Effects of Gender and Vitamin D on Vascular Reactivity of the Carotid Artery on a Testosterone-Induced PCOS Model
Abstract
1. Introduction
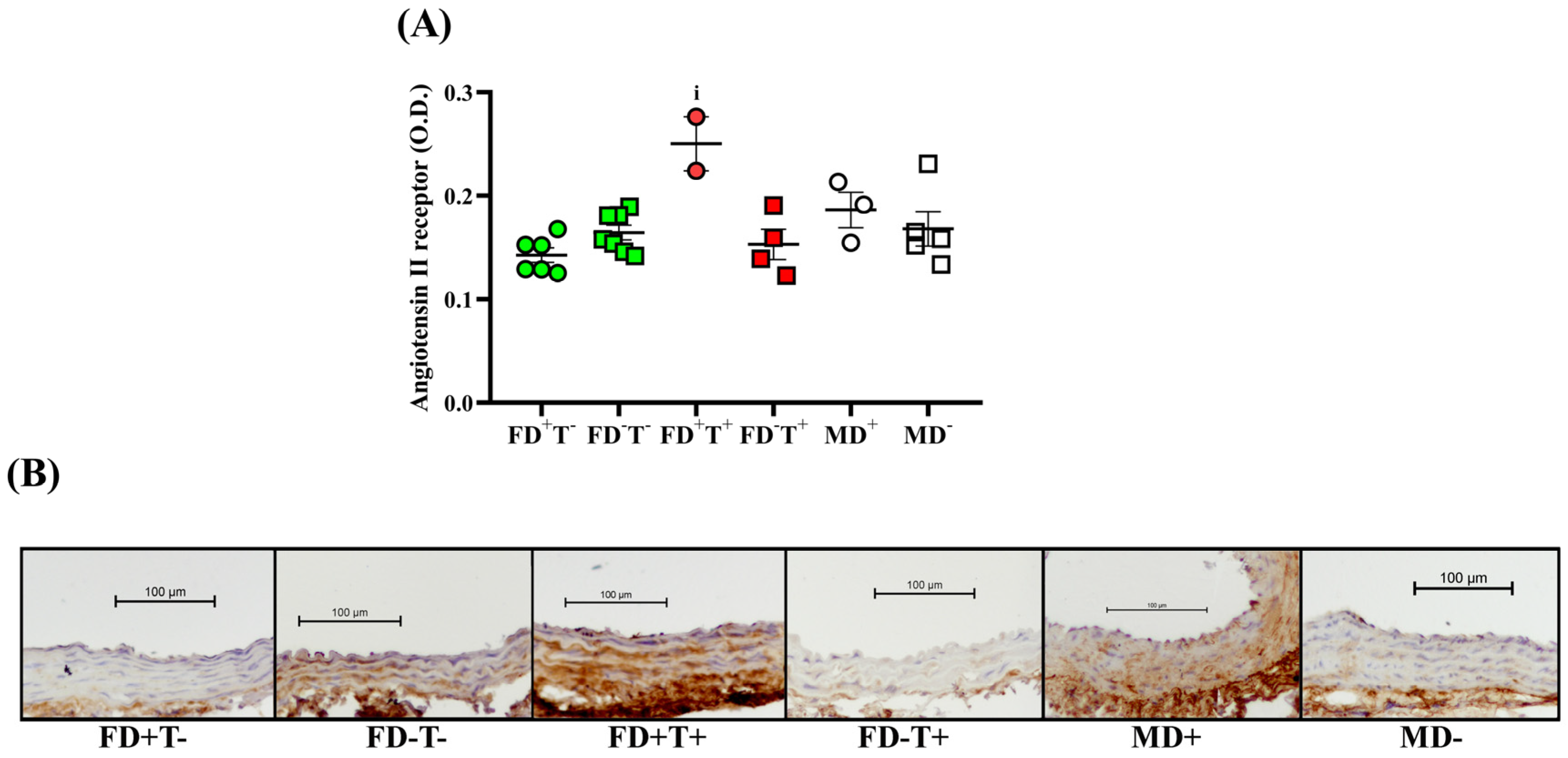
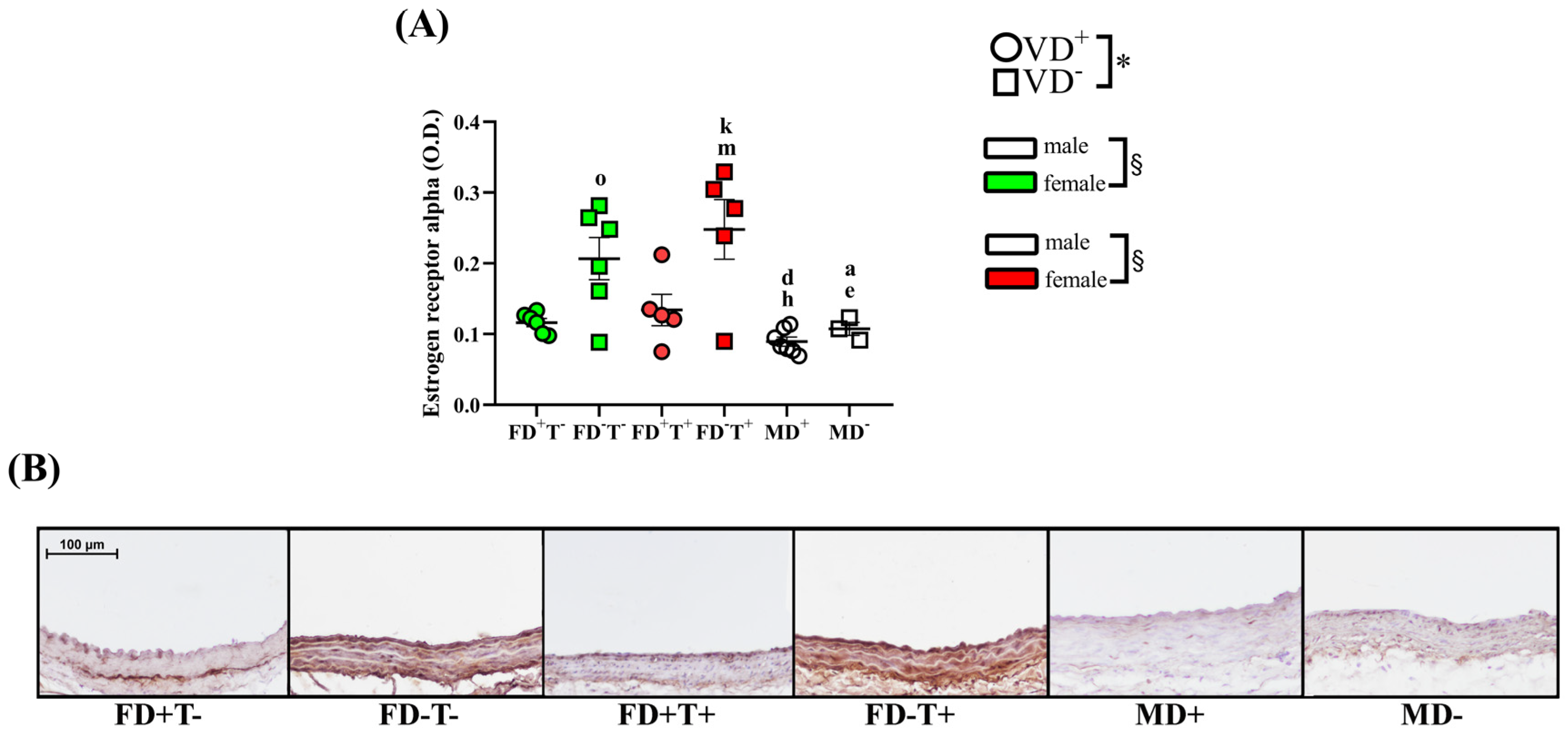
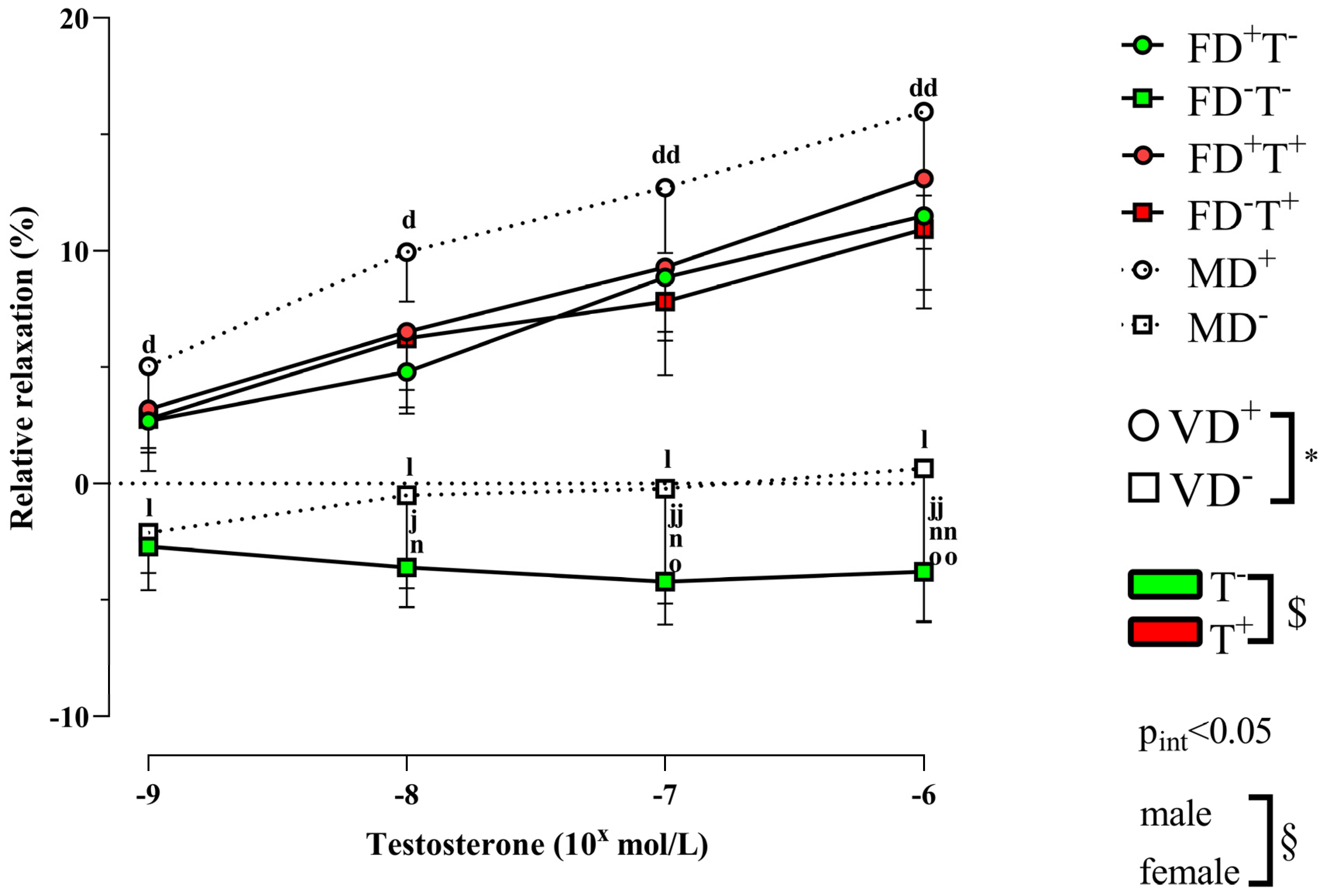
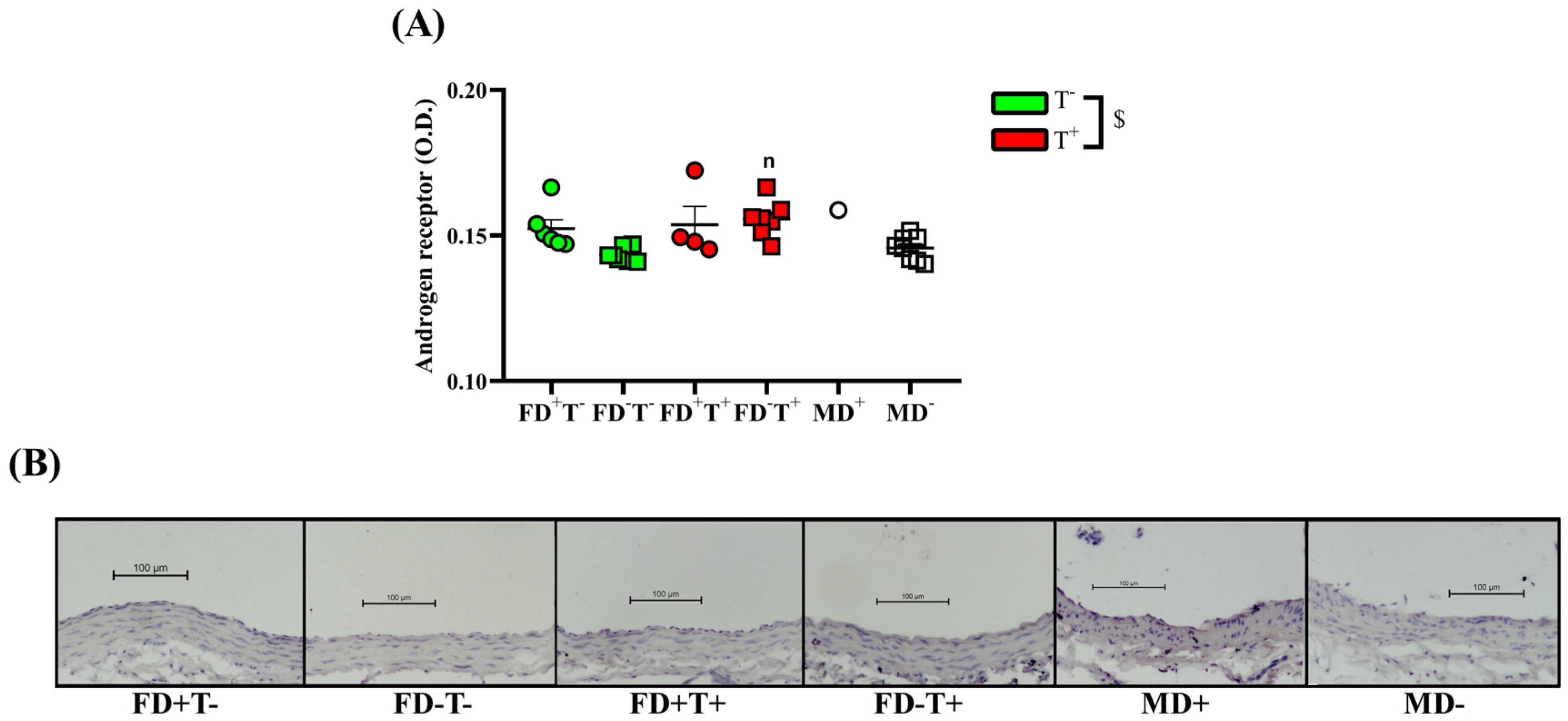
2. Results
2.1. Effects of Vitamin D Deficiency and PCOS in Female Groups
2.2. Effects of Vitamin D Deficiency in Male Animals
2.3. Sex Differences
3. Discussion
3.1. Effects of PCOS on Carotid Artery
3.2. Effects of Vitamin D Deficiency on Carotid Artery
3.3. Sex Differences in Carotid Artery
3.4. Strengths and Limitations
4. Materials and Methods
4.1. Chemicals
4.2. Animals
4.3. Chronic Treatment of the Rats
4.3.1. Vitamin D Deficiency and Supplementation
4.3.2. Hyperandrogenism
4.4. Myography
4.5. Immunohistochemistry
4.6. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khalil, R.A. Estrogen, vascular estrogen receptor and hormone therapy in postmenopausal vascular disease. Biochem. Pharmacol. 2013, 86, 1627–1642. [Google Scholar] [CrossRef]
- Somani, Y.B.; Pawelczyk, J.A.; De Souza, M.J.; Kris-Etherton, P.M.; Proctor, D.N. Aging women and their endothelium: Probing the relative role of estrogen on vasodilator function. Am. J. Physiol.-Heart Circ. Physiol. 2019, 317, H395–H404. [Google Scholar] [CrossRef]
- Botelho, J.; Machado, V.; Proença, L.; Delgado, A.S.; Mendes, J.J. Vitamin D Deficiency and Oral Health: A Comprehensive Review. Nutrients 2020, 12, 1471. [Google Scholar] [CrossRef]
- de la Guía-Galipienso, F.; Martínez-Ferran, M.; Vallecillo, N.; Lavie, C.J.; Sanchis-Gomar, F.; Pareja-Galeano, H. Vitamin D and cardiovascular health. Clin. Nutr. 2021, 40, 2946–2957. [Google Scholar] [CrossRef]
- Latic, N.; Erben, R.G. Vitamin D and Cardiovascular Disease, with Emphasis on Hypertension, Atherosclerosis, and Heart Failure. Int. J. Mol. Sci. 2020, 21, 6483. [Google Scholar] [CrossRef]
- Martyniak, A.; Tomasik, P.J. A New Perspective on the Renin-Angiotensin System. Diagnostics 2022, 13, 16. [Google Scholar] [CrossRef]
- Chen, S.; Sun, Y.; Agrawal, D.K. Vitamin D deficiency and essential hypertension. J. Am. Soc. Hypertens. 2015, 9, 885–901. [Google Scholar] [CrossRef]
- Jia, J.; Tao, X.; Tian, Z.; Liu, J.; Ye, X.; Zhan, Y. Vitamin D receptor deficiency increases systolic blood pressure by upregulating the renin-angiotensin system and autophagy. Exp. Ther. Med. 2022, 23, 314. [Google Scholar] [CrossRef]
- Jensen, N.S.; Wehland, M.; Wise, P.M.; Grimm, D. Latest Knowledge on the Role of Vitamin D in Hypertension. Int. J. Mol. Sci. 2023, 24, 4679. [Google Scholar] [CrossRef]
- Kim, D.H.; Meza, C.A.; Clarke, H.; Kim, J.S.; Hickner, R.C. Vitamin D and Endothelial Function. Nutrients 2020, 12, 575. [Google Scholar] [CrossRef]
- Alhayaza, R.; Haque, E.; Karbasiafshar, C.; Sellke, F.W.; Abid, M.R. The Relationship Between Reactive Oxygen Species and Endothelial Cell Metabolism. Front. Chem. 2020, 8, 592688. [Google Scholar] [CrossRef]
- Dadaei, T.; Safapoor, M.H.; Asadzadeh Aghdaei, H.; Balaii, H.; Pourhoseingholi, M.A.; Naderi, N.; Zojaji, H.; Azimzadeh, P.; Mohammadi, P.; Zali, M.R. Effect of vitamin D3 supplementation on TNF-α serum level and disease activity index in Iranian IBD patients. Gastroenterol. Hepatol. Bed Bench 2015, 8, 49–55. [Google Scholar]
- Oncul, M.; Albayrak, M.; Sozer, V.; Karakus, B.; Gelisgen, R.; Karatas, S.; Simsek, G.; Uzun, H. Polycystic ovary syndrome and endothelial dysfunction: A potential role for soluble lectin-like oxidized low density lipoprotein receptor-1. Reprod. Biol. 2020, 20, 396–401. [Google Scholar] [CrossRef]
- Meng, C. Nitric oxide (NO) levels in patients with polycystic ovary syndrome (PCOS): A meta-analysis. J. Int. Med. Res. 2019, 47, 4083–4094. [Google Scholar] [CrossRef]
- Dokras, A. Heart health in polycystic ovary syndrome: Time to act on the data. Fertil. Steril. 2022, 117, 885–886. [Google Scholar] [CrossRef]
- Kim, H.A.; Perrelli, A.; Ragni, A.; Retta, F.; De Silva, T.M.; Sobey, C.G.; Retta, S.F. Vitamin D Deficiency and the Risk of Cerebrovascular Disease. Antioxidants 2020, 9, 327. [Google Scholar] [CrossRef]
- Li, J.; Lai, H.; Yang, L.; Zhu, H.; Chen, S.; Lai, S. Age and Gender Differences in the Association between Serum 25-Hydroxyvitamin D and Stroke in the General US Population: The National Health and Nutrition Examination Survey, 2001–2006. J. Stroke Cerebrovasc. Dis. 2017, 26, 2510–2518. [Google Scholar] [CrossRef]
- Rad, R.E.; Zarbakhsh, M.; Sarabi, S. The relationship of vitamin D deficiency with severity and outcome of acute stroke. Rom. J. Intern. Med. 2021, 59, 351–358. [Google Scholar] [CrossRef]
- Stewart, C.E.; Sohrabji, F. Gonadal hormones and stroke risk: PCOS as a case study. Front. Neuroendocrinol. 2020, 58, 100853. [Google Scholar] [CrossRef]
- Xu, T.; Zhong, C.; Peng, Y.; Chen, C.S.; Wang, J.; Ju, Z.; Li, Q.; Geng, D.; Sun, Y.; Zhang, D.; et al. Serum 25-hydroxyvitamin D deficiency predicts poor outcome amongst acute ischaemic stroke patients with low high density lipoprotein cholesterol. Eur. J. Neurol. 2016, 23, 1763–1768. [Google Scholar] [CrossRef]
- Bushnell, C.D.; Chaturvedi, S.; Gage, K.R.; Herson, P.S.; Hurn, P.D.; Jiménez, M.C.; Kittner, S.J.; Madsen, T.E.; McCullough, L.D.; McDermott, M.; et al. Sex differences in stroke: Challenges and opportunities. J. Cereb. Blood Flow Metab. 2018, 38, 2179–2191. [Google Scholar] [CrossRef]
- Reeves, M.J.; Bushnell, C.D.; Howard, G.; Gargano, J.W.; Duncan, P.W.; Lynch, G.; Khatiwoda, A.; Lisabeth, L. Sex differences in stroke: Epidemiology, clinical presentation, medical care, and outcomes. Lancet Neurol. 2008, 7, 915–926. [Google Scholar] [CrossRef]
- Roy-O’Reilly, M.; McCullough, L.D. Age and Sex Are Critical Factors in Ischemic Stroke Pathology. Endocrinology 2018, 159, 3120–3131. [Google Scholar] [CrossRef]
- Várbíró, S.; Takács, I.; Tűű, L.; Nas, K.; Sziva, R.E.; Hetthéssy, J.R.; Török, M. Effects of Vitamin D on Fertility, Pregnancy and Polycystic Ovary Syndrome-A Review. Nutrients 2022, 14, 1649. [Google Scholar] [CrossRef]
- Tarszabó, R.; Bányai, B.; Ruisanchez, É.; Péterffy, B.; Korsós-Novák, Á.; Lajtai, K.; Sziva, R.E.; Gerszi, D.; Hosszú, Á.; Benkő, R.; et al. Influence of Vitamin D on the Vasoactive Effect of Estradiol in a Rat Model of Polycystic Ovary Syndrome. Int. J. Mol. Sci. 2021, 22, 9404. [Google Scholar] [CrossRef]
- Mishra, J.S.; More, A.S.; Hankins, G.D.V.; Kumar, S. Hyperandrogenemia reduces endothelium-derived hyperpolarizing factor-mediated relaxation in mesenteric artery of female rats. Biol. Reprod. 2017, 96, 1221–1230. [Google Scholar] [CrossRef]
- Lajtai, K.; Tarszabo, R.; Banyai, B.; Peterffy, B.; Gerszi, D.; Ruisanchez, E.; Sziva, R.E.; Korsos-Novak, A.; Benko, R.; Hadjadj, L.; et al. Effect of Vitamin D Status on Vascular Function of the Aorta in a Rat Model of PCOS. Oxid. Med. Cell. Longev. 2021, 2021, 8865979. [Google Scholar] [CrossRef]
- Hadjadj, L.; Várbíró, S.; Horváth, E.M.; Monori-Kiss, A.; Pál, É.; Karvaly, G.B.; Heinzlmann, A.; Magyar, A.; Szabó, I.; Sziva, R.E.; et al. Insulin resistance in an animal model of polycystic ovary disease is aggravated by vitamin D deficiency: Vascular consequences. Diabetes Vasc. Dis. Res. 2018, 15, 294–301. [Google Scholar] [CrossRef]
- Chinnathambi, V.; Blesson, C.S.; Vincent, K.L.; Saade, G.R.; Hankins, G.D.; Yallampalli, C.; Sathishkumar, K. Elevated testosterone levels during rat pregnancy cause hypersensitivity to angiotensin II and attenuation of endothelium-dependent vasodilation in uterine arteries. Hypertension 2014, 64, 405–414. [Google Scholar] [CrossRef]
- Komukai, K.; Mochizuki, S.; Yoshimura, M. Gender and the renin-angiotensin-aldosterone system. Fundam. Clin. Pharmacol. 2010, 24, 687–698. [Google Scholar] [CrossRef]
- Stanhewicz, A.E.; Wenner, M.M.; Stachenfeld, N.S. Sex differences in endothelial function important to vascular health and overall cardiovascular disease risk across the lifespan. Am. J. Physiol.-Heart Circ. Physiol. 2018, 315, H1569–H1588. [Google Scholar] [CrossRef]
- Chen, Y.F.; Naftilan, A.J.; Oparil, S. Androgen-dependent angiotensinogen and renin messenger RNA expression in hypertensive rats. Hypertension 1992, 19, 456–463. [Google Scholar] [CrossRef]
- de Groot, P.C.; Dekkers, O.M.; Romijn, J.A.; Dieben, S.W.; Helmerhorst, F.M. PCOS, coronary heart disease, stroke and the influence of obesity: A systematic review and meta-analysis. Hum. Reprod. Update 2011, 17, 495–500. [Google Scholar] [CrossRef]
- Macut, D.; Mladenović, V.; Bjekić-Macut, J.; Livadas, S.; Stanojlović, O.; Hrnčić, D.; Rašić-Marković, A.; Milutinović, D.V.; Andrić, Z. Hypertension in Polycystic Ovary Syndrome: Novel Insights. Curr. Hypertens. Rev. 2020, 16, 55–60. [Google Scholar] [CrossRef]
- Villavicencio, A.; Bacallao, K.; Avellaira, C.; Gabler, F.; Fuentes, A.; Vega, M. Androgen and estrogen receptors and co-regulators levels in endometria from patients with polycystic ovarian syndrome with and without endometrial hyperplasia. Gynecol. Oncol. 2006, 103, 307–314. [Google Scholar] [CrossRef]
- Hulchiy, M.; Nybacka, Å.; Sahlin, L.; Hirschberg, A.L. Endometrial Expression of Estrogen Receptors and the Androgen Receptor in Women With Polycystic Ovary Syndrome: A Lifestyle Intervention Study. J. Clin. Endocrinol. Metab. 2016, 101, 561–571. [Google Scholar] [CrossRef]
- Xu, X.L.; Huang, Z.Y.; Yu, K.; Li, J.; Fu, X.W.; Deng, S.L. Estrogen Biosynthesis and Signal Transduction in Ovarian Disease. Front. Endocrinol. 2022, 13, 827032. [Google Scholar] [CrossRef]
- Berger, L.; El-Alfy, M.; Labrie, F. Effects of intravaginal dehydroepiandrosterone on vaginal histomorphology, sex steroid receptor expression and cell proliferation in the rat. J. Steroid Biochem. Mol. Biol. 2008, 109, 67–80. [Google Scholar] [CrossRef]
- Eriksen, M.B.; Glintborg, D.; Nielsen, M.F.; Jakobsen, M.A.; Brusgaard, K.; Tan, Q.; Gaster, M. Testosterone treatment increases androgen receptor and aromatase gene expression in myotubes from patients with PCOS and controls, but does not induce insulin resistance. Biochem. Biophys. Res. Commun. 2014, 451, 622–626. [Google Scholar] [CrossRef]
- Zang, H.; Sahlin, L.; Masironi, B.; Hirschberg, A.L. Effects of testosterone and estrogen treatment on the distribution of sex hormone receptors in the endometrium of postmenopausal women. Menopause 2008, 15, 233–239. [Google Scholar] [CrossRef]
- Baldassarre, M.; Perrone, A.M.; Giannone, F.A.; Armillotta, F.; Battaglia, C.; Costantino, A.; Venturoli, S.; Meriggiola, M.C. Androgen receptor expression in the human vagina under different physiological and treatment conditions. Int. J. Impot. Res. 2013, 25, 7–11. [Google Scholar] [CrossRef][Green Version]
- Masszi, G.; Novak, A.; Tarszabo, R.; Horvath, E.M.; Buday, A.; Ruisanchez, E.; Tokes, A.M.; Sara, L.; Benko, R.; Nadasy, G.L.; et al. Effects of vitamin D3 derivative--calcitriol on pharmacological reactivity of aortic rings in a rodent PCOS model. Pharmacol. Rep. 2013, 65, 476–483. [Google Scholar] [CrossRef]
- Gangula, P.R.; Dong, Y.L.; Al-Hendy, A.; Richard-Davis, G.; Montgomery-Rice, V.; Haddad, G.; Millis, R.; Nicholas, S.B.; Moseberry, D. Protective cardiovascular and renal actions of vitamin D and estrogen. Front. Biosci. 2013, 5, 134–148. [Google Scholar] [CrossRef]
- Pelham, C.J.; Drews, E.M.; Agrawal, D.K. Vitamin D controls resistance artery function through regulation of perivascular adipose tissue hypoxia and inflammation. J. Mol. Cell. Cardiol. 2016, 98, 1–10. [Google Scholar] [CrossRef]
- Sipos, M.; Peterffy, B.; Sziva, R.E.; Magyar, P.; Hadjadj, L.; Banyai, B.; Suli, A.; Soltesz-Katona, E.; Gerszi, D.; Kiss, J.; et al. Vitamin D Deficiency Cause Gender Specific Alterations of Renal Arterial Function in a Rodent Model. Nutrients 2021, 13, 704. [Google Scholar] [CrossRef]
- Del Pinto, R.; Wright, J.T.; Monaco, A.; Pietropaoli, D.; Ferri, C. Vitamin D and blood pressure control among hypertensive adults: Results from NHANES 2001–2014. J. Hypertens 2020, 38, 150–158. [Google Scholar] [CrossRef]
- Pál, É.; Ungvári, Z.; Benyó, Z.; Várbíró, S. Role of Vitamin D Deficiency in the Pathogenesis of Cardiovascular and Cerebrovascular Diseases. Nutrients 2023, 15, 334. [Google Scholar] [CrossRef]
- Crescioli, C. The Role of Estrogens and Vitamin D in Cardiomyocyte Protection: A Female Perspective. Biomolecules 2021, 11, 1815. [Google Scholar] [CrossRef]
- Fontányi, Z.; Sziva, R.E.; Pál, É.; Hadjadj, L.; Monori-Kiss, A.; Horváth, E.M.; Benkő, R.; Magyar, A.; Heinzlmann, A.; Benyó, Z.; et al. Vitamin D Deficiency Reduces Vascular Reactivity of Coronary Arterioles in Male Rats. Curr. Issues Mol. Biol. 2021, 43, 79–92. [Google Scholar] [CrossRef]
- Hasan, N.; Sonnenschein, C.; Soto, A.M. Vitamin D3 constrains estrogen’s effects and influences mammary epithelial organization in 3D cultures. Sci. Rep. 2019, 9, 7423. [Google Scholar] [CrossRef]
- Krishnan, A.V.; Swami, S.; Feldman, D. The potential therapeutic benefits of vitamin D in the treatment of estrogen receptor positive breast cancer. Steroids 2012, 77, 1107–1112. [Google Scholar] [CrossRef]
- Masszi, G.; Horvath, E.M.; Tarszabo, R.; Benko, R.; Novak, A.; Buday, A.; Tokes, A.M.; Nadasy, G.L.; Hamar, P.; Benyo, Z.; et al. Reduced estradiol-induced vasodilation and poly-(ADP-ribose) polymerase (PARP) activity in the aortas of rats with experimental polycystic ovary syndrome (PCOS). PLoS ONE 2013, 8, e55589. [Google Scholar] [CrossRef]
- Krause, D.N.; Duckles, S.P.; Pelligrino, D.A. Influence of sex steroid hormones on cerebrovascular function. J. Appl. Physiol. 2006, 101, 1252–1261. [Google Scholar] [CrossRef]
- Al-Bayyari, N.; Al-Domi, H.; Zayed, F.; Hailat, R.; Eaton, A. Androgens and hirsutism score of overweight women with polycystic ovary syndrome improved after vitamin D treatment: A randomized placebo controlled clinical trial. Clin. Nutr. 2021, 40, 870–878. [Google Scholar] [CrossRef]
- Pál, É.; Hadjadj, L.; Fontányi, Z.; Monori-Kiss, A.; Mezei, Z.; Lippai, N.; Magyar, A.; Heinzlmann, A.; Karvaly, G.; Monos, E.; et al. Vitamin D deficiency causes inward hypertrophic remodeling and alters vascular reactivity of rat cerebral arterioles. PLoS ONE 2018, 13, e0192480. [Google Scholar] [CrossRef]
- Masszi, G.; Benko, R.; Csibi, N.; Horvath, E.M.; Tokes, A.M.; Novak, A.; Beres, N.J.; Tarszabo, R.; Buday, A.; Repas, C.; et al. Endothelial relaxation mechanisms and nitrative stress are partly restored by Vitamin D3 therapy in a rat model of polycystic ovary syndrome. Life Sci. 2013, 93, 133–138. [Google Scholar] [CrossRef][Green Version]
- Masszi, G.; Buday, A.; Novak, A.; Horvath, E.M.; Tarszabo, R.; Sara, L.; Revesz, C.; Benko, R.; Nadasy, G.L.; Benyó, Z.; et al. Altered insulin-induced relaxation of aortic rings in a dihydrotestosterone-induced rodent model of polycystic ovary syndrome. Fertil. Steril. 2013, 99, 573–578. [Google Scholar] [CrossRef]
- Sara, L.; Antal, P.; Masszi, G.; Buday, A.; Horvath, E.M.; Hamar, P.; Monos, E.; Nadasy, G.L.; Varbiro, S. Arteriolar insulin resistance in a rat model of polycystic ovary syndrome. Fertil. Steril. 2012, 97, 462–468. [Google Scholar] [CrossRef]
- Várbíró, S.; Sára, L.; Antal, P.; Monori-Kiss, A.; Tőkés, A.M.; Monos, E.; Benkő, R.; Csibi, N.; Szekeres, M.; Tarszabo, R.; et al. Lower-limb veins are thicker and vascular reactivity is decreased in a rat PCOS model: Concomitant vitamin D3 treatment partially prevents these changes. Am. J. Physiol.-Heart Circ. Physiol. 2014, 307, H848–H857. [Google Scholar] [CrossRef]
- Sipos, M.; Gerszi, D.; Dalloul, H.; Bányai, B.; Sziva, R.E.; Kollarics, R.; Magyar, P.; Török, M.; Ács, N.; Szekeres, M.; et al. Vitamin D Deficiency and Gender Alter Vasoconstrictor and Vasodilator Reactivity in Rat Carotid Artery. Int. J. Mol. Sci. 2021, 22, 8029. [Google Scholar] [CrossRef]
- Medina, D.; Mehay, D.; Arnold, A.C. Sex differences in cardiovascular actions of the renin-angiotensin system. Clin. Auton. Res. 2020, 30, 393–408. [Google Scholar] [CrossRef]
- Fernández-Atucha, A.; Izagirre, A.; Fraile-Bermúdez, A.B.; Kortajarena, M.; Larrinaga, G.; Martinez-Lage, P.; Echevarría, E.; Gil, J. Sex differences in the aging pattern of renin-angiotensin system serum peptidases. Biol. Sex Differ. 2017, 8, 5. [Google Scholar] [CrossRef]
- De Silva, T.M.; Broughton, B.R.; Drummond, G.R.; Sobey, C.G.; Miller, A.A. Gender influences cerebral vascular responses to angiotensin II through Nox2-derived reactive oxygen species. Stroke 2009, 40, 1091–1097. [Google Scholar] [CrossRef]
- Faraci, F.M.; Lamping, K.G.; Modrick, M.L.; Ryan, M.J.; Sigmund, C.D.; Didion, S.P. Cerebral vascular effects of angiotensin II: New insights from genetic models. J. Cereb. Blood Flow. Metab. 2006, 26, 449–455. [Google Scholar] [CrossRef]
- Santos, R.L.; Abreu, G.R.; Bissoli, N.S.; Moysés, M.R. Endothelial mediators of 17 beta-estradiol-induced coronary vasodilation in the isolated rat heart. Braz. J. Med. Biol. Res. 2004, 37, 569–575. [Google Scholar] [CrossRef]
- Santos, R.L.; Lima, J.T.; Rouver, W.N.; Moysés, M.R. Deficiency of sex hormones does not affect 17-ß-estradiol-induced coronary vasodilation in the isolated rat heart. Braz. J. Med. Biol. Res. 2016, 49, e5058. [Google Scholar] [CrossRef]
- Yıldırım, E.; Erol, K. The effects of testosterone on isolated sheep coronary artery. Anadolu Kardiyol. Derg. 2011, 11, 343–350. [Google Scholar] [CrossRef]
- Pal, E.; Hadjadj, L.; Fontanyi, Z.; Monori-Kiss, A.; Lippai, N.; Horvath, E.M.; Magyar, A.; Horvath, E.; Monos, E.; Nadasy, G.L.; et al. Gender, hyperandrogenism and vitamin D deficiency related functional and morphological alterations of rat cerebral arteries. PLoS ONE 2019, 14, e0216951. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Süli, A.; Magyar, P.; Vezér, M.; Bányai, B.; Szekeres, M.; Sipos, M.; Mátrai, M.; Hetthéssy, J.R.; Dörnyei, G.; Ács, N.; et al. Effects of Gender and Vitamin D on Vascular Reactivity of the Carotid Artery on a Testosterone-Induced PCOS Model. Int. J. Mol. Sci. 2023, 24, 16577. https://doi.org/10.3390/ijms242316577
Süli A, Magyar P, Vezér M, Bányai B, Szekeres M, Sipos M, Mátrai M, Hetthéssy JR, Dörnyei G, Ács N, et al. Effects of Gender and Vitamin D on Vascular Reactivity of the Carotid Artery on a Testosterone-Induced PCOS Model. International Journal of Molecular Sciences. 2023; 24(23):16577. https://doi.org/10.3390/ijms242316577
Chicago/Turabian StyleSüli, Anita, Péter Magyar, Márton Vezér, Bálint Bányai, Mária Szekeres, Miklós Sipos, Máté Mátrai, Judit Réka Hetthéssy, Gabriella Dörnyei, Nándor Ács, and et al. 2023. "Effects of Gender and Vitamin D on Vascular Reactivity of the Carotid Artery on a Testosterone-Induced PCOS Model" International Journal of Molecular Sciences 24, no. 23: 16577. https://doi.org/10.3390/ijms242316577
APA StyleSüli, A., Magyar, P., Vezér, M., Bányai, B., Szekeres, M., Sipos, M., Mátrai, M., Hetthéssy, J. R., Dörnyei, G., Ács, N., Horváth, E. M., Nádasy, G. L., Várbíró, S., & Török, M. (2023). Effects of Gender and Vitamin D on Vascular Reactivity of the Carotid Artery on a Testosterone-Induced PCOS Model. International Journal of Molecular Sciences, 24(23), 16577. https://doi.org/10.3390/ijms242316577







