Abstract
Dinucleoside polyphosphates (NpnNs) are considered novel signalling molecules involved in the induction of plant defence mechanisms. However, NpnN signal recognition and transduction are still enigmatic. Therefore, the aim of our research was the identification of the NpnN receptor and signal transduction pathways evoked by these nucleotides. Earlier, we proved that purine and pyrimidine NpnNs differentially affect the phenylpropanoid pathway in Vitis vinifera suspension-cultured cells. Here, we report, for the first time, that both diadenosine tetraphosphate (Ap4A) and dicytidine tetraphosphate (Cp4C)-induced stomatal closure in Arabidopsis thaliana. Moreover, we showed that plasma membrane purinoreceptor P2K1/DORN1 (does not respond to nucleotide 1) is essential for Ap4A-induced stomata movements but not for Cp4C. Wild-type Col-0 and the dorn1-3 A. thaliana knockout mutant were used. Examination of the leaf epidermis dorn1-3 mutant provided evidence that P2K1/DORN1 is a part of the signal transduction pathway in stomatal closure evoked by extracellular Ap4A but not by Cp4C. Reactive oxygen species (ROS) are involved in signal transduction caused by Ap4A and Cp4C, leading to stomatal closure. Ap4A induced and Cp4C suppressed the transcriptional response in wild-type plants. Moreover, in dorn1-3 leaves, the effect of Ap4A on gene expression was impaired. The interaction between P2K1/DORN1 and Ap4A leads to changes in the transcription of signalling hubs in signal transduction pathways.
1. Introduction
Regulation of plant metabolic processes takes place at a molecular level. The defence reactions are among the processes in which signal transduction plays a key role. Based on the criterion of the distance that a given signal molecule can cover, short-distance molecules cause local intercellular responses, and long-distance molecules trigger systemic responses. Signalling molecules regulate many processes throughout various signal transduction pathways and specific or unspecific receptors [1]. Unlike animals, the ability of extracellular nucleotides to initiate diverse signalling responses in plants remained enigmatic for years. A growing number of nucleotides classified as signalling molecules have been identified in plants [2]. Among them, extracellular ATP (eATP) plays an essential role in plant growth [3,4,5,6,7,8] and development [9,10]. Extracellular ATP regulates responses to biotic stress [11,12,13,14] and abiotic stress [15,16,17,18]. One of the reactions that eATP can control is stomatal movements [12,19,20]. In this reaction, the cytoplasmic Ca2+ ions ([Ca2+]cyt) and the complex signalling cross-talk between second messengers, such as nitric oxide (NO) [7,21,22], and reactive oxygen species (ROS) [12,23,24,25] plays a crucial role as a mediator in the signal transduction pathway. Consequently, these messenger agents affect the phosphorylation of mitogen-activated protein kinase (MAPK) and the expression of defence-related genes [12,26,27].
We have a longstanding interest in the function of dinucleoside polyphosphates (NpnNs) in plant cells. Our papers describe changes in gene expression profile and metabolism in Arabidopsis thaliana and Vitis vinifera treated with a broad spectrum of NpnNs. We postulated the participation of NpnNs in the plant defence responses since they induce synthesis of the phenylpropanoid pathway-delivered secondary metabolites [28,29,30]. The phenylpropanoid pathway participates in plant defence responses [31,32]. Identification of Ap4A and other NpnNs across prokaryotic and eukaryotic cells testifies to their universality [33]. Due to the dramatic increase in levels of various NpnNs observed in cells subjected to abiotic stress factors [34,35,36,37,38], these compounds have been termed “alarmones”, triggering stress adaptive processes. Our latest findings confirmed the induction of the phenylpropanoid pathway by purine, pyrimidine, and purine–pyrimidine hybrids of NpnNs. Moreover, we observed that diadenosine polyphosphates (ApnA) induced stilbene biosynthesis. In contrast, dicytidine polyphosphates (CpnC) strongly inhibited this reaction but markedly induced the expression of the cinnamoyl-CoA reductase gene that controls lignin biosynthesis [30]. Nonetheless, the underlying mechanism of NpnN signal recognition and transduction in plants remains elusive. The growing number of plant enzymes found to be involved in NpnN biosynthesis and degradation strengthens the hypothesis of their signalling function [2,33].
Plants can respond to extracellular purine nucleotides, such as eATP, through plasma membrane receptors. So far, two plant receptors with an eATP binding domain have been identified. They are P2K1/DORN1 (does not respond to nucleotides 1) [26] and P2K2/DORN2, which belong to the L-type lectin receptor kinase (LecRK) protein family [39,40]. LecRK proteins activate the processes controlling stress responses, development, growth, and disease resistance [41]. Although eATP sensing and action in plants have been elucidated, the mechanisms of signal perception and transduction evoked by NpnNs, such as Ap4A and Cp4C, remain enigmatic. In animal cells, among the different nucleotides and nucleosides, eATP, together with Ap4A, shares access to the same receptors that belong to the P2 group, which is divided into two classes, namely ligand-gated ion channels (P2Xs) and G protein-coupled (P2Ys) receptors [42,43,44,45,46]. Therefore, we hypothesise that the purinoreceptor P2K1/DORN1, a receptor of eATP, is also necessary for sensing Ap4A in plant cells. Moreover, we wondered whether P2K1/DORN1 is also engaged in the effects evoked by the pyrimidine nucleotide Cp4C.
Here, we present, for the first time, evidence for the involvement of the P2K1/DORN1 receptor in the sensing of Ap4A in plants. All experiments were conducted on 4-week-old Arabidopsis thaliana wild-type Col-0 and dorn1-3 knockout mutant leaves. Our research showed that extracellular Ap4A and Cp4C evoked stomatal closure in Col-0 plants. This effect was abolished in the dorn1-3 mutant by Ap4A but not Cp4C. This result confirms the requirement of P2K1/DORN1 for Ap4A-induced stomatal closure. Nevertheless, our research indicates the involvement of superoxide (•O2−) and hydrogen peroxide (H2O2) in the signal transduction evoked by Ap4A and Cp4C, leading to stomatal closure. Furthermore, we analysed the expression of genes encoding selected proteins integrated within the signalling hubs. It concerns NADPH oxidases (RBOHD and RBOHF), MAPK cascades, SNF1/AMPK-related protein kinases (SnRKs), and transcriptional factors, such as ZAT6 and ZAT12. Notably, Ap4A induced the expression of the tested genes. Moreover, the gene expression in dorn1-3 was almost abolished by the Ap4A effect.
2. Results
2.1. Ap4A and Cp4C Induce Stomatal Closure
Our previous research showed that exogenous NpnNs induce the biosynthesis of secondary metabolites that play an essential role in the plant defence strategy [28,29,30]. We wondered how the signal evoked by NpnNs could be sensed and transduced in plant cells and whether plants contain cell membrane receptor(s) for these molecules. It is known that eATP, one of the exogenous purine nucleotides, evokes stomatal closure with the involvement of the purinoreceptor P2K1/DORN1 in Arabidopsis thaliana [12,26]. Therefore, based on similarities in the ATP and Ap4A structures, we tested the effect of these nucleotides on stomatal movements. Moreover, we also included cytidine nucleotides in our research because of the different effects of purine and pyrimidine NpnNs on the phenylpropanoid pathway in Vitis vinifera cells [30]. To trace stomatal movement under the nucleotide treatment, we examined the ability of purine NpnNs such as Ap3A and Ap4A to stimulate stomatal closure. Additionally, for the positive control, we tested the effects of ADP and ATP, as described earlier [12,26], as well as ABA—a well-known molecule controlling stomatal movements [47,48]. Exogenous Ap4A significantly reduced the stomatal aperture in the light. It was at a similar level compared to the effect evoked by ATP and ADP. However, Ap3A did not evoke such an effect (Figure 1). We also examined stomatal movement under the treatment of cytidine mono- and dinucleotides (CDP, CTP, Cp3C, Cp4C). Interestingly, only Cp4C triggered significant stomatal closure among tested cytidine nucleotides. As expected, ABA closed stomata [49] (Figure 1).
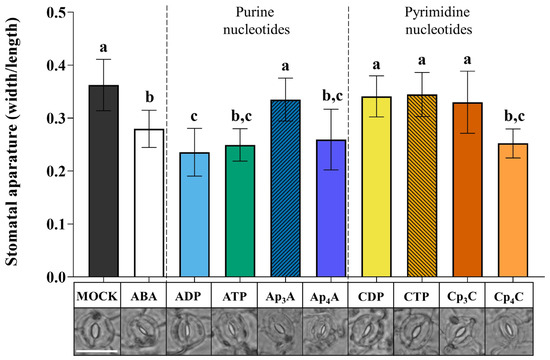
Figure 1.
Diadenosine tetraphosphate (Ap4A) and dicytidine tetraphosphate (Cp4C), similar to adenosine diphosphate (ADP) and adenosine triphosphate (ATP), induce stomatal closure in Arabidopsis thaliana Col-0 plants. Images represent stomata in the abaxial epidermis of a leaf treated for 2 h with the MOCK solution MES/KOH opening buffer, 10 µM abscisic acid (ABA), and 2 mM purine and pyrimidine nucleotides. White bar = 25 μm. Bars represent mean values ± SD, n ≥ 20, three biological replicates. Different letters above the error bars indicate statistically significant differences according to the ANOVA analysis with the post-hoc Tukey’s HSD multiple comparisons test (p < 0.05).
In plant cells, there are enzymes degrading NpnNs to mononucleotides [50]. To confirm that Ap4A and Cp4C evoke stomatal closure but not by the products of their degradation (AMP, ADP, ATP, and CMP, CDP, CTP, respectively), we collected samples of leaf epidermis from the microscope slides after incubation of nucleotides, and application of the HPLC assay (Method S2) proved that Ap4A was not degraded to the corresponding mononucleotide. Only a trace amount of CTP was detected in a solution of Cp4C after the investigation (Figure S1).
2.2. P2K1/DORN1 Is Involved in Signal Perception Evoked by Ap4A but Not Cp4C
Plants respond to eATP by the induction of a complex signalling network after signal recognition by the P2K1/DORN1 and P2K2 receptors [26,39]. Similarities in stomatal movements evoked by eATP, Ap4A, and Cp4C led us to hypothesise that those nucleotides could interact with P2K1/DORN1. Based on the results presented in Figure 1, Ap4A and Cp4C were chosen for further experiments. The dorn1-3 mutant, having a T-DNA insertion in the extracellular legume-type lectin domain, was selected based on literature data [12,26]. We found that Ap4A and eATP did not close stomata in dorn1-3 mutant leaves. Contrary to this, Cp4C significantly closed stomata in dorn1-3 mutant leaves. As expected, ABA-treated mutant leaves also showed closed stomata [12] (Figure 2). Thus, the results strongly suggest that besides eATP, P2K1/DORN1 may also be involved in signal perception elicited by Ap4A but not Cp4C.
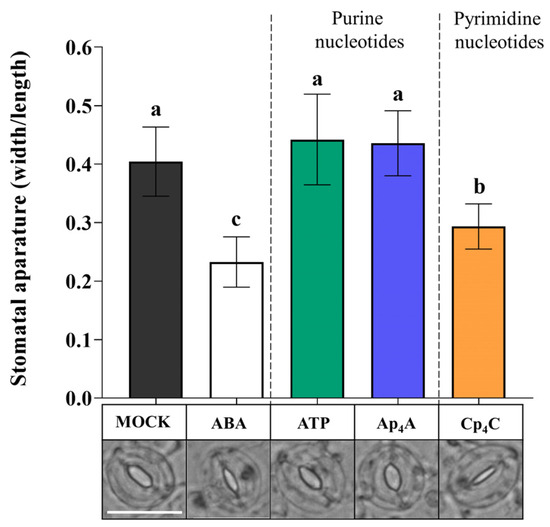
Figure 2.
Diadenosine tetraphosphate (Ap4A), similar to extracellular (eATP), did not induce stomatal closure in the dorn1-3 Arabidopsis thaliana mutant. However, dicytidine tetraphosphate (Cp4C) and abscisic acid (ABA) evoked stomatal closing. Images represent stomata in the abaxial epidermis of dorn1-3 leaf treated for 2 h with MOCK solution opening buffer, 10 µM ABA and 2 mM adenosine triphosphate (ATP), Ap4A, and Cp4C. White bar = 25 μm. Bars represent mean values ± SD, n ≥ 20, three biological replicates. Different letters above the error bars indicate statistically significant differences according to the ANOVA analysis with the post-hoc Tukey’s HSD multiple comparisons test (p < 0.05).
2.3. ROS Are Produced in Leaves under Nucleotide Treatment
It was previously found that the elevated production of ROS and stomatal closure are mediated by eATP recognition by the receptor P2K1/DORN1, followed by direct phosphorylation of the NADPH oxidase RBOHD [12]. This phosphorylation causes an increase in the generation of extracellular ROS, such as •O2−, which is then converted into H2O2 in the extracellular environment [51,52]. Notably, the apoplastic production of ROS is one of the fastest physiologically common responses to external stimuli observed in plants [53,54]. Considering all the above-described information, we decided to investigate the accumulation of •O2− and H2O2 in Arabidopsis thaliana leaves in response to 2 mM ATP, CTP, Ap4A, and Cp4C. Our experiments revealed that the NBT staining of leaves, indicating •O2− accumulation, was increased in Col-0 leaves treated with CTP, Ap4A, and Cp4C but not by eATP, while in the dorn1-3 mutant, only Cp4C evoked an accumulation of •O2− (Figure 3a). DAB staining representing the concentration of H2O2 in leaves was increased in Col-0 leaves under eATP, Ap4A, and Cp4C, while CTP caused only slight DAB staining. In the dorn1-3 mutant, only CTP and Cp4C evoked an accumulation of H2O2 in the leaves. Nevertheless, only weak DAB staining was caused by CTP (Figure 3b).
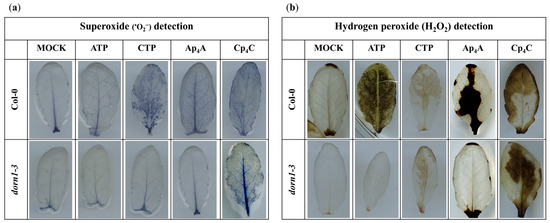
Figure 3.
Histochemical detection of •O2− (a) and H2O2 (b) in leaves of Arabidopsis thaliana Col-0 and the dorn1-3 mutant triggered by 2 mM adenosine triphosphate (ATP), cytidine triphosphate (CTP), diadenosine tetraphosphate (Ap4A), dicytidine tetraphosphate (Cp4C) after 2 h treatment. Leaves were stained with nitroblue tetrazolium (NBT) and 3,3′-diaminobenzidine tetrahydrochloride (DAB) for •O2− and H2O2 detection, respectively. The experiment was repeated six times, and representative leaves were chosen.
2.4. ROS Are Involved in Signal Transduction Evoked by eATP, Ap4A and Cp4C, Leading to Stomatal Closure
Based on the results indicating that Ap4A and Cp4C induced the production of ROS (Figure 3a,b), we wondered whether these key signalling molecules are components of signal transduction pathways evoked by NpnNs leading to stomatal closure. We simultaneously applied superoxide dismutase (SOD) and catalase (CAT), enzymes scavenging ROS [54,55], and thereby sought to confirm the role of •O2− and H2O2 in the transduction pathway of the signal generated by Ap4A and Cp4C. Interestingly, CAT and SOD eliminated the effect of stomatal closure under simultaneous nucleotide treatment, so our observations showed the direct involvement of •O2− and H2O2 in stomatal closure evoked by eATP, Ap4A, and Cp4C. However, the plants did close their stomata upon adding ABA (Figure 4).
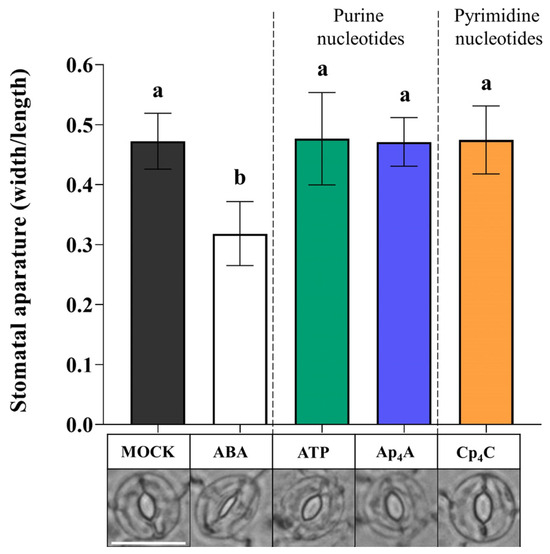
Figure 4.
Reactive oxygen species (ROS) enzyme scavengers, catalase (CAT) and superoxide dismutase (SOD), eliminate the effect of stomatal closure after the 2 mM adenosine triphosphate (ATP), diadenosine tetraphosphate (Ap4A), dicytidine tetraphosphate (Cp4C) treatment in Arabidopsis thaliana Col-0 leaves. White bar = 25 μm. Bars represent mean values ± SD, n ≥ 20, three biological replicates. Different letters above the error bars indicate statistically significant differences according to the ANOVA analysis with the post-hoc Tukey’s HSD multiple comparisons test (p < 0.05).
2.5. P2K1/DORN1 Is Implicated in Ap4A- and eATP-Responsive Gene Expression
It is known that transcriptional upregulation of defence-related and wound-response genes by eATP is P2K1/DORN1-dependent [26,56]. Thus, we decided to investigate whether Ap4A also changes the expression of the defence-related genes and whether the plasma membrane receptor P2K1/DORN1 is engaged in this regulation. To understand the signal transduction pathway evoked by Ap4A, we tested the gene expression coding for proteins as a component of signalling hubs known as key points in response to stresses. First, we studied the NADPH oxidase respiratory burst oxidase homologs (RBOHs), RBOHD, and RBOHF, which generate ROS [54]. We found that Ap4A up-regulated RBOHF but not by eATP in Col-0 plants. Interestingly, both eATP and Ap4A downregulated RBOHF expression in the dorn1-3 mutant (Figure 5a). The expression of RBOHD was drastically induced (the most among all studied genes) by eATP but only in Col-0 plants. In contrast, in the dorn1-3 plants, this effect was weak. Ap4A evoked slight changes in expression levels of RBOHD in Col-0 and dorn1-3 plants (Figure 5a).
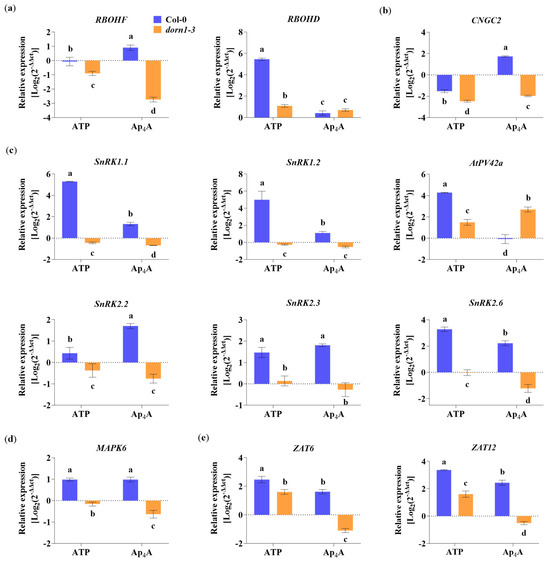
Figure 5.
The purinoceptor P2K1/DORN1 is involved in the Ap4A-induced transcriptional response in Arabidopsis thaliana Col-0 leaves. Graphs present the changes in the gene expression level for NADPH oxidase respiratory burst homologs (RBOHD and RBOHF) (a), cyclic nucleotide-gated channel 2 (CNGC2) (b), SNF1/AMPK-related protein kinases (SnRKs) (c), mitogen-activated protein kinase 6 (MAPK6) (d), and transcription factors (ZAT6 and ZAT12) (e). Leaves taken from Col-0 and the dorn1-3 mutant were treated for 2 h with 2 mM adenosine triphosphate (ATP) and diadenosine tetraphosphate (Ap4A). Transcript levels are represented as Log2(2–ΔΔCt) compared to the MOCK-treated (control) plants. The housekeeping gene AtACT2 was used for data normalisation as an endogenous control. Data are mean ± SD from 3 biological replicates. Different letters above the error bars indicate statistically significant differences according to the ANOVA analysis with the post-hoc Tukey’s HSD multiple comparisons test (p < 0.05).
Other components involved in a variety of signalling pathways, ranging from development to stress responses, are cyclic nucleotide-gated channels (CNGCs) [57,58]. Moreover, AtCNGC2 mediates eATP signal transduction in cells of the root epidermis [20]. We found that Ap4A induced CNGC2 expression in Col-0 plants and decreased the expression in the dorn1-3 mutant. Extracellular ATP decreased the expression of CNGC2 in both Col-0 and dorn1-3 mutant plants (Figure 5b). We also focused on essential protein kinases, such as SnRKs, that regulate cellular energy homeostasis, stress response, and growth [59]. Thus, we checked the changes in the expression of SnRK1.1, SnRK1.2, SnRK2.1, SnRK2.2, and SnRK2.6. We also tested the expression of PV42a encoding cystathionine-β-synthase (CBS) domain-containing protein belonging to the PV42 class of γ-type subunits of the plant SnRK1 complexes. It is known that CBS domains generally act as regulatory domains of protein activity through adenosyl ligand binding [60]. Our experiments showed that eATP strongly induced the expression of SnRK1.1, SnRK1.2, and PV42a in Col-0 plants. Although Ap4A causes a lower effect than eATP, the elevation in the expression of SnRK1.1 and SnRK1.2 was statistically significant. Interestingly, in Col-0 plants, only eATP up-regulates the transcription of PV42a. Still, in the dorn1-3 mutant compared to Col-0, only Ap4A treatment caused induction of the expression (Figure 5c). Extracellular ATP and Ap4A increased the expression of SnRK2.2, SnRK2.3, and SnRK2.6 in Col-0 plants. In the dorn1-3 mutant plants, Ap4A down-regulated SnRK2.2, SnRK2.3, and SnRK2.6. Still, the effect of eATP in the mutant was not the same for the expression of the three SnRK2 genes; namely, the expression of SnRK2.2 was decreased, SnRK2.3 was slightly increased, and there was no effect on SnRK2.6 expression (Figure 5c). The strong relationships between secondary messengers, such as ROS and MAPKs, are often highlighted in the literature [61,62]. MAPK6, among its roles in various metabolic processes in plants, can regulate the activities of diverse targets, including transcription factors [63]. We observed an up-regulation of MAPK6 expression by both eATP and Ap4A in Col-0 plants and down-regulation in the dorn1-3 mutant (Figure 5d). Among the transcription factors that MAPKs regulate, we tested the regulation of expression of the zinc-finger transcription factors (ZAT6 and ZAT12) and found that eATP and Ap4A up-regulated the expression of both genes, as mentioned above, in Col-0 plants. Extracellular ATP increased the expression of ZAT6 and ZAT12 also in the dorn1-3 mutant, but Ap4A downregulated the expression of both genes in the mutant plants (Figure 5e).
3. Discussion
Plants are exposed to continuous changes in environmental conditions that lead to an imbalance in cellular homeostasis. It is known that in response to various stresses in prokaryotic and eukaryotic cells, NpnNs accumulate. The accumulation of such uncommon nucleotides can be considered in the context of the “friend hypothesis” (alarmone) and “foe hypothesis” regarding critically damaged cells as a result of internal and external stresses [33,64]. Although there are identified Ap4A-binding protein targets in cells [33], the signalling pathways are still unclear. We reported previously that extracellular NpnNs regulate the phenylpropanoid pathway, producing secondary metabolites—key molecules in response to abiotic stress in Arabidopsis thaliana and Vitis vinifera [28,29,30]. Notably, one of the phenylpropanoid pathway enzymes, 4-coumarate:CoA ligase, is known to catalyse the synthesis of Ap4A [65], and its activity was increased by Ap4A [28]. It is known that some extracellular ApnN may become internalised and operate intracellularly [33]. Despite this obvious evidence of the signalling function of uncommon nucleotides in regulating phenylpropanoid synthesis, no receptors or signalling pathways have been identified in plants until now. Here, we demonstrated, for the first time, that Ap4A and Cp4C evoked stomatal closure in Arabidopsis thaliana leaves (Figure 1). We did not observe such an effect in dorn1-3 plants under the Ap4A effect (Figure 2). Thus, we can conclude that plasma membrane purinoreceptor P2K1/DORN1 is essential in Ap4A perception. However, our research also indicates that P2K1/DORN1 is not involved in signal perception elicited by Cp4C (Figure 2). Such results suggest that in plants, P2K1/DORN1 is not Cp4C-binding, or there are other protein(s) interacting with this nucleotide. After Ap4A signal recognition, P2K1/DORN1 stimulates ROS burst and the defence-related response. Our data indicating ROS involvement in the plant response to Ap4A and Cp4C support the hypothesis concerning the signalling function of Np4Ns (Figure 3 and Figure 4). Moreover, the HPLC assay proved that Ap4A was not degraded to corresponding mononucleotides, which could evoke stomatal closure during the experiment (Figure S1). Only a tiny amount of CTP was detected in a solution of Cp4C after the investigation. Still, as we proved, CTP did not evoke stomatal closure (Figure 1). Therefore, it confirms that the observed stomatal closure and ROS accumulation were caused by Ap4A and Cp4C but not by their decomposition products.
The upregulation of defence-related genes encoding proteins involved in signalling hubs was reported [59]. The expression of the genes described in this research was mostly abolished or down-regulated in the dorn1-3 mutant (Figure 5). Recent studies consider cross-talk between diverse plant defence response markers such as ROS, hormones, and kinase cascades, leading to transcriptional, translational, and metabolic reprogramming [54]. Our transcriptional analysis focused on elements that integrate various signals and included cyclic nucleotide-gated channels (CNGCs) and NADPH oxidases—respiratory burst oxidase homologs (RBOHD and RBOHF) that generate ROS. Moreover, our studies are focused on SNF1-related protein kinases (SnRKs) and PV42a, a cystathionine-β-synthase (CBS) domain-containing protein, belongs to the PV42 class of γ-type subunits of the plant SnRK1 complexes. The next elements of signal transduction pathways that we tested concern MAPK6 and transcription factors (ZATs) (Figure 5). The transcript level of CGNC2 increased only under Ap4A in Col-0 plant leaves (Figure 5b). Involving CGNC2 in another purine nucleotide, eATP, signal transduction in the root epidermis and eATP-induced Ca2+ influx were described by Wang [20]. This result suggests that CNGC channels can be a part of signal transduction evoked by Ap4A.
Rapid systemic signalling in response to stress can be stimulated by RBOHD and RBOHF, producing apoplastic ROS [66]. It is known that the elevated production of ROS and stomatal closure are mediated by eATP recognition by the receptor P2K1/DORN1, followed by direct phosphorylation of RBOHD [12], while RBOHD expression was significantly reduced in dorn1-3 mutant plants. Our studies showed that transcriptomic changes in both RBOHD and RBOHF evoked by Ap4A are similar, but in the dorn1-3 plants, the expression of RBOHF was also strongly inhibited (Figure 5a). This observation correlated with the accumulation of ROS in Arabidopsis thaliana leaves (Figure 3). Stress signalling in plants also involves different families of kinases, including the MAPK module, that can be activated by ROS [67]. Moreover, it was previously shown that MAPKs are activated by eATP [26,68,69,70]. We observed the induction of MAPK6 expression evoked by eATP and Ap4A (Figure 5d), and it is known that MPK6 modulates actin remodelling to activate stomatal defence in Arabidopsis thaliana [71]. MAPK pathways are necessary for several ABA responses in many plant species, including antioxidant defence and guard cell signalling [47,48]. Protein complexes SNF1-related protein kinase 1s (SnRK1s) and SnRK2s play a prominent role in ABA signalling [72,73]. Numerous studies indicate SnRK1s and SnRK2s as regulators of the target of rapamycin (TOR) kinase activity in controlling autophagy [74,75]. We observed that Ap4A induced the expression of both SnRK1s and SnRK2s at a similar level in Col-0 plants. However, induction evoked by eATP was much higher for SnRK1s than SnRK2s in wild-type plants. In the dorn1-3 mutant, the expression of SnRK1s and SnRK2s was decreased (Figure 5c). Also, neither of the tested pyrimidine nucleotides, CTP and Cp4C, affected the expression of SnRKs in Col-0 plants (Figure S2). It is known that SnRKs can regulate RBOH, which is engaged in ROS production [54]. The SnRK1s and SnRK2s were identified as critical nodes for stress and growth signalling pathways [59]. Moreover, it was suggested that under normal conditions, cytosol-localised SnRK1.1, in response to high-ammonium or low-pH stress, migrates to the nucleus and promotes the phosphorylation of the transcription factors regulating the expression of responsive genes [76]. Studies on AKINβ1, subunit SnRK1, showed its regulatory effect on secondary metabolic processes (e.g., flavonoid metabolism) [77]. Another SnRK1 subunit is PV42a, which is the CBS domain protein. Ap4A did not change the expression of the gene encoding AtPV42a in Col-0 plants (Figure 5c). It is known that enzymes containing CBS domains can be regulated by Ap4A binding [33]. Therefore, we postulate that AtPV42a regulates SnRK1s in response to Ap4A. Moreover, SnRK1, SnRK2, and MAPK interact with transcriptional factors [78,79]. The induction of ZAT12 and ZAT6 transcription factors in which MAPK6 is involved in an abiotic stress marker was described [63]. In the present research, we found that Ap4A and eATP induced both ZAT6 and ZAT12 gene expression in Col-0 plants, and lack of the P2K1/DORN1 receptor in the dorn1-3 mutants diminished this effect (Figure 5e). It is known that the transcript level of ZAT6 positively affected the concentrations of phenylpropanoids, including anthocyanin and total flavonoids [80]. Moreover, it was proved that ZAT6 and ZAT12 are involved in the response to cadmium stress and abiotic stress in plants [81,82,83,84], and the expression of ZAT12 was strictly dependent on the ROS wave [85,86].
The results of our research presented here shed more light on the signalling function of Ap4A, its perception and signal transduction pathway in plants. We had previously proposed a hypothetical NpnN signalling network in a plant cell [2]. Then, we strongly suggested the existence of some receptor and signalling transduction pathways involving signalling hubs and transcription factors resulting in gene expression changes, including genes coding for enzymes catalysing the phenylpropanoid pathway [2,28,29,30]. Here, we fill a few gaps in this network (Figure 6).
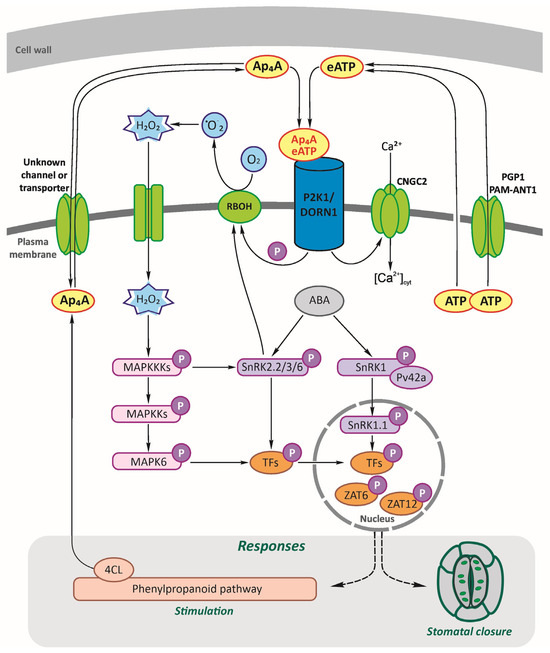
Figure 6.
Hypothetical working model of diadenosine tetraphosphate (Ap4A) signalling network in a plant cell. Ap4A, similar to extracellular adenosine triphosphate (eATP) [26], can be recognised by the purinoreceptor P2K1/DORN1 and lead to stomatal closure. As our study showed, Ap4A triggered the reactive oxygen species (ROS) wave, which evoked changes in the expression of the defence-related genes encoding proteins involved in signalling hubs, such as CNGC2; RBOHD and RBOHF generate ROS; SnRKs; AtPV42a, γ-type subunits of the plant SnRK1 complexes; MAPK cascades; and transcription factors, ZATs. The wounded cell membrane and transporters can release ATP to the extracellular space matrix: PGP1, p-glycoprotein belonging to ATP-binding cassette ABC transporters, and PM-ANT1, plasma membrane-localised nucleotide transporters [15,87]. Extracellular ATP recognition by P2K1/DORN1 evoked phosphorylation of RBOHD [12]. Also, CNGC2 [20] and MAPK cascades are involved in eATP signal transduction [26,68,69,70]. We previously described that 4-coumarate:CoA ligase (4CL), the branch point of the phenylpropanoid pathway, can synthesise Ap4A [65], and its activity is induced by Ap4A [28]. As yet, no channel or transporter for Ap4A in plants is known. P, phosphate.
4. Materials and Methods
4.1. Nucleotides
Ap4A and Cp4C were synthesised following previously reported procedures, purified by reverse-phase HPLC, and isolated as ammonium (NH4+) salts. The purities (>95%) were confirmed by analytical HPLC, 1H NMR and 31P NMR [30].
4.2. Plant Material
Arabidopsis thaliana lines were in the Columbia (Col-0) ecotype. A T-DNA insertion line of LecRK-I.9 (Salk_042209; dorn1-3) was obtained from NASC (Nottingham Arabidopsis Stock Centre, Nottingham, UK). Surface-sterilised seeds were stratified in darkness at 4 °C for 48 h and transferred to a growth chamber. Plants were grown for four weeks on the soil at 21–23 °C, 60–70% humidity, under a long-day photoperiod (16 h light and 8 h dark), 120 µmol m−2 s−1 light intensity. Genotyping of insertional mutants is described in Methods S1. Primers are listed in Table S1.
4.3. Stomatal Aperture Measurement
To ensure fully open stomata, plants were placed for 3 h under light intensity 120 µmol m−2 s−1. Samples of leaf epidermis were obtained from the abaxial side. They were placed on a microscope slide for 2 h of incubation in (i) MOCK solution MES/KOH opening buffer containing 10 mM MES pH 6.15, 10 mM KCl, 10 μM CaCl2 (control), (ii) 10 µM abscisic acid (ABA, Sigma Aldrich, St. Louis, MO, USA, A1049) dissolved in the MOCK solution MES/KOH buffer, and (iii) 2 mM ADP (Sigma, A2754), ATP (Sigma, AA8937), Ap3A, Ap4A, and CDP (Sigma, C9755), CTP, Cp3C, Cp4C dissolved in the MOCK solution MES/KOH buffer. We chose 2 mM concentration of nucleotides based on literature data concerning the effect of eATP on regulation of stomatal aperture [12].
CTP and NpnNs were synthesised as described previously [30]. Stomata were observed using the ZOE Fluorescent Cell Imager (Bio-Rad, Hercules, CA, USA, 1450031EDU). Measurements, including stomatal aperture width and length, were performed with ImageJ 1.54g software. The involvement of ROS in stomatal movement under nucleotide treatment was examined by the simultaneous addition of ROS enzyme scavengers to the nucleotide solutions. Catalase (CAT) (Sigma Aldrich, C100) and superoxide dismutase (SOD) (Sigma Aldrich, S9697), in a concentration of 100 units mL−1 and 500 units mL−1, respectively, were used together in an incubation mixture.
4.4. Detection of Intracellular ROS Burst in Leaves
Two leaves were incubated in 3 mL of MOCK solution MES/KOH opening buffer or the buffer enriched in 2 mM concentrations of tested nucleotides. After 2 h, the incubating buffers were gently replaced with 3 mL of staining solutions, and submerged leaves were vacuum infiltrated three times (1 min each time). The staining solution for •O2− detection was composed of 0.5% nitroblue tetrazolium (NBT, Sigma-Aldrich, N6876) dissolved in 10 mM potassium phosphate buffer, pH 7.8 [88], and the staining solution for H2O2 synthesis was composed of 3,3′-diaminobenzidine tetrahydrochloride (DAB, Sigma-Aldrich, D5905) (1 mg ml−1 DAB) dissolved in 10 mM potassium phosphate buffer, pH 7.4, and 0.05% Tween [89]. Samples were incubated at room temperature for the next 2 h in the dark with continuous shaking. Then, leaves were incubated in 96% ethanol overnight for bleaching, and the photographs were taken with an Epson Perfection V700 scanner.
4.5. Gene Expression Analyses
According to the manufacturer’s instructions, total RNA was extracted from leaves using the RNeasy Plant Mini Kit (Qiagen, Germantown MD, USA). Evaluation of RNA purity, cDNA synthesis, reverse transcription, and RT-qPCR were performed as described previously by Pietrowska-Borek and co-workers [28,90,91]. The qRT-PCR reactions were performed using a CFX96 Real-Time PCR Detection System (Bio-Rad). The specific primers for Arabidopsis thaliana genes are listed in Table S1. The 2−ΔΔCt method [92] was applied to calculate the relative gene expression. The data were normalised against the reference gene, ACTIN2 (ACT2). For statistical analysis, the gene expression data were Log2-transformed to meet distribution and variance assumptions.
4.6. Statistical Analysis
All experiments were performed at least three times. The results are shown as the mean ± SD. The statistical significance of the differences among the means was analysed by the ANOVA with the post-hoc Tukey’s HSD multiple comparisons test (p < 0.05) using Statistica, Version 13 (TIBCO Software Inc., Palo Alto, CA, USA).
5. Conclusions
In the present work, we confirmed that in plants, an Ap4A receptor exists, and we found that it is purine receptor P2K1/DORN1. Moreover, we indicated ROSs as second messengers, kinases, and transcription factors engaged in the Ap4A signal transduction pathway. Nevertheless, further studies, both in silico and in vitro, on the binding of Ap4A to the P2K1/DORN1, including key residues that modulate Ap4A affinity, are required. We believe that the presented results in this paper contribute to the description of the role of NpnNs in signalling hubs and can help better understand the function of uncommon nucleotides in plants.
Supplementary Materials
The supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms242316688/s1. Refs. [93,94,95,96,97,98,99,100] are cited in the supplementary materials.
Author Contributions
J.D. co-designed the studies, carried out experiments, analysed results, and was involved in statistical analysis, visualisation, and writing of the original draft of the manuscript; V.H.N. and J.K. synthesised the dinucleoside polyphosphates and participated in reviewing and editing of the manuscript; S.B. participated in writing and critically reviewing the manuscript and co-designed and prepared Figure 6; M.P.-B. conceived the topic of the research, planned and supervised all experiments, analysed all results, performed the statistical analysis, participated in writing the draft of the manuscript, co-designed and co-created all figures, and prepared the final version of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The publication was co-financed within the framework of the Polish Ministry of Science and Higher Education’s programme “Regional Excellence Initiative” in the years 2019–2023 (No. 005/RID/2018/19)”, financing amounting PLN 12 000 000 (M.P.-B.); the statutory activity of Poznań University of Life Sciences, no. 506.181.01 (M.P.-B. and J.D.) and no. 506.181.09 (M.P.-B.); and the funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No 861381 (NATURE-ETN) (V.H.N.).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original data obtained during this research are available from the corresponding authors on reasonable request, and some of them are also accessible in the Supplementary materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sun, T.; Zhang, Y. Short- and Long-distance Signaling in Plant Defense. Plant J. 2021, 105, 505–517. [Google Scholar] [CrossRef] [PubMed]
- Pietrowska-Borek, M.; Dobrogojski, J.; Sobieszczuk-Nowicka, E.; Borek, S. New Insight into Plant Signaling: Extracellular ATP and Uncommon Nucleotides. Cells 2020, 9, 345. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Sivaguru, M.; Stacey, G. Extracellular ATP in Plants. Visualization, Localization, and Analysis of Physiological Significance in Growth and Signaling. Plant Physiol. 2006, 142, 984–992. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Steinebrunner, I.; Sun, Y.; Butterfield, T.; Torres, J.; Arnold, D.; Gonzalez, A.; Jacob, F.; Reichler, S.; Roux, S.J. Apyrases (Nucleoside Triphosphate-Diphosphohydrolases) Play a Key Role in Growth Control in Arabidopsis. Plant Physiol. 2007, 144, 961–975. [Google Scholar] [CrossRef]
- Riewe, D.; Grosman, L.; Fernie, A.R.; Wucke, C.; Geigenberger, P. The Potato-Specific Apyrase Is Apoplastically Localized and Has Influence on Gene Expression, Growth, and Development. Plant Physiol. 2008, 147, 1092–1109. [Google Scholar] [CrossRef]
- Tonón, C.; Cecilia Terrile, M.; José Iglesias, M.; Lamattina, L.; Casalongué, C. Extracellular ATP, Nitric Oxide and Superoxide Act Coordinately to Regulate Hypocotyl Growth in Etiolated Arabidopsis Seedlings. J. Plant Physiol. 2010, 167, 540–546. [Google Scholar] [CrossRef]
- Clark, G.; Wu, M.; Wat, N.; Onyirimba, J.; Pham, T.; Herz, N.; Ogoti, J.; Gomez, D.; Canales, A.A.; Aranda, G.; et al. Both the Stimulation and Inhibition of Root Hair Growth Induced by Extracellular Nucleotides in Arabidopsis Are Mediated by Nitric Oxide and Reactive Oxygen Species. Plant Mol. Biol. 2010, 74, 423–435. [Google Scholar] [CrossRef]
- Zhu, R.; Dong, X.; Xue, Y.; Xu, J.; Zhang, A.; Feng, M.; Zhao, Q.; Xia, S.; Yin, Y.; He, S.; et al. Redox-Responsive Transcription Factor 1 (RRFT1) Is Involved in Extracellular ATP-Regulated Arabidopsis thaliana Seedling Growth. Plant Cell Physiol. 2020, 61, 685–698. [Google Scholar] [CrossRef]
- Reichler, S.A.; Torres, J.; Rivera, A.L.; Cintolesi, V.A.; Clark, G.; Roux, S.J. Intersection of Two Signalling Pathways: Extracellular Nucleotides Regulate Pollen Germination and Pollen Tube Growth via Nitric Oxide. J. Exp. Bot. 2009, 60, 2129–2138. [Google Scholar] [CrossRef]
- Wu, Y.; Qin, B.; Feng, K.; Yan, R.; Kang, E.; Liu, T.; Shang, Z. Extracellular ATP Promoted Pollen Germination and Tube Growth of Nicotiana Tabacum through Promoting K+ and Ca2+ Absorption. Plant Reprod. 2018, 31, 399–410. [Google Scholar] [CrossRef]
- Chivasa, S.; Ndimba, B.K.; Simon, W.J.; Lindsey, K.; Slabas, A.R. Extracellular ATP Functions as an Endogenous External Metabolite Regulating Plant Cell Viability. Plant Cell 2005, 17, 3019–3034. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Cao, Y.; Li, H.; Kim, D.; Ahsan, N.; Thelen, J.; Stacey, G. Extracellular ATP Elicits DORN1-Mediated RBOHD Phosphorylation to Regulate Stomatal Aperture. Nat. Commun. 2017, 8, 2265. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, D.; Zhang, T.; Koo, A.J.; Stacey, G.; Tanaka, K. Extracellular ATP Acts on Jasmonate Signaling to Reinforce Plant Defense. Plant Physiol. 2018, 176, 511–523. [Google Scholar] [CrossRef] [PubMed]
- Goodman, H.L.; Kroon, J.T.M.; Tomé, D.F.A.; Hamilton, J.M.U.; Alqarni, A.O.; Chivasa, S. Extracellular ATP Targets Arabidopsis RIBONUCLEASE 1 to Suppress Mycotoxin Stress-Induced Cell Death. New Phytol. 2022, 235, 1531–1542. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.; Rajagopal, A.; Windsor, B.; Dudler, R.; Lloyd, A.; Roux, S.J. A Role for Ectophosphatase in Xenobiotic Resistance. Plant Cell 2000, 12, 519–533. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Yang, S.H.; Kim, T.J.; Han, J.S.; Suh, J.W. Hypertonic Stress Increased Extracellular ATP Levels and the Expression of Stress-Responsive Genes in Arabidopsis thaliana Seedlings. Biosci. Biotechnol. Biochem. 2009, 73, 1252–1256. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhang, X.; Deng, S.; Zhang, C.; Wang, M.; Ding, M.; Zhao, R.; Shen, X.; Zhou, X.; Lu, C.; et al. Extracellular ATP Signaling Is Mediated by H2O2 and Cytosolic Ca2+ in the Salt Response of Populus euphratica Cells. PLoS ONE 2012, 7, e53136. [Google Scholar] [CrossRef]
- Hou, Q.Z.; Sun, K.; Zhang, H.; Su, X.; Fan, B.Q.; Feng, H.Q. The Responses of Photosystem II and Intracellular ATP Production of Arabidopsis Leaves to Salt Stress Are Affected by Extracellular ATP. J. Plant Res. 2018, 131, 331–339. [Google Scholar] [CrossRef]
- Duong, H.N.; Cho, S.H.; Wang, L.; Pham, A.Q.; Davies, J.M.; Stacey, G. Cyclic Nucleotide-Gated Ion Channel 6 Is Involved in Extracellular ATP Signaling and Plant Immunity. Plant J. 2021, 109, 1386–1396. [Google Scholar] [CrossRef]
- Wang, L.; Ning, Y.; Sun, J.; Wilkins, K.A.; Matthus, E.; McNelly, R.E.; Dark, A.; Rubio, L.; Moeder, W.; Yoshioka, K.; et al. Arabidopsis thaliana CYCLIC NUCLEOTIDE-GATED CHANNEL2 Mediates Extracellular ATP Signal Transduction in Root Epidermis. New Phytol. 2022, 234, 412–421. [Google Scholar] [CrossRef]
- Foresi, N.P.; Laxalt, A.M.; Tonón, C.V.; Casalongué, C.A.; Lamattina, L. Extracellular ATP Induces Nitric Oxide Production in Tomato Cell Suspensions. Plant Physiol. 2007, 145, 589–592. [Google Scholar] [CrossRef]
- Wu, S.-J.; Wu, J.-Y. Extracellular ATP-Induced NO Production and Its Dependence on Membrane Ca2+ Flux in Salvia miltiorrhiza Hairy Roots. J. Exp. Bot. 2008, 59, 4007–4016. [Google Scholar] [CrossRef]
- Song, C.J.; Steinebrunner, I.; Wang, X.; Stout, S.C.; Roux, S.J. Extracellular ATP Induces the Accumulation of Superoxide via NADPH Oxidases in Arabidopsis. Plant Physiol. 2006, 140, 1222–1232. [Google Scholar] [CrossRef]
- Wu, S.-J.; Liu, Y.-S.; Wu, J.-Y. The Signaling Role of Extracellular ATP and Its Dependence on Ca2+ Flux in Elicitation of Salvia miltiorrhiza Hairy Root Cultures. Plant Cell Physiol. 2008, 49, 617–624. [Google Scholar] [CrossRef]
- Demidchik, V.; Shang, Z.; Shin, R.; Thompson, E.; Rubio, L.; Laohavisit, A.; Mortimer, J.C.; Chivasa, S.; Slabas, A.R.; Glover, B.J.; et al. Plant Extracellular ATP Signalling by Plasma Membrane NADPH Oxidase and Ca2+ Channels. Plant J. 2009, 58, 903–913. [Google Scholar] [CrossRef]
- Choi, J.; Tanaka, K.; Cao, Y.; Qi, Y.; Qiu, J.; Liang, Y.; Lee, S.Y.; Stacey, G. Identification of a Plant Receptor for Extracellular ATP. Science 2014, 343, 290–294. [Google Scholar] [CrossRef]
- Li, P.; Zhao, L.; Qi, F.; Htwe, N.M.P.S.; Li, Q.; Zhang, D.; Lin, F.; Shang-Guan, K.; Liang, Y. The Receptor-like Cytoplasmic Kinase RIPK Regulates Broad-Spectrum ROS Signaling in Multiple Layers of Plant Immune System. Mol. Plant 2021, 14, 1652–1667. [Google Scholar] [CrossRef]
- Pietrowska-Borek, M.; Nuc, K.; Zielezińska, M.; Guranowski, A. Diadenosine Polyphosphates (Ap3A and Ap4A) Behave as Alarmones Triggering the Synthesis of Enzymes of the Phenylpropanoid Pathway in Arabidopsis thaliana. FEBS Open Bio 2011, 1, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Pietrowska-Borek, M.; Czekała, Ł.; Belchí-Navarro, S.; Pedreño, M.A.; Guranowski, A. Diadenosine Triphosphate Is a Novel Factor Which in Combination with Cyclodextrins Synergistically Enhances the Biosynthesis of Trans-Resveratrol in Vitis vinifera Cv. Monastrell Suspension Cultured Cells. Plant Physiol. Biochem. 2014, 84, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Pietrowska-Borek, M.; Wojdyła-Mamoń, A.; Dobrogojski, J.; Młynarska-Cieślak, A.; Baranowski, M.R.; Dąbrowski, J.M.; Kowalska, J.; Jemielity, J.; Borek, S.; Pedreño, M.A.; et al. Purine and Pyrimidine Dinucleoside Polyphosphates Differentially Affect the Phenylpropanoid Pathway in Vitis vinifera L. Cv. Monastrell Suspension Cultured Cells. Plant Physiol. Biochem. 2020, 147, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Dixon, R.A.; Paiva, N.L. Stress-Induced Phenylpropanoid Metabolism. Plant Cell 1995, 7, 1085–1097. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of Phenylpropanoid Pathway and the Role of Polyphenols in Plants under Abiotic Stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, F.; McLennan, A.G.; Urbaniak, M.D.; Jones, N.J.; Copeland, N.A. Re-Evaluation of Diadenosine Tetraphosphate (Ap4A) from a Stress Metabolite to Bona Fide Secondary Messenger. Front. Mol. Biosci. 2020, 7, 606807. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.C.; Bochner, B.R.; Ames, B.N. AppppA, Heat-Shock Stress, and Cell Oxidation. Proc. Natl. Acad. Sci. USA 1983, 80, 7496–7500. [Google Scholar] [CrossRef]
- Bochner, B.R.; Lee, P.C.; Wilson, S.W.; Cutler, C.W.; Ames, B.N. AppppA and Related Adenylylated Nucleotides Are Synthesized as a Consequence of Oxidation Stress. Cell 1984, 37, 225–232. [Google Scholar] [CrossRef]
- Baltzinger, M.; Ebel, J.-P.; Remy, P. Accumulation of Dinucleoside Polyphosphates in Saccharomyces cerevisiae under Stress Conditions. High Levels Are Associated with Cell Death. Biochimie 1986, 68, 1231–1236. [Google Scholar] [CrossRef]
- Coste, H.; Brevet, A.; Plateau, P.; Blanquet, S. Non-Adenylylated Bis(5’-Nucleosidyl) Tetraphosphates Occur in Saccharomyces cerevisiae and in Escherichia coli and Accumulate upon Temperature Shift or Exposure to Cadmium. J. Biol. Chem. 1987, 262, 12096–12103. [Google Scholar] [CrossRef]
- Pálfi, Z.; Surányi, G.; Borbély, G. Alterations in the Accumulation of Adenylylated Nucleotides in Heavy-Metal-Ion-Stressed and Heat-Stressed Synechococcus Sp. Strain PCC 6301, a Cyanobacterium, in Light and Dark. Biochem. J. 1991, 276, 487–491. [Google Scholar] [CrossRef][Green Version]
- Pham, A.Q.; Cho, S.-H.; Nguyen, C.T.; Stacey, G. Arabidopsis Lectin Receptor Kinase P2K2 Is a Second Plant Receptor for Extracellular ATP and Contributes to Innate Immunity. Plant Physiol. 2020, 183, 1364–1375. [Google Scholar] [CrossRef]
- Cho, S.-H.; Nguyen, C.T.; Pham, A.Q.; Stacey, G. Computational Prediction and in Vitro Analysis of the Potential Ligand Binding Site within the Extracellular ATP Receptor, P2K2. Plant Signal. Behav. 2023, 18, e2173146. [Google Scholar] [CrossRef]
- Jose, J.; Ghantasala, S.; Choudhury, S.R. Arabidopsis Transmembrane Receptor-like Kinases (RLKS): A Bridge between Extracellular Signal and Intracellular Regulatory Machinery. Int. J. Mol. Sci. 2020, 21, 4000. [Google Scholar] [CrossRef] [PubMed]
- Vigne, P.; Breittmayer, J.P.; Frelin, C. Diadenosine Polyphosphates as Antagonists of the Endogenous P2Y1 Receptor in Rat Brain Capillary Endothelial Cells of the B7 and B10 Clones: APnAs as Antagonists of P2Y1 Receptors. Br. J. Pharmacol. 2000, 129, 1506–1512. [Google Scholar] [CrossRef] [PubMed][Green Version]
- McDonald, H.A.; Chu, K.L.; Bianchi, B.R.; McKenna, D.G.; Briggs, C.A.; Burgard, E.C.; Lynch, K.J.; Faltynek, C.; Cartmell, J.; Jarvis, M.F. Potent Desensitization of Human P2X3 Receptors by Diadenosine Polyphosphates. Eur. J. Pharmacol. 2002, 435, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chang, C.-F.; Morales, M.; Chiang, Y.-H.; Harvey, B.K.; Su, T.-P.; Tsao, L.-I.; Chen, S.; Thiemermann, C. Diadenosine Tetraphosphate Protects against Injuries Induced by Ischemia and 6-Hydroxydopamine in Rat Brain. J. Neurosci. 2003, 23, 7958–7965. [Google Scholar] [CrossRef] [PubMed]
- Verspohl, E.J.; Johannwille, B.; Kaiserling-Buddemeier, I.; Schlüter, H.; Hagemann, J. Diadenosine Polyphosphates in Cultured Vascular Smooth-Muscle Cells and Endothelium Cells—Their Interaction with Specific Receptors and Their Degradation. J. Pharm. Pharmacol. 2010, 51, 1175–1181. [Google Scholar] [CrossRef]
- Burnstock, G. Purine and Purinergic Receptors. Brain Neurosci. Adv. 2018, 2, 1–10. [Google Scholar] [CrossRef]
- Hsu, P.-K.; Dubeaux, G.; Takahashi, Y.; Schroeder, J.I. Signaling Mechanisms in Abscisic Acid-Mediated Stomatal Closure. Plant J. Cell Mol. Biol. 2021, 105, 307–321. [Google Scholar] [CrossRef]
- Danquah, A.; De Zelicourt, A.; Colcombet, J.; Hirt, H. The Role of ABA and MAPK Signaling Pathways in Plant Abiotic Stress Responses. Biotechnol. Adv. 2014, 32, 40–52. [Google Scholar] [CrossRef]
- Bharath, P.; Gahir, S.; Raghavendra, A.S. Abscisic Acid-Induced Stomatal Closure: An Important Component of Plant Defense against Abiotic and Biotic Stress. Front. Plant Sci. 2021, 12, 615114. [Google Scholar] [CrossRef]
- Guranowski, A. Metabolism of Diadenosine Tetraphosphate (Ap4A) and Related Nucleotides in Plants; Review with Historical and General Perspective. Front. Biosci. 2004, 9, 1398. [Google Scholar] [CrossRef]
- Waszczak, C.; Carmody, M.; Kangasjarvi, J. Reactive Oxygen Species in Plant Signaling. Annu. Rev. Plant Biol. 2018, 69, 209–236. [Google Scholar] [CrossRef]
- Smirnoff, N.; Arnaud, D. Hydrogen Peroxide Metabolism and Functions in Plants. New Phytol. 2019, 221, 1197–1214. [Google Scholar] [CrossRef] [PubMed]
- Macho, A.P.; Zipfel, C. Plant PRRs and the Activation of Innate Immune Signaling. Mol. Cell 2014, 54, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R.; Zandalinas, S.I.; Fichman, Y.; Van Breusegem, F. Reactive Oxygen Species Signalling in Plant Stress Responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 663–679. [Google Scholar] [CrossRef] [PubMed]
- Khokon, A.R.; Okuma, E.; Hossain, M.A.; Munemasa, S.; Uraji, M.; Nakamura, Y.; Mori, I.C.; Murata, Y. Involvement of Extracellular Oxidative Burst in Salicylic Acid-Induced Stomatal Closure in Arabidopsis: Extracellular ROS Mediate SA-Induced Stomatal Closure. Plant Cell Environ. 2011, 34, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Jewell, J.B.; Sowders, J.M.; He, R.; Willis, M.A.; Gang, D.R.; Tanaka, K. Extracellular ATP Shapes a Defense-Related Transcriptome Both Independently and along with Other Defense Signaling Pathways. Plant Physiol. 2019, 179, 1144–1158. [Google Scholar] [CrossRef] [PubMed]
- Duszyn, M.; Świeżawska, B.; Szmidt-Jaworska, A.; Jaworski, K. Cyclic Nucleotide Gated Channels (CNGCs) in Plant Signalling-Current Knowledge and Perspectives. J. Plant Physiol. 2019, 241, 153035. [Google Scholar] [CrossRef]
- Jarratt-Barnham, E.; Wang, L.; Ning, Y.; Davies, J.M. The Complex Story of Plant Cyclic Nucleotide-Gated Channels. Int. J. Mol. Sci. 2021, 22, 874. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Y.; Zhu, J.-K. Thriving under Stress: How Plants Balance Growth and the Stress Response. Dev. Cell 2020, 55, 529–543. [Google Scholar] [CrossRef]
- Baudry, K.; Barbut, F.; Domenichini, S.; Guillaumot, D.; Thy, M.P.; Vanacker, H.; Majeran, W.; Krieger-Liszkay, A.; Issakidis-Bourguet, E.; Lurin, C. Adenylates Regulate Arabidopsis Plastidial Thioredoxin Activities through the Binding of a CBS Domain Protein. Plant Physiol. 2022, 189, 2298–2314. [Google Scholar] [CrossRef]
- Matsushita, M.; Nakamura, T.; Moriizumi, H.; Miki, H.; Takekawa, M. Stress-Responsive MTK1 SAPKKK Serves as a Redox Sensor That Mediates Delayed and Sustained Activation of SAPKs by Oxidative Stress. Sci. Adv. 2020, 6, eaay9778. [Google Scholar] [CrossRef] [PubMed]
- Byrne, D.P.; Shrestha, S.; Galler, M.; Cao, M.; Daly, L.A.; Campbell, A.E.; Eyers, C.E.; Veal, E.A.; Kannan, N.; Eyers, P.A. Aurora A Regulation by Reversible Cysteine Oxidation Reveals Evolutionarily Conserved Redox Control of Ser/Thr Protein Kinase Activity. Sci. Signal. 2020, 13, eaax2713. [Google Scholar] [CrossRef] [PubMed]
- Smékalová, V.; Doskočilová, A.; Komis, G.; Šamaj, J. Crosstalk between Secondary Messengers, Hormones and MAPK Modules during Abiotic Stress Signalling in Plants. Biotechnol. Adv. 2014, 32, 2–11. [Google Scholar] [CrossRef] [PubMed]
- McLennan, A. Dinucleoside Polyphosphates—Friend or Foe? Pharmacol. Ther. 2000, 87, 73–89. [Google Scholar] [CrossRef] [PubMed]
- Pietrowska-Borek, M.; Stuible, H.-P.; Kombrink, E.; Guranowski, A. 4-Coumarate:Coenzyme A Ligase Has the Catalytic Capacity to Synthesize and Reuse Various (Di)Adenosine Polyphosphates. Plant Physiol. 2003, 131, 1401–1410. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.-G.; Hilleary, R.; Swanson, S.J.; Kim, S.-H.; Gilroy, S. Rapid, Long-Distance Electrical and Calcium Signaling in Plants. Annu. Rev. Plant Biol. 2016, 67, 287–307. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, S. MAPK Cascades in Plant Disease Resistance Signaling. Annu. Rev. Phytopathol. 2013, 51, 245–266. [Google Scholar] [CrossRef]
- Medina-Castellanos, E.; Esquivel-Naranjo, E.U.; Heil, M.; Herrera-Estrella, A. Extracellular ATP Activates MAPK and ROS Signaling during Injury Response in the Fungus Trichoderma atroviride. Front. Plant Sci. 2014, 5, 659. [Google Scholar] [CrossRef]
- Chen, D.; Hao, F.; Mu, H.; Ahsan, N.; Thelen, J.J.; Stacey, G. S-Acylation of P2K1 Mediates Extracellular ATP-Induced Immune Signaling in Arabidopsis. Nat. Commun. 2021, 12, 2750. [Google Scholar] [CrossRef]
- Cho, S.-H.; Tóth, K.; Kim, D.; Vo, P.H.; Lin, C.-H.; Handakumbura, P.P.; Ubach, A.R.; Evans, S.; Paša-Tolić, L.; Stacey, G. Activation of the Plant Mevalonate Pathway by Extracellular ATP. Nat. Commun. 2022, 13, 450. [Google Scholar] [CrossRef]
- Zou, M.; Guo, M.; Zhou, Z.; Wang, B.; Pan, Q.; Li, J.; Zhou, J.-M.; Li, J. MPK3- and MPK6-Mediated VLN3 Phosphorylation Regulates Actin Dynamics during Stomatal Immunity in Arabidopsis. Nat. Commun. 2021, 12, 6474. [Google Scholar] [CrossRef] [PubMed]
- Jossier, M.; Bouly, J.-P.; Meimoun, P.; Arjmand, A.; Lessard, P.; Hawley, S.; Grahame Hardie, D.; Thomas, M. SnRK1 (SNF1-Related Kinase 1) Has a Central Role in Sugar and ABA Signalling in Arabidopsis thaliana. Plant J. 2009, 59, 316–328. [Google Scholar] [CrossRef] [PubMed]
- Ou, X.; Li, T.; Zhao, Y.; Chang, Y.; Wu, L.; Chen, G.; Day, B.; Jiang, K. Calcium-Dependent ABA Signaling Functions in Stomatal Immunity by Regulating Rapid SA Responses in Guard Cells. J. Plant Physiol. 2022, 268, 153585. [Google Scholar] [CrossRef]
- Signorelli, S.; Tarkowski, Ł.P.; Van den Ende, W.; Bassham, D.C. Linking Autophagy to Abiotic and Biotic Stress Responses. Trends Plant Sci. 2019, 24, 413–430. [Google Scholar] [CrossRef]
- Belda-Palazón, B.; Adamo, M.; Valerio, C.; Ferreira, L.J.; Confraria, A.; Reis-Barata, D.; Rodrigues, A.; Meyer, C.; Rodriguez, P.L.; Baena-González, E. A Dual Function of SnRK2 Kinases in the Regulation of SnRK1 and Plant Growth. Nat. Plants 2020, 6, 1345–1353. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Fang, X.; Xiao, C.; Ma, Z.; Huang, X.; Su, J.; Li, J.; Wang, J.; Wang, S.; Luan, S.; et al. Kinase SnRK1.1 Regulates Nitrate Channel SLAH3 Engaged in Nitrate-Dependent Alleviation of Ammonium Toxicity. Plant Physiol. 2021, 186, 731–749. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, L.; Micallef, B.J.; Tetlow, I.J.; Mullen, R.T.; Feil, R.; Lunn, J.E.; Emes, M.J. AKINβ1, a Subunit of SnRK1, Regulates Organic Acid Metabolism and Acts as a Global Modulator of Genes Involved in Carbon, Lipid, and Nitrogen Metabolism. J. Exp. Bot. 2020, 71, 1010–1028. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, Q.; Tian, C.; Liu, G.; Pan, Y.; Xu, X.; Shi, X.; Zhang, Z.; Meng, L. Physiological and Transcriptome Analyses of CaCl2 Treatment to Alleviate Chilling Injury in Pineapple. Plants 2022, 11, 2215. [Google Scholar] [CrossRef]
- Son, S.; Im, J.H.; Ko, J.-H.; Han, K.-H. SNF1-Related Protein Kinase 1 Represses Arabidopsis Growth through Post-Translational Modification of E2Fa in Response to Energy Stress. New Phytol. 2023, 237, 823–839. [Google Scholar] [CrossRef]
- Shi, H.; Liu, G.; Wei, Y.; Chan, Z. The Zinc-Finger Transcription Factor ZAT6 Is Essential for Hydrogen Peroxide Induction of Anthocyanin Synthesis in Arabidopsis. Plant Mol. Biol. 2018, 97, 165–176. [Google Scholar] [CrossRef]
- Opdenakker, K.; Remans, T.; Keunen, E.; Vangronsveld, J.; Cuypers, A. Exposure of Arabidopsis thaliana to Cd or Cu Excess Leads to Oxidative Stress Mediated Alterations in MAPKinase Transcript Levels. Environ. Exp. Bot. 2012, 83, 53–61. [Google Scholar] [CrossRef]
- Shi, H.; Wang, X.; Ye, T.; Chen, F.; Deng, J.; Yang, P.; Zhang, Y.; Chan, Z. The Cysteine2/Histidine2-Type Transcription Factor ZINC FINGER OF ARABIDOPSIS THALIANA6 Modulates Biotic and Abiotic Stress Responses by Activating Salicylic Acid-Related Genes and C-REPEAT-BINDING FACTOR Genes in Arabidopsis. Plant Physiol. 2014, 165, 1367–1379. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yang, L.; Yan, X.; Liu, Y.; Wang, R.; Fan, T.; Ren, Y.; Tang, X.; Xiao, F.; Liu, Y.; et al. Zinc-Finger Transcription Factor ZAT6 Positively Regulates Cadmium Tolerance through the Glutathione-Dependent Pathway in Arabidopsis. Plant Physiol. 2016, 171, 707–719. [Google Scholar] [CrossRef] [PubMed]
- Dang, F.; Li, Y.; Wang, Y.; Lin, J.; Du, S.; Liao, X. ZAT10 Plays Dual Roles in Cadmium Uptake and Detoxification in Arabidopsis. Front. Plant Sci. 2022, 13, 994100. [Google Scholar] [CrossRef]
- Brumbarova, T.; Le, C.T.T.; Ivanov, R.; Bauer, P. Regulation of ZAT12 Protein Stability: The Role of Hydrogen Peroxide. Plant Signal. Behav. 2016, 11, e1137408. [Google Scholar] [CrossRef][Green Version]
- Myers, R.J., Jr.; Fichman, Y.; Stacey, G.; Mittler, R. Extracellular ATP Plays an Important Role in Systemic Wound Response Activation. Plant Physiol. 2022, 189, 1314–1325. [Google Scholar] [CrossRef]
- Rieder, B.; Neuhaus, H.E. Identification of an Arabidopsis Plasma Membrane-Located ATP Transporter Important for Anther Development. Plant Cell 2011, 23, 1932–1944. [Google Scholar] [CrossRef]
- Müller, K.; Carstens, A.C.; Linkies, A.; Torres, M.A.; Leubner-Metzger, G. The NADPH-oxidase AtrbohB Plays a Role in Arabidopsis Seed After-ripening. New Phytol. 2009, 184, 885–897. [Google Scholar] [CrossRef]
- Daudi, A.; O’Brien, J.A. Detection of Hydrogen Peroxide by DAB Staining in Arabidopsis Leaves. Bio-Protocol 2012, 2, e263. [Google Scholar] [CrossRef]
- Pietrowska-Borek, M.; Nuc, K.; Guranowski, A. Exogenous Adenosine 5’-Phosphoramidate Behaves as a Signal Molecule in Plants; It Augments Metabolism of Phenylpropanoids and Salicylic Acid in Arabidopsis thaliana Seedlings. Plant Physiol. Biochem. 2015, 94, 144–152. [Google Scholar] [CrossRef]
- Pietrowska-Borek, M.; Nuc, K. Both Cyclic-AMP and Cyclic-GMP Can Act as Regulators of the Phenylpropanoid Pathway in Arabidopsis thaliana Seedlings. Plant Physiol. Biochem. 2013, 70, 142–149. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing Real-Time PCR Data by the Comparative CT Method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Doyle, J.L.; Doyle, J.M. A Rapid DNA Isolation Procedure for Small Quantities of Fresh Leaf Tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- Nakashima, K.; Fujita, Y.; Kanamori, N.; Katagiri, T.; Umezawa, T.; Kidokoro, S.; Maruyama, K.; Yoshida, T.; Ishiyama, K.; Kobayashi, M.; et al. Three Arabidopsis SnRK2 Protein Kinases, SRK2D/SnRK2.2, SRK2E/SnRK2.6/OST1 and SRK2I/SnRK2.3, Involved in ABA Signaling Are Essential for the Control of Seed Development and Dormancy. Plant Cell Physiol. 2009, 50, 1345–1363. [Google Scholar] [CrossRef]
- Fang, L.; Hou, X.; Lee, L.Y.C.; Liu, L.; Yan, X.; Yu, H. AtPV42a and AtPV42b Redundantly Regulate Reproductive Development in Arabidopsis thaliana. PLoS ONE 2011, 6, e19033. [Google Scholar] [CrossRef] [PubMed]
- Kannan, P.; Pandey, D.; Gupta, A.K.; Punetha, H.; Taj, G.; Kumar, A. Expression Analysis of MAP2K9 and MAPK6 during Pathogenesis of Alternaria Blight in Arabidopsis thaliana Ecotype Columbia. Mol. Biol. Rep. 2012, 39, 4439–4444. [Google Scholar] [CrossRef] [PubMed]
- Le, C.T.T.; Brumbarova, T.; Ivanov, R.; Stoof, C.; Weber, E.; Mohrbacher, J.; Fink-Straube, C.; Bauer, P. ZINC FINGER OF ARABIDOPSIS THALIANA12 (ZAT12) Interacts with FER-LIKE IRON DEFICIENCY-INDUCED TRANSCRIPTION FACTOR (FIT) Linking Iron Deficiency and Oxidative Stress Responses. Plant Physiol. 2016, 170, 540–557. [Google Scholar] [CrossRef] [PubMed]
- Morales, J.; Kadota, Y.; Zipfel, C.; Molina, A.; Torres, M.-A. The Arabidopsis NADPH Oxidases RbohD and RbohF Display Differential Expression Patterns and Contributions during Plant Immunity. EXBOTJ 2016, 67, 1663–1676. [Google Scholar] [CrossRef] [PubMed]
- Guranowski, A.; Starzyńska, E.; Pietrowska-Borek, M.; Rejman, D.; Blackburn, G.M. Novel Diadenosine Polyphosphate Analogs with Oxymethylene Bridges Replacing Oxygen in the Polyphosphate Chain: Potential Substrates and/or Inhibitors of Ap4A Hydrolases. FEBS J. 2009, 276, 1546–1553. [Google Scholar] [CrossRef]
- Guranowski, A.; Wojdyła, A.M.; Pietrowska-Borek, M.; Bieganowski, P.; Khurs, E.N.; Cliff, M.J.; Blackburn, G.M.; Błaziak, D.; Stec, W.J. Fhit Proteins Can Also Recognize Substrates Other than Dinucleoside Polyphosphates. FEBS Lett. 2008, 582, 3152–3158. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).