Astroglial S100B Secretion Is Mediated by Ca2+ Mobilization from Endoplasmic Reticulum: A Study Using Forskolin and DMSO as Secretagogues
Abstract
:1. Introduction
2. Results
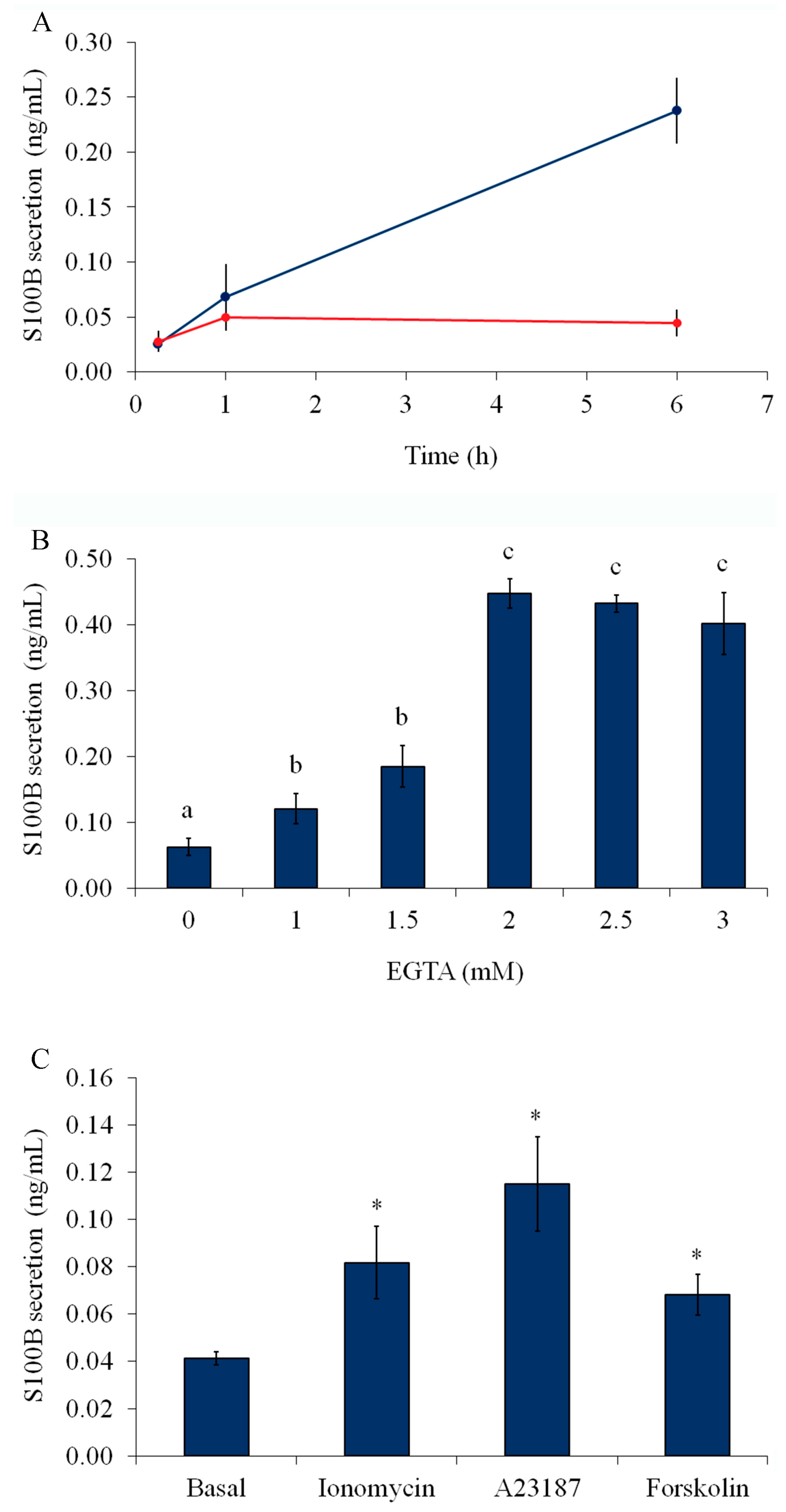
2.1. Serum Deprivation, External Calcium, and Forskolin Stimulate S100B Secretion
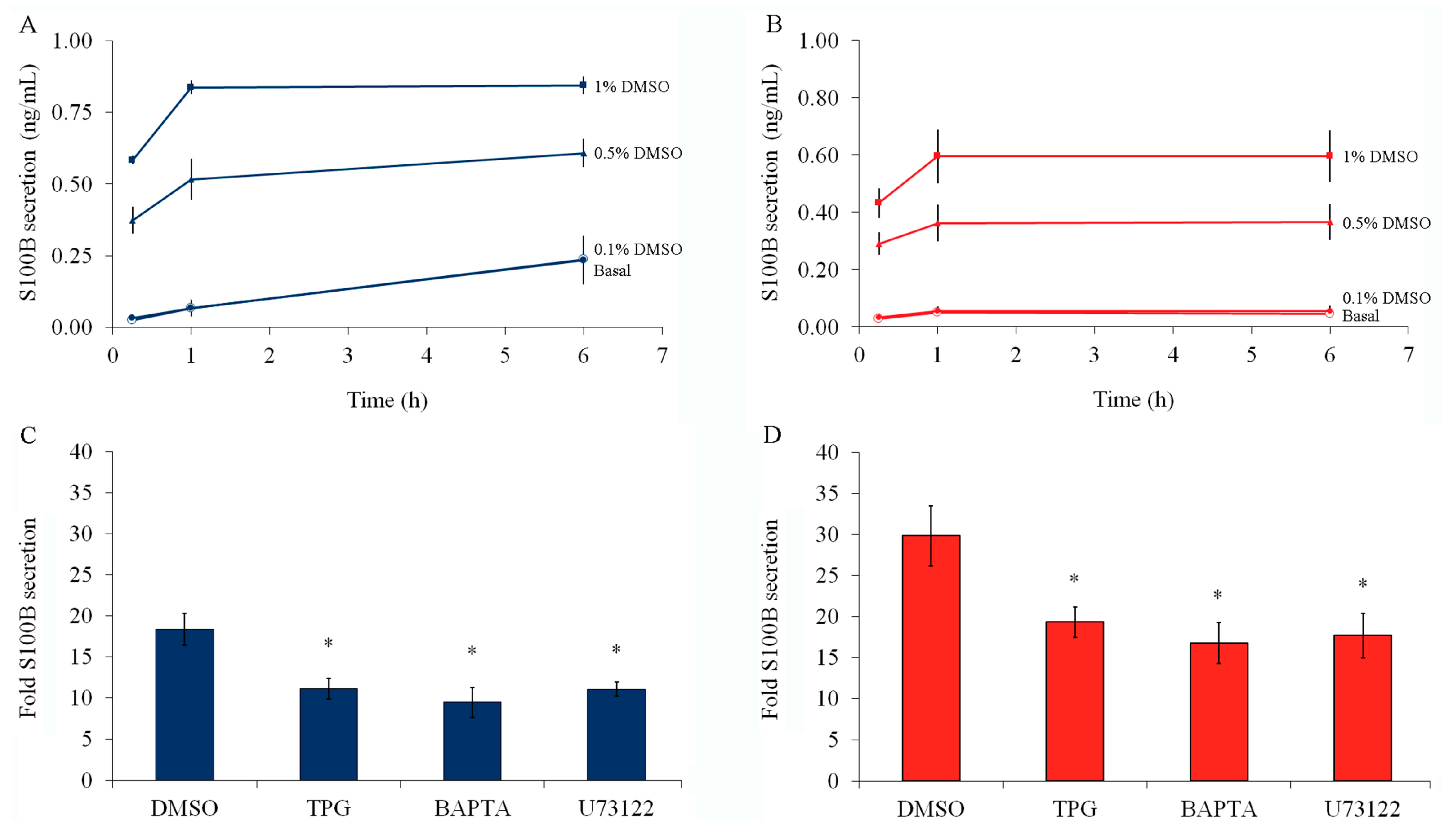
2.2. S100B Secretion Is Stimulated by DMSO, and This Effect Is Prevented by Thapsigargin or BAPTA-AM
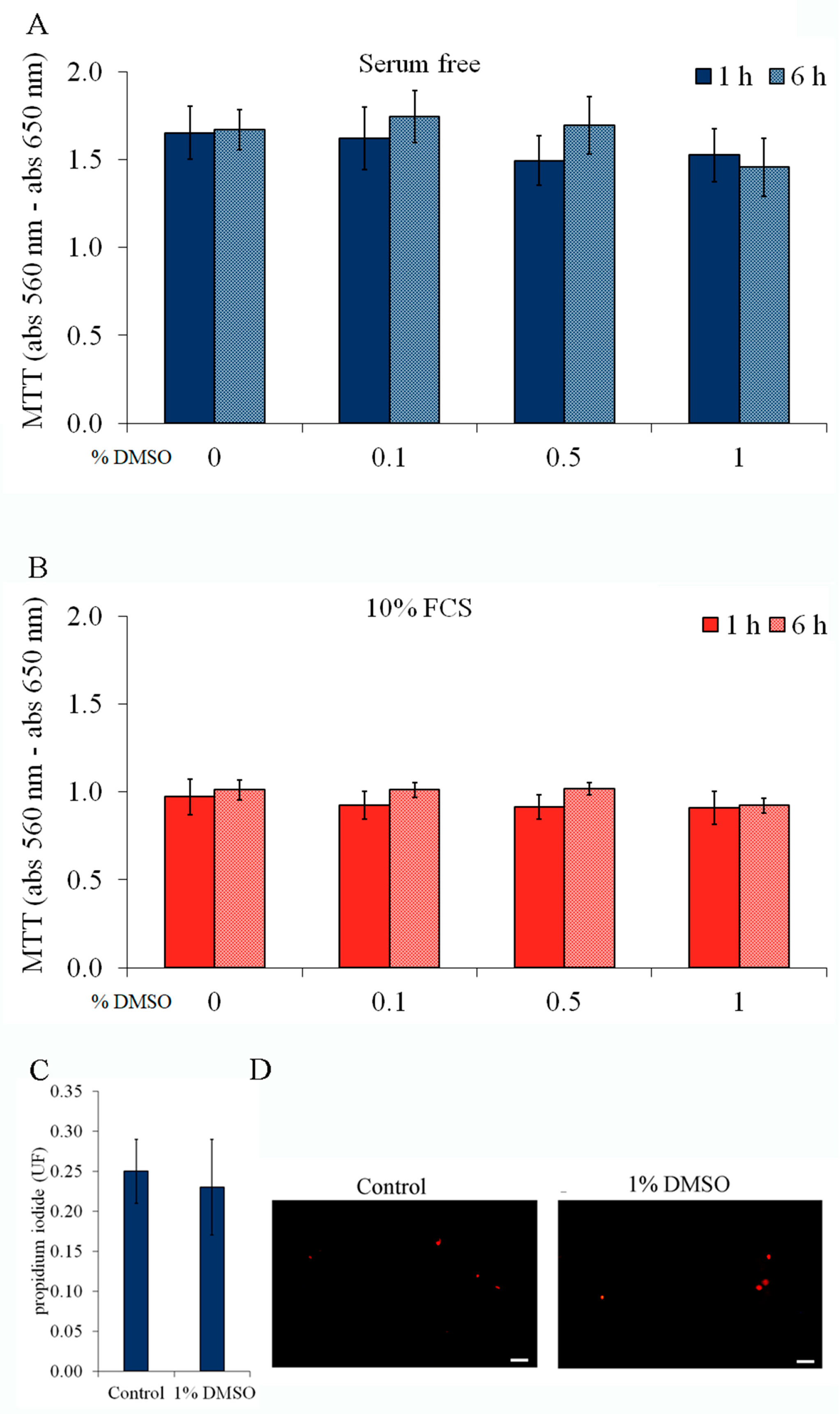
2.3. Cell Viability and Integrity Are Not Affected by Forskolin or DMSO
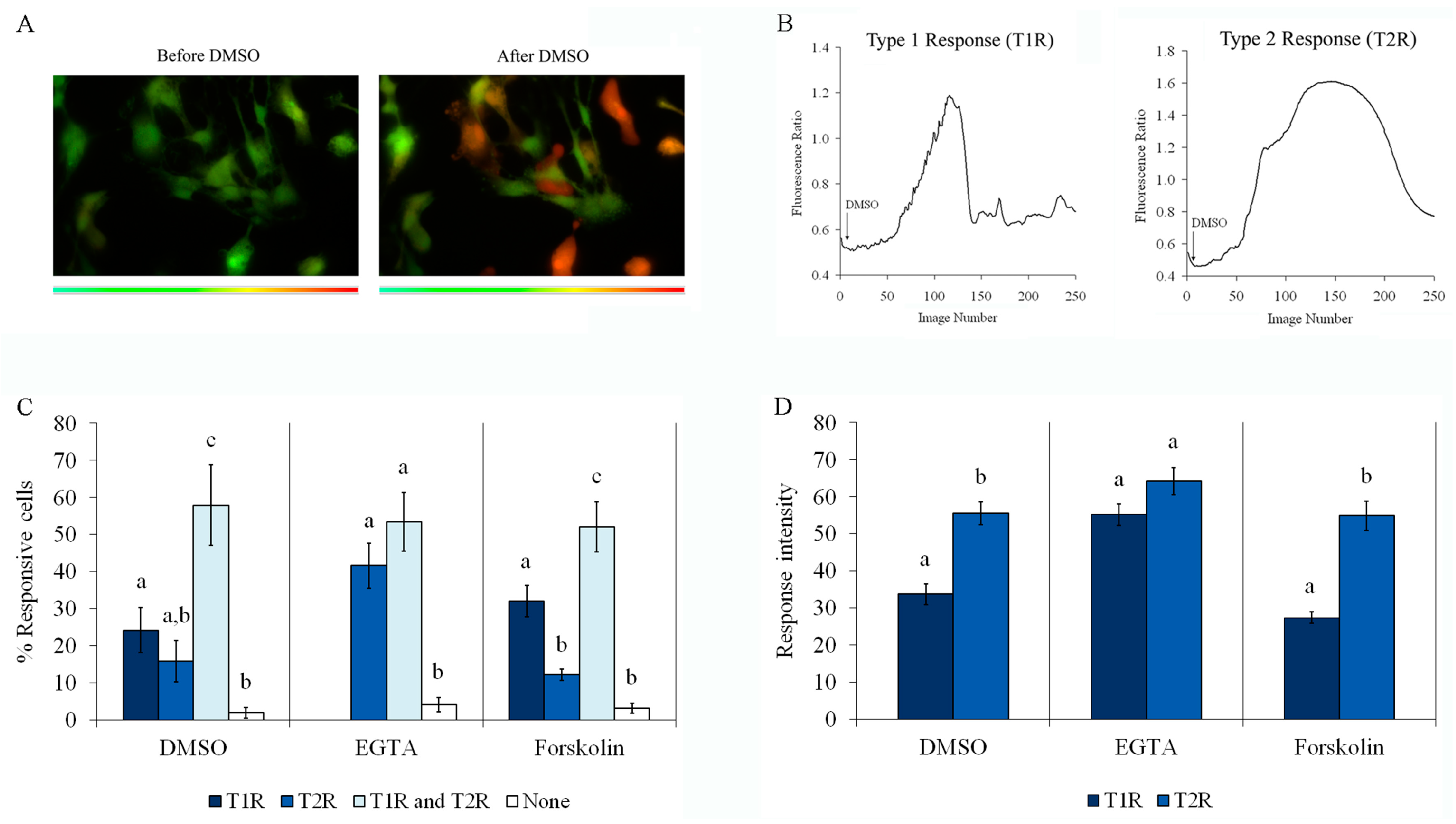
2.4. Forskolin, EGTA, and DMSO Induce an Increase in Intracellular Ca2+
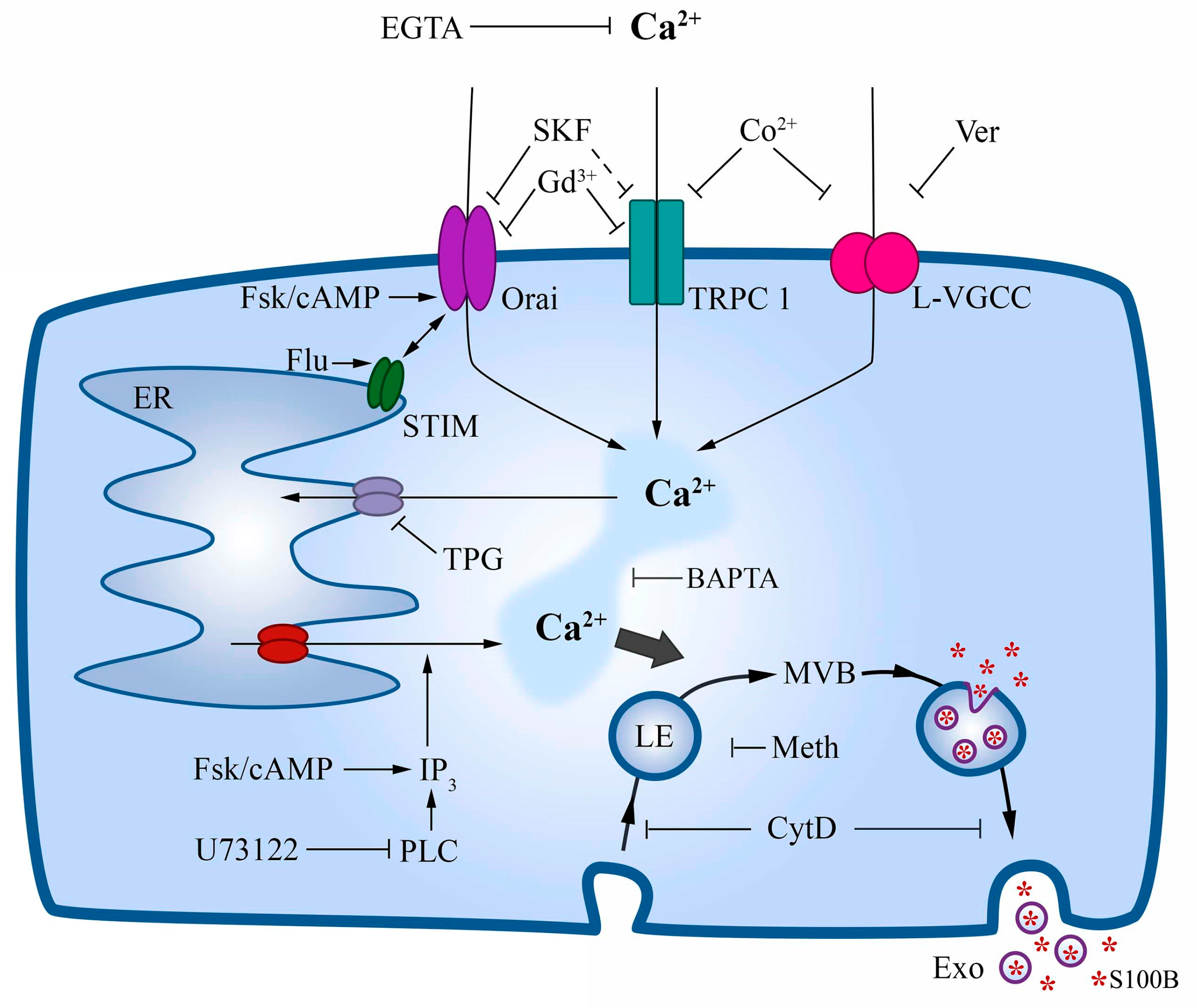
2.5. DMSO-Induced Ca2+ Increase Involves Mobilization from the Endoplasmic Reticulum
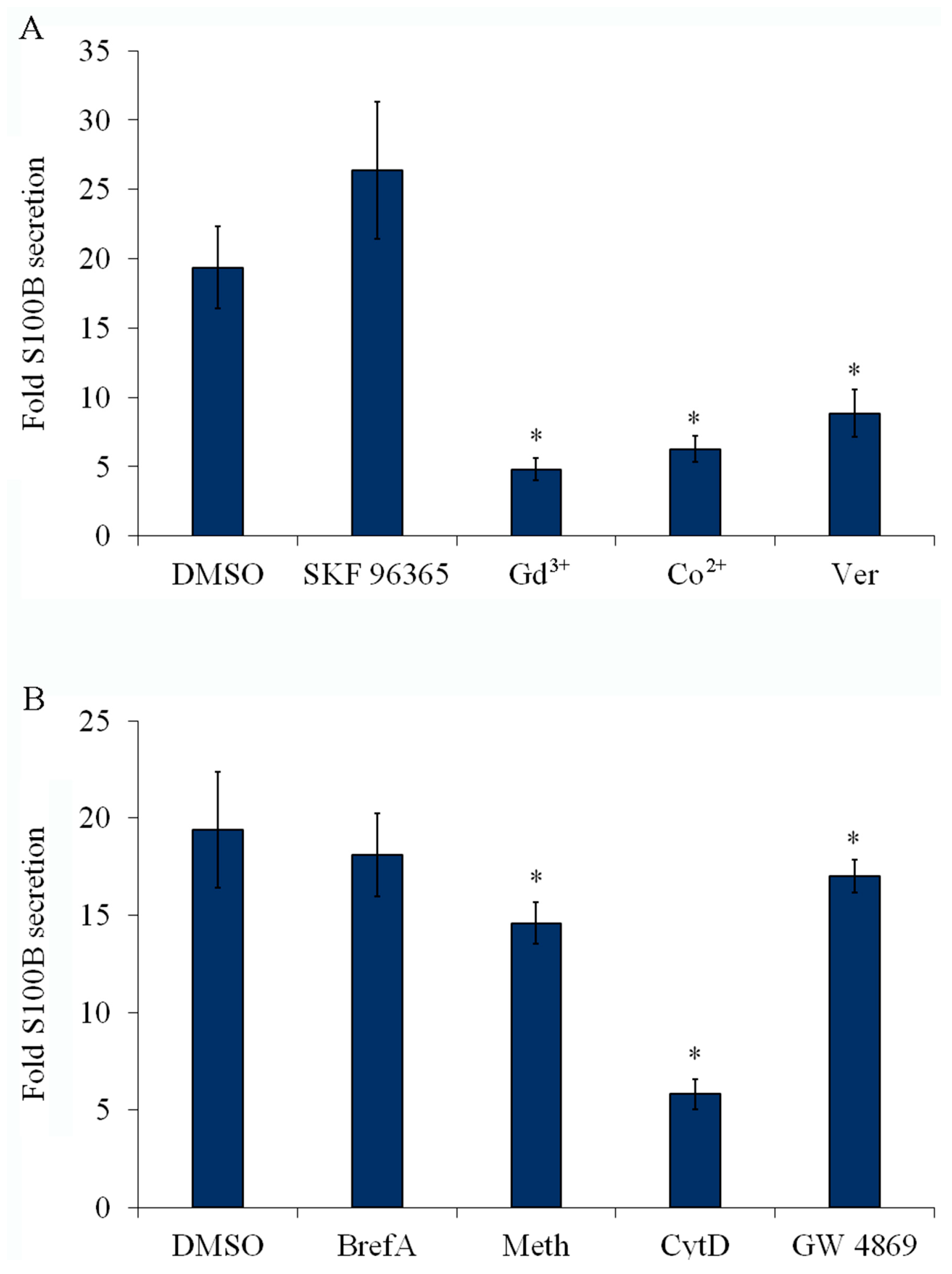
2.6. Inhibition of Plasma Membrane Ca2+ Channels Reduces S100B Secretion
2.7. S100B Secretion Involves a Vesicle-Exporting Mechanism
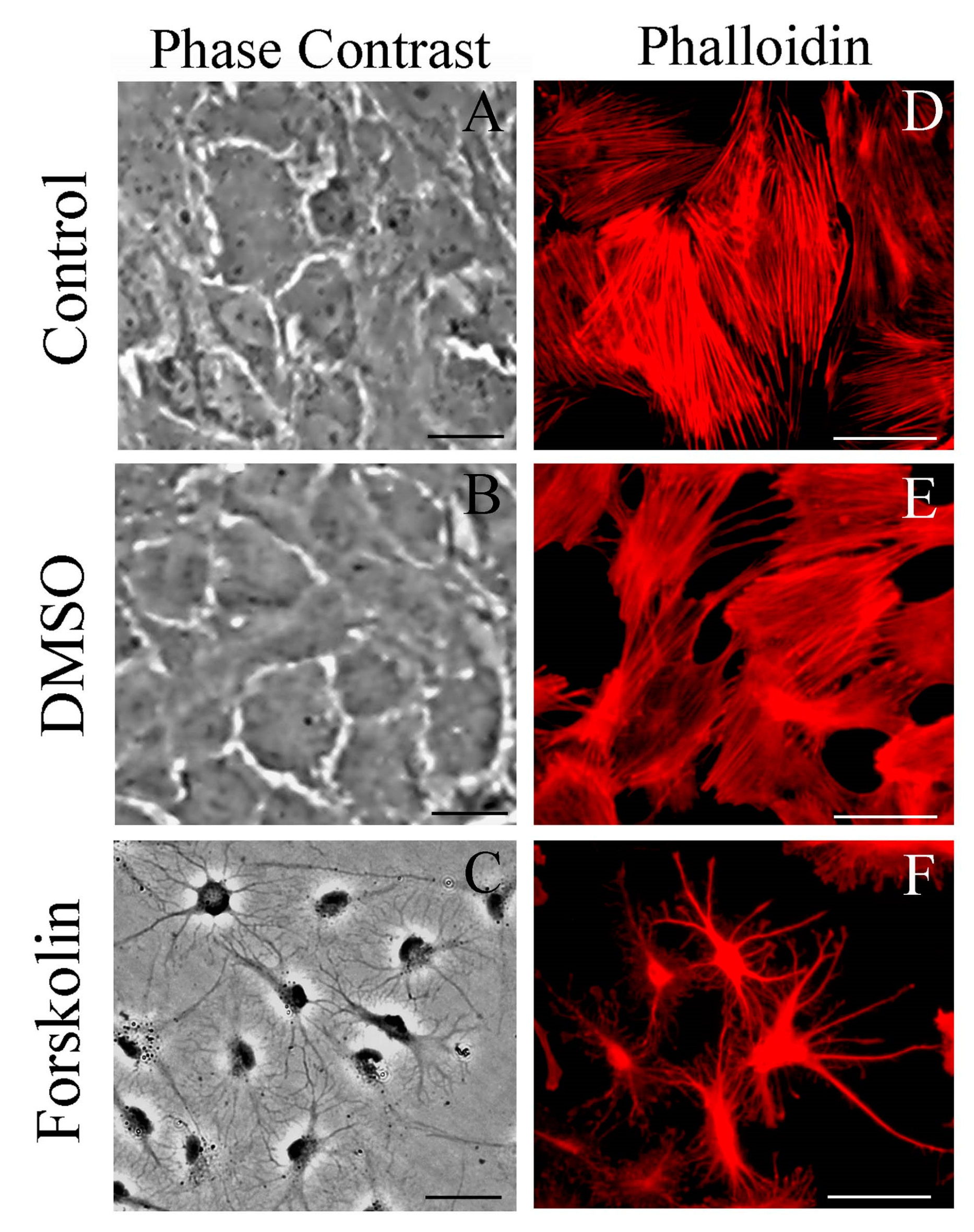
2.8. Different from Forskolin, DMSO Does Not Induce Morphological Changes Associated with S100B Secretion
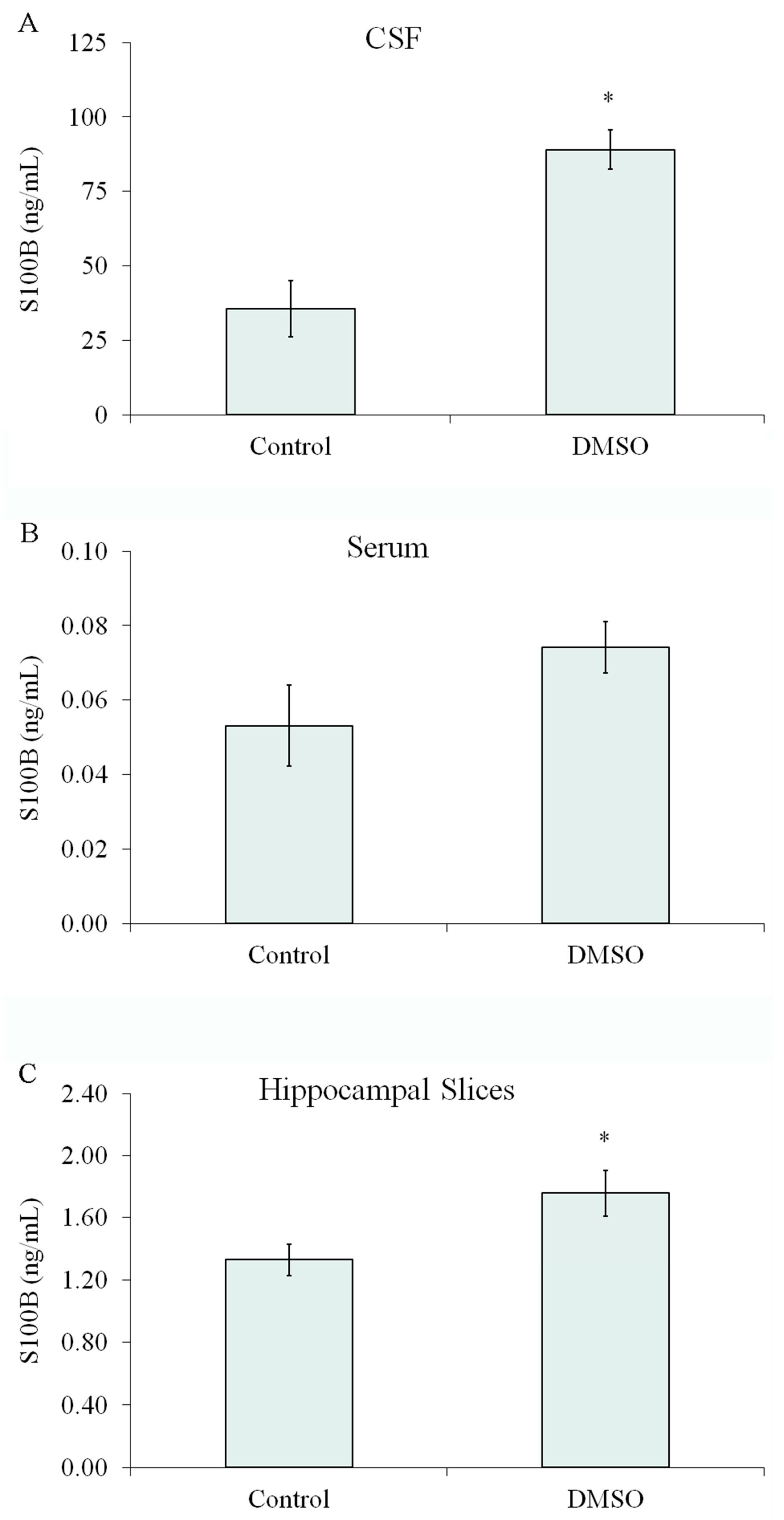
2.9. The Effect of DMSO on S100B Secretion Was Also Observed In Vivo and in Acute Hippocampal Slices
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goncalves, C.A.; Leite, M.C.; Nardin, P. Biological and Methodological Features of the Measurement of S100B, a Putative Marker of Brain Injury. Clin. Biochem. 2008, 41, 755–763. [Google Scholar] [CrossRef]
- Michetti, F.; Di Sante, G.; Clementi, M.E.; Sampaolese, B.; Casalbore, P.; Volonté, C.; Romano Spica, V.; Parnigotto, P.P.; Di Liddo, R.; Amadio, S.; et al. Growing Role of S100B Protein as a Putative Therapeutic Target for Neurological- and Nonneurological-Disorders. Neurosci. Biobehav. Rev. 2021, 127, 446–458. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, Y.; Deng, S.; Mo, Y.; Huang, Y.; Li, W.; Ge, C.; Ren, X.; Zhang, H.; Zhang, X.; et al. S100B/RAGE/Ceramide Signaling Pathway Is Involved in Sepsis-Associated Encephalopathy. Life Sci. 2021, 277, 119490. [Google Scholar] [CrossRef]
- Barateiro, A.; Afonso, V.; Santos, G.; Cerqueira, J.J.; Brites, D.; van Horssen, J.; Fernandes, A. S100B as a Potential Biomarker and Therapeutic Target in Multiple Sclerosis. Mol. Neurobiol. 2016, 53, 3976–3991. [Google Scholar] [CrossRef]
- Kellermann, I.; Kleindienst, A.; Hore, N.; Buchfelder, M.; Brandner, S. Early CSF and Serum S100B Concentrations for Outcome Prediction in Traumatic Brain Injury and Subarachnoid Hemorrhage. Clin. Neurol. Neurosurg. 2016, 145, 79–83. [Google Scholar] [CrossRef]
- Marenholz, I.; Heizmann, C.W. S100 Proteins in Mouse and Man: From Evolution to Function and Pathology (Including an Update of the Nomenclature). Biochem. Biophys. Res. Commun. 2004, 322, 1111–1122. [Google Scholar] [CrossRef]
- Donato, R.; Sorci, G.; Riuzzi, F.; Arcuri, C.; Bianchi, R.; Brozzi, F.; Tubaro, C.; Giambanco, I. S100B’s Double Life: Intracellular Regulator and Extracellular Signal. Biochim. Biophys. Acta 2009, 1793, 1008–1022. [Google Scholar] [CrossRef]
- Donato, R.; Cannon, B.R.; Sorci, G.; Riuzzi, F.; Hsu, K.; Weber, D.J.; Geczy, C.L. Functions of S100 Proteins. Curr. Mol. Med. 2012, 13, 24–57. [Google Scholar] [CrossRef]
- Steiner, J.; Bernstein, H.G.; Bielau, H.; Berndt, A.; Brisch, R.; Mawrin, C.; Keilhoff, G.; Bogerts, B. Evidence for a Wide Extra-Astrocytic Distribution of S100B in Human Brain. BMC Neurosci. 2007, 8, 2. [Google Scholar] [CrossRef]
- Pichiule, P.; Chavez, J.C.; Schmidt, A.M.; Vannucci, S.J. Hypoxia-Inducible Factor-1 Mediates Neuronal Expression of the Receptor for Advanced Glycation End Products Following Hypoxia/Ischemia. J. Biol. Chem. 2007, 282, 36330–36340. [Google Scholar] [CrossRef]
- Tramontina, A.C.; Tramontina, F.; Bobermin, L.D.; Zanotto, C.; Souza, D.F.; Leite, M.C.; Nardin, P.; Gottfried, C.; Goncalves, C.A. Secretion of S100B, an Astrocyte-Derived Neurotrophic Protein, Is Stimulated by Fluoxetine via a Mechanism Independent of Serotonin. Prog. Neuropsychopharmacol. Biol. Psychiatry 2008, 32, 1580–1583. [Google Scholar] [CrossRef]
- Sakatani, S.; Seto-Ohshima, A.; Shinohara, Y.; Yamamoto, Y.; Yamamoto, H.; Itohara, S.; Hirase, H. Neural-Activity-Dependent Release of S100B from Astrocytes Enhances Kainate-Induced Gamma Oscillations In Vivo. J. Neurosci. 2008, 28, 10928–10936. [Google Scholar] [CrossRef]
- Lunardi, P.; Nardin, P.; Guerra, M.C.; Abib, R.; Leite, M.C.; Gonçalves, C.A. Huperzine A, but Not Tacrine, Stimulates S100B Secretion in Astrocyte Cultures. Life Sci. 2013, 92, 701–707. [Google Scholar] [CrossRef]
- Leite, M.C.; Galland, F.; de Souza, D.F.; Guerra, M.C.; Bobermin, L.; Biasibetti, R.; Gottfried, C.; Goncalves, C.A. Gap Junction Inhibitors Modulate S100B Secretion in Astrocyte Cultures and Acute Hippocampal Slices. J. Neurosci. Res. 2009, 87, 2439–2446. [Google Scholar] [CrossRef]
- Buyukuysal, R.L. Protein S100B Release from Rat Brain Slices during and after Ischemia: Comparison with Lactate Dehydrogenase Leakage. Neurochem. Int. 2005, 47, 580–588. [Google Scholar] [CrossRef]
- Davey, G.E.; Murmann, P.; Heizmann, C.W. Intracellular Ca2+ and Zn2+ Levels Regulate the Alternative Cell Density-Dependent Secretion of S100B in Human Glioblastoma Cells. J. Biol. Chem. 2001, 276, 30819–30826. [Google Scholar] [CrossRef]
- Eriksen, J.L.; Gillespie, R.; Druse, M.J. Effects of Ethanol and 5-HT1A Agonists on Astroglial S100B. Brain Res. Dev. Brain Res. 2002, 139, 97–105. [Google Scholar] [CrossRef]
- Goncalves, D.; Karl, J.; Leite, M.; Rotta, L.; Salbego, C.; Rocha, E.; Wofchuk, S.; Goncalves, C.A. High Glutamate Decreases S100B Secretion Stimulated by Serum Deprivation in Astrocytes. Neuroreport 2002, 13, 1533–1535. [Google Scholar] [CrossRef]
- Matsunaga, H.; Ueda, H. Evidence for Serum-Deprivation-Induced Co-Release of FGF-1 and S100A13 from Astrocytes. Neurochem. Int. 2006, 49, 294–303. [Google Scholar] [CrossRef]
- Nardin, P.; Tortorelli, L.; Quincozes-Santos, A.; de Almeida, L.M.; Leite, M.C.; Thomazi, A.P.; Gottfried, C.; Wofchuk, S.T.; Donato, R.; Goncalves, C.A. S100B Secretion in Acute Brain Slices: Modulation by Extracellular Levels of Ca2+ and K+. Neurochem. Res. 2009, 34, 1603–1611. [Google Scholar] [CrossRef]
- Penazzi, L.; Lorengel, J.; Sündermann, F.; Golovyashkina, N.; Marre, S.; Mathis, C.M.B.; Lewejohann, L.; Brandt, R.; Bakota, L. DMSO Modulates CNS Function in a Preclinical Alzheimer’s Disease Model. Neuropharmacology 2017, 113, 434–444. [Google Scholar] [CrossRef]
- Steiner, J.; Bernstein, H.G.; Bogerts, B.; Gos, T.; Richter-Landsberg, C.; Wunderlich, M.T.; Keilhoff, G. S100B Is Expressed in, and Released from, OLN-93 Oligodendrocytes: Influence of Serum and Glucose Deprivation. Neuroscience 2008, 154, 496–503. [Google Scholar] [CrossRef]
- Galland, F.; Seady, M.; Taday, J.; Smaili, S.S.; Gonçalves, C.A.; Leite, M.C. Astrocyte Culture Models: Molecular and Function Characterization of Primary Culture, Immortalized Astrocytes and C6 Glioma Cells. Neurochem. Int. 2019, 131, 104538. [Google Scholar] [CrossRef]
- Simpson, P.B.; Russell, J.T. Role of Mitochondrial Ca2+ Regulation in Neuronal and Glial Cell Signalling. Brain Res Rev. 1998, 26, 72–81. [Google Scholar] [CrossRef]
- Heidemann, A.C.; Schipke, C.G.; Kettenmann, H. Extracellular Application of Nicotinic Acid Adenine Dinucleotide Phosphate Induces Ca2+ Signaling in Astrocytes in Situ. J. Biol. Chem. 2005, 280, 35630–35640. [Google Scholar] [CrossRef]
- Li, D.; Ropert, N.; Koulakoff, A.; Giaume, C.; Oheim, M. Lysosomes Are the Major Vesicular Compartment Undergoing Ca2+-Regulated Exocytosis from Cortical Astrocytes. J. Neurosci. 2008, 28, 7648–7658. [Google Scholar] [CrossRef]
- Hian Tan, K.; Sim Tan, S.; Sze, S.K.; Ryan Lee, W.K.; Jack Ng, M.; Kiang Lim, S. Plasma Biomarker Discovery in Preeclampsia Using a Novel Differential Isolation Technology for Circulating Extracellular Vesicles. Am. J. Obstet. Gynecol. 2014, 211, 380.e1–380.e13. [Google Scholar] [CrossRef]
- Wang, G.; Dinkins, M.; He, Q.; Zhu, G.; Poirier, C.; Campbell, A.; Mayer-Proschel, M.; Bieberich, E. Astrocytes Secrete Exosomes Enriched with Proapoptotic Ceramide and Prostate Apoptosis Response 4 (PAR-4): Potential Mechanism of Apoptosis Induction in Alzheimer Disease (AD). J. Biol. Chem. 2012, 287, 21384–21395. [Google Scholar] [CrossRef]
- Huang, Y.L.; Saljo, A.; Suneson, A.; Hansson, H.A. Comparison among Different Approaches for Sampling Cerebrospinal Fluid in Rats. Brain Res. Bull. 1996, 41, 273–279. [Google Scholar] [CrossRef]
- Michetti, F.; Corvino, V.; Geloso, M.C.; Lattanzi, W.; Bernardini, C.; Serpero, L.; Gazzolo, D. The S100B Protein in Biological Fluids: More than a Lifelong Biomarker of Brain Distress. J. Neurochem. 2012, 120, 644–659. [Google Scholar] [CrossRef]
- Van Eldik, L.J.; Wainwright, M.S. The Janus Face of Glial-Derived S100B: Beneficial and Detrimental Functions in the Brain. Restor. Neurol. Neurosci. 2003, 21, 97–108. [Google Scholar]
- Goncalves, D.S.; Lenz, G.; Karl, J.; Goncalves, C.A.; Rodnight, R. Extracellular S100B Protein Modulates ERK in Astrocyte Cultures. Neuroreport 2000, 11, 807–809. [Google Scholar] [CrossRef]
- Suzuki, F.; Kato, K.; Kato, T.; Ogasawara, N. S-100 Protein in Clonal Astroglioma Cells Is Released by Adrenocorticotropic Hormone and Corticotropin-like Intermediate-Lobe Peptide. J. Neurochem. 1987, 49, 1557–1563. [Google Scholar] [CrossRef]
- Verkhratsky, N.; Toescu, E.C. Calcium Excitability of Glial Cells. In The Tripartite Synapse. Glia in Synaptic Transmission, 1st ed.; Oxford University Press: New York, NY, USA, 2002; ISBN 0198508549. [Google Scholar]
- Santos, N.C.; Figueira-Coelho, J.; Martins-Silva, J.; Saldanha, C. Multidisciplinary Utilization of Dimethyl Sulfoxide: Pharmacological, Cellular, and Molecular Aspects. Biochem. Pharmacol. 2003, 65, 1035–1041. [Google Scholar] [CrossRef]
- Xing, L.; Remick, D.G. Mechanisms of Dimethyl Sulfoxide Augmentation of IL-1 Beta Production. J. Immunol. 2005, 174, 6195–6202. [Google Scholar] [CrossRef]
- Kemp, D.M.; Habener, J.F. Synergistic Effect of Dimethyl Sulfoxide on Glucagon-like Peptide 1 (GLP-1)-Stimulated Insulin Secretion and Gene Transcription in INS-1 Cells: Characterization and Implications. Biochem. Pharmacol. 2002, 64, 689–697. [Google Scholar] [CrossRef]
- Zeng, X.; Dai, J.; Remick, D.G.; Wang, X. Homocysteine Mediated Expression and Secretion of Monocyte Chemoattractant Protein-1 and Interleukin-8 in Human Monocytes. Circ. Res. 2003, 93, 311–320. [Google Scholar] [CrossRef]
- Bezprozvanny, I. The Inositol 1,4,5-Trisphosphate Receptors. Cell Calcium 2005, 38, 261–272. [Google Scholar] [CrossRef]
- Agulhon, C.; Petravicz, J.; McMullen, A.B.; Sweger, E.J.; Minton, S.K.; Taves, S.R.; Casper, K.B.; Fiacco, T.A.; McCarthy, K.D. What Is the Role of Astrocyte Calcium in Neurophysiology? Neuron 2008, 59, 932–946. [Google Scholar] [CrossRef]
- Soulsby, M.D.; Wojcikiewicz, R.J. Calcium Mobilization via Type III Inositol 1,4,5-Trisphosphate Receptors Is Not Altered by PKA-Mediated Phosphorylation of Serines 916, 934, and 1832. Cell Calcium 2007, 42, 261–270. [Google Scholar] [CrossRef]
- Kanno, T.; Nishizaki, T. A2a Adenosine Receptor Mediates PKA-Dependent Glutamate Release from Synaptic-like Vesicles and Ca2+ Efflux from an IP3- and Ryanodine-Insensitive Intracellular Calcium Store in Astrocytes. Cell Physiol. Biochem. 2012, 30, 1398–1412. [Google Scholar] [CrossRef]
- Grimaldi, M.; Favit, A.; Alkon, D.L. CAMP-Induced Cytoskeleton Rearrangement Increases Calcium Transients through the Enhancement of Capacitative Calcium Entry. J. Biol. Chem. 1999, 274, 33557–33564. [Google Scholar] [CrossRef]
- Wu, M.L.; Chen, W.H.; Liu, I.H.; Tseng, C.D.; Wang, S.M. A Novel Effect of Cyclic AMP on Capacitative Ca2+ Entry in Cultured Rat Cerebellar Astrocytes. J. Neurochem. 1999, 73, 1318–1328. [Google Scholar]
- Zeng, B.; Chen, G.-L.; Xu, S.-Z. Store-Independent Pathways for Cytosolic STIM1 Clustering in the Regulation of Store-Operated Ca2+ Influx. Biochem. Pharmacol. 2012, 84, 1024–1035. [Google Scholar] [CrossRef]
- Verkhratsky, A.; Rodríguez, J.J.; Parpura, V. Calcium Signalling in Astroglia. Mol. Cell. Endocrinol. 2012, 353, 45–56. [Google Scholar] [CrossRef]
- Kraft, R. STIM and ORAI Proteins in the Nervous System. Channels 2015, 9, 245–252. [Google Scholar] [CrossRef]
- Molnár, T.; Yarishkin, O.; Iuso, A.; Barabas, P.; Jones, B.; Marc, R.E.; Phuong, T.T.T.; Križaj, D. Store-Operated Calcium Entry in Müller Glia Is Controlled by Synergistic Activation of TRPC and Orai Channels. J. Neurosci. 2016, 36, 3184–3198. [Google Scholar] [CrossRef]
- Song, M.; Chen, D.; Yu, S.P. The TRPC Channel Blocker SKF 96365 Inhibits Glioblastoma Cell Growth by Enhancing Reverse Mode of the Na + /Ca 2+ Exchanger and Increasing Intracellular Ca2+. Br. J. Pharmacol. 2014, 171, 3432–3447. [Google Scholar] [CrossRef]
- Hou, P.P.; Chen, H.Z. Extracellular Vesicles in the Tumor Immune Microenvironment. Cancer Lett. 2021, 516, 48–56. [Google Scholar] [CrossRef]
- Perrone, L.; Peluso, G.; Melone, M.A. RAGE Recycles at the Plasma Membrane in S100B Secretory Vesicles and Promotes Schwann Cells Morphological Changes. J. Cell. Physiol. 2008, 217, 60–71. [Google Scholar] [CrossRef]
- Lasič, E.; Galland, F.; Vardjan, N.; Šribar, J.; Križaj, I.; Leite, M.C.; Zorec, R.; Stenovec, M. Time-Dependent Uptake and Trafficking of Vesicles Capturing Extracellular S100B in Cultured Rat Astrocytes. J. Neurochem. 2016, 139, 309–323. [Google Scholar] [CrossRef]
- Dinkins, M.B.; Enasko, J.; Hernandez, C.; Wang, G.; Kong, J.; Helwa, I.; Liu, Y.; Terry, A.V.; Bieberich, E. Neutral Sphingomyelinase-2 Deficiency Ameliorates Alzheimer’s Disease Pathology and Improves Cognition in the 5XFAD Mouse. J. Neurosci. 2016, 36, 8653–8667. [Google Scholar] [CrossRef]
- Alegre, E.; Zubiri, L.; Perez-Gracia, J.L.; González-Cao, M.; Soria, L.; Martín-Algarra, S.; González, A. Circulating Melanoma Exosomes as Diagnostic and Prognosis Biomarkers. Clin. Chim. Acta 2016, 454, 28–32. [Google Scholar] [CrossRef]
- Fitzgerald, W.; Freeman, M.L.; Lederman, M.M.; Vasilieva, E.; Romero, R.; Margolis, L. A System of Cytokines Encapsulated in ExtraCellular Vesicles. Sci. Rep. 2018, 8, 8973. [Google Scholar] [CrossRef]
- Dallwig, R.; Deitmer, J.W. Cell-Type Specific Calcium Responses in Acute Rat Hippocampal Slices. J. Neurosci. Methods 2002, 116, 77–87. [Google Scholar] [CrossRef]
- Kleindienst, A.; McGinn, M.J.; Harvey, H.B.; Colello, R.J.; Hamm, R.J.; Bullock, M.R. Enhanced Hippocampal Neurogenesis by Intraventricular S100B Infusion Is Associated with Improved Cognitive Recovery after Traumatic Brain Injury. J. Neurotrauma 2005, 22, 645–655. [Google Scholar] [CrossRef]
- Leite, M.C.; Galland, F.; Brolese, G.; Guerra, M.C.; Bortolotto, J.W.; Freitas, R.; de Almeida, L.M.V.; Gottfried, C.; Goncalves, C.A.; Gonçalves, C.A.; et al. A Simple, Sensitive and Widely Applicable ELISA for S100B: Methodological Features of the Measurement of This Glial Protein. J. Neurosci. Methods 2008, 169, 93–99. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leite, M.C.; Galland, F.; Guerra, M.C.; Rodrigues, L.; Taday, J.; Monteforte, P.T.; Hirata, H.; Gottfried, C.; Donato, R.; Smaili, S.; et al. Astroglial S100B Secretion Is Mediated by Ca2+ Mobilization from Endoplasmic Reticulum: A Study Using Forskolin and DMSO as Secretagogues. Int. J. Mol. Sci. 2023, 24, 16576. https://doi.org/10.3390/ijms242316576
Leite MC, Galland F, Guerra MC, Rodrigues L, Taday J, Monteforte PT, Hirata H, Gottfried C, Donato R, Smaili S, et al. Astroglial S100B Secretion Is Mediated by Ca2+ Mobilization from Endoplasmic Reticulum: A Study Using Forskolin and DMSO as Secretagogues. International Journal of Molecular Sciences. 2023; 24(23):16576. https://doi.org/10.3390/ijms242316576
Chicago/Turabian StyleLeite, Marina C., Fabiana Galland, Maria Cristina Guerra, Letícia Rodrigues, Jéssica Taday, Priscila T. Monteforte, Hanko Hirata, Carmem Gottfried, Rosario Donato, Soraya Smaili, and et al. 2023. "Astroglial S100B Secretion Is Mediated by Ca2+ Mobilization from Endoplasmic Reticulum: A Study Using Forskolin and DMSO as Secretagogues" International Journal of Molecular Sciences 24, no. 23: 16576. https://doi.org/10.3390/ijms242316576
APA StyleLeite, M. C., Galland, F., Guerra, M. C., Rodrigues, L., Taday, J., Monteforte, P. T., Hirata, H., Gottfried, C., Donato, R., Smaili, S., & Gonçalves, C.-A. (2023). Astroglial S100B Secretion Is Mediated by Ca2+ Mobilization from Endoplasmic Reticulum: A Study Using Forskolin and DMSO as Secretagogues. International Journal of Molecular Sciences, 24(23), 16576. https://doi.org/10.3390/ijms242316576







