Effects of Coverlys TF150® on the Photosynthetic Characteristics of Grape
Abstract
:1. Introduction
2. Results
2.1. Effects of Coverlys TF150® on the Grape Canopy Microdomain Environment
2.2. Effects of Coverlys TF150® on the Grape Canopy Microdomain Environment
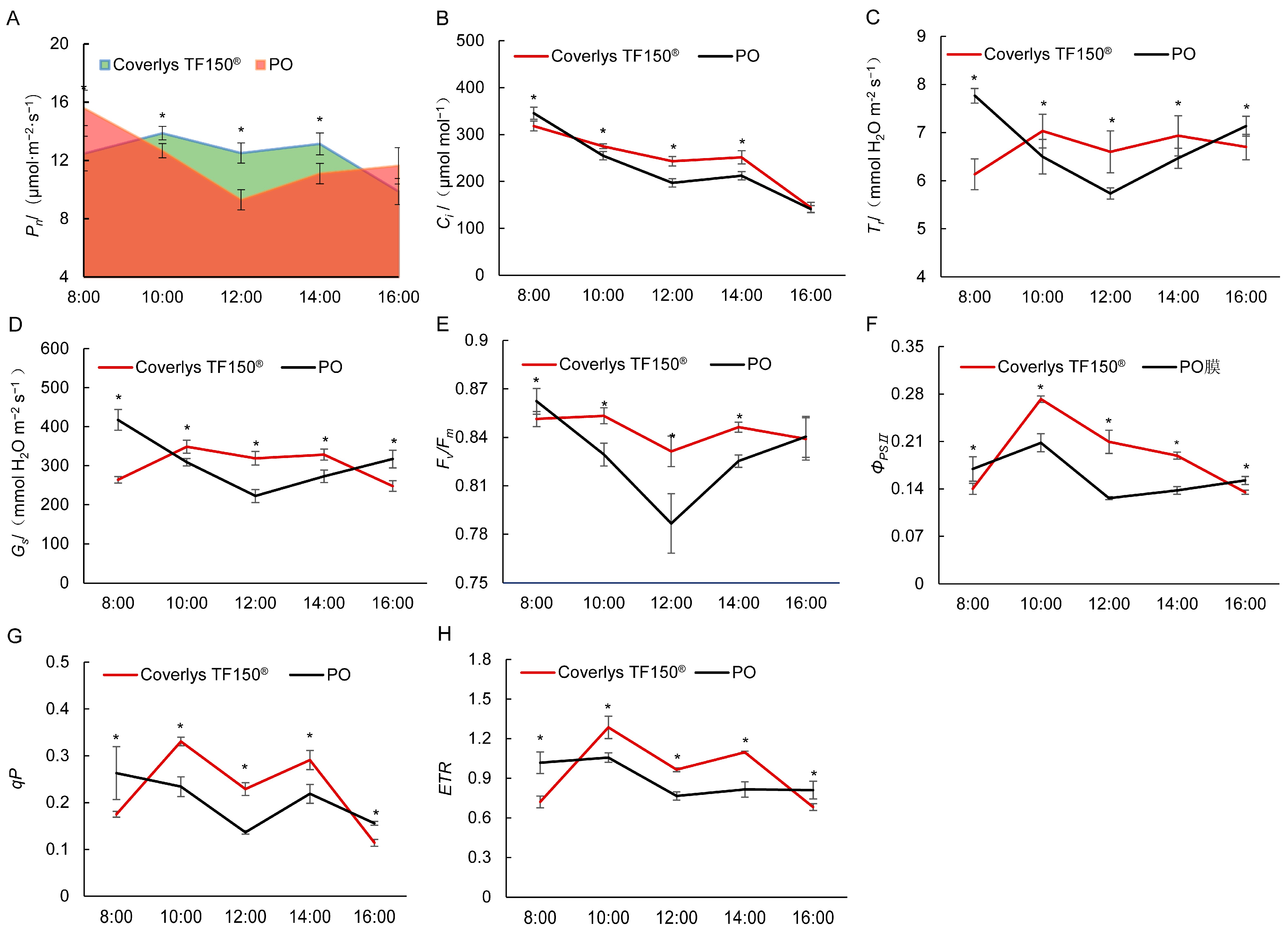
2.3. Effects of Coverlys TF150® on Photosynthetic Characteristics of Grape Leaves
2.4. Analysis of Differentially Expressed Genes
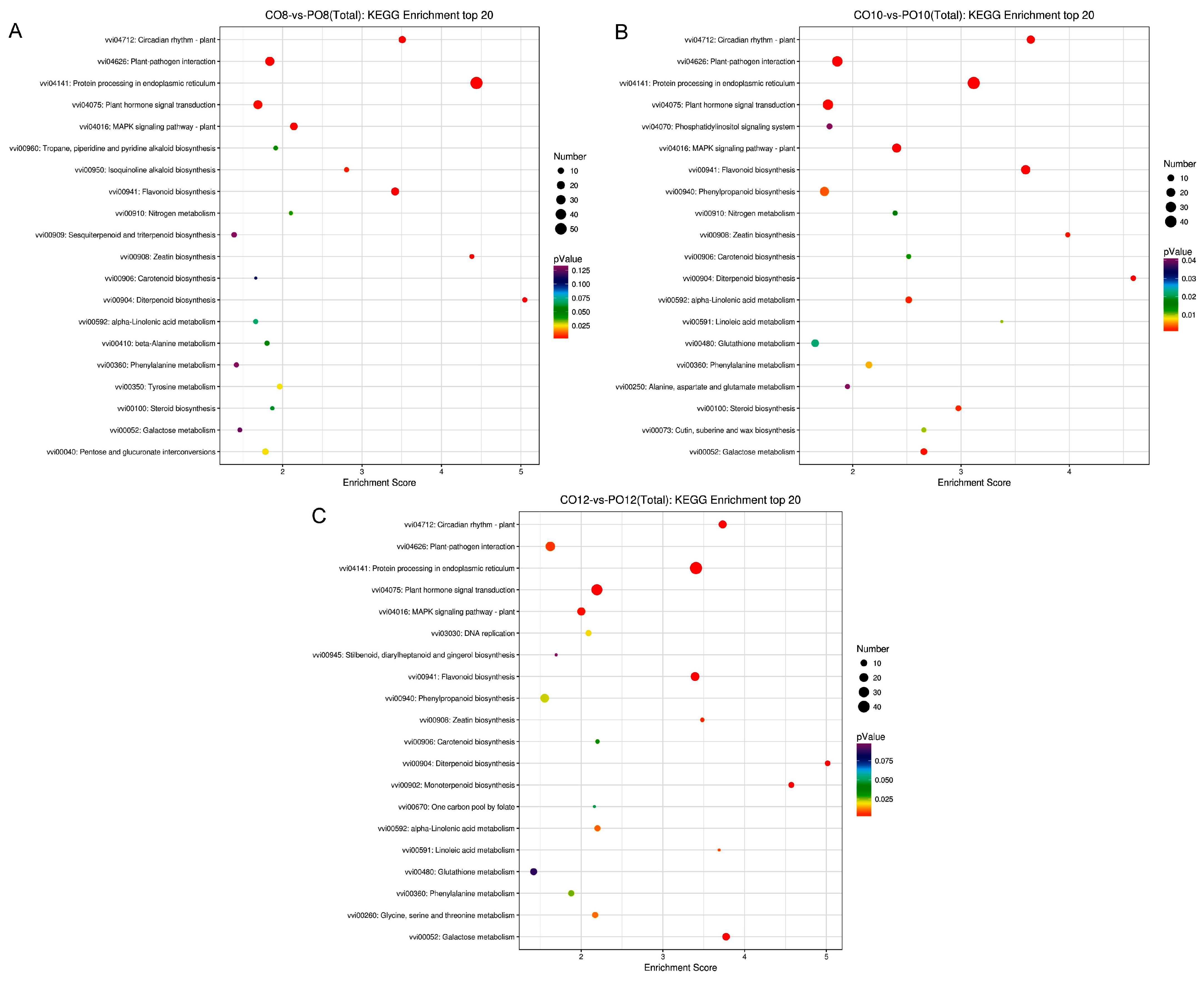
2.5. KEGG Enrichment Analysis of Differential Genes Expression
2.6. KEGG Enrichment Analysis of Differential Genes
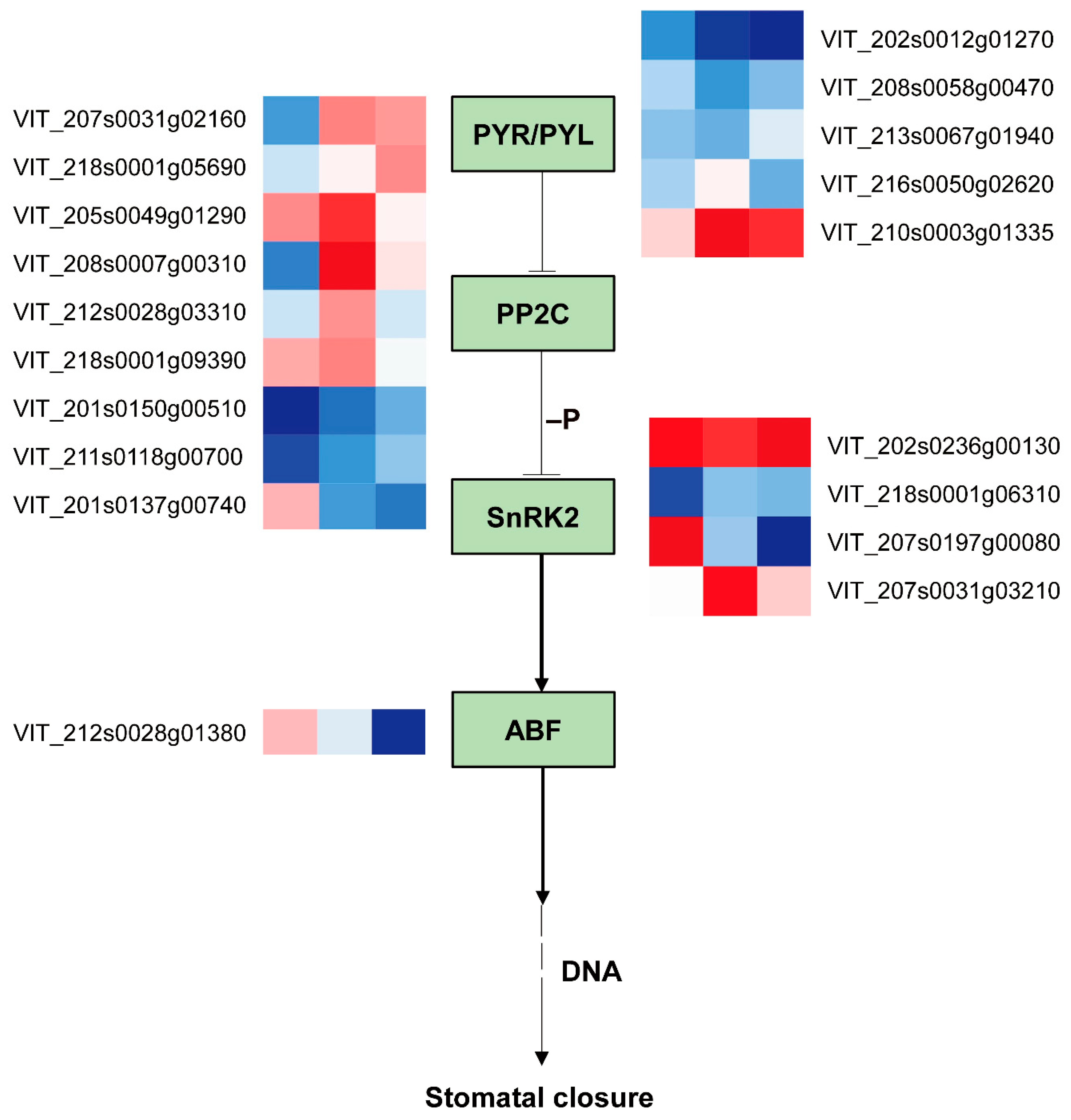
2.7. Comparison of ABA Pathway Gene Expression in Grape Leaves under Coverlys TF150® and PO Film
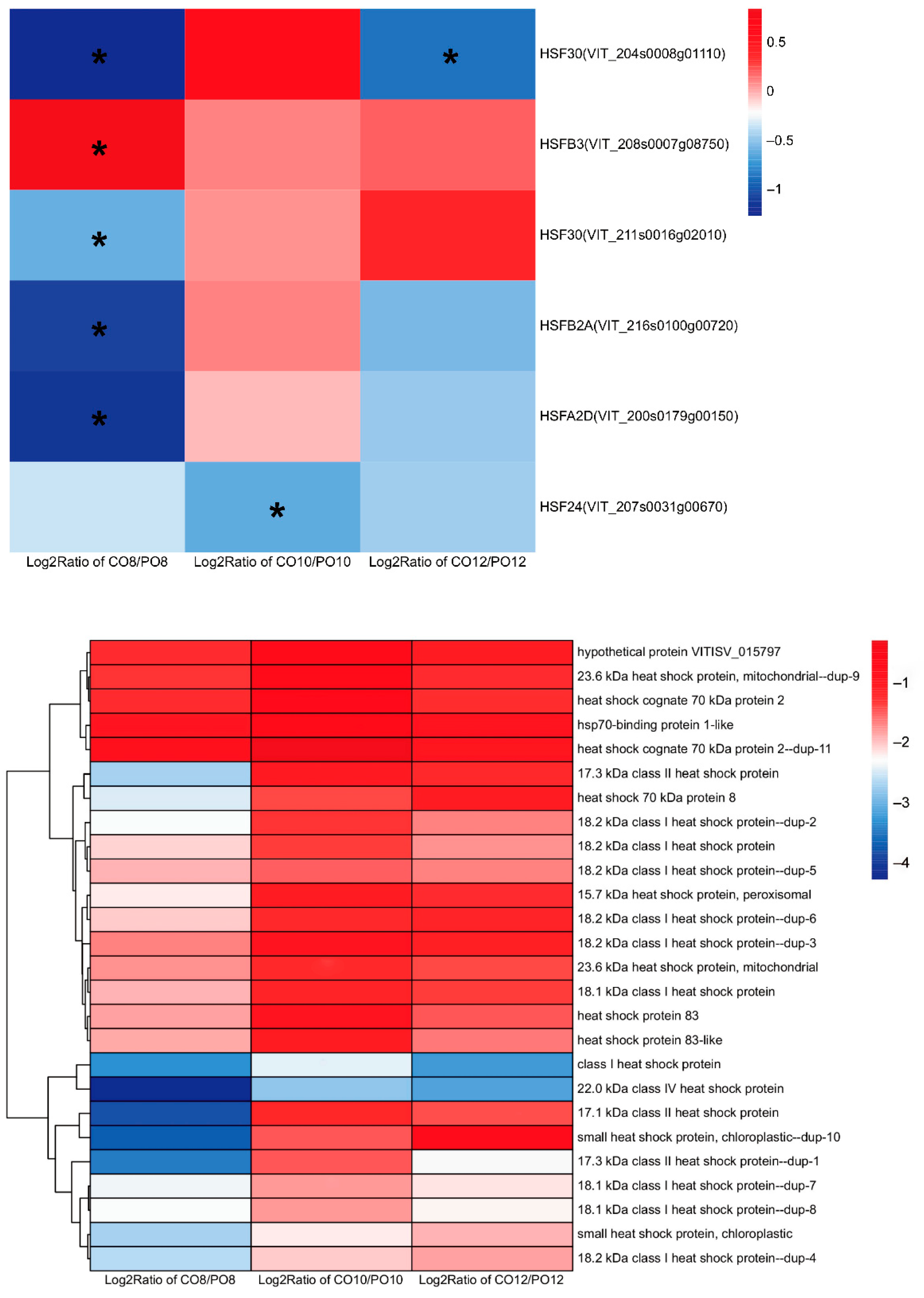
2.8. Comparison of Heat Shock Protein and Heat Shock Protein Transcription Factor Gene Expression in Grape Leaves under Coverlys TF150® and PO Film
3. Discussion
3.1. Effects of Coverlys TF150® on Leaf Morphology, Anatomical Structure, and Stomata of Grape
3.2. Effects of Coverlys TF150® on Chlorophyll Fluorescence and HSPs in Grape Leaves
3.3. Effects of Coverlys TF150® on Chlorophyll Fluorescence and HSPs in Grape Leaves
4. Materials and Methods
4.1. Test Site and Materials
4.2. Microdomain Environmental Monitoring
4.3. Determination of Grape Leaf Area, Leaf Thickness, Leaf Weight, and Chlorophyll Content
4.3.1. Determination of Grape Leaf Area, Leaf Thickness, and Leaf Weight
4.3.2. Determination of Chlorophyll Content in Grape Leaves
- Chla = 12.21A663 − 2.81A646
- Chlb = 20.13A646 − 5.03A663
- Chl(a + b) = Chla + Chlb
4.4. Preparation and Observation of Paraffin Sections of Grape Leaves
4.5. Determination of the Photosynthetic Characteristics of Grape
4.5.1. Determination of Grape Leaf Photosynthesis
4.5.2. Determination of Chlorophyll Fluorescence in Grape Leaves
4.6. RNA Extraction, cDNA Library Preparation, and Sequencing
4.7. Data Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Meng, J.F.; Ning, P.F.; Xu, T.F.; Zhang, Z.W. Effect of rain-shelter cultivation of Vitis vinifera cv. Cabernet Gernischet on the phenolic profile of berry skins and the incidence of grape diseases. Molecules 2013, 18, 381–397. [Google Scholar] [CrossRef] [PubMed]
- Meng, N.; Ren, Z.Y.; Yang, X.F.; Pan, Q.H. Effects of simple rain-shelter cultivation on fatty acid and amino acid accumulation in ‘Chardonnay’ grape berries. J. Sci. Food Agric. 2018, 98, 1222–1231. [Google Scholar] [CrossRef] [PubMed]
- Du, F.; Deng, W.; Yang, M.; Wang, H.; Mao, R.; Shao, J.; Zhu, S. Protecting grapevines from rainfall in rainy conditions reduces disease severity and enhances profitability. Crop Prot. 2015, 67, 261–268. [Google Scholar] [CrossRef]
- Ajmi, A.; Vázquez, S.; Morales, F.; Chaari, A.; El-Jendoubi, H.; Abadía, A.; Larbi, A. Prolonged artificial shade affects morphological, anatomical, biochemical and ecophysiological behavior of young olive trees (cv. Arbosana). Sci. Hortic. 2018, 241, 275–284. [Google Scholar] [CrossRef]
- Zhang, H.; Hou, Q.; Tu, K.; Qiao, G.; Li, Q.; Wen, X. The Effects of Rain-shelter Cultivation on the Photosynthetic Characteristics and Chlorophyll Fluorescence of Sweet Cherry (Prunus avium L.). Erwerbs-Obstbau 2021, 63, 359–368. [Google Scholar] [CrossRef]
- Zha, Q.; Xi, X.; Jiang, A.; Wang, S.; Tian, Y. Changes in the protective mechanism of photosystem II and molecular regulation in response to high temperature stress in grapevines. Plant Physiol. Biochem. 2016, 101, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, X.; Zhai, H.; Gao, H.; Yao, Y.; Du, Y. Responses of photosystem II photochemistry and the alternative oxidase pathway to heat stress in grape leaves. Acta Physiol. Plant. 2016, 38, 232. [Google Scholar] [CrossRef]
- Camejo, D.; Rodríguez, P.; Morales, M.A.; Dell’Amico, J.M.; Torrecillas, A.; Alarcón, J.J. High temperature effects on photosynthetic activity of two tomato cultivars with different heat susceptibility. J. Plant Physiol. 2005, 162, 281–289. [Google Scholar] [CrossRef]
- Tang, R.; Gupta, S.K.; Niu, S.; Li, X.Q.; Yang, Q.; Che, G.; Zhu, W.; Haroon, M. Transcriptome analysis of heat stress response genes in potato leaves. Mol. Biol. Rep. 2020, 47, 4311–4321. [Google Scholar] [CrossRef]
- Greer, D.H.; Weedon, M.M. Interactions between light and growing season temperatures on, growth and development and gas exchange of Semillon (Vitis vinifera L.) vines grown in an irrigated vineyard. Plant Physiol. Biochem. 2012, 54, 59–69. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Oxidant and antioxidant signalling in plants: A re-evaluation of the concept of oxidative stress in a physiological context. Plant Cell Environ. 2010, 28, 1056–1071. [Google Scholar] [CrossRef]
- Chen, L.S.; Li, P.; Cheng, L. Effects of high temperature coupled with high light on the balance between photooxidation and photoprotection in the sun-exposed peel of apple. Planta 2008, 228, 745–756. [Google Scholar] [CrossRef] [PubMed]
- Schrameyer, V.; Krämer, W.; Hill, R.; Jeans, J.; Larkum, A.; Bischof, K.; Campbel, D.; Ralpph, P. Under high light stress two Indo-Pacific coral species display differential photodamage and photorepair dynamics. Mar. Biol. 2016, 163, 168. [Google Scholar] [CrossRef]
- Li, X.X.; He, F.; Wang, J.; Li, Z.; Pan, Q.H. Simple rain-shelter cultivation prolongs accumulation period of anthocyanins in wine grape berries. Molecules 2014, 19, 14843–14861. [Google Scholar] [CrossRef] [PubMed]
- Al-Mahdouri, A.; Baneshi, M.; Gonome, H.; Okajima, J.; Maruyama, S. Evaluation of optical properties and thermal performances of different greenhouse covering materials. Sol. Energy 2013, 96, 21–32. [Google Scholar] [CrossRef]
- Tian, T.; Qiao, G.; Deng, B.; Wen, Z.; Hong, Y.; Wen, X. The effects of rain shelter coverings on the vegetative growth and fruit characteristics of Chinese cherry (Prunus pseudocerasus Lindl.). Sci. Hortic. 2019, 254, 228–235. [Google Scholar] [CrossRef]
- Palma, L.D.; Limosani, P.; Vox, G.; Schettini, E.; Antoniciello, D.; Laporta, F.; Novello, V. Technical properties of new agrotextile fabrics improving vineyard microclimate, table grape yield and quality. Acta Hortic. 2020, 1276, 271–278. [Google Scholar] [CrossRef]
- Sun, Y.; Gao, Y.; Wang, H.; Yang, X.; Zhai, H.; Du, Y. Stimulation of cyclic electron flow around PSI as a response to the combined stress of high light and high temperature in grape leaves. Funct. Plant Biol. 2018, 45, 1038–1045. [Google Scholar] [CrossRef]
- Jin, B.; Wang, L.; Wang, J.; Jiang, K.; Wang, Y.; Jiang, X.; Ni, C.; Wang, Y.; Teng, N. The effect of experimental warming on leaf functional traits, leaf structure and leaf biochemistry in Arabidopsis thaliana. BMC Plant Biol. 2011, 11, 35. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.M.; Roychowdhury, R.; Fujita, M. Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int. J. Mol. Sci. 2013, 14, 9643–9684. [Google Scholar] [CrossRef]
- Luo, T.Q.; Zhao, W.Z.; Zhang, Y.Z.; Xu, S.R.; Xiao, W.; Li, L. Effect of Strong Light on Chlorophyll Degradation in Strawberry Leaves. Russ. J. Plant Physiol. 2022, 69, 163. [Google Scholar] [CrossRef]
- Zhang, H.; Pan, C.; Gu, S.; Ma, Q.; Zhang, Y.; Li, X.; Shi, K. Stomatal movements are involved in elevated CO2-mitigated high temperature stress in tomato. Physiol. Plant. 2019, 165, 569–583. [Google Scholar] [CrossRef] [PubMed]
- O’Carrigan, A.; Hinde, E.; Lu, N.; Xu, X.Q.; Duan, H.; Huang, G.; Mak, M.; Bellotti, B.; Chen, Z.H. Effects of light irradiance on stomatal regulation and growth of tomato. Environ. Exp. Bot. 2014, 98, 65–73. [Google Scholar] [CrossRef]
- Leng, P.; Yuan, B.; Guo, Y. The role of abscisic acid in fruit ripening and responses to abiotic stress. J. Exp. Bot. 2014, 65, 4577–4588. [Google Scholar] [CrossRef]
- Xiang, N.; Hu, J.; Ya, S.; Guo, X. Plant hormones and volatiles response to temperature stress in sweet corn (Zea mays L.) seedlings. J. Agric. Food Chem. 2021, 69, 6779–6790. [Google Scholar] [CrossRef]
- Nishimura, N.; Sarkeshik, A.; Nito, K.; Park, S.Y.; Wang, A.; Carvalho, P.C.; Lee, S.; Caddell, D.F.; Cutler, S.R.; Chory, J.; et al. PYR/PYL/RCAR family members are major in-vivo ABI1 protein phosphatase 2C-interacting proteins in Arabidopsis. Plant J. 2010, 61, 290–299. [Google Scholar] [CrossRef]
- Santiago, J.; Dupeux, F.; Betz, K.; Antoni, R.; Gonzalez-Guzman, M.; Rodriguez, L.; Márquez, J.A.; Rodriguez, P.L. Structural insights into PYR/PYL/RCAR ABA receptors and PP2Cs. Plant Sci. 2012, 182, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Hartung, W.; Leport, L.; Ratcliffe, R.G.; Sauter, A.; Duda, R.; Turner, N.C. Abscisic acid concentration, root pH and anatomy do not explain growth differences of chickpea (Cicer arietinum L.) and lupin (Lupinus angustifolius L.) on acid and alkaline soils. Plant Soil 2002, 240, 191–199. [Google Scholar] [CrossRef]
- Danquah, A.; De Zélicourt, A.; Colcombet, J.; Hirt, H. The role of ABA and MAPK signaling pathways in plant abiotic stress responses. Biotechnol. Adv. 2014, 32, 40–52. [Google Scholar] [CrossRef]
- Gao, Y.; Li, X.X.; Han, M.M.; Yang, X.F.; Li, Z.; Wang, J.; Pan, Q.H. Rain-shelter cultivation modifies carbon allocation in the polyphenolic and volatile metabolism of Vitis vinifera L. Chardonnay grapes. PLoS ONE 2016, 11, e0156117. [Google Scholar] [CrossRef]
- Tu, W.; Li, Y.; Liu, W.; Wu, L.; Xie, X.; Zhang, Y.; Wilhelm, C.; Yang, C. Spring Ephemerals Adapt to Extremely High Light Conditions via an Unusual Stabilization of Photosystem II. Front. Plant Sci. 2016, 6, 1189. [Google Scholar] [CrossRef] [PubMed]
- Allakhverdiev, S.I.; Kreslavski, V.D.; Klimov, V.V.; Los, D.A.; Carpentier, R.; Mohanty, P. Heat stress: An overview of molecular responses in photosynthesis. Photosynth. Res. 2008, 98, 541–550. [Google Scholar] [CrossRef]
- Gao, Y.B.; Zheng, W.W.; Zhang, C.; Zhang, L.L.; Xu, K. High temperature and high light intensity induced photoinhibition of bayberry (Myrica rubra Sieb. et Zucc.) by disruption of D1 turnover in photosystem II. Sci. Hortic. 2019, 248, 132–137. [Google Scholar] [CrossRef]
- Yamamoto, Y. Quality control of photosystem II. Plant Cell Physiol. 2001, 42, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Sakamoto, W. Protein quality control in chloroplasts: A current model of D1 protein degradation in the photosystem II repair cycle. J. Biochem. 2009, 146, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Hirata, K.; Kikuchi, A.; Sasaki, T.; Kuroda, S.; Kaibuchi, K.; Matsuura, Y.; Takai, Y. Involvement of rho p21 in the GTP-enhanced calcium ion sensitivity of smooth muscle contraction. J. Biol. Chem. 1992, 267, 8719–8722. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Zhang, S.B.; Zhang, J.L.; Hu, H. Photoinhibition of photosystem I under high light in the shade-established tropical tree species Psychotria rubra. Front. Plant Sci. 2015, 6, 801. [Google Scholar] [CrossRef]
- Schultz, H. Climate change and viticulture: A European perspective on climatology, carbon dioxide and UV-B effect. Aust. J. Grape Wine Res. 2000, 6, 2–12. [Google Scholar] [CrossRef]
- Wise, R.R.; Olson, A.J.; Schrader, S.M.; Sharkey, T.D. Electron transport is the functional limitation of photosynthesis in field-grown pima cotton plants at high temperature. Plant Cell Environ. 2004, 27, 717–724. [Google Scholar] [CrossRef]
- Crafts-Brandner, S.J.; Salvucci, M.E. Rubisco activase constrains the photosynthetic potential of leaves at high temperature and CO2. Proc. Natl. Acad. Sci. USA 2000, 97, 13430–13435. [Google Scholar] [CrossRef]
- Demirevska-Kepova, K.; Holzer, R.; Simova-Stoilova, L.; Feller, U. Heat stress effects on ribulose-1, 5-bisphosphate carboxylase/oxygenase, Rubisco binding protein and Rubisco activase in wheat leaves. Biol. Plant. 2005, 49, 521–525. [Google Scholar] [CrossRef]
- Luo, H.B.; Ma, L.; Xi, H.F.; Duan, W.; Li, S.H.; Loescher, W.; Wang, J.F.; Wang, L.J. Photosynthetic responses to heat treatments at different temperatures and following recovery in grapevine (Vitis amurensis L.) leaves. PLoS ONE 2011, 6, e23033. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Chen, J.; Wang, Z.; Tan, T.; Li, S.; Li, J. Soybean (Glycine max L. Merr.) seedlings response to shading: Leaf structure, photosynthesis and proteomic analysis. BMC Plant Biol. 2019, 19, 34. [Google Scholar] [CrossRef] [PubMed]
- Kooten, O.; Snel, J.F.H. The use of chlorophyll fluorescence nomenclature in plant stress physiology. Photosynth. Res. 1990, 25, 147–150. [Google Scholar] [CrossRef] [PubMed]

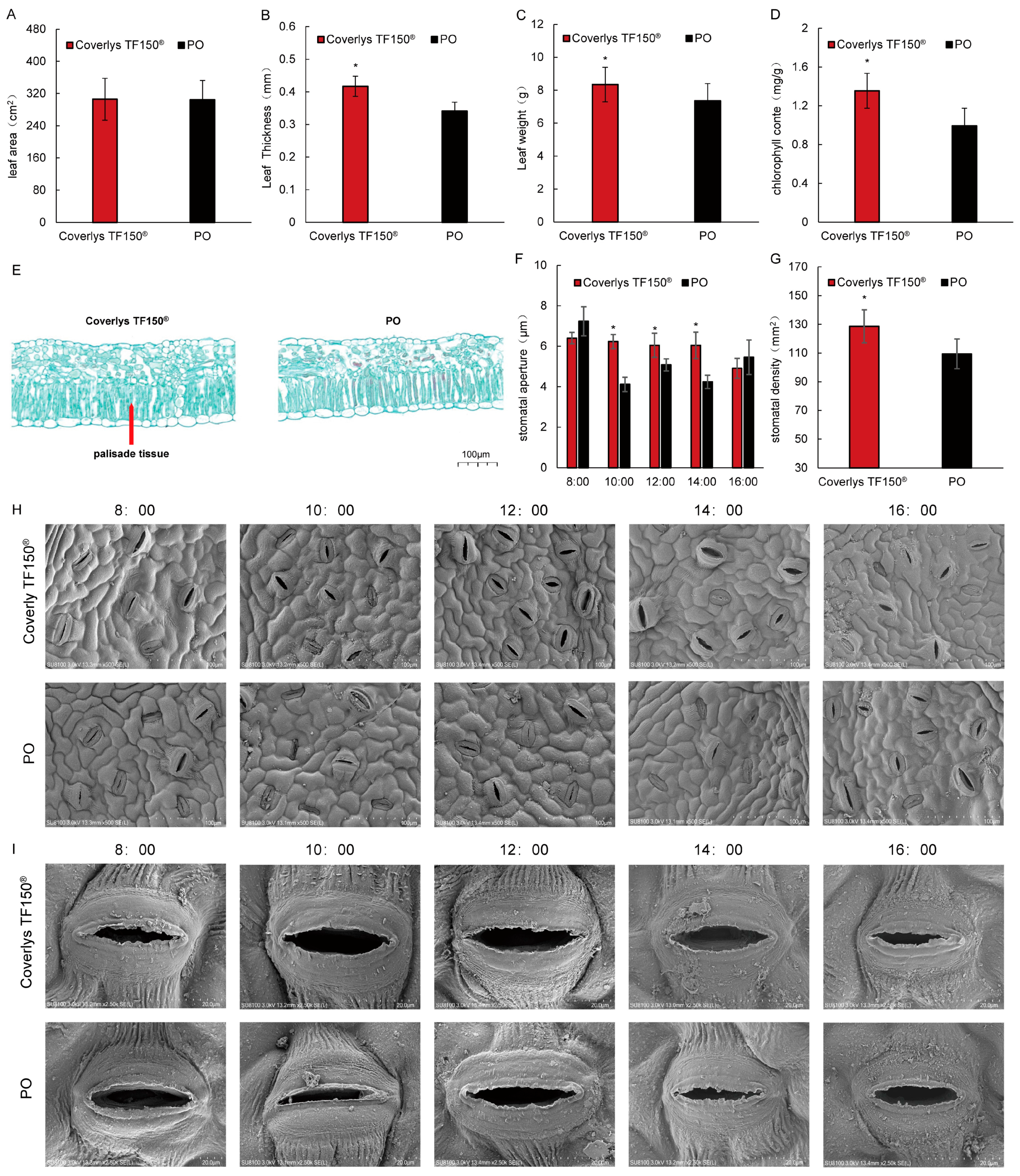


| Treatment | Coverlys TF150® | PO |
|---|---|---|
| Upper epidermal thickness (μm) | 26.88 ± 2.41 a | 24.09 ± 2.52 a |
| Lower epidermal thickness (μm) | 19.46 ± 1.66 a | 22.25 ± 2.53 a |
| Palisade tissue thickness (μm) | 101.32 ± 3.92 a | 87.89 ± 5.08 b |
| Spongy tissue thickness (μm) | 84.45 ± 10.22 a | 78.76 ± 4.85 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Jiang, E.; Liu, M.; Sun, Q.; Gao, Z.; Du, Y. Effects of Coverlys TF150® on the Photosynthetic Characteristics of Grape. Int. J. Mol. Sci. 2023, 24, 16659. https://doi.org/10.3390/ijms242316659
Li Z, Jiang E, Liu M, Sun Q, Gao Z, Du Y. Effects of Coverlys TF150® on the Photosynthetic Characteristics of Grape. International Journal of Molecular Sciences. 2023; 24(23):16659. https://doi.org/10.3390/ijms242316659
Chicago/Turabian StyleLi, Zhonghan, Enshun Jiang, Minghui Liu, Qinghua Sun, Zhen Gao, and Yuanpeng Du. 2023. "Effects of Coverlys TF150® on the Photosynthetic Characteristics of Grape" International Journal of Molecular Sciences 24, no. 23: 16659. https://doi.org/10.3390/ijms242316659
APA StyleLi, Z., Jiang, E., Liu, M., Sun, Q., Gao, Z., & Du, Y. (2023). Effects of Coverlys TF150® on the Photosynthetic Characteristics of Grape. International Journal of Molecular Sciences, 24(23), 16659. https://doi.org/10.3390/ijms242316659






