Thermodynamic Characterization of the Interaction of Biofunctionalized Gold Nanoclusters with Serum Albumin Using Two- and Three-Dimensional Methods
Abstract
:1. Introduction
2. Results
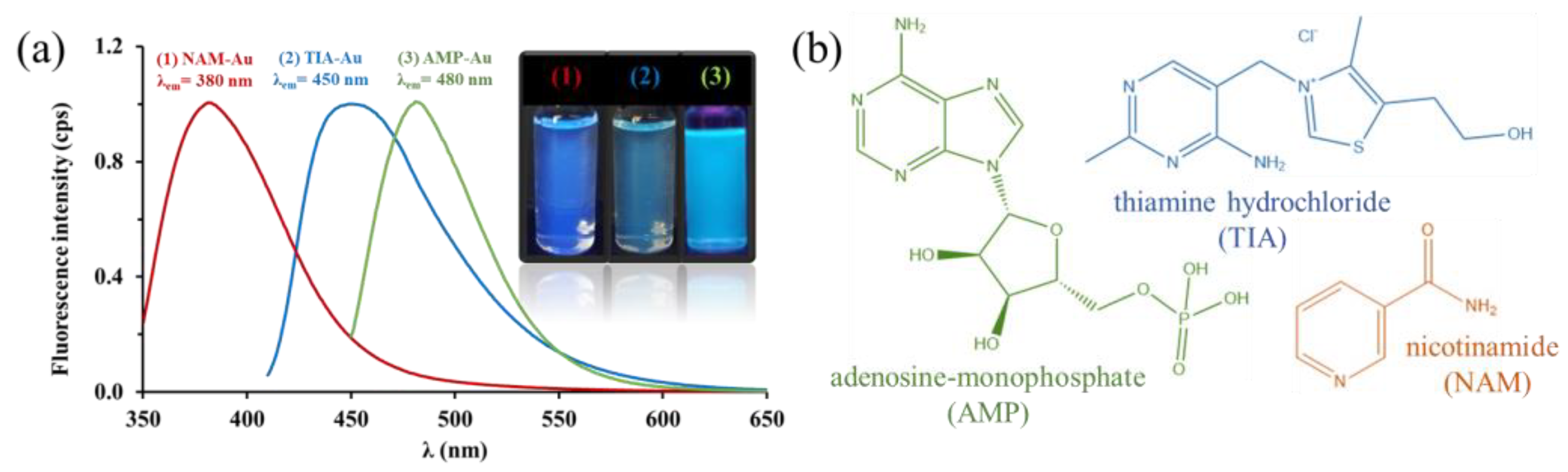
2.1. Optical and Structural Properties of the Biofunctionalized AuNCs
2.2. Surface Plasmon Resonance (SPR) Spectroscopy
2.3. Measurement of Ligand Binding Energetics using Isothermal Titration Calorimetry (ITC)
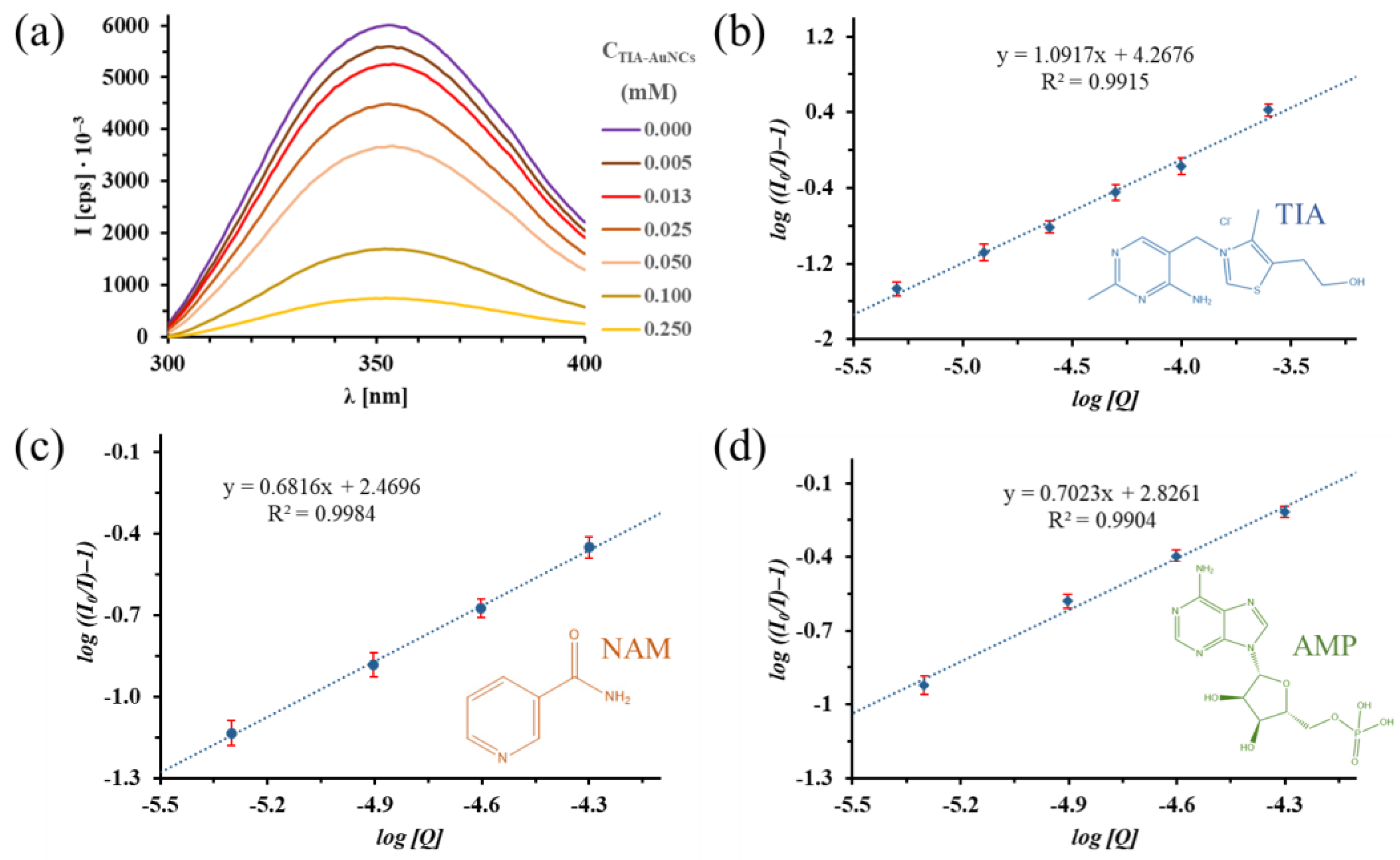
2.4. Photoluminescence (PL) Spectroscopy Investigation of Albumin Fluorescence Quenching
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Methods
4.2.1. Surface Plasmon Resonance (SPR) Spectroscopy
4.2.2. Isothermal Titration Calorimetry (ITC)
4.2.3. Photoluminescence (PL) Spectroscopy
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zheng, Y.; Lai, L.; Liu, W.; Jiang, H.; Wang, X. Recent advances in biomedical applications of fluorescent gold nanoclusters. Adv. Colloid Interface Sci. 2017, 242, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Aikens, C.M.; Hollander, F.J.; Schatz, G.C.; Jin, R. Correlating the Crystal Structure of A Thiol-Protected Au25 Cluster and Optical Properties. J. Am. Chem. Soc. 2008, 130, 5883–5885. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Qian, H.; Jin, R. Gold nanocluster-catalyzed selective oxidation of sulfide to sulfoxide. Nanoscale 2012, 4, 6714–6717. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhu, W.; Wan, A.; Liu, L. The mechanism and application of the protein-stabilized gold nanocluster sensing system. Anal. 2016, 142, 567–581. [Google Scholar] [CrossRef] [PubMed]
- Ungor, D.; Gombár, G.; Juhász, Á.; Samu, G.F.; Csapó, E. Promising Bioactivity of Vitamin B1-Au Nanocluster: Structure, Enhanced Antioxidant Behavior, and Serum Protein Interaction. Antioxidants 2023, 12, 874. [Google Scholar] [CrossRef] [PubMed]
- Gombár, G.; Ungor, D.; Samu, G.F.; Dömötör, O.; Csapó, E. Synthesis and characterization of novel blue-emitting nicotinamide-gold nanoclusters with “chain-breaker” antioxidant property. J. Mol. Liq. 2022, 359. [Google Scholar] [CrossRef]
- Csapó, E.; Ungor, D.; Kele, Z.; Baranyai, P.; Deák, A.; Juhász, Á.; Janovák, L.; Dékány, I. Influence of pH and aurate/amino acid ratios on the tuneable optical features of gold nanoparticles and nanoclusters. Colloids Surf. A Physicochem. Eng. Asp. 2017, 532, 601–608. [Google Scholar] [CrossRef]
- Gombár, G.; Simon, P.; Ungor, D.; Szatmári, I.; Csapó, E. Histidinehydroxamic acid as new biomolecule to produce molecular-like fluorescent gold nanoclusters: Possible mechanisms for metal ion sensing. J. Mol. Liq. 2023, 387. [Google Scholar] [CrossRef]
- Marjomäki, V.; Lahtinen, T.; Martikainen, M.; Koivisto, J.; Malola, S.; Salorinne, K.; Pettersson, M.; Häkkinen, H. Site-specific targeting of enterovirus capsid by functionalized monodisperse gold nanoclusters. Proc. Natl. Acad. Sci. USA 2014, 111, 1277–1281. [Google Scholar] [CrossRef]
- Chen, L.-Y.; Wang, C.-W.; Yuan, Z.; Chang, H.-T. Fluorescent Gold Nanoclusters: Recent Advances in Sensing and Imaging. Anal. Chem. 2014, 87, 216–229. [Google Scholar] [CrossRef]
- Chen, D.; Li, B.; Cai, S.; Wang, P.; Peng, S.; Sheng, Y.; He, Y.; Gu, Y.; Chen, H. Dual targeting luminescent gold nanoclusters for tumor imaging and deep tissue therapy. Biomaterials 2016, 100, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Govindaraju, S.; Ankireddy, S.R.; Viswanath, B.; Kim, J.; Yun, K. Fluorescent Gold Nanoclusters for Selective Detection of Dopamine in Cerebrospinal fluid. Sci. Rep. 2017, 7, 40298. [Google Scholar] [CrossRef]
- Kennedy, L.C.; Bickford, L.R.; Lewinski, N.A.; Coughlin, A.J.; Hu, Y.; Day, E.S.; West, J.L.; Drezek, R.A. A New Era for Cancer Treatment: Gold-Nanoparticle-Mediated Thermal Therapies. Small 2010, 7, 169–183. [Google Scholar] [CrossRef]
- Geng, T.; Zhao, L.; Wu, D.; Zhang, H.; Zhao, X.; Jiao, M.; Zeng, L. Bovine Serum Albumin-Encapsulated Ultrasmall Gold Nanoclusters for Photodynamic Therapy of Tumors. ACS Appl. Nano Mater. 2021, 4, 13818–13825. [Google Scholar] [CrossRef]
- Gao, L.-X.; Chen, W.-Q.; Liu, Y.; Jiang, F.-L. Fluorescent Labeling of Human Serum Albumin by Thiol-Cyanimide Addition and Its Application in the Fluorescence Quenching Method for Nanoparticle–Protein Interactions. Anal. Chem. 2022, 94, 3111–3119. [Google Scholar] [CrossRef]
- Shao, Q.; Hall, C.K. Allosteric effects of gold nanoparticles on human serum albumin. Nanoscale 2016, 9, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Anand, K.; Rajamanikandan, R.; Sharma, A.S.; Ilanchelian, M.; Khan, F.I.; Tiloke, C.; Katari, N.K.; Boomi, P.; Balakumar, C.; Saravanan, M.; et al. Human serum albumin interaction, in silico and anticancer evaluation of Pine-Gold nanoparticles. Process. Biochem. 2019, 89, 98–109. [Google Scholar] [CrossRef]
- Wang, G.; Yan, C.; Gao, S.; Liu, Y. Surface chemistry of gold nanoparticles determines interactions with bovine serum albumin. Mater. Sci. Eng. C 2019, 103, 109856. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, M.; Yang, W.; Zhou, Z.; Liu, L.; Zhang, Q. Interaction of bovine serum albumin with self-assembled nanoparticles of 6-O-cholesterol modified chitosan. Colloids Surf. B: Biointerfaces 2012, 92, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H. The enhanced permeability and retention (EPR) effect in tumor vasculature: The key role of tumor-selective macromolecular drug targeting. Adv. Enzym. Regul. 2001, 41, 189–207. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Matsumura, Y. Tumoritropic and lymphotropic principles of macromolecular drugs. Crit. Rev. Ther. Drug Carr. Syst. 1989, 6, 193–210. [Google Scholar] [PubMed]
- van de Looij, S.M.; Hebels, E.R.; Viola, M.; Hembury, M.; Oliveira, S.; Vermonden, T. Gold Nanoclusters: Imaging, Therapy, and Theranostic Roles in Biomedical Applications. Bioconjugate Chem. 2021, 33, 4–23. [Google Scholar] [CrossRef] [PubMed]
- Popov, A.; Lisyte, V.; Kausaite-Minkstimiene, A.; Bernotiene, E.; Ramanaviciene, A. Experimental Evaluation of Quantum Dots and Antibodies Conjugation by Surface Plasmon Resonance Spectroscopy. Int. J. Mol. Sci. 2022, 23, 12626. [Google Scholar] [CrossRef]
- Komorek, P.; Martin, E.; Jachimska, B. Adsorption and Conformation Behavior of Lysozyme on a Gold Surface Determined by QCM-D, MP-SPR, and FTIR. Int. J. Mol. Sci. 2021, 22, 1322. [Google Scholar] [CrossRef] [PubMed]
- Ungor, D.; Szilágyi, I.; Csapó, E. Yellow-emitting Au/Ag bimetallic nanoclusters with high photostability for detection of folic acid. J. Mol. Liq. 2021, 338, 116695. [Google Scholar] [CrossRef]
- Sápi, A.; Kéri, A.; Kálomista, I.; Dobó, D.G.; Szamosvölgyi, S.; Juhász, K.L.; Kukovecz, K.; Kónya, Z.; Galbács, G. Determination of the platinum concentration of a Pt/silica nanocomposite decorated with ultra small Pt nanoparticles using single particle inductively coupled plasma mass spectrometry. J. Anal. At. Spectrom. 2017, 32, 996–1003. [Google Scholar] [CrossRef]
- Kálomista, I.; Kéri, A.; Galbács, G. On the applicability and performance of the single particle ICP-MS nano-dispersion characterization method in cases complicated by spectral interferences. J. Anal. At. Spectrom. 2016, 31, 1112–1122. [Google Scholar] [CrossRef]
- Kajner, G.; Kéri, A.; Bélteki, Á.; Valkai, S.; Dér, A.; Geretovszky, Z.; Galbács, G. Multifunctional microfluidic chips for the single particle inductively coupled plasma mass spectrometry analysis of inorganic nanoparticles. Lab Chip 2022, 22, 2766–2776. [Google Scholar] [CrossRef]
- Kéri, A.; Sápi, A.; Ungor, D.; Sebők, D.; Csapó, E.; Kónya, Z.; Galbács, G. Porosity determination of nano- and sub-micron particles by single particle inductively coupled plasma mass spectrometry. J. Anal. At. Spectrom. 2020, 35, 1139–1147. [Google Scholar] [CrossRef]
- Myszka, D.G. Kinetic analysis of macromolecular interactions using surface plasmon resonance biosensors. Curr. Opin. Biotechnol. 1997, 8, 50–57. [Google Scholar] [CrossRef]
- Nguyen, B.; Tanious, F.A.; Wilson, W.D. Biosensor-surface plasmon resonance: Quantitative analysis of small molecule–nucleic acid interactions. Methods 2007, 42, 150–161. [Google Scholar] [CrossRef]
- Juhász, Á.; Luty-Błocho, M.; Wojnicki, M.; Tóth, G.K.; Csapó, E. General method for kinetic and thermodynamic evaluation of a receptor model peptide-drug molecule interaction studied by surface plasmon resonance. Microchem. J. 2019, 147, 311–318. [Google Scholar] [CrossRef]
- Coelho, Y.L.; Aguiar, C.d.D.; de Paula, H.M.C.; Marques, I.A.; Rosa, L.N.S.; Virtuoso, L.S.; Duarte, A.; Pires, A.C.d.S.; da Silva, L.H.M. Exploring the interaction between lactoferrin and CdTe quantum dots: Energetic and molecular dynamic study. J. Mol. Liq. 2022, 356, 119005. [Google Scholar] [CrossRef]
- Jugl, A.; Pekař, M. Hyaluronan-Arginine Interactions—An Ultrasound and ITC Study. Polymers 2020, 12, 2069. [Google Scholar] [CrossRef]
- Mitra, D. Use of isothermal titration calorimetry to study various systems. Mater. Today Proc. 2020, 23, 284–300. [Google Scholar] [CrossRef]
- Herrera, I.; Winnik, M.A. Differential Binding Models for Isothermal Titration Calorimetry: Moving beyond the Wiseman Isotherm. J. Phys. Chem. B 2013, 117, 8659–8672. [Google Scholar] [CrossRef] [PubMed]
- Ross, P.D.; Subramanian, S. Thermodynamics of protein association reactions: Forces contributing to stability. Biochemistry 1981, 20, 3096–3102. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.-Y.; Song, L.-N.; Zhao, Y.; Zang, F.-L.; Zhao, Z.-H.; Chen, N.-H.; Xu, X.-J.; Wang, C.-J. Spectroscopic Study on the Interaction between Naphthalimide-Polyamine Conjugates and Bovine Serum Albumin (BSA). Molecules 2015, 20, 16491–16523. [Google Scholar] [CrossRef]
- Scatchard, G. The Attractions of Proteins for Small Molecules and Ions. Ann. N. Y. Acad. Sci. 1949, 51, 660–672. [Google Scholar] [CrossRef]
- Sandu, N.; Chilom, C.G.; David, M.; Florescu, M. Evaluation of the interaction of levothyroxine with bovine serum albumin using spectroscopic and molecular docking studies. J. Biomol. Struct. Dyn. 2020, 40, 1139–1151. [Google Scholar] [CrossRef]
- Chilom, C.G.; David, M.; Florescu, M. Monitoring biomolecular interaction between folic acid and bovine serum albumin. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 230, 118074. [Google Scholar] [CrossRef]
- Shang, L.; Brandholt, S.; Stockmar, F.; Trouillet, V.; Bruns, M.; Nienhaus, G.U. Effect of Protein Adsorption on the Fluorescence of Ultrasmall Gold Nanoclusters. Small 2011, 8, 661–665. [Google Scholar] [CrossRef]
- Shang, L.; Yang, L.; Seiter, J.; Heinle, M.; Brenner-Weiss, G.; Gerthsen, D.; Nienhaus, G.U. Nanoparticles Interacting with Proteins and Cells: A Systematic Study of Protein Surface Charge Effects. Adv. Mater. Interfaces 2013, 1, 1300079. [Google Scholar] [CrossRef]
- Chen, L.; Gharib, M.; Zeng, Y.; Roy, S.; Nandi, C.K.; Chakraborty, I. Advances in bovine serum albumin-protected gold nanoclusters: From understanding the formation mechanisms to biological applications. Mater. Today Chem. 2023, 29. [Google Scholar] [CrossRef]
- Adhav, V.A.; Saikrishnan, K. The Realm of Unconventional Noncovalent Interactions in Proteins: Their Significance in Structure and Function. ACS Omega 2023, 8, 22268–22284. [Google Scholar] [CrossRef] [PubMed]
- Dyrda-Terniuk, T.; Sugajski, M.; Pryshchepa, O.; Śliwiak, J.; Buszewska-Forajta, M.; Pomastowski, P.; Buszewski, B. The Study of Protein–Cyclitol Interactions. Int. J. Mol. Sci. 2022, 23, 2940. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Li, Y.; Xia, Y.-L.; Ai, S.-M.; Liang, J.; Sang, P.; Ji, X.-L.; Liu, S.-Q. Insights into Protein–Ligand Interactions: Mechanisms, Models, and Methods. Int. J. Mol. Sci. 2016, 17, 144. [Google Scholar] [CrossRef] [PubMed]
- Russell, B.A.; Kubiak-Ossowska, K.; Mulheran, P.A.; Birch, D.J.S.; Chen, Y. Locating the nucleation sites for protein encapsulated gold nanoclusters: A molecular dynamics and fluorescence study. Phys. Chem. Chem. Phys. 2015, 17, 21935–21941. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.-M.; Dong, P.; Chen, W.-Q.; Xu, S.-P.; Yang, L.-Y.; Jiang, F.-L.; Liu, Y. Thermodynamics and Mechanisms of the Interactions between Ultrasmall Fluorescent Gold Nanoclusters and Human Serum Albumin, γ-Globulins, and Transferrin: A Spectroscopic Approach. Langmuir 2017, 33, 5108–5116. [Google Scholar] [CrossRef] [PubMed]
- Gaudreault, J.; Forest-Nault, C.; De Crescenzo, G.; Durocher, Y.; Henry, O. On the Use of Surface Plasmon Resonance-Based Biosensors for Advanced Bioprocess Monitoring. Processes 2021, 9, 1996. [Google Scholar] [CrossRef]
- Homola, J. Surface Plasmon Resonance Sensors for Detection of Chemical and Biological Species. Chem. Rev. 2008, 108, 462–493. [Google Scholar] [CrossRef]
- Zhang, Y.; Akilesh, S.; Wilcox, D.E. Isothermal Titration Calorimetry Measurements of Ni(II) and Cu(II) Binding to His, GlyGlyHis, HisGlyHis, and Bovine Serum Albumin: A Critical Evaluation. Inorg. Chem. 2000, 39, 3057–3064. [Google Scholar] [CrossRef] [PubMed]
- Omanovic-Miklicanin, E.; Manfield, I.; Wilkins, T. Application of isothermal titration calorimetry in evaluation of protein–nanoparticle interactions. J. Therm. Anal. Calorim. 2016, 127, 605–613. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juhász, Á.; Gombár, G.; Várkonyi, E.F.; Wojnicki, M.; Ungor, D.; Csapó, E. Thermodynamic Characterization of the Interaction of Biofunctionalized Gold Nanoclusters with Serum Albumin Using Two- and Three-Dimensional Methods. Int. J. Mol. Sci. 2023, 24, 16760. https://doi.org/10.3390/ijms242316760
Juhász Á, Gombár G, Várkonyi EF, Wojnicki M, Ungor D, Csapó E. Thermodynamic Characterization of the Interaction of Biofunctionalized Gold Nanoclusters with Serum Albumin Using Two- and Three-Dimensional Methods. International Journal of Molecular Sciences. 2023; 24(23):16760. https://doi.org/10.3390/ijms242316760
Chicago/Turabian StyleJuhász, Ádám, Gyöngyi Gombár, Egon F. Várkonyi, Marek Wojnicki, Ditta Ungor, and Edit Csapó. 2023. "Thermodynamic Characterization of the Interaction of Biofunctionalized Gold Nanoclusters with Serum Albumin Using Two- and Three-Dimensional Methods" International Journal of Molecular Sciences 24, no. 23: 16760. https://doi.org/10.3390/ijms242316760
APA StyleJuhász, Á., Gombár, G., Várkonyi, E. F., Wojnicki, M., Ungor, D., & Csapó, E. (2023). Thermodynamic Characterization of the Interaction of Biofunctionalized Gold Nanoclusters with Serum Albumin Using Two- and Three-Dimensional Methods. International Journal of Molecular Sciences, 24(23), 16760. https://doi.org/10.3390/ijms242316760









