Transluminal Pillars—Their Origin and Role in the Remodelling of the Zebrafish Caudal Vein Plexus
Abstract
1. Introduction
2. Results
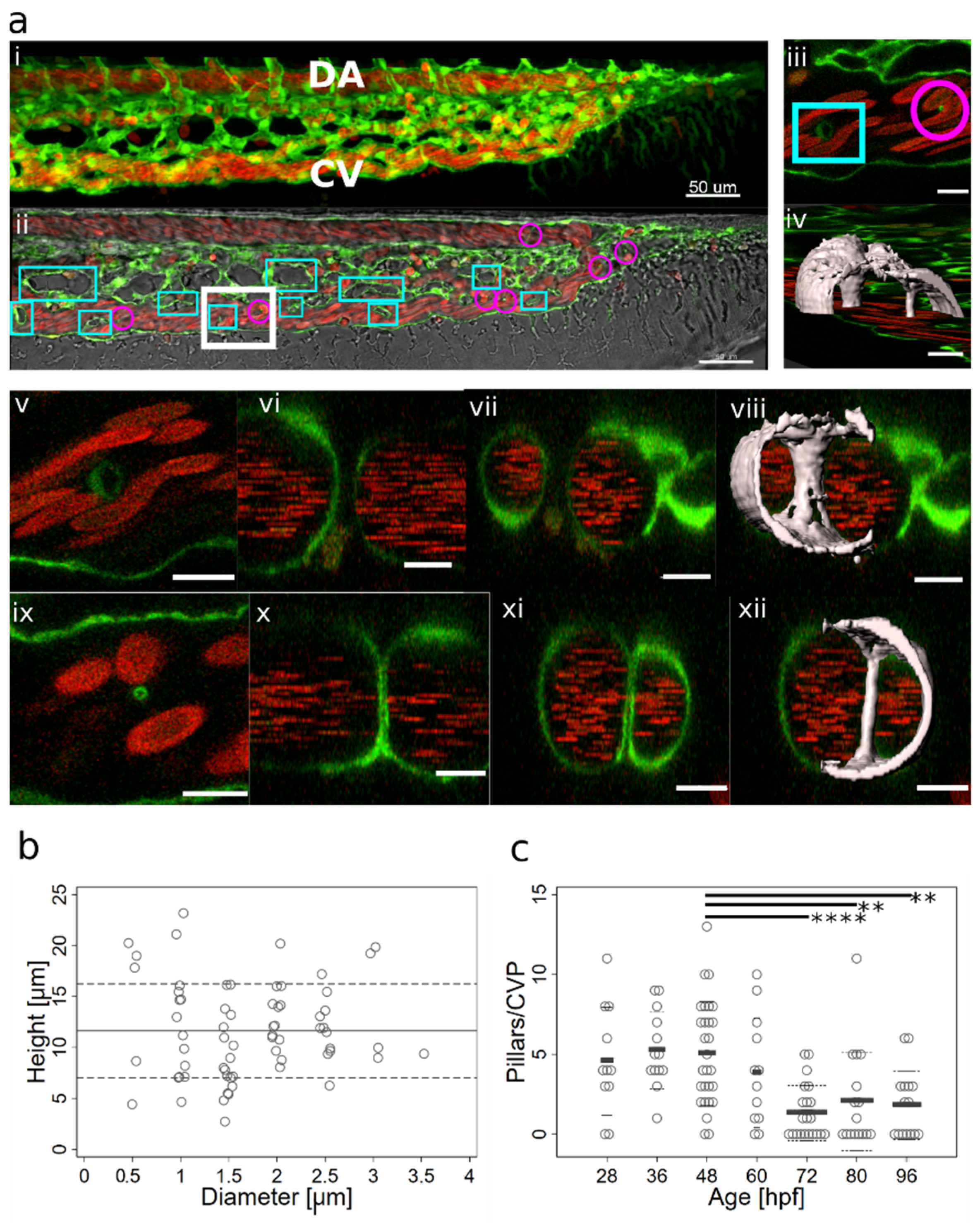
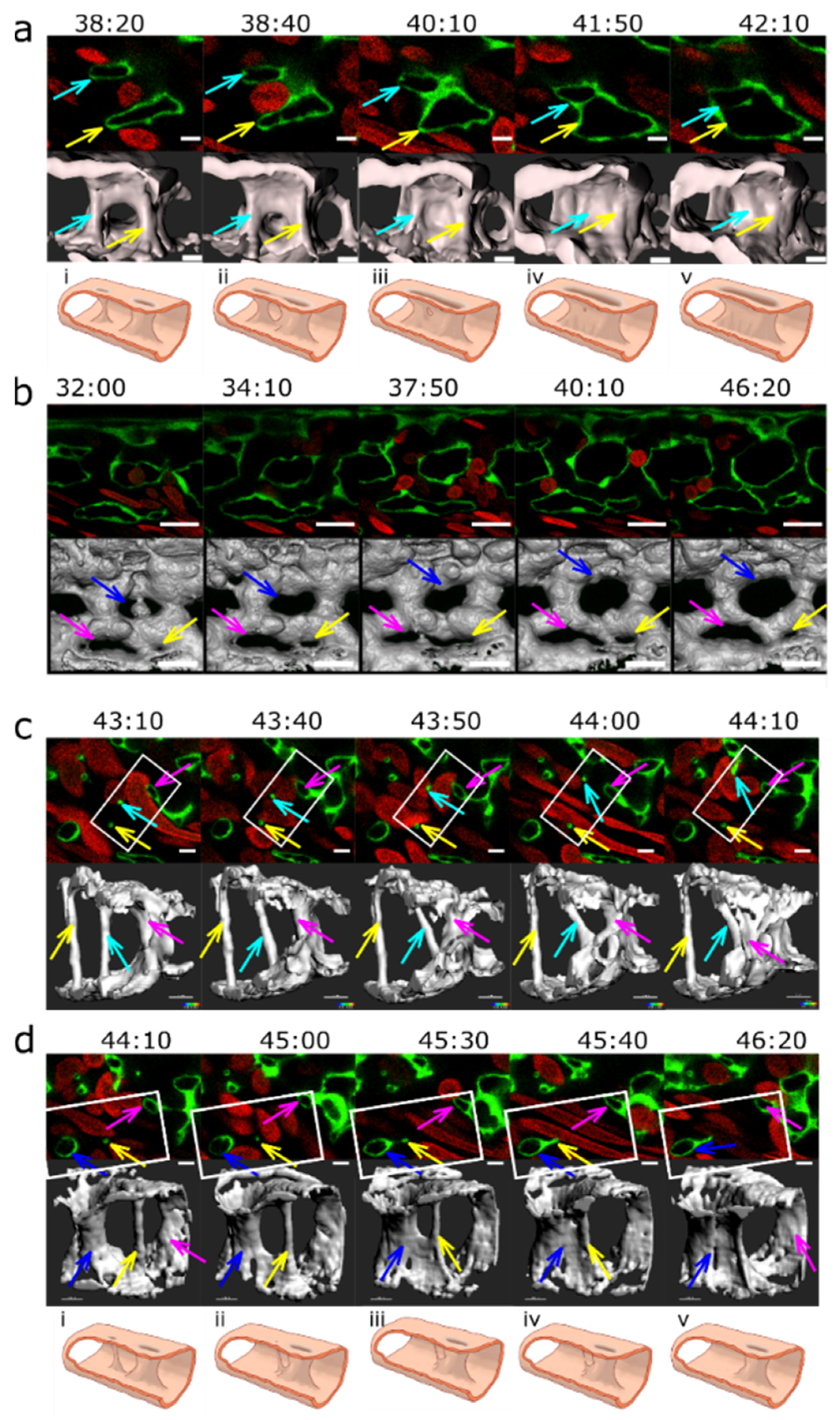
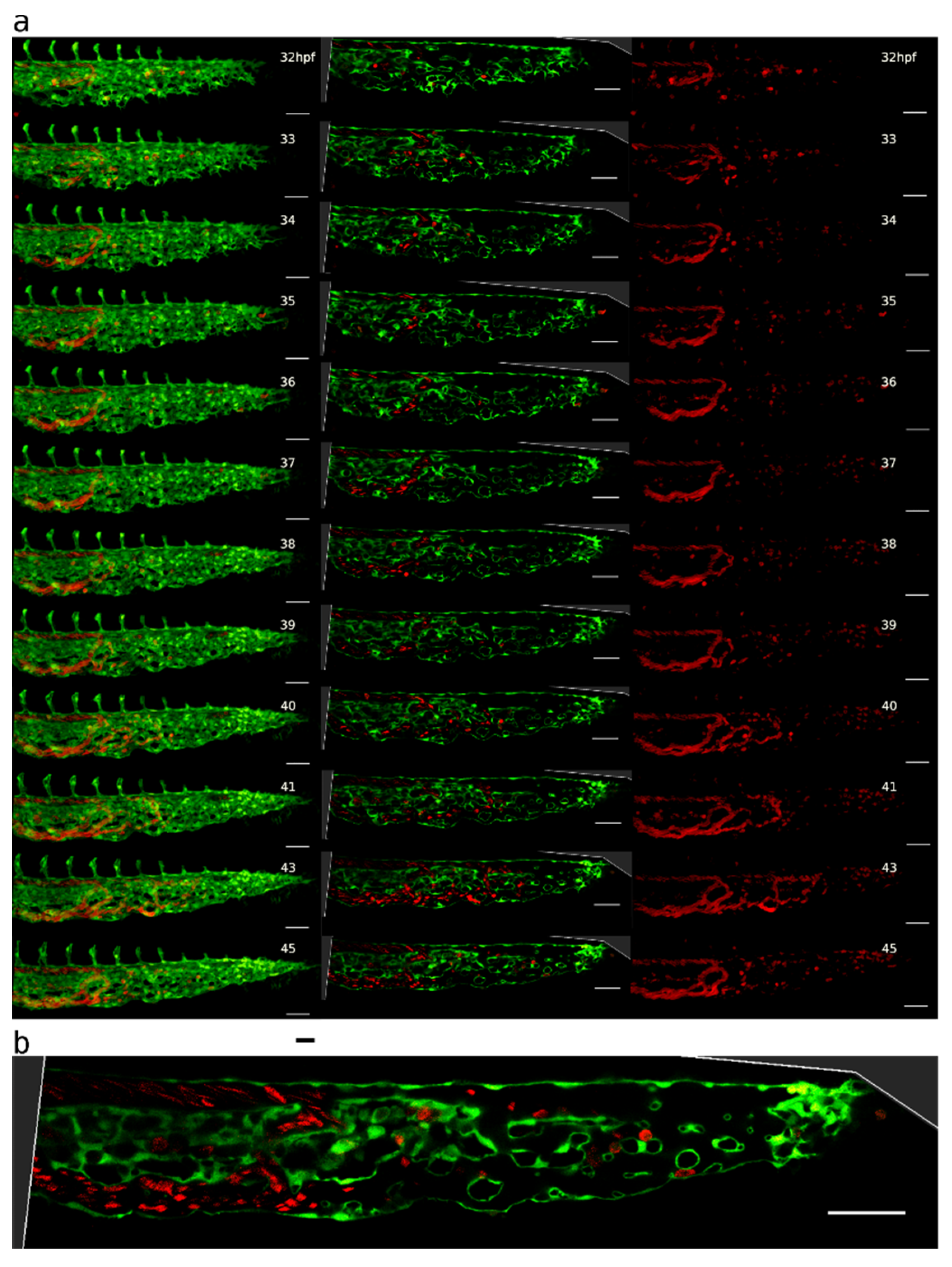
2.1. Appearance, Morphology, and Distribution of Pillars in the Developing CVP
2.2. Mechanisms of Pillar Formation
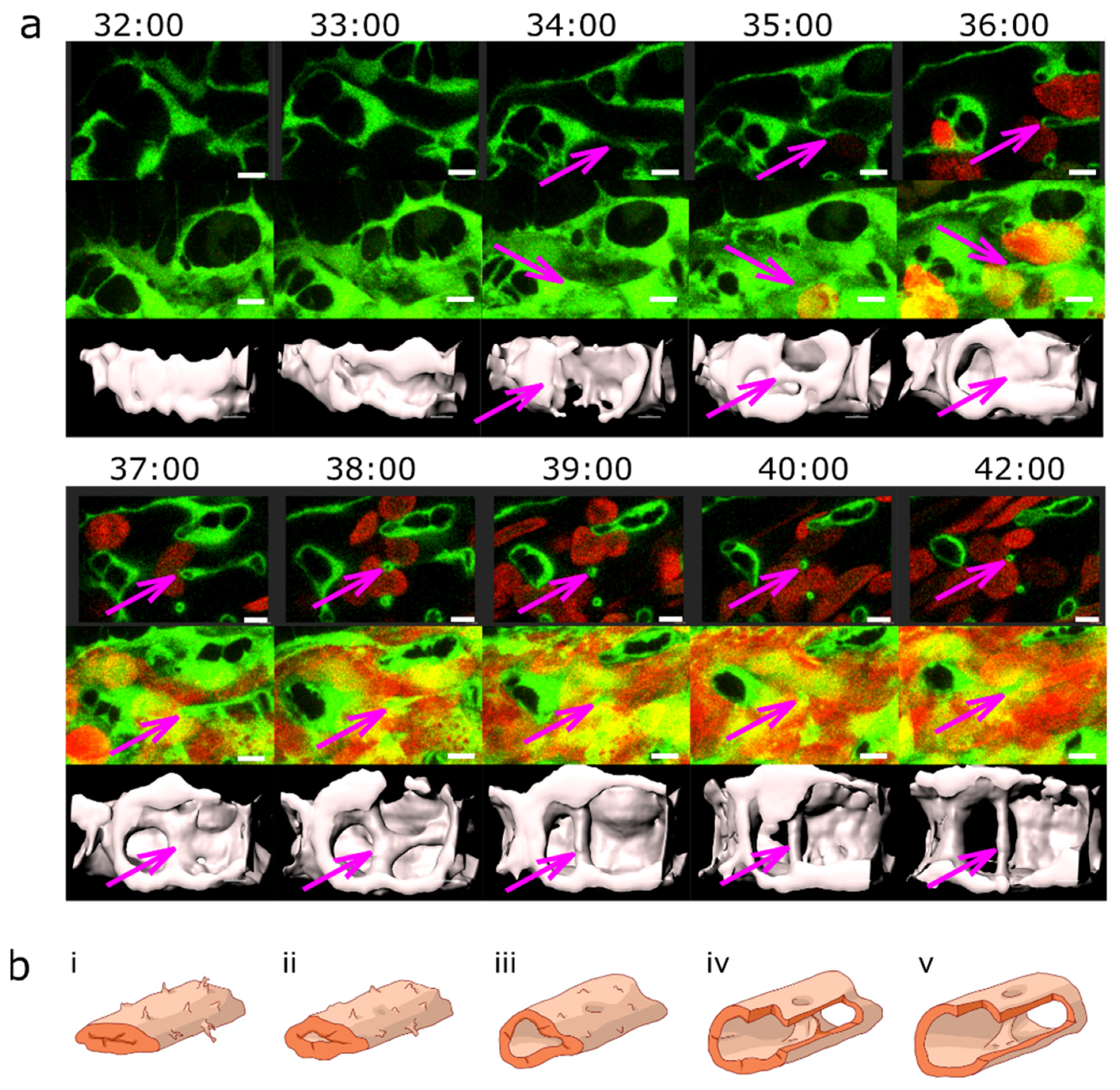

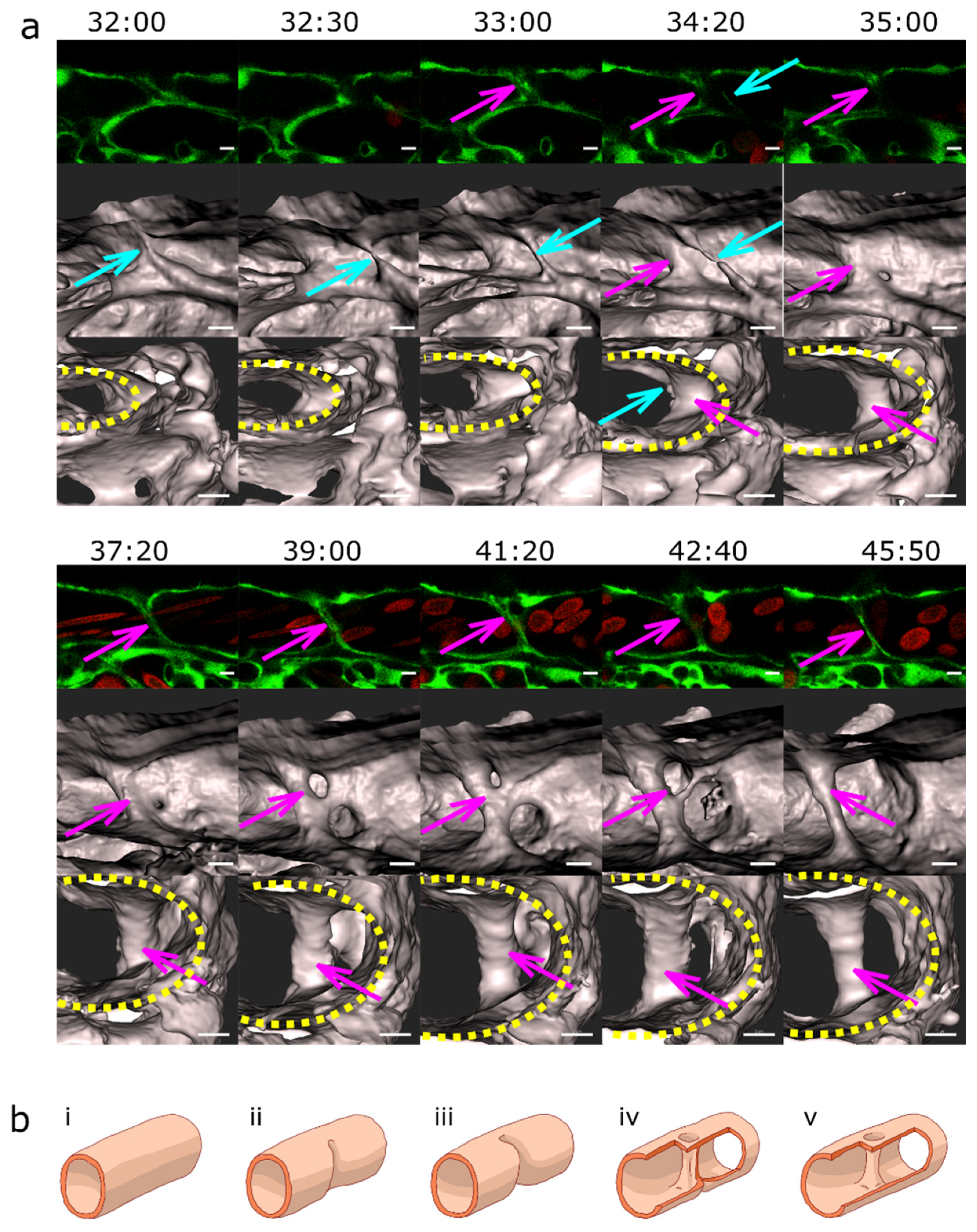
2.3. Blood Flow and Pillar Formation
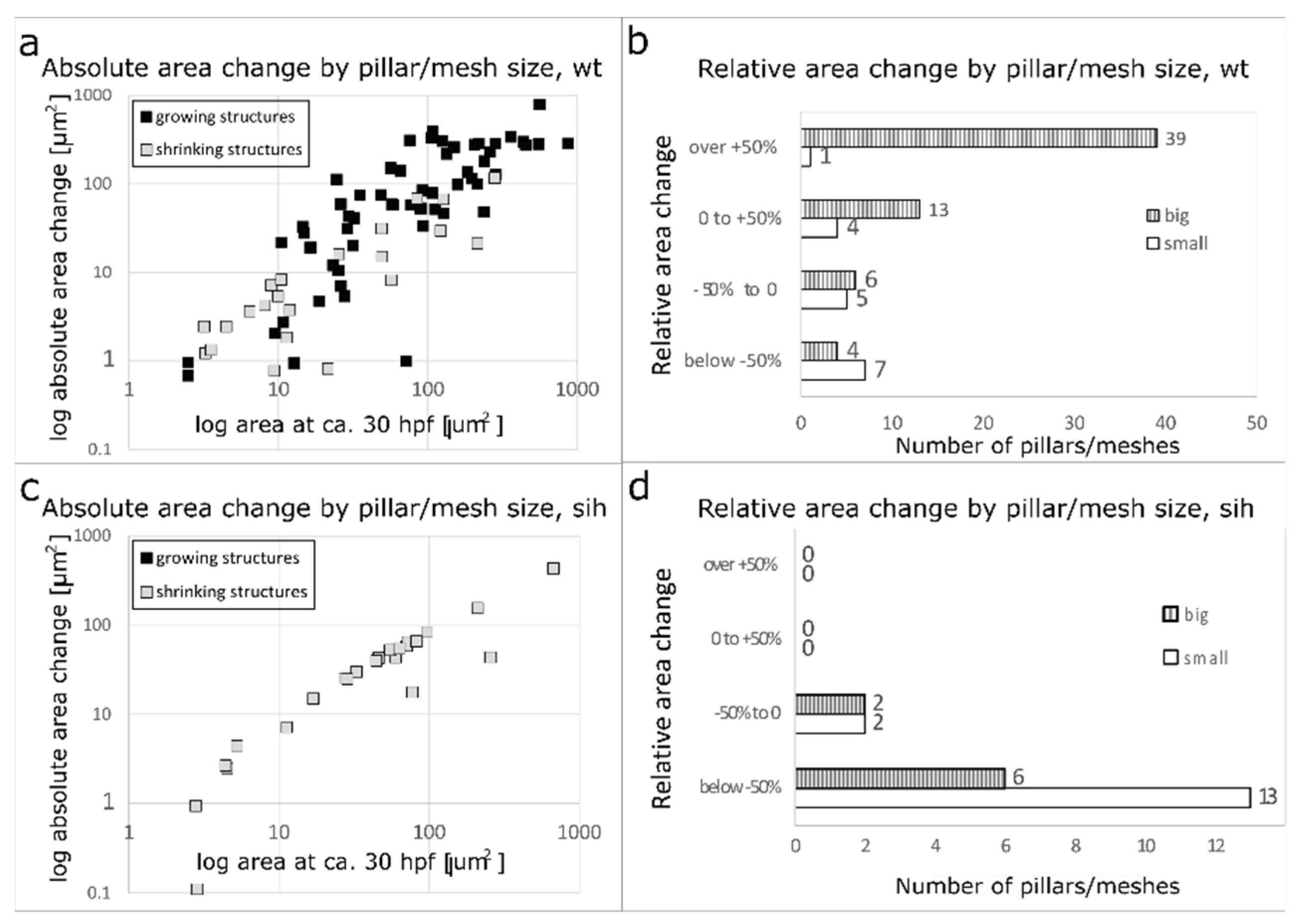
2.4. Further Fate of Pillars and Intercapillary Meshes
2.5. Merging of Adjacent Pillars/Meshes
2.6. Plexus Simplification and Establishment of Hierarchy
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Live Imaging
4.3. Serial Block Face Scanning Electron Microscopy
4.4. Pillar Tracking
4.5. Image Analysis, Statistics, and Artwork
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burri, P.H.; Djonov, V. Intussusceptive angiogenesis––The alternative to capillary sprouting. Mol. Aspects Med. 2002, 23, 1–27. [Google Scholar] [CrossRef]
- Schlatter, P.; König, M.F.; Karlsson, L.M.; Burri, P.H. Quantitative Study of Intussusceptive Capillary Growth in the Chorioallantoic Membrane (CAM) of the Chicken Embryo. Microvasc. Res. 1997, 54, 65–73. [Google Scholar] [CrossRef]
- Belle, J.; Ysasi, A.; Bennett, R.D.; Filipovic, N.; Nejad, M.I.; Trumper, D.L.; Ackermann, M.; Wagner, W.; Tsuda, A.; Konerding, M.A.; et al. Stretch-induced intussuceptive and sprouting angiogenesis in the chick chorioallantoic membrane. Microvasc. Res. 2014, 95, 60–67. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Djonov, V.G.; Kurz, H.; Burri, P.H. Optimality in the developing vascular system: Branching remodeling by means of intussusception as an efficient adaptation mechanism. Dev. Dyn. 2002, 224, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Patan, S. TIE1 and TIE2 Receptor Tyrosine Kinases Inversely Regulate Embryonic Angiogenesis by the Mechanism of Intussusceptive Microvascular Growth. Microvasc. Res. 1998, 56, 1–21. [Google Scholar] [CrossRef]
- Gorczyca, J.; Litwin, J.A.; Nowogrodzka-Zagórska, M.; Skawina, A.; Miodoński, A.J. Architecture of blood vessels in human fetal gastric corpus: A corrosion casting study. Ann. Anat.-Anat. Anz. 1999, 181, 353–358. [Google Scholar] [CrossRef]
- Lametschwandtner, A.; Lametschwandtner, U.; Radner, C.; Minnich, B. Spatial growth and pattern formation in the small intestine microvascular bed from larval to adult Xenopus laevis: A scanning electron microscope study of microvascular corrosion casts. Anat. Embryol. 2006, 211, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Karthik, S.; Djukic, T.; Kim, J.-D.; Zuber, B.; Makanya, A.; Odriozola, A.; Hlushchuk, R.; Filipovic, N.; Jin, S.W.; Djonov, V. Synergistic interaction of sprouting and intussusceptive angiogenesis during zebrafish caudal vein plexus development. Sci. Rep. 2018, 8, 9840. [Google Scholar] [CrossRef]
- Leonard, E.V.; Hasan, S.S.; Siekmann, A.F. Temporally and regionally distinct morphogenetic processes govern zebrafish caudal fin blood vessel network expansion. Development 2023, 150, dev201030. [Google Scholar] [CrossRef]
- Hanahan, D.; Folkman, J. Patterns and Emerging Mechanisms of the Angiogenic Switch during Tumorigenesis. Cell 1996, 86, 353–364. [Google Scholar] [CrossRef]
- Cockerill, G.W.; Gamble, J.R.; Vadas, M.A. Angiogenesis: Models and Modulators. Int. Rev. Cytol. 1995, 159, 113–160. [Google Scholar]
- Van Hinsbergh, V.W.M.; Koolwijk, P. Endothelial sprouting and angiogenesis: Matrix metalloproteinases in the lead. Cardiovasc. Res. 2008, 78, 203–212. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, J.; Zhou, H.; Fan, G.; Li, Q. Molecular Mechanisms and Anticancer Therapeutic Strategies in Vasculogenic Mimicry. J. Cancer 2019, 10, 6327–6340. [Google Scholar] [CrossRef]
- Hendrix, M.J.C.; Seftor, E.A.; Seftor, R.E.B.; Chao, J.-T.; Chien, D.-S.; Chu, Y.-W. Tumor cell vascular mimicry: Novel targeting opportunity in melanoma. Pharmacol. Ther. 2016, 159, 83–92. [Google Scholar] [CrossRef]
- Djonov, V.; Baum, O.; Burri, P.H. Vascular remodeling by intussusceptive angiogenesis. Cell Tissue Res. 2003, 314, 107–117. [Google Scholar] [CrossRef]
- Polykandriotis, E.; Euler, S.; Arkudas, A.; Pryymachuk, G.; Beier, J.P.; Greil, P.; Dragu, A.; Lametschwandtner, A.; Kneser, U.; Horch, R.E. Regression and persistence: Remodelling in a tissue engineered axial vascular assembly. J. Cell. Mol. Med. 2009, 13, 4166–4175. [Google Scholar] [CrossRef]
- Szczerba, D.; Székely, G. A Computational Model of Micro-vascular Growth. In Computational Science–ICCS 2005; Sunderam, V.S., van Albada, G.D., Sloot, P.M.A., Dongarra, J., Eds.; Lecture Notes in Computer Science; Springer: Berlin/Heidelberg, Germany, 2005; Volume 3516, pp. 17–24. ISBN 978-3-540-26044-8. [Google Scholar]
- Filipovic, N.; Tsuda, A.; Lee, G.S.; Miele, L.F.; Lin, M.; Konerding, M.A.; Mentzer, S.J. Computational flow dynamics in a geometric model of intussusceptive angiogenesis. Microvasc. Res. 2009, 78, 286–293. [Google Scholar] [CrossRef]
- Mentzer, S.J.; Konerding, M.A. Intussusceptive angiogenesis: Expansion and remodeling of microvascular networks. Angiogenesis 2014, 17, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Du Cheyne, C.; Smeets, M.; De Spiegelaere, W. Techniques used to assess intussusceptive angiogenesis: A systematic review. Dev. Dyn. 2021, 250, 1704–1716. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Solimando, A.G.; Pezzella, F. The Anti-VEGF(R) Drug Discovery Legacy: Improving Attrition Rates by Breaking the Vicious Cycle of Angiogenesis in Cancer. Cancers 2021, 13, 3433. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Crivellato, E. “Sprouting angiogenesis”, a reappraisal. Dev. Biol. 2012, 372, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Patan, S.; Haenni, B.; Burri, P.H. Implementation of Intussusceptive Microvascular Growth in the Chicken Chorioallantoic Membrane (CAM): 2. Pillar Formation by Capillary Fusion. Microvasc. Res. 1997, 53, 33–52. [Google Scholar] [CrossRef] [PubMed]
- Patan, S.; Haenni, B.; Burri, P.H. Implementation of Intussusceptive Microvascular Growth in the Chicken Chorioallantoic Membrane (CAM): 1. pillar formation by folding of the capillary wall. Microvasc. Res. 1996, 51, 80–98. [Google Scholar] [CrossRef] [PubMed]
- Paku, S.; Dezső, K.; Bugyik, E.; Tóvári, J.; Tímár, J.; Nagy, P.; Laszlo, V.; Klepetko, W.; Döme, B. A New Mechanism for Pillar Formation during Tumor-Induced Intussusceptive Angiogenesis: Inverse Sprouting. Am. J. Pathol. 2011, 179, 1573–1585. [Google Scholar] [CrossRef] [PubMed]
- Djonov, V.; Schmid, M.; Tschanz, S.A.; Burri, P.H. Intussusceptive angiogenesis: Its role in embryonic vascular network formation. Circ. Res. 2000, 86, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, A.; Mickoleit, M.; Weber, M.; Huisken, J. Multilayer mounting enables long-term imaging of zebrafish development in a light sheet microscope. Dev. Camb. Engl. 2012, 139, 3242–3247. [Google Scholar] [CrossRef] [PubMed]
- Wiley, D.M.; Kim, J.-D.; Hao, J.; Hong, C.C.; Bautch, V.L.; Jin, S.-W. Distinct signalling pathways regulate sprouting angiogenesis from the dorsal aorta and the axial vein. Nat. Cell Biol. 2011, 13, 686–692. [Google Scholar] [CrossRef]
- Murayama, E.; Kissa, K.; Zapata, A.; Mordelet, E.; Briolat, V.; Lin, H.-F.; Handin, R.I.; Herbomel, P. Tracing hematopoietic precursor migration to successive hematopoietic organs during zebrafish development. Immunity 2006, 25, 963–975. [Google Scholar] [CrossRef]
- Isogai, S.; Horiguchi, M.; Weinstein, B.M. The vascular anatomy of the developing zebrafish: An atlas of embryonic and early larval development. Dev. Biol. 2001, 230, 278–301. [Google Scholar] [CrossRef]
- De Spiegelaere, W.; Casteleyn, C.; Van den Broeck, W.; Plendl, J.; Bahramsoltani, M.; Simoens, P.; Djonov, V.; Cornillie, P. Intussusceptive Angiogenesis: A Biologically Relevant Form of Angiogenesis. J. Vasc. Res. 2012, 49, 390–404. [Google Scholar] [CrossRef]
- Chouinard-Pelletier, G.; Jahnsen, E.D.; Jones, E.A.V. Increased shear stress inhibits angiogenesis in veins and not arteries during vascular development. Angiogenesis 2013, 16, 71–83. [Google Scholar] [CrossRef]
- Baum, O.; Suter, F.; Gerber, B.; Tschanz, S.; Buergy, R.; Blank, F.; Hlushchuk, R.; Djonov, V. VEGF-A Promotes Intussusceptive Angiogenesis in the Developing Chicken Chorioallantoic Membrane. Microcirculation 2010, 17, 447–457. [Google Scholar] [CrossRef]
- Ackermann, M.; Kim, Y.O.; Wagner, W.L.; Schuppan, D.; Valenzuela, C.D.; Mentzer, S.J.; Kreuz, S.; Stiller, D.; Wollin, L.; Konerding, M.A. Effects of nintedanib on the microvascular architecture in a lung fibrosis model. Angiogenesis 2017, 20, 359–372. [Google Scholar] [CrossRef]
- Polykandriotis, E.; Arkudas, A.; Beier, J.; Dragu, A.; Rath, S.N.; Pryymachuk, G.; Schmidt, V.; Lametschwandtner, A.; Horch, R.; Kneser, U. The impact of VEGF and bFGF on vascular stereomorphology in the context of angiogenic neo-arborisation after vascular induction. J. Electron Microsc. 2011, 60, 267–274. [Google Scholar] [CrossRef]
- Mukwaya, A.; Peebo, B.; Xeroudaki, M.; Ali, Z.; Lennikov, A.; Jensen, L.; Lagali, N. Factors regulating capillary remodeling in a reversible model of inflammatory corneal angiogenesis. Sci. Rep. 2016, 6, 32137. [Google Scholar] [CrossRef]
- Bugyik, E.; Dezső, K.; Reiniger, L.; László, V.; Tóvári, J.; Tímár, J.; Nagy, P.; Klepetko, W.; Döme, B.; Paku, S. Lack of Angiogenesis in Experimental Brain Metastases. J. Neuropathol. Exp. Neurol. 2011, 70, 979–991. [Google Scholar] [CrossRef]
- Sarı Kılıçaslan, S.M.; Coşkun Cevher, Ş.; Güleç Peker, E.G. Ultrastructural changes in blood vessels in epidermal growth factor treated experimental cutaneous wound model. Pathol.-Res. Pract. 2013, 209, 710–715. [Google Scholar] [CrossRef]
- Giacomini, A.; Ackermann, M.; Belleri, M.; Coltrini, D.; Nico, B.; Ribatti, D.; Konerding, M.A.; Presta, M.; Righi, M. Brain angioarchitecture and intussusceptive microvascular growth in a murine model of Krabbe disease. Angiogenesis 2015, 18, 499–510. [Google Scholar] [CrossRef]
- Ceauşu, R.A.; Cîmpean, A.M.; Gaje, P.; Gurzu, S.; Jung, I.; Raica, M. CD105/Ki67 double immunostaining expression in liver metastasis from colon carcinoma. Romanian J. Morphol. Embryol. Rev. Roum. Morphol. Embryol. 2011, 52, 613–616. [Google Scholar]
- Bai, L.; Wu, D.; Xu, J.; Liu, H.; Xie, M.; Guan, G.; Sun, Z.; Tan, X. On model of angiogenesis and the mechanism in porous silk fibroin films. J. Mater. Sci. Mater. Med. 2011, 22, 927–933. [Google Scholar] [CrossRef]
- Dimova, I.; Hlushchuk, R.; Makanya, A.; Styp-Rekowska, B.; Ceauşu, R.; Flueckiger, S.; Lang, S.; Semela, D.; Noble, F.; Chatterjee, S.; et al. Inhibition of Notch signaling induces extensive intussusceptive neo-angiogenesis by recruitment of mononuclear cells. Angiogenesis 2013, 16. [Google Scholar] [CrossRef]
- Ackermann, M.; Morse, B.A.; Delventhal, V.; Carvajal, I.M.; Konerding, M.A. Anti-VEGFR2 and anti-IGF-1R-Adnectins inhibit Ewing’s sarcoma A673-xenograft growth and normalize tumor vascular architecture. Angiogenesis 2012, 15, 685–695. [Google Scholar] [CrossRef]
- Rossi-Schneider, T.; Verli, F.; Marinho, S.; Yurgel, L.; Souza, M. Study of Intussusceptive Angiogenesis in Inflammatory Regional Lymph Nodes by Scanning Electron Microscopy. Microsc. Res. Tech. 2009, 73, 14–19. [Google Scholar] [CrossRef]
- Lee, G.S.; Filipovic, N.; Miele, L.F.; Lin, M.; Simpson, D.C.; Giney, B.; Konerding, M.A.; Tsuda, A.; Mentzer, S.J. Blood flow shapes intravascular pillar geometry in the chick chorioallantoic membrane. J. Angiogenesis Res. 2010, 2, 11. [Google Scholar] [CrossRef]
- Li, W.; Tran, V.; Shaked, I.; Xue, B.; Moore, T.; Lightle, R.; Kleinfeld, D.; Awad, I.A.; Ginsberg, M.H. Abortive intussusceptive angiogenesis causes multi-cavernous vascular malformations. eLife 2021, 10, e62155. [Google Scholar] [CrossRef]
- Carmeliet, P. Angiogenesis in health and disease. Nat. Med. 2003, 9, 653–660. [Google Scholar] [CrossRef]
- Gerhardt, H.; Golding, M.; Fruttiger, M.; Ruhrberg, C.; Lundkvist, A.; Abramsson, A.; Jeltsch, M.; Mitchell, C.; Alitalo, K.; Shima, D.; et al. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J. Cell Biol. 2003, 161, 1163–1177. [Google Scholar] [CrossRef]
- Blanco, R.; Gerhardt, H. VEGF and Notch in Tip and Stalk Cell Selection. Cold Spring Harb. Perspect. Med. 2013, 3, a006569. [Google Scholar] [CrossRef] [PubMed]
- Phng, L.-K.; Stanchi, F.; Gerhardt, H. Filopodia are dispensable for endothelial tip cell guidance. Development 2013, 140, 4031–4040. [Google Scholar] [CrossRef]
- Gianni-Barrera, R.; Trani, M.; Fontanellaz, C.; Heberer, M.; Djonov, V.; Hlushchuk, R.; Banfi, A. VEGF over-expression in skeletal muscle induces angiogenesis by intussusception rather than sprouting. Angiogenesis 2013, 16, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Arpino, J.-M.; Yin, H.; Prescott, E.K.; Staples, S.C.R.; Nong, Z.; Li, F.; Chevalier, J.; Balint, B.; O’Neil, C.; Mortuza, R.; et al. Low-flow intussusception and metastable VEGFR2 signaling launch angiogenesis in ischemic muscle. Sci. Adv. 2021, 7, eabg9509. [Google Scholar] [CrossRef]
- Wnuk, M.; Hlushchuk, R.; Tuffin, G.; Huynh-Do, U.; Djonov, V. The Effects of PTK787/ZK222584, an Inhibitor of VEGFR and PDGFRβ Pathways, on Intussusceptive Angiogenesis and Glomerular Recovery from Thy1.1 Nephritis. Am. J. Pathol. 2011, 178, 1899–1912. [Google Scholar] [CrossRef] [PubMed]
- Wnuk, M.; Hlushchuk, R.; Janot, M.; Tuffin, G.; Martiny-Baron, G.; Holzer, P.; Imbach-Weese, P.; Djonov, V.; Huynh-Do, U. Podocyte EphB4 signaling helps recovery from glomerular injury. Kidney Int. 2012, 81, 1212–1225. [Google Scholar] [CrossRef]
- Hagedorn, M.; Balke, M.; Schmidt, A.; Bloch, W.; Kurz, H.; Javerzat, S.; Rousseau, B.; Wilting, J.; Bikfalvi, A. VEGF coordinates interaction of pericytes and endothelial cells during vasculogenesis and experimental angiogenesis. Dev. Dyn. 2004, 230, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Groppa, E.; Brkic, S.; Uccelli, A.; Wirth, G.; Korpisalo-Pirinen, P.; Filippova, M.; Dasen, B.; Sacchi, V.; Muraro, M.G.; Trani, M.; et al. EphrinB2/EphB4 signaling regulates non-sprouting angiogenesis by VEGF. EMBO Rep. 2018, 19, e45054. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.U.; Chen, Z.-F.; Anderson, D.J. Molecular Distinction and Angiogenic Interaction between Embryonic Arteries and Veins Revealed by ephrin-B2 and Its Receptor Eph-B4. Cell 1998, 93, 741–753. [Google Scholar] [CrossRef] [PubMed]
- Du, E.; Li, X.; He, S.; Li, X.; He, S. The critical role of the interplays of EphrinB2/EphB4 and VEGF in the induction of angiogenesis. Mol. Biol. Rep. 2020, 47, 4681–4690. [Google Scholar] [CrossRef]
- Chen, D.; Van Der Ent, M.A.; Lartey, N.L.; King, P.D. EPHB4-RASA1-Mediated Negative Regulation of Ras-MAPK Signaling in the Vasculature: Implications for the Treatment of EPHB4- and RASA1-Related Vascular Anomalies in Humans. Pharmaceuticals 2023, 16, 165. [Google Scholar] [CrossRef]
- Phng, L.-K.; Belting, H.-G. Endothelial cell mechanics and blood flow forces in vascular morphogenesis. Semin. Cell Dev. Biol. 2021, 120, 32–43. [Google Scholar] [CrossRef]
- Lawson, N.D.; Weinstein, B.M. In vivo imaging of embryonic vascular development using transgenic zebrafish. Dev. Biol. 2002, 248, 307–318. [Google Scholar] [CrossRef]
- Traver, D.; Paw, B.H.; Poss, K.D.; Penberthy, W.T.; Lin, S.; Zon, L.I. Transplantation and in vivo imaging of multilineage engraftment in zebrafish bloodless mutants. Nat. Immunol. 2003, 4, 1238–1246. [Google Scholar] [CrossRef] [PubMed]
- Sehnert, A.J.; Huq, A.; Weinstein, B.M.; Walker, C.; Fishman, M.; Stainier, D.Y.R. Cardiac troponin T is essential in sarcomere assembly and cardiac contractility. Nat. Genet. 2002, 31, 106–110. [Google Scholar] [CrossRef]
- Xie, X.; Zhou, T.; Wang, Y.; Chen, H.; Lei, D.; Huang, L.; Wang, Y.; Jin, X.; Sun, T.; Tan, J.; et al. Blood Flow Regulates Zebrafish Caudal Vein Plexus Angiogenesis by ERK5-klf2a-nos2b Signaling. Curr. Mol. Med. 2018, 18, 3–14. [Google Scholar] [CrossRef]
- Caduff, J.H.; Fischer, L.C.; Burri, P.H. Scanning electron microscope study of the developing microvasculature in the postnatal rat lung. Anat. Rec. 1986, 216, 154–164. [Google Scholar] [CrossRef]
- Konerding, M.A.; Gibney, B.C.; Houdek, J.P.; Chamoto, K.; Ackermann, M.; Lee, G.S.; Lin, M.; Tsuda, A.; Mentzer, S.J. Spatial dependence of alveolar angiogenesis in post-pneumonectomy lung growth. Angiogenesis 2012, 15, 23–32. [Google Scholar] [CrossRef]
- Föhst, S.; Wagner, W.; Ackermann, M.; Redenbach, C.; Schladitz, K.; Wirjadi, O.; Ysasi, A.B.; Mentzer, S.J.; Konerding, M.A. Three-dimensional image analytical detection of intussusceptive pillars in murine lung. J. Microsc. 2015, 260, 326–337. [Google Scholar] [CrossRef]
- Burri, P.H.; Hlushchuk, R.; Djonov, V. Intussusceptive angiogenesis: Its emergence, its characteristics, and its significance. Dev. Dyn. 2004, 231, 474–488. [Google Scholar] [CrossRef]
- Wakayama, Y.; Fukuhara, S.; Ando, K.; Matsuda, M.; Mochizuki, N. Cdc42 Mediates Bmp-Induced Sprouting Angiogenesis through Fmnl3-Driven Assembly of Endothelial Filopodia in Zebrafish. Dev. Cell 2015, 32, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Davis, G.E.; Senger, D.R. Endothelial extracellular matrix: Biosynthesis, remodeling, and functions during vascular morphogenesis and neovessel stabilization. Circ. Res. 2005, 97, 1093–1107. [Google Scholar] [CrossRef]
- Díaz-Flores, L.; Gutiérrez, R.; García, M.D.P.; Sáez, F.J.; Díaz-Flores, L.; Madrid, J.F. Piecemeal Mechanism Combining Sprouting and Intussusceptive Angiogenesis in Intravenous Papillary Formation Induced by PGE2 and Glycerol. Anat. Rec. 2017, 300, 1781–1792. [Google Scholar] [CrossRef] [PubMed]
- Greysson-Wong, J.; Rode, R.; Ryu, J.-R.; Rinker, K.D.; Childs, S.J. Venous activation of MEK/ERK drives development of arteriovenous malformation and blood flow anomalies with loss of Rasa1. Dev. Biol. 2021. [Google Scholar] [CrossRef]
- Kawasaki, J.; Aegerter, S.; Fevurly, R.D.; Mammoto, A.; Mammoto, T.; Sahin, M.; Mably, J.D.; Fishman, S.J.; Chan, J. RASA1 functions in EPHB4 signaling pathway to suppress endothelial mTORC1 activity. J. Clin. Invest. 2014, 124, 2774–2784. [Google Scholar] [CrossRef] [PubMed]
- Walton, J. Lead asparate, an en bloc contrast stain particularly useful for ultrastructural enzymology. J. Histochem. Cytochem. 1979, 27, 1337–1342. [Google Scholar] [CrossRef] [PubMed]
- Vladymyrov, M.; Abe, J.; Moalli, F.; Stein, J.V.; Ariga, A. Real-time tissue offset correction system for intravital multiphoton microscopy. J. Immunol. Methods 2016, 438, 35–41. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Röss, H.; Aaldijk, D.; Vladymyrov, M.; Odriozola, A.; Djonov, V. Transluminal Pillars—Their Origin and Role in the Remodelling of the Zebrafish Caudal Vein Plexus. Int. J. Mol. Sci. 2023, 24, 16703. https://doi.org/10.3390/ijms242316703
Röss H, Aaldijk D, Vladymyrov M, Odriozola A, Djonov V. Transluminal Pillars—Their Origin and Role in the Remodelling of the Zebrafish Caudal Vein Plexus. International Journal of Molecular Sciences. 2023; 24(23):16703. https://doi.org/10.3390/ijms242316703
Chicago/Turabian StyleRöss, Helena, Dea Aaldijk, Mykhailo Vladymyrov, Adolfo Odriozola, and Valentin Djonov. 2023. "Transluminal Pillars—Their Origin and Role in the Remodelling of the Zebrafish Caudal Vein Plexus" International Journal of Molecular Sciences 24, no. 23: 16703. https://doi.org/10.3390/ijms242316703
APA StyleRöss, H., Aaldijk, D., Vladymyrov, M., Odriozola, A., & Djonov, V. (2023). Transluminal Pillars—Their Origin and Role in the Remodelling of the Zebrafish Caudal Vein Plexus. International Journal of Molecular Sciences, 24(23), 16703. https://doi.org/10.3390/ijms242316703




