Evaluation of the Continuous Positive Airway Pressure Effect on Neurotrophins’ Gene Expression and Protein Levels
Abstract
:1. Introduction
2. Results
3. Discussion
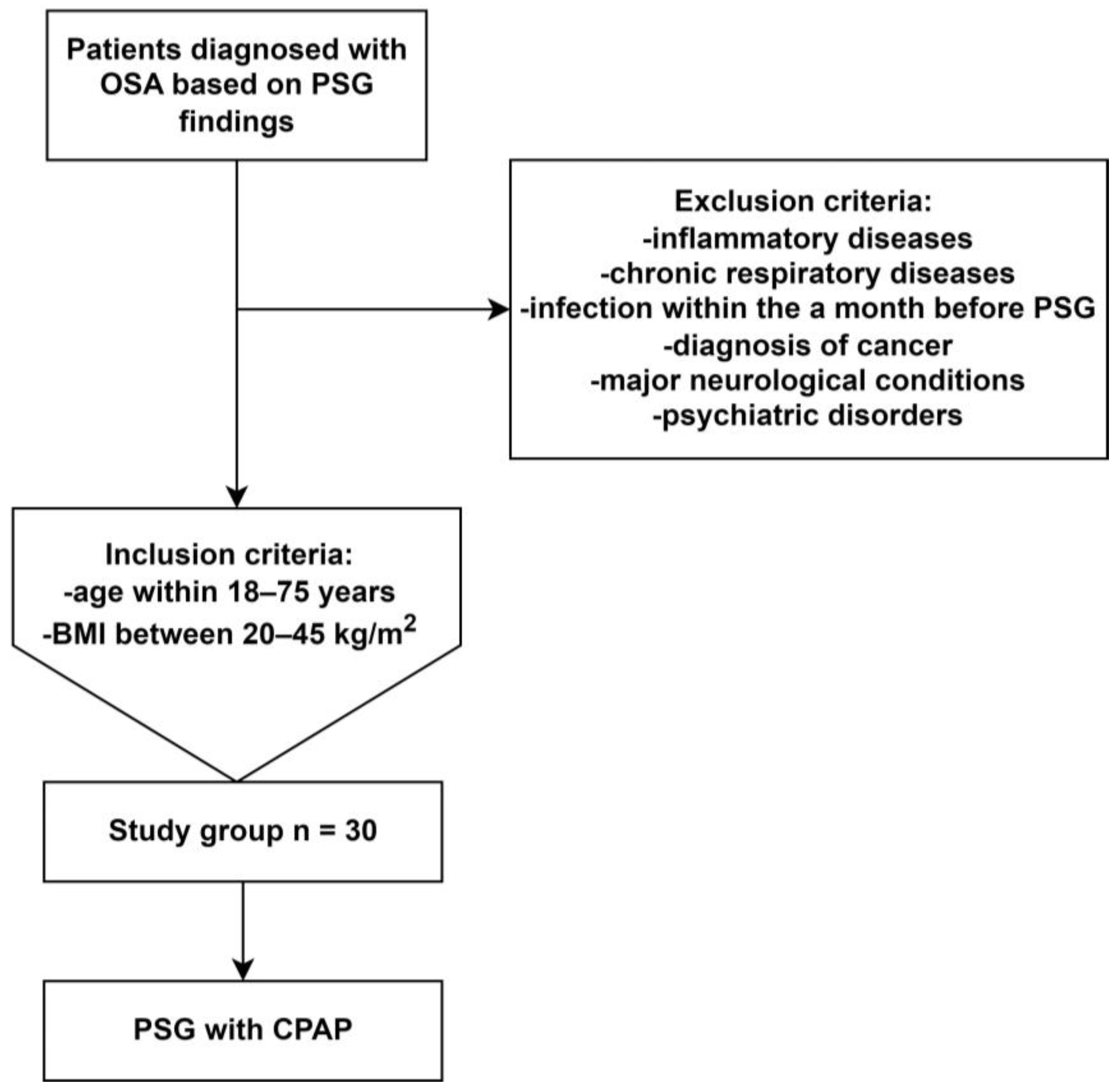
4. Materials and Methods
4.1. Polysomnography and CPAP Treatment
4.2. Assessment of Protein and mRNA Level
4.3. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gabryelska, A.; Turkiewicz, S.; Ditmer, M.; Sochal, M. Neurotrophins in the Neuropathophysiology, Course, and Complications of Obstructive Sleep Apnea—A Narrative Review. Int. J. Mol. Sci. 2023, 24, 1808. [Google Scholar] [CrossRef] [PubMed]
- Gabryelska, A.; Chrzanowski, J.; Sochal, M.; Kaczmarski, P.; Turkiewicz, S.; Ditmer, M.; Karuga, F.F.; Czupryniak, L.; Białasiewicz, P. Nocturnal Oxygen Saturation Parameters as Independent Risk Factors for Type 2 Diabetes Mellitus among Obstructive Sleep Apnea Patients. J. Clin. Med. 2021, 10, 3770. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.A.; Simpson, F.C. Obstructive sleep apnea and psychiatric disorders: A systematic review. J. Clin. Sleep Med. 2015, 11, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, D.J.; Punjabi, N.M. Diagnosis and Management of Obstructive Sleep Apnea: A Review. JAMA 2020, 323, 1389–1400. [Google Scholar] [CrossRef]
- Calik, M.W. Treatments for Obstructive Sleep Apnea. J. Clin. Outcomes Manag. 2016, 23, 181–192. [Google Scholar]
- Vijayan, V.K. Morbidities associated with obstructive sleep apnea. Expert Rev. Respir. Med. 2012, 6, 557–566. [Google Scholar] [CrossRef]
- Song, S.O.; He, K.; Narla, R.R.; Kang, H.G.; Ryu, H.U.; Boyko, E.J. Metabolic Consequences of Obstructive Sleep Apnea Especially Pertaining to Diabetes Mellitus and Insulin Sensitivity. Diabetes Metab. J. 2019, 43, 144–155. [Google Scholar] [CrossRef]
- Ayas, N.T.; Taylor, C.M.; Laher, I. Cardiovascular consequences of obstructive sleep apnea. Curr. Opin. Cardiol. 2016, 31, 599–605. [Google Scholar] [CrossRef]
- Gabryelska, A.; Turkiewicz, S.; Ditmer, M.; Karuga, F.F.; Strzelecki, D.; Bialasiewicz, P.; Sochal, M. BDNF and proBDNF Serum Protein Levels in Obstructive Sleep Apnea Patients and Their Involvement in Insomnia and Depression Symptoms. J. Clin. Med. 2022, 11, 7135. [Google Scholar] [CrossRef]
- Makhout, S.; Vermeiren, E.; Van De Maele, K.; Bruyndonckx, L.; De Winter, B.Y.; Van Hoorenbeeck, K.; Verhulst, S.L.; Van Eyck, A. The Role of Brain-Derived Neurotrophic Factor in Obstructive Sleep Apnea and Endothelial Function in a Pediatric Population with Obesity. Front. Med. 2022, 8, 835515. [Google Scholar] [CrossRef]
- Szaulinska, K.; Plywaczewski, R.; Sikorska, O.; Holka-Pokorska, J.; Wierzbicka, A.; Wichniak, A.; Sliwinski, P. Obstructive sleep apnea in severe mental disorders. Psychiatr. Pol. 2015, 49, 883–895. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-Y.; Ko, I.; Kim, D.-K. Association of Obstructive Sleep Apnea with the Risk of Affective Disorders. JAMA Otolaryngol.–Head Neck Surg. 2019, 145, 1020–1026. [Google Scholar] [CrossRef] [PubMed]
- Braitman, D.V. Screening for Sleep Apnea in Psychiatry. Am. J. Psychiatry Resid. J. 2018, 13, 5–7. [Google Scholar] [CrossRef]
- Schröder, C.M.; O’Hara, R. Depression and Obstructive Sleep Apnea (OSA). Ann. Gen. Psychiatry 2005, 4, 13. [Google Scholar] [CrossRef]
- McPhee, G.M.; Downey, L.A.; Stough, C. Neurotrophins as a reliable biomarker for brain function, structure and cognition: A systematic review and meta-analysis. Neurobiol. Learn. Mem. 2020, 175, 107298. [Google Scholar] [CrossRef]
- Kowiański, P.; Lietzau, G.; Czuba, E.; Waśkow, M.; Steliga, A.; Moryś, J. BDNF: A Key Factor with Multipotent Impact on Brain Signaling and Synaptic Plasticity. Cell. Mol. Neurobiol. 2018, 38, 579–593. [Google Scholar] [CrossRef]
- Pöyhönen, S.; Er, S.; Domanskyi, A.; Airavaara, M. Effects of Neurotrophic Factors in Glial Cells in the Central Nervous System: Expression and Properties in Neurodegeneration and Injury. Front. Physiol. 2019, 10, 486. [Google Scholar] [CrossRef]
- Shah, F.; Forsgren, S.; Holmlund, T.; Levring Jäghagen, E.; Berggren, D.; Franklin, K.A.; Stål, P. Neurotrophic factor BDNF is upregulated in soft palate muscles of snorers and sleep apnea patients. Laryngoscope Investig. Otolaryngol. 2019, 4, 174–180. [Google Scholar] [CrossRef]
- McGregor, C.E.; English, A.W. The Role of BDNF in Peripheral Nerve Regeneration: Activity-Dependent Treatments and Val66Met. Front. Cell. Neurosci. 2019, 12, 522. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, P.; Chen, F.; Zhao, Y.; Li, Y.; He, X.; Huselstein, C.; Ye, Q.; Tong, Z.; Chen, Y. Brain Derived Neurotrophic Factor and Glial Cell Line-Derived Neurotrophic Factor-Transfected Bone Mesenchymal Stem Cells for the Repair of Periphery Nerve Injury. Front. Bioeng. Biotechnol. 2020, 8, 874. [Google Scholar] [CrossRef]
- Flores, K.R.; Viccaro, F.; Aquilini, M.; Scarpino, S.; Ronchetti, F.; Mancini, R.; Di Napoli, A.; Scozzi, D.; Ricci, A. Protective role of brain derived neurotrophic factor (BDNF) in obstructive sleep apnea syndrome (OSAS) patients. PLoS ONE 2020, 15, e0227834. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.; Müller, P.; Dordevic, M.; Lessmann, V.; Brigadski, T.; Müller, N.G. Daily Intermittent Normobaric Hypoxia Over 2 Weeks Reduces BDNF Plasma Levels in Young Adults—A Randomized Controlled Feasibility Study. Front. Physiol. 2018, 9, 1337. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Xiong, L.J.; Tong, Y.; Mao, M. The neuroprotective roles of BDNF in hypoxic ischemic brain injury. Biomed. Rep. 2013, 1, 167–176. [Google Scholar] [CrossRef]
- Bothwell, M. NGF, BDNF, NT3, and NT4. Handb. Exp. Pharmacol. 2014, 220, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Goldbart, A.D.; Mager, E.; Veling, M.C.; Goldman, J.L.; Kheirandish-Gozal, L.; Serpero, L.D.; Piedimonte, G.; Gozal, D. Neurotrophins and Tonsillar Hypertrophy in Children with Obstructive Sleep Apnea. Pediatr. Res. 2007, 62, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Minnone, G.; De Benedetti, F.; Bracci-Laudiero, L. NGF and Its Receptors in the Regulation of Inflammatory Response. Int. J. Mol. Sci. 2017, 18, 1028. [Google Scholar] [CrossRef] [PubMed]
- Larkin, E.K.; Patel, S.R.; Goodloe, R.J.; Li, Y.; Zhu, X.; Gray-McGuire, C.; Adams, M.D.; Redline, S. A candidate gene study of obstructive sleep apnea in European Americans and African Americans. Am. J. Respir. Crit. Care Med. 2010, 182, 947–953. [Google Scholar] [CrossRef]
- Cao, Y.; Zhu, Q.; Cai, X.; Wu, T.; Aierken, X.; Ahmat, A.; Liu, S.; Li, N. Glial Cell-Derived Neurotrophic Factor Functions as a Potential Candidate Gene in Obstructive Sleep Apnea Based on a Combination of Bioinformatics and Targeted Capture Sequencing Analyses. Biomed. Res. Int. 2021, 2021, 6656943. [Google Scholar] [CrossRef]
- Staats, R.; Stoll, P.; Zingler, D.; Virchow, J.C.; Lommatzsch, M. Regulation of brain-derived neurotrophic factor (BDNF) during sleep apnoea treatment. Thorax 2005, 60, 688. [Google Scholar] [CrossRef]
- Dewan, N.A.; Nieto, F.J.; Somers, V.K. Intermittent hypoxemia and OSA: Implications for comorbidities. Chest 2015, 147, 266–274. [Google Scholar] [CrossRef]
- Huang, E.J.; Reichardt, L.F. Neurotrophins: Roles in neuronal development and function. Annu. Rev. Neurosci. 2001, 24, 677–736. [Google Scholar] [CrossRef]
- Gabryelska, A.; Sochal, M.; Turkiewicz, S.; Białasiewicz, P. Relationship between HIF-1 and Circadian Clock Proteins in Obstructive Sleep Apnea Patients-Preliminary Study. J. Clin. Med. 2020, 9, 1599. [Google Scholar] [CrossRef]
- Xiao, Q.-X.; Chen, J.-J.; Fang, C.-L.; Su, Z.-Y.; Wang, T.-H. Neurotrophins-3 plays a vital role in anti-apoptosis associated with NGF and BDNF regulation in neonatal rats with hypoxic-ischemic brain injury. Ibrain 2020, 6, 12–17. [Google Scholar] [CrossRef]
- Takei, N.; Tanaka, O.; Endo, Y.; Lindholm, D.; Hatanaka, H. BDNF and NT-3 but not CNTF counteract the Ca2+ ionophore-induced apoptosis of cultured cortical neurons: Involvement of dual pathways. Neuropharmacology 1999, 38, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Erickson, K.I.; Prakash, R.S.; Voss, M.W.; Chaddock, L.; Heo, S.; McLaren, M.; Pence, B.D.; Martin, S.A.; Vieira, V.J.; Woods, J.A.; et al. Brain-derived neurotrophic factor is associated with age-related decline in hippocampal volume. J. Neurosci. 2010, 30, 5368–5375. [Google Scholar] [CrossRef]
- Oh, H.; Lewis, D.A.; Sibille, E. The Role of BDNF in Age-Dependent Changes of Excitatory and Inhibitory Synaptic Markers in the Human Prefrontal Cortex. Neuropsychopharmacology 2016, 41, 3080–3091. [Google Scholar] [CrossRef]
- Von Bohlen Und Halbach, O. Involvement of BDNF in Age-Dependent Alterations in the Hippocampus. Front. Aging Neurosci. 2010, 2, 36. [Google Scholar] [CrossRef] [PubMed]
- Gabryelska, A.; Sochal, M. Evaluation of HIF-1 Involvement in the BDNF and ProBDNF Signaling Pathways among Obstructive Sleep Apnea Patients. Int. J. Mol. Sci. 2022, 23, 14876. [Google Scholar] [CrossRef]
- Li, Y.; Chen, J.; Yu, H.; Ye, J.; Wang, C.; Kong, L. Serum brain-derived neurotrophic factor as diagnosis clue for Alzheimer’s disease: A cross-sectional observational study in the elderly. Front. Psychiatry 2023, 14, 1127658. [Google Scholar] [CrossRef]
- Arslan, B.; Şemsi, R.; İriz, A.; Sepici Dinçel, A. The evaluation of serum brain-derived neurotrophic factor and neurofilament light chain levels in patients with obstructive sleep apnea syndrome. Laryngoscope Investig. Otolaryngol. 2021, 6, 1466–1473. [Google Scholar] [CrossRef]
- Wang, W.-H.; He, G.-P.; Xiao, X.-P.; Gu, C.; Chen, H.-Y. Relationship between brain–derived neurotrophic factor and cognitive function of obstructive sleep apnea/hypopnea syndrome patients. Asian Pac. J. Trop. Med. 2012, 5, 906–910. [Google Scholar] [CrossRef]
- Kaminska, M.; O’Sullivan, M.; Mery, V.P.; Lafontaine, A.L.; Robinson, A.; Gros, P.; Martin, J.G.; Benedetti, A.; Kimoff, R.J. Inflammatory markers and BDNF in obstructive sleep apnea (OSA) in Parkinson’s disease (PD). Sleep Med. 2022, 90, 258–261. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, J.M.; Garcia, A.J., 3rd; Anderson, T.M.; Koschnitzky, J.E.; Peng, Y.J.; Kumar, G.K.; Prabhakar, N.R. Central and peripheral factors contributing to obstructive sleep apneas. Respir. Physiol. Neurobiol. 2013, 189, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Deuschle, M.; Schredl, M.; Wisch, C.; Schilling, C.; Gilles, M.; Geisel, O.; Hellweg, R. Serum brain-derived neurotrophic factor (BDNF) in sleep-disordered patients: Relation to sleep stage N3 and rapid eye movement (REM) sleep across diagnostic entities. J. Sleep Res. 2018, 27, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Halonen, R.; Kuula, L.; Lahti, J.; Räikkönen, K.; Pesonen, A.-K. The association between overnight recognition accuracy and slow oscillation-spindle coupling is moderated by BDNF Val66Met. Behav. Brain Res. 2022, 428, 113889. [Google Scholar] [CrossRef] [PubMed]
- Kapur, V.K.; Auckley, D.H.; Chowdhuri, S.; Kuhlmann, D.C.; Mehra, R.; Ramar, K.; Harrod, C.G. Clinical Practice Guideline for Diagnostic Testing for Adult Obstructive Sleep Apnea: An American Academy of Sleep Medicine Clinical Practice Guideline. J. Clin. Sleep Med. 2017, 13, 479–504. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
| PSG Parameter | Median (IQR) |
|---|---|
| Sleep Efficiency [%] | 86.20 (74.80–89.70) |
| Sleep Maintenance [%] | 91.70 (80.00–93.20) |
| Sleep Onset Latency [min] | 16.00 (9.00–27.00) |
| Stage 1 nREM [h] | 2.10 (1.65–3.41) |
| Stage 2 nREM [h] | 1.94 (1.07–2.78) |
| Stage 3 nREM [h] | 0.56 (0.13–1.14) |
| TST [h] | 6.55 (5.75–7.24) |
| REM [h] | 1.17 (0.72–1.43) |
| nREM [h] | 5.31 (4.88–5.81) |
| Arousal Index [events/h] | 22.30 (14.95–31.20) |
| AHI [events/h] | 47.95 (24.75–67.20) |
| Desaturation Index [events/h] | 50.60 (27.13–78.65) |
| Total Number of Desaturations | 303.00 (137.75–349.50) |
| Minimum Oxygen Saturation [%] | 71.40 (64.95–76.00) |
| Time Point | Difference between after PSG with CPAP and after PSG | Increase from after PSG to PSG with CPAP (n; %) | p-Value | |||
|---|---|---|---|---|---|---|
| After PSG | After PSG with CPAP | |||||
| Proteins | BDNF [ng/mL] | 14.80 (10.40–20.61) | 6.53 (2.96–12.80) | −12.63 ((−12.63)–2.32) | 9 (30.00%) | 0.002 |
| proBDNF [ng/mL] | 6.27 (4.95–8.76) | 3.31 (1.62–5.26) | −3.10 ((−5.83)–1.19) | 10 (33.33%) | 0.003 | |
| GDNF [ng/mL] | 96.91 (85.93–129.38) | 92.47 (82.89–97.14) | 0.00 ((−35.16)–7.13) | 15 (50.00%) | 0.047 | |
| NTF3 [ng/mL] | 148.43 (130.23–172.96) | 169.00 (140.04–219.16) | 18.04 (1.74–50.16) | 23 (76.67%) | 0.001 | |
| NTF4 [pg/mL] | 2.35 (1.80–3.26) | 1.78 (0.80–2.48) | −0.53 ((−1.13)–0.14) | 8 (26.67%) | 0.009 | |
| Time Point | Difference between after PSG with CPAP and after PSG | Increase from after PSG to PSG with CPAP (n; %) | p-Value | |||
|---|---|---|---|---|---|---|
| After PSG | After PSG with CPAP | |||||
| Gene Expression (ΔCt) × 100 | BDNF | 19.20 (6.02–84.38) | 72.32 (30.27–107.23) | 8.72 ((−46.00)–82.97) | 17 (56.67%) | 0.338 |
| GDNF | 10.16 (0.97–41.08) | 26.21 (17.26–39.91) | 17.56 ((−25.20–29.23) | 17 (56.67%) | 0.432 | |
| NTF3 | 18.89 (3.07–87.56) | 39.96 (20.55–80.81) | 9.13 ((−53.40)–43.34) | 16 (53.33%) | 0.347 | |
| NTF4 | 33.35 (1.16–17.37) | 69.29 (34.72–246.63) | 18.30 ((−93.10)–163.58) | 18 (60.00%) | 0.622 | |
| R | p | ||
|---|---|---|---|
| Sleep Efficiency | BDNF mRNA after PSG | −0.644 | 0.003 |
| GDNF mRNA after PSG | −0.458 | 0.049 | |
| NTF3 mRNA after PSG | −0.489 | 0.033 | |
| NTF4 mRNA after PSG | −0.484 | 0.036 | |
| Difference between BDNF mRNA expression after PSG with CPAP and after PSG | 0.486 | 0.041 | |
| Sleep Maintenance Efficiency | BDNF mRNA after PSG | −0.693 | 0.001 |
| GDNF mRNA after PSG | −0.561 | 0.012 | |
| NTF3 mRNA after PSG | −0.440 | 0.040 | |
| NTF4 mRNA after PSG | −0.488 | 0.038 | |
| Difference between BDNF mRNA expression after PSG with CPAP and after PSG | 0.631 | 0.005 | |
| Sleep Onset Latency | Difference between BDNF mRNA expression after PSG with CPAP and after PSG | 0.387 | 0.038 |
| Difference between NTF3 mRNA expression after PSG with CPAP and after PSG | 0.440 | 0.019 | |
| Difference between NTF4 mRNA expression after PSG with CPAP and after PSG | 0.424 | 0.025 | |
| Stage 2 NREM duration | proBDNF serum protein concentration | −0.465 | 0.010 |
| BDNF serum protein concentration | −0.379 | 0.039 | |
| Difference between BDNF serum protein concentration after PSG with CPAP and after PSG | 0.398 | 0.029 | |
| Stage 2 NREM percentage | proBDNF serum protein concentration | −0.463 | 0.010 |
| BDNF serum protein concentration | −0.390 | 0.033 | |
| Difference between BDNF serum protein concentration after PSG with CPAP and after PSG | 0.395 | 0.031 | |
| NTF3 serum protein concentrations after PSG with CPAP and after PSG | 0.720 | <0.001 | |
| NTF4 serum protein concentrations after PSG with CPAP and after PSG | 0.569 | 0.001 | |
| NTF3 mRNA and serum protein concentration after PSG with CPAP | 0.537 | 0.003 | |
| NTF3 mRNA and the difference between NTF3 serum protein concentration after PSG with CPAP and after PSG | 0.595 | <0.001 | |
| GDNF mRNA and the difference between GDNF serum protein concentration after PSG with CPAP and after PSG | −0.397 | 0.033 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gabryelska, A.; Turkiewicz, S.; Ditmer, M.; Gajewski, A.; Białasiewicz, P.; Strzelecki, D.; Chałubiński, M.; Sochal, M. Evaluation of the Continuous Positive Airway Pressure Effect on Neurotrophins’ Gene Expression and Protein Levels. Int. J. Mol. Sci. 2023, 24, 16599. https://doi.org/10.3390/ijms242316599
Gabryelska A, Turkiewicz S, Ditmer M, Gajewski A, Białasiewicz P, Strzelecki D, Chałubiński M, Sochal M. Evaluation of the Continuous Positive Airway Pressure Effect on Neurotrophins’ Gene Expression and Protein Levels. International Journal of Molecular Sciences. 2023; 24(23):16599. https://doi.org/10.3390/ijms242316599
Chicago/Turabian StyleGabryelska, Agata, Szymon Turkiewicz, Marta Ditmer, Adrian Gajewski, Piotr Białasiewicz, Dominik Strzelecki, Maciej Chałubiński, and Marcin Sochal. 2023. "Evaluation of the Continuous Positive Airway Pressure Effect on Neurotrophins’ Gene Expression and Protein Levels" International Journal of Molecular Sciences 24, no. 23: 16599. https://doi.org/10.3390/ijms242316599
APA StyleGabryelska, A., Turkiewicz, S., Ditmer, M., Gajewski, A., Białasiewicz, P., Strzelecki, D., Chałubiński, M., & Sochal, M. (2023). Evaluation of the Continuous Positive Airway Pressure Effect on Neurotrophins’ Gene Expression and Protein Levels. International Journal of Molecular Sciences, 24(23), 16599. https://doi.org/10.3390/ijms242316599





