Insights into the Serum Metabolic Adaptations in Response to Inspiratory Muscle Training: A Metabolomic Approach Based on 1H NMR and UHPLC-HRMS/MS
Abstract
:1. Introduction
2. Results
2.1. Participants
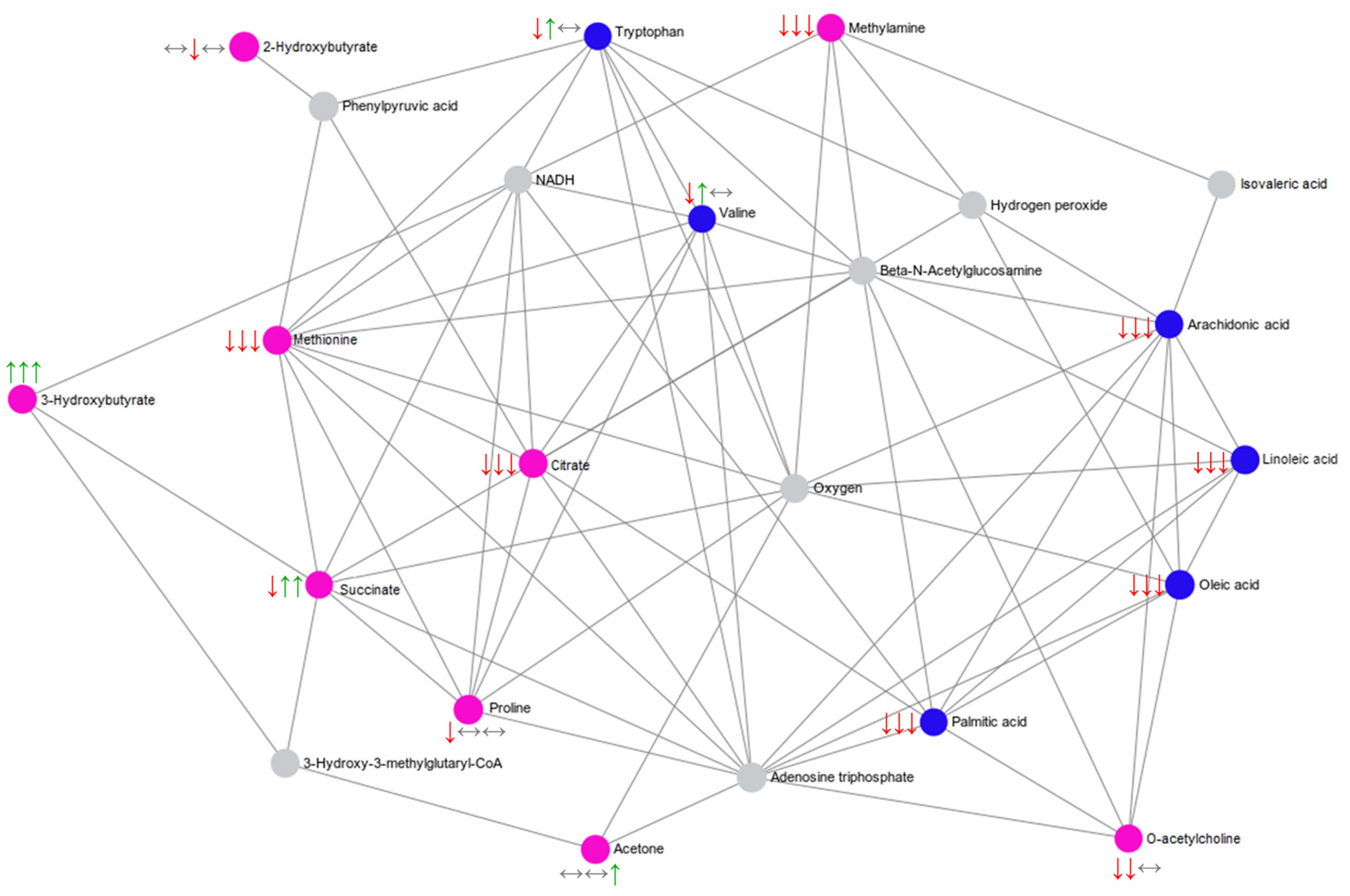
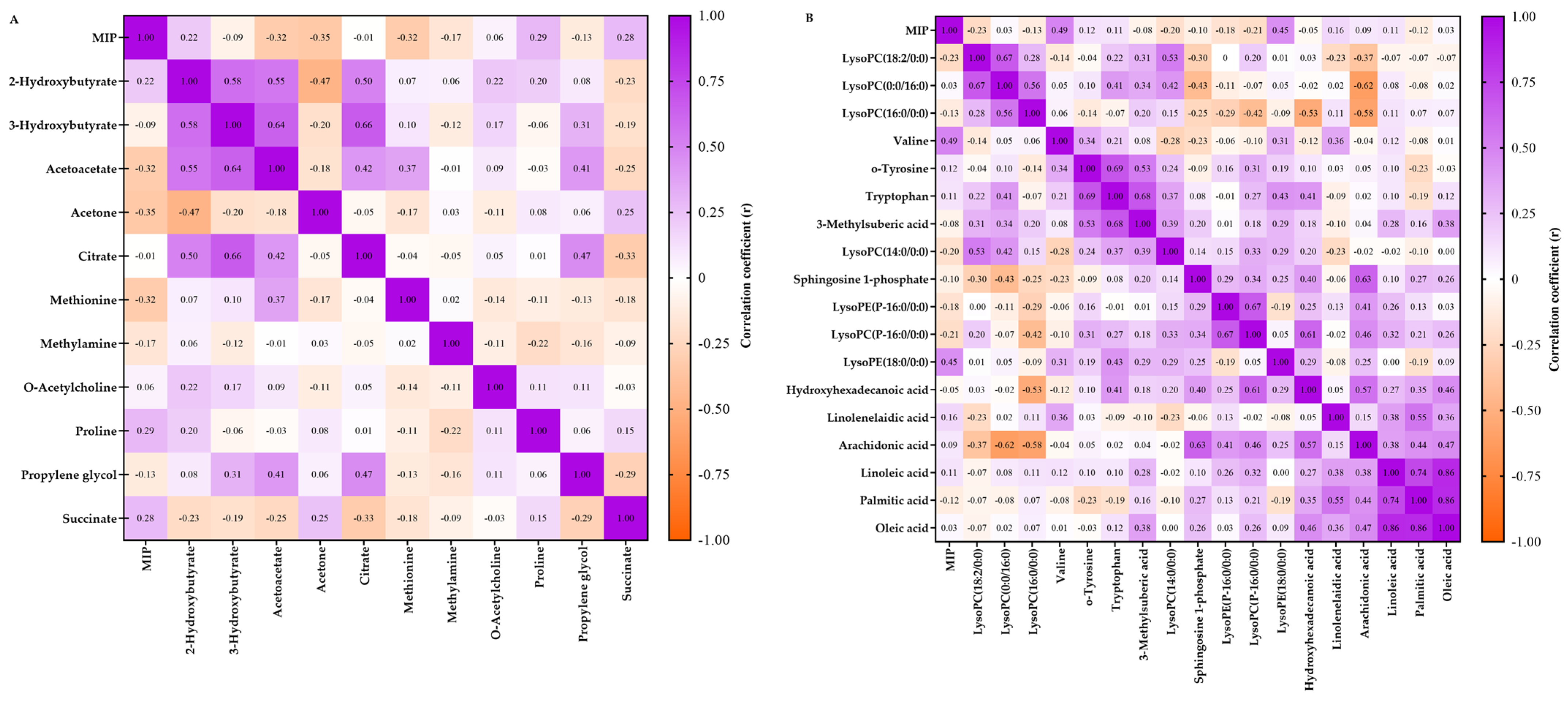
2.2. Metabolomics Data
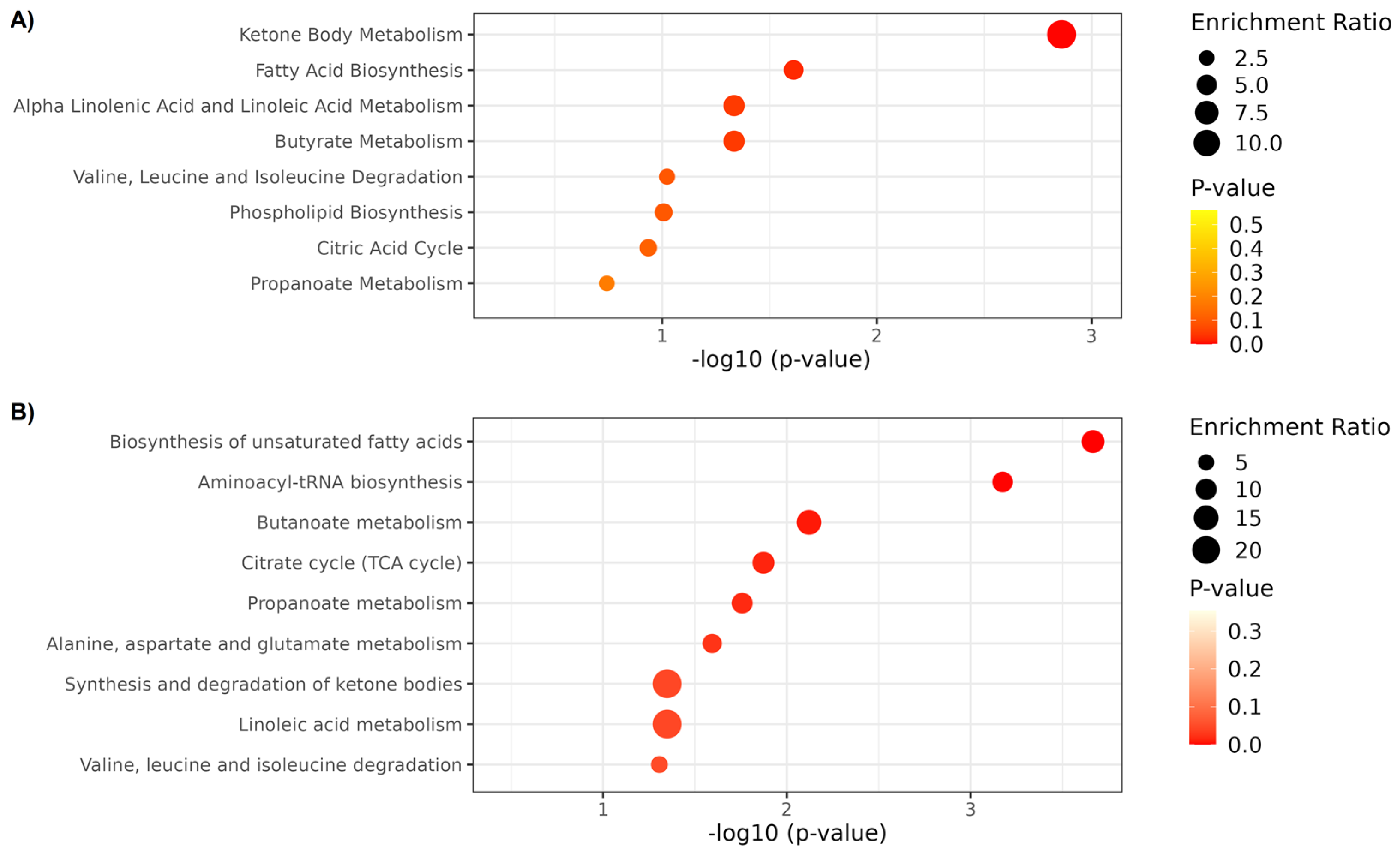
2.3. Metabolite Set Enrichment Analysis
2.4. Summary of the Main Findings
3. Discussion
4. Materials and Methods
4.1. Participants
4.2. Experimental Design
4.3. Blood Sample Collection
4.4. Respiratory Muscle Strength
4.5. Aerobic Power (O2peak)
4.6. Inspiratory Muscle Training
4.7. 1H NMR-Based Metabolomics
4.8. UHPLC-HRMS-Based Metabolomics
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Janssens, L.; Brumagne, S.; McConnell, A.K.; Raymaekers, J.; Goossens, N.; Gayan-Ramirez, G.; Hermans, G.; Troosters, T. The Assessment of Inspiratory Muscle Fatigue in Healthy Individuals: A Systematic Review. Respir. Med. 2013, 107, 331–346. [Google Scholar] [CrossRef]
- Forster, H.V.; Haouzi, P.; Dempsey, J.A. Control of Breathing during Exercise. Compr. Physiol. 2012, 2, 743–777. [Google Scholar] [CrossRef]
- Fernández-Lázaro, D.; Corchete, L.A.; García, J.F.; Jerves Donoso, D.; Lantarón-Caeiro, E.; Cobreros Mielgo, R.; Mielgo-Ayuso, J.; Gallego-Gallego, D.; Seco-Calvo, J. Effects on Respiratory Pressures, Spirometry Biomarkers, and Sports Performance after Inspiratory Muscle Training in a Physically Active Population by Powerbreath®: A Systematic Review and Meta-Analysis. Biology 2022, 12, 56. [Google Scholar] [CrossRef]
- Fernández-Lázaro, D.; Gallego-Gallego, D.; Corchete, L.A.; Fernández Zoppino, D.; González-Bernal, J.J.; García Gómez, B.; Mielgo-Ayuso, J. Inspiratory Muscle Training Program Using the PowerBreath®: Does It Have Ergogenic Potential for Respiratory and/or Athletic Performance? A Systematic Review with Meta-Analysis. Int. J. Environ. Res. Public Health 2021, 18, 6703. [Google Scholar] [CrossRef]
- Shei, R.-J. Recent Advancements in Our Understanding of the Ergogenic Effect of Respiratory Muscle Training in Healthy Humans: A Systematic Review. J. Strength Cond. Res. 2018, 32, 2665–2676. [Google Scholar] [CrossRef]
- McConnell, A.K. Respiratory Muscle Training as an Ergogenic Aid. J. Exerc. Sci. Fit. 2009, 7, S18–S27. [Google Scholar] [CrossRef]
- Azambuja, A.D.C.M.; De Oliveira, L.Z.; Sbruzzi, G. Inspiratory Muscle Training in Patients with Heart Failure: What Is New? Systematic Review and Meta-Analysis. Phys. Ther. 2020, 100, 2099–2109. [Google Scholar] [CrossRef] [PubMed]
- Beaumont, M.; Forget, P.; Couturaud, F.; Reychler, G. Effects of Inspiratory Muscle Training in COPD Patients: A Systematic Review and Meta-Analysis. Clin. Respir. J. 2018, 12, 2178–2188. [Google Scholar] [CrossRef] [PubMed]
- Manifield, J.; Winnard, A.; Hume, E.; Armstrong, M.; Baker, K.; Adams, N.; Vogiatzis, I.; Barry, G. Inspiratory Muscle Training for Improving Inspiratory Muscle Strength and Functional Capacity in Older Adults: A Systematic Review and Meta-Analysis. Age Ageing 2021, 50, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Karsten, M.; Ribeiro, G.S.; Esquivel, M.S.; Matte, D.L. The Effects of Inspiratory Muscle Training with Linear Workload Devices on the Sports Performance and Cardiopulmonary Function of Athletes: A Systematic Review and Meta-Analysis. Phys. Ther. Sport 2018, 34, 92–104. [Google Scholar] [CrossRef] [PubMed]
- Lorca-Santiago, J.; Jiménez, S.L.; Pareja-Galeano, H.; Lorenzo, A. Inspiratory Muscle Training in Intermittent Sports Modalities: A Systematic Review. Int. J. Environ. Res. Public Health 2020, 17, 4448. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, J.A.; Romer, L.; Rodman, J.; Miller, J.; Smith, C. Consequences of Exercise-Induced Respiratory Muscle Work. Respir. Physiol. Neurobiol. 2006, 151, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Illi, S.K.; Held, U.; Frank, I.; Spengler, C.M. Effect of Respiratory Muscle Training on Exercise Performance in Healthy Individuals. Sport. Med. 2012, 42, 707–724. [Google Scholar] [CrossRef] [PubMed]
- Martins de Abreu, R.; Porta, A.; Rehder-Santos, P.; Cairo, B.; Donisete da Silva, C.; De Favari Signini, É.; Sakaguchi, C.A.; Catai, A.M. Effects of Inspiratory Muscle-Training Intensity on Cardiovascular Control in Amateur Cyclists. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2019, 317, R891–R902. [Google Scholar] [CrossRef]
- Sheel, A.W. Respiratory Muscle Training in Healthy Individuals: Physiological Rationale and Implications for Exercise Performance. Sports Med. 2002, 32, 567–581. [Google Scholar] [CrossRef] [PubMed]
- HajGhanbari, B.; Yamabayashi, C.; Buna, T.R.; Coelho, J.D.; Freedman, K.D.; Morton, T.A.; Palmer, S.A.; Toy, M.A.; Walsh, C.; Sheel, A.W.; et al. Effects of Respiratory Muscle Training on Performance in Athletes: A Systematic Review with Meta-Analyses. J. Strength Cond. Res. 2013, 27, 1643–1663. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Chang, H.-Y.; Ho, C.-C.; Lee, P.-F.; Chou, Y.-C.; Tsai, M.-W.; Chou, L.-W. Effects of 4-Week Inspiratory Muscle Training on Sport Performance in College 800-Meter Track Runners. Medicina 2021, 57, 72. [Google Scholar] [CrossRef]
- Rehder-Santos, P.; Abreu, R.M.; Signini, É.D.F.; da Silva, C.D.; Sakaguchi, C.A.; Dato, C.C.; Catai, A.M. Moderate- and High-Intensity Inspiratory Muscle Training Equally Improves Inspiratory Muscle Strength and Endurance-A Double-Blind Randomized Controlled Trial. Int. J. Sports Physiol. Perform. 2021, 16, 1111–1119. [Google Scholar] [CrossRef]
- Craighead, D.H.; Heinbockel, T.C.; Freeberg, K.A.; Rossman, M.J.; Jackman, R.A.; Jankowski, L.R.; Hamilton, M.N.; Ziemba, B.P.; Reisz, J.A.; D’Alessandro, A.; et al. Time-Efficient Inspiratory Muscle Strength Training Lowers Blood Pressure and Improves Endothelial Function, NO Bioavailability, and Oxidative Stress in Midlife/Older Adults With Above-Normal Blood Pressure. J. Am. Heart Assoc. 2021, 10, e020980. [Google Scholar] [CrossRef]
- Sakaguchi, C.A.; Nieman, D.C.; Signini, E.F.; de Abreu, R.M.; Silva, C.D.; Rehder-Santos, P.; Carosio, M.G.A.; Maria, R.M.; Dato, C.C.; de Araújo, H.S.S.; et al. Chronic Influence of Inspiratory Muscle Training at Different Intensities on the Serum Metabolome. Metabolites 2020, 10, 78. [Google Scholar] [CrossRef]
- Khoramipour, K.; Sandbakk, Ø.; Hassanzadeh, A.; Abbas, K.; Gaeini, A. Metabolomics in Exercise and Sports: A Systematic Review. Sports Med. 2022, 52, 547–583. [Google Scholar] [CrossRef] [PubMed]
- Jaguri, A.; Al Thani, A.A.; Elrayess, M.A. Exercise Metabolome: Insights for Health and Performance. Metabolites 2023, 13, 694. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.M.; Carter, H. The Effect of Endurance Training on Parameters of Aerobic Fitness. Sport. Med. 2000, 29, 373–386. [Google Scholar] [CrossRef] [PubMed]
- Kelly, R.S.; Kelly, M.P.; Kelly, P. Metabolomics, Physical Activity, Exercise and Health: A Review of the Current Evidence. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165936. [Google Scholar] [CrossRef] [PubMed]
- Duft, R.G.; Chacon-mikahil, M.P.T. Metabolomics and Exercise: Possibilities and Perspectives. Motriz Rev. Educ. Fis. 2017, 23, 1–13. [Google Scholar] [CrossRef]
- Wood, P.; Schlierf, G.; Kinsell, L. Plasma Free Oleic and Palmitic Acid Levels during Vigorous Exercise. Metabolism 1965, 14, 1095–1100. [Google Scholar] [CrossRef]
- Vadász, I.; Morty, R.E.; Kohstall, M.G.; Olschewski, A.; Grimminger, F.; Seeger, W.; Ghofrani, H.A. Oleic Acid Inhibits Alveolar Fluid Reabsorption: A Role in Acute Respiratory Distress Syndrome? Am. J. Respir. Crit. Care Med. 2005, 171, 469–479. [Google Scholar] [CrossRef]
- Magder, S.; Erian, R.; Roussos, C. Respiratory Muscle Blood Flow in Oleic Acid-Induced Pulmonary Edema. J. Appl. Physiol. 1986, 60, 1849–1856. [Google Scholar] [CrossRef]
- Nethery, D.; Callahan, L.A.; Stofan, D.; Mattera, R.; DiMarco, A.; Supinski, G. PLA(2) Dependence of Diaphragm Mitochondrial Formation of Reactive Oxygen Species. J. Appl. Physiol. 2000, 89, 72–80. [Google Scholar] [CrossRef]
- Das, U.N. Ageing: Is There a Role for Arachidonic Acid and Other Bioactive Lipids? A Review. J. Adv. Res. 2018, 11, 67–79. [Google Scholar] [CrossRef]
- Shen, C.-L.; Watkins, B.A.; Kahathuduwa, C.; Chyu, M.-C.; Zabet-Moghaddam, M.; Elmassry, M.M.; Luk, H.-Y.; Brismée, J.-M.; Knox, A.; Lee, J.; et al. Tai Chi Improves Brain Functional Connectivity and Plasma Lysophosphatidylcholines in Postmenopausal Women With Knee Osteoarthritis: An Exploratory Pilot Study. Front. Med. 2021, 8, 775344. [Google Scholar] [CrossRef] [PubMed]
- Knuplez, E.; Marsche, G. An Updated Review of Pro- and Anti-Inflammatory Properties of Plasma Lysophosphatidylcholines in the Vascular System. Int. J. Mol. Sci. 2020, 21, 4501. [Google Scholar] [CrossRef] [PubMed]
- Law, S.-H.; Chan, M.-L.; Marathe, G.K.; Parveen, F.; Chen, C.-H.; Ke, L.-Y. An Updated Review of Lysophosphatidylcholine Metabolism in Human Diseases. Int. J. Mol. Sci. 2019, 20, 1149. [Google Scholar] [CrossRef] [PubMed]
- Pararasa, C.; Ikwuobe, J.; Shigdar, S.; Boukouvalas, A.; Nabney, I.T.; Brown, J.E.; Devitt, A.; Bailey, C.J.; Bennett, S.J.; Griffiths, H.R. Age-Associated Changes in Long-Chain Fatty Acid Profile during Healthy Aging Promote pro-Inflammatory Monocyte Polarization via PPARγ. Aging Cell 2016, 15, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.U.; Yoon, K.J.; Park, S.; Lee, J.C.; Moon, H.Y.; Moon, M.H. Exercise-Induced Recovery of Plasma Lipids Perturbed by Ageing with Nanoflow UHPLC-ESI-MS/MS. Anal. Bioanal. Chem. 2020, 412, 8003–8014. [Google Scholar] [CrossRef]
- Hodun, K.; Chabowski, A.; Baranowski, M. Sphingosine-1-Phosphate in Acute Exercise and Training. Scand. J. Med. Sci. Sports 2021, 31, 945–955. [Google Scholar] [CrossRef]
- Ebenezer, D.L.; Fu, P.; Natarajan, V. Targeting Sphingosine-1-Phosphate Signaling in Lung Diseases. Pharmacol. Ther. 2016, 168, 143–157. [Google Scholar] [CrossRef]
- Milara, J.; Navarro, R.; Juan, G.; Peiró, T.; Serrano, A.; Ramón, M.; Morcillo, E.; Cortijo, J. Sphingosine-1-Phosphate Is Increased in Patients with Idiopathic Pulmonary Fibrosis and Mediates Epithelial to Mesenchymal Transition. Thorax 2012, 67, 147–156. [Google Scholar] [CrossRef]
- Yang, Y.; Uhlig, S. The Role of Sphingolipids in Respiratory Disease. Ther. Adv. Respir. Dis. 2011, 5, 325–344. [Google Scholar] [CrossRef]
- Evans, M.; Cogan, K.E.; Egan, B. Metabolism of Ketone Bodies during Exercise and Training: Physiological Basis for Exogenous Supplementation. J. Physiol. 2017, 595, 2857–2871. [Google Scholar] [CrossRef]
- Brunengraber, H.; Boutry, M.; Lowenstein, J.M. Fatty Acid and 3-β-Hydroxysterol Synthesis in the Perfused Rat Liver: Including Measurements on the Production of Lactate, Pyruvate, β-Hydroxybutyrate, and Acetoacetate by the Fed Liver. J. Biol. Chem. 1973, 248, 2656–2669. [Google Scholar] [CrossRef] [PubMed]
- Sansone, M.; Sansone, A.; Borrione, P.; Romanelli, F.; Di Luigi, L.; Sgrò, P. Effects of Ketone Bodies on Endurance Exercise. Curr. Sports Med. Rep. 2018, 17, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Sousa, A.P.; Cunha, D.M.; Franco, C.; Teixeira, C.; Gojon, F.; Baylina, P.; Fernandes, R. Which Role Plays 2-Hydroxybutyric Acid on Insulin Resistance? Metabolites 2021, 11, 835. [Google Scholar] [CrossRef]
- Castro, A.; Duft, R.G.; Ferreira, M.V.; Silva, L.D.M.; De Andrade, L.; Ga, A.F.; De Oliveira-Nunes, S.G.; Cavaglieri, C.; Sujoy, G.; Bouchard, C.; et al. Association of Skeletal Muscle and Serum Metabolites with Maximum Power Output Gains in Response to Continuous Endurance or High-Intensity Interval Training Programs: The TIMES Study—A Randomized Controlled Trial. PLoS ONE 2019, 14, e0212115. [Google Scholar] [CrossRef]
- Castro, A.; Duft, R.G.; de Oliveira-Nunes, S.G.; de Andrade, A.L.L.; Cavaglieri, C.R.; Chacon-Mikahil, M.P.T. Association Between Changes in Serum and Skeletal Muscle Metabolomics Profile With Maximum Power Output Gains in Response to Different Aerobic Training Programs: The Times Study. Front. Physiol. 2021, 12, 756618. [Google Scholar] [CrossRef] [PubMed]
- Harper, A.E.; Miller, R.H.; Block, K.P. Branched-Chain Amino Acid Metabolism. Annu. Rev. Nutr. 1984, 4, 409–454. [Google Scholar] [CrossRef]
- Canfield, C.-A.; Bradshaw, P.C. Amino Acids in the Regulation of Aging and Aging-Related Diseases. Transl. Med. Aging 2019, 3, 70–89. [Google Scholar] [CrossRef]
- Castro, A.; Signini, É.F.; De Oliveira, J.M.; Di Medeiros Leal, M.C.B.; Rehder-Santos, P.; Millan-Mattos, J.C.; Minatel, V.; Pantoni, C.B.F.; Oliveira, R.V.; Catai, A.M.; et al. The Aging Process: A Metabolomics Perspective. Molecules 2022, 27, 8656. [Google Scholar] [CrossRef]
- Ninomiya, S.; Nakamura, N.; Nakamura, H.; Mizutani, T.; Kaneda, Y.; Yamaguchi, K.; Matsumoto, T.; Kitagawa, J.; Kanemura, N.; Shiraki, M.; et al. Low Levels of Serum Tryptophan Underlie Skeletal Muscle Atrophy. Nutrients 2020, 12, 978. [Google Scholar] [CrossRef]
- Overmyer, K.A.; Evans, C.R.; Qi, N.R.; Minogue, C.E.; Carson, J.J.; Chermside-Scabbo, C.J.; Koch, L.G.; Britton, S.L.; Pagliarini, D.J.; Coon, J.J.; et al. Maximal Oxidative Capacity during Exercise Is Associated with Skeletal Muscle Fuel Selection and Dynamic Changes in Mitochondrial Protein Acetylation. Cell Metab. 2015, 21, 468–478. [Google Scholar] [CrossRef]
- Wishart, D.S.; Guo, A.; Oler, E.; Wang, F.; Anjum, A.; Peters, H.; Dizon, R.; Sayeeda, Z.; Tian, S.; Lee, B.L.; et al. HMDB 5.0: The Human Metabolome Database for 2022. Nucleic Acids Res. 2022, 50, D622–D631. [Google Scholar] [CrossRef]
- Stryeck, S.; Gastrager, M.; Degoricija, V.; Trbušić, M.; Potočnjak, I.; Radulović, B.; Pregartner, G.; Berghold, A.; Madl, T.; Frank, S. Serum Concentrations of Citrate, Tyrosine, 2- and 3- Hydroxybutyrate Are Associated with Increased 3-Month Mortality in Acute Heart Failure Patients. Sci. Rep. 2019, 9, 6743. [Google Scholar] [CrossRef]
- Labaki, W.W.; Gu, T.; Murray, S.; Curtis, J.L.; Yeomans, L.; Bowler, R.P.; Barr, R.G.; Comellas, A.P.; Hansel, N.N.; Cooper, C.B.; et al. Serum Amino Acid Concentrations and Clinical Outcomes in Smokers: SPIROMICS Metabolomics Study. Sci. Rep. 2019, 9, 11367. [Google Scholar] [CrossRef]
- Wu, K.K. Extracellular Succinate: A Physiological Messenger and a Pathological Trigger. Int. J. Mol. Sci. 2023, 24, 11165. [Google Scholar] [CrossRef] [PubMed]
- Zafeiridis, A.; Chatziioannou, A.C.; Sarivasiliou, H.; Kyparos, A.; Nikolaidis, M.G.; Vrabas, I.S.; Pechlivanis, A.; Zoumpoulakis, P.; Baskakis, C.; Dipla, K.; et al. Global Metabolic Stress of Isoe Ff Ort Continuous and High Intensity Interval Aerobic Exercise: A Comparative 1 H NMR Metabonomic Study. J. Proteome Res. 2016, 15, 4452–4463. [Google Scholar] [CrossRef] [PubMed]
- Gibala, M.J.; MacLean, D.A.; Graham, T.E.; Saltin, B. Tricarboxylic Acid Cycle Intermediate Pool Size and Estimated Cycle Flux in Human Muscle during Exercise. Am. J. Physiol. 1998, 275, E235–E242. [Google Scholar] [CrossRef] [PubMed]
- Rehder-Santos, P.; Minatel, V.; Milan-Mattos, J.C.; Signini, É.D.F.; de Abreu, R.M.; Dato, C.C.; Catai, A.M. Critical Inspiratory Pressure—A New Methodology for Evaluating and Training the Inspiratory Musculature for Recreational Cyclists: Study Protocol for a Randomized Controlled Trial. Trials 2019, 20, 258. [Google Scholar] [CrossRef] [PubMed]
- Hautmann, H.; Hefele, S.; Schotten, K.; Huber, R.M. Maximal Inspiratory Mouth Pressures (PIMAX) in Healthy Subjects--What Is the Lower Limit of Normal? Respir. Med. 2000, 94, 689–693. [Google Scholar] [CrossRef] [PubMed]
- Schulz, K.F.; Altman, D.G.; Moher, D. CONSORT 2010 Statement: Updated Guidelines for Reporting Parallel Group Randomised Trials. J. Pharmacol. Pharmacother. 2010, 1, 100–107. [Google Scholar] [CrossRef] [PubMed]
- National Academies of Sciences, Engineering, and Medicine. Dietary Reference Intakes for Energy; National Academies Press: Washington, DC, USA, 2023; ISBN 978-0-309-69723-1. [Google Scholar]
- De Favari Signini, É.; Castro, A.; Rehder-Santos, P.; Cristina Millan-Mattos, J.; Magalhães de Oliveira, J.; Minatel, V.; Bianca Falasco Pantoni, C.; Sobreiro Selistre de Araújo, H.; Fabrizzi, F.; Porta, A.; et al. Integrative Perspective of the Healthy Aging Process Considering the Metabolome, Cardiac Autonomic Modulation and Cardiorespiratory Fitness Evaluated in Age Groups. Sci. Rep. 2022, 12, 21314. [Google Scholar] [CrossRef]
- Souza, R. Pressões Respiratórias Estáticas Máximas. J. Pneumol. 2002, 28, S155–S165. [Google Scholar]
- Neder, J.A.; Andreoni, S.; Lerario, M.C.; Nery, L.E. Reference Values for Lung Function Tests. II. Maximal Respiratory Pressures and Voluntary Ventilation. Braz. J. Med. Biol. Res. 1999, 32, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Wasserman, K. Principles of Exercise Testing and Interpretation: Including Pathophysiology and Clinical Applications; Wolters Kluwer Health/Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2012; ISBN 9781609138998. [Google Scholar]
- Balady, G.J.; Arena, R.; Sietsema, K.; Myers, J.; Coke, L.; Fletcher, G.F.; Forman, D.; Franklin, B.; Guazzi, M.; Gulati, M.; et al. Clinician’s Guide to Cardiopulmonary Exercise Testing in Adults: A Scientific Statement from the American Heart Association. Circulation 2010, 122, 191–225. [Google Scholar] [CrossRef] [PubMed]
- Florindo, A.A.; Latorre, M.d.R.D.d.O.; Jaime, P.C.; Tanaka, T.; de Freitas Zerbini, C.A. Methodology to evaluation the habitual physical activity in men aged 50 years or more. Rev. Saude Publica 2004, 38, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Wang, Y.; Zhang, Y.; Li, F.; Xia, W.; Zhou, Y.; Qiu, Y.; Li, H.; Zhu, F. NOREVA: Enhanced Normalization and Evaluation of Time-Course and Multi-Class Metabolomic Data. Nucleic Acids Res. 2020, 48, W436–W448. [Google Scholar] [CrossRef]
- Box, G.E.P.; Cox, D.R. An Analysis of Transformations. J. R. Stat. Soc. Ser. B 1964, 26, 211–252. [Google Scholar] [CrossRef]
- Benjamini, Y.; Ochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Xia, J.; Wishart, D.S. MSEA: A Web-Based Tool to Identify Biologically Meaningful Patterns in Quantitative Metabolomic Data. Nucleic Acids Res. 2010, 38, W71–W77. [Google Scholar] [CrossRef]


| Variable | Sham (n = 7) | MI (n = 11) | HI (n = 10) | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) | 28.3 | ± | 5.3 | 32.8 | ± | 6.4 | 30.4 | ± | 7.4 | 0.384 |
| Height (m) | 1.79 | ± | 0.04 | 1.77 | ± | 0.06 | 1.76 | ± | 0.06 | 0.481 |
| Body mass (kg) | 76.9 | ± | 10.4 | 75.4 | ± | 8.3 | 78.6 | ± | 10.9 | 0.781 |
| BMI (kg·m−2) | 24.0 | ± | 3.8 | 24.1 | ± | 1.9 | 25.3 | ± | 3.7 | 0.630 |
| Body fat (%) | 21.9 | ± | 6.0 | 20.8 | ± | 3.3 | 22.6 | ± | 4.6 | 0.680 |
| MIP (cm·H2O) | 149.9 | ± | 14.1 | 159.4 | ± | 24.8 | 146.8 | ± | 12.8 | 0.307 |
| O2 peak (ml·kg−1·min−1) | 41.4 | ± | 3.4 | 50.7 | ± | 9.4 | 50.2 | ± | 13.1 | 0.140 |
| Compound | LMM Effects | Metabolomic Platform | Group | Enriched Pathways | ||
|---|---|---|---|---|---|---|
| Sham | MI | HI | ||||
| 2-Hydroxybutyrate | Interaction | 1H NMR | ↔ | ↓ | ↔ | Propanoate metabolism |
| 3-Hydroxybutyrate | Interaction | 1H NMR | ↑ | ↑ | ↑ | Fatty acid biosynthesis |
| Acetoacetate | Interaction | 1H NMR | ↔ | ↓ | ↓ | Ketone body metabolism; Butanoate metabolism; Butyrate metabolism; Fatty acid biosynthesis. |
| Acetone | Interaction | 1H NMR | ↔ | ↔ | ↑ | Ketone body metabolism |
| Citrate | Interaction | 1H NMR | ↓ | ↓ | ↓ | Alanine, aspartate and glutamate metabolism; Citrate cycle |
| Methionine | Interaction | 1H NMR | ↑ | ↔ | ↔ | Aminoacyl-tRNA biosynthesis |
| Methylamine | Interaction | 1H NMR | ↓ | ↓ | ↓ | |
| O-Acetylcholine | Interaction | 1H NMR | ↓ | ↓ | ↔ | |
| Proline | Interaction | 1H NMR | ↓ | ↔ | ↔ | Aminoacyl-tRNA biosynthesis |
| Propylene glycol | Interaction | 1H NMR | ↑ | ↑ | ↑ | |
| Succinate | Interaction | 1H NMR | ↓ | ↑ # | ↑ | Ketone body metabolism; Butanoate metabolism; Butyrate metabolism; Fatty acid biosynthesis; Propanoate metabolism; Citrate cycle; Alanine, aspartate and glutamate metabolism. |
| 3-Methylsuberic acid | Time | UHPL-HRMS/MS | ↑ | ↑ | ↑ | |
| Arachidonic acid | Time | UHPL-HRMS/MS | ↓ | ↓ | ↓ | Biosynthesis of unsaturated fatty acids; Alpha linolenic acid and linoleic acid metabolism |
| Hydroxyhexadecanoic acid | Time | UHPL-HRMS/MS | ↑ | ↑ | ↑ | |
| Linoleic acid | Time | UHPL-HRMS/MS | ↓ | ↓ | ↓ | Biosynthesis of unsaturated fatty acids; Alpha linolenic acid and linoleic acid metabolism |
| Linolenelaidic acid | Time | UHPL-HRMS/MS | ↓ | ↓ | ↓ | |
| LysoPC (0:0/16:0) | Interaction | UHPL-HRMS/MS | ↓ | ↔ | ↔ | |
| LysoPC (14:0/0:0) | Time | UHPL-HRMS/MS | ↑ | ↑ | ↑ | |
| LysoPC (16:0/0:0) | Time | UHPL-HRMS/MS | ↓ | ↓ | ↓ | |
| LysoPC (18:2/0:0) | Interaction | UHPL-HRMS/MS | ↔ | ↔ | ↔ | |
| LysoPE (18:0/0:0) | Time | UHPL-HRMS/MS | ↓ | ↓ | ↓ | |
| LysoPE (P-16:0/0:0) | Interaction | UHPL-HRMS/MS | ↔ | ↔ | ↓ | |
| LysoPC (P-16:0/0:0) | Group | UHPL-HRMS/MS | ↔ | ↔ | ↓ | |
| Oleic acid | Time | UHPL-HRMS/MS | ↓ | ↓ | ↓ | Biosynthesis of unsaturated fatty acids |
| o-Tyrosine | Interaction | UHPL-HRMS/MS | ↔ | ↑ | ↑ | Biosynthesis of unsaturated fatty acids |
| Palmitic acid | Time | UHPL-HRMS/MS | ↓ | ↓ | ↓ | |
| Sphingosine 1-phosphate | Interaction | UHPL-HRMS/MS | ↑ | ↓ ‡ | ↓ | |
| Tryptophan | Interaction | UHPL-HRMS/MS | ↓ | ↑ | ↔ | Aminoacyl-tRNA biosynthesis |
| Valine | Interaction | UHPL-HRMS/MS | ↓ | ↑ | ↔ | Aminoacyl-tRNA biosynthesis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro, A.; Catai, A.M.; Rehder-Santos, P.; Signini, É.F.; de Abreu, R.M.; Da Silva, C.D.; Dato, C.C.; Oliveira, R.V.; Ferreira, A.G. Insights into the Serum Metabolic Adaptations in Response to Inspiratory Muscle Training: A Metabolomic Approach Based on 1H NMR and UHPLC-HRMS/MS. Int. J. Mol. Sci. 2023, 24, 16764. https://doi.org/10.3390/ijms242316764
Castro A, Catai AM, Rehder-Santos P, Signini ÉF, de Abreu RM, Da Silva CD, Dato CC, Oliveira RV, Ferreira AG. Insights into the Serum Metabolic Adaptations in Response to Inspiratory Muscle Training: A Metabolomic Approach Based on 1H NMR and UHPLC-HRMS/MS. International Journal of Molecular Sciences. 2023; 24(23):16764. https://doi.org/10.3390/ijms242316764
Chicago/Turabian StyleCastro, Alex, Aparecida M. Catai, Patrícia Rehder-Santos, Étore F. Signini, Raphael Martins de Abreu, Claudio Donisete Da Silva, Carla Cristina Dato, Regina V. Oliveira, and Antônio G. Ferreira. 2023. "Insights into the Serum Metabolic Adaptations in Response to Inspiratory Muscle Training: A Metabolomic Approach Based on 1H NMR and UHPLC-HRMS/MS" International Journal of Molecular Sciences 24, no. 23: 16764. https://doi.org/10.3390/ijms242316764
APA StyleCastro, A., Catai, A. M., Rehder-Santos, P., Signini, É. F., de Abreu, R. M., Da Silva, C. D., Dato, C. C., Oliveira, R. V., & Ferreira, A. G. (2023). Insights into the Serum Metabolic Adaptations in Response to Inspiratory Muscle Training: A Metabolomic Approach Based on 1H NMR and UHPLC-HRMS/MS. International Journal of Molecular Sciences, 24(23), 16764. https://doi.org/10.3390/ijms242316764








