot analysis of cell metabolism and phenotype in hypoxic gradients. Nat. Mater. 2016, 15, 227–234. [Google Scholar] [CrossRef]
- Ii Timberlake, M.; Dwivedi, Y. Linking unfolded protein response to inflammation and depression: Potential pathologic and therapeutic implications. Mol. Psychiatry 2019, 24, 987–994. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Y.; Tan, J.W.; Hu, T.; Zhang, H.F.; Sorenson, C.M.; Smith, J.A.; Sheibani, N. Tunicamycin-induced photoreceptor atrophy precedes degeneration of retinal capillaries with minimal effects on retinal ganglion and pigment epithelium cells. Exp. Eye Res. 2019, 187, 107756. [Google Scholar] [CrossRef]
- Yang, Y.; Pei, X.; Jin, Y.; Wang, Y.; Zhang, C. The roles of endoplasmic reticulum stress response in female mammalian reproduction. Cell Tissue Res. 2016, 363, 589–597. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Li, S.; Li, Y.; Wang, X.; Liu, B.; Fu, Q.; Ma, S. Curcumin attenuates glutamate neurotoxicity in the hippocampus by suppression of ER stress-associated TXNIP/NLRP3 inflammasome activation in a manner dependent on AMPK. Toxicol. Appl. Pharmacol. 2015, 286, 53–63. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Q.; Zhao, W.; Li, J.; Sun, Y.; Liu, K.; Liu, B.; Zhang, N. Astragaloside IV and cycloastragenol are equally effective in inhibition of endoplasmic reticulum stress-associated TXNIP/NLRP3 inflammasome activation in the endothelium. J. Ethnopharmacol. 2015, 169, 210–218. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Wang, Y.; Wen, X.; Ma, X.N.; Chen, W.; Huang, F.; Kou, J.; Qi, L.W.; Liu, B.; et al. Pharmacological activation of AMPK prevents Drp1-mediated mitochondrial fission and alleviates endoplasmic reticulum stress-associated endothelial dysfunction. J. Mol. Cell. Cardiol. 2015, 86, 62–74. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, Y.; Xu, Y.; Ruan, W.; Wang, H.; Zhang, Y.; Saavedra, J.M.; Zhang, L.; Huang, Z.; Pang, T. A Dual AMPK/Nrf2 Activator Reduces Brain Inflammation After Stroke by Enhancing Microglia M2 Polarization. Antioxid. Redox Signal. 2018, 28, 141–163. [Google Scholar] [CrossRef]
- Wu, X.; Luo, P.; Rao, W.; Dai, S.; Zhang, L.; Ma, W.; Pu, J.; Yu, Y.; Wang, J.; Fei, Z. Homer1a Attenuates Hydrogen Peroxide-Induced Oxidative Damage in HT-22 Cells through AMPK-Dependent Autophagy. Front. Neurosci. 2018, 12, 51. [Google Scholar] [CrossRef]
- Zhang, R.X.; Wen, Y.; Guo, D.D.; Xu, F.R.; Wang, G.M.; Wang, X.R.; Shi, Y.W.; Ding, J.; Jiang, Q.; Jiang, W.J.; et al. Intravitreal injection of fibrillin 2 (Fbn2) recombinant protein for therapy of retinopathy in a retina-specific Fbn2 knock-down mouse model. Sci. Rep. 2023, 13, 6865. [Google Scholar] [CrossRef]
- Liu, X.; Hu, R.; Pei, L.; Si, P.; Wang, C.; Tian, X.; Wang, X.; Liu, H.; Wang, B.; Xia, Z.; et al. Regulatory T cell is critical for interleukin-33-mediated neuroprotection against stroke. Exp. Neurol. 2020, 328, 113233. [Google Scholar] [CrossRef]
- Dvoriantchikova, G.; Ivanov, D.; Barakat, D.; Grinberg, A.; Wen, R.; Slepak, V.Z.; Shestopalov, V.I. Genetic ablation of Pannexin1 protects retinal neurons from ischemic injury. PLoS ONE 2012, 7, e31991. [Google Scholar] [CrossRef]
- Fan, W.; Xing, Y.; Zhong, Y.; Chen, C.; Shen, Y. Expression of NMDA receptor subunit 1 in the rat retina. Acta Histochem. 2013, 115, 42–47. [Google Scholar] [CrossRef]
- Kapupara, K.; Wen, Y.T.; Tsai, R.K.; Huang, S.P. Soluble P-selectin promotes retinal ganglion cell survival through activation of Nrf2 signaling after ischemia injury. Cell Death Dis. 2017, 8, e3172. [Google Scholar] [CrossRef]
- Jiang, N.; Li, Z.; Li, Z.; Zhang, Y.; Yu, Z.; Wan, P.; Zhu, Y.; Li, Y.; Su, W.; Zhuo, Y. Laquinimod exerts anti-inflammatory and antiapoptotic effects in retinal ischemia/reperfusion injury. Int. Immunopharmacol. 2020, 88, 106989. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, W.; Wu, X.; Dou, Y.; Yan, Y.; Chen, L.; Fei, Z.; Fei, F. Homer1 Protects against Retinal Ganglion Cell Pyroptosis by Inhibiting Endoplasmic Reticulum Stress-Associated TXNIP/NLRP3 Inflammasome Activation after Middle Cerebral Artery Occlusion-Induced Retinal Ischemia. Int. J. Mol. Sci. 2023, 24, 16811. https://doi.org/10.3390/ijms242316811
Lv W, Wu X, Dou Y, Yan Y, Chen L, Fei Z, Fei F. Homer1 Protects against Retinal Ganglion Cell Pyroptosis by Inhibiting Endoplasmic Reticulum Stress-Associated TXNIP/NLRP3 Inflammasome Activation after Middle Cerebral Artery Occlusion-Induced Retinal Ischemia. International Journal of Molecular Sciences. 2023; 24(23):16811. https://doi.org/10.3390/ijms242316811
Chicago/Turabian StyleLv, Weihao, Xiuquan Wu, Yanan Dou, Yiwen Yan, Leiying Chen, Zhou Fei, and Fei Fei. 2023. "Homer1 Protects against Retinal Ganglion Cell Pyroptosis by Inhibiting Endoplasmic Reticulum Stress-Associated TXNIP/NLRP3 Inflammasome Activation after Middle Cerebral Artery Occlusion-Induced Retinal Ischemia" International Journal of Molecular Sciences 24, no. 23: 16811. https://doi.org/10.3390/ijms242316811
APA StyleLv, W., Wu, X., Dou, Y., Yan, Y., Chen, L., Fei, Z., & Fei, F. (2023). Homer1 Protects against Retinal Ganglion Cell Pyroptosis by Inhibiting Endoplasmic Reticulum Stress-Associated TXNIP/NLRP3 Inflammasome Activation after Middle Cerebral Artery Occlusion-Induced Retinal Ischemia. International Journal of Molecular Sciences, 24(23), 16811. https://doi.org/10.3390/ijms242316811



