Ion Channel Disturbances in Migraine Headache: Exploring the Potential Role of the Kynurenine System in the Context of the Trigeminovascular System
Abstract
1. Introduction
2. Migraine Pathogenesis and the Impact of the Ion Channels
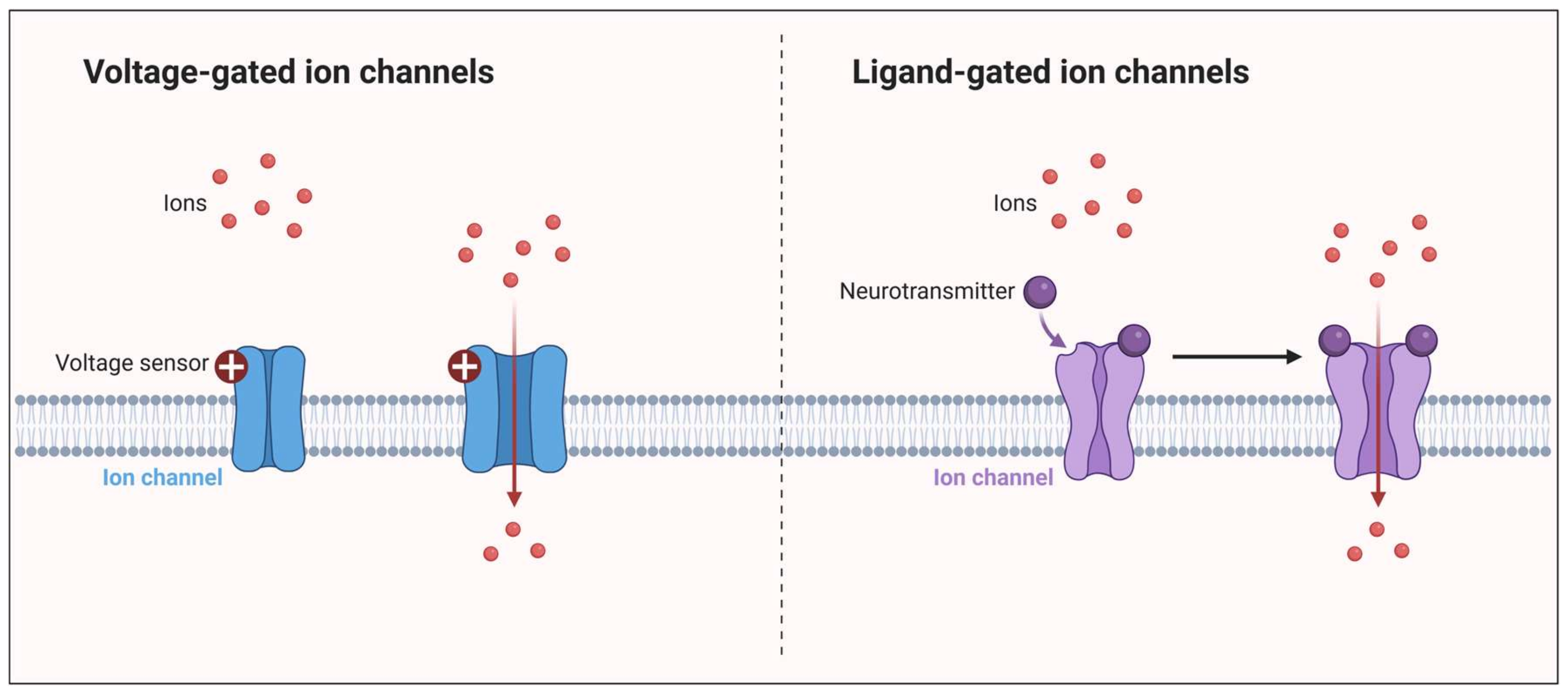
3. Ion Channels in Migraine: Unraveling Pathogenesis and Therapeutic Implications
3.1. Potassium Channels
3.1.1. Adenosine Triphosphate-Sensitive Potassium (KATP) Channels
3.1.2. Large-Conductance Calcium-Activated Potassium (BKCa) Channels

3.1.3. Two-Pore Domain (K2P) Potassium Channel
3.2. Acid-Sensing Ion Channels (ASICs)
3.3. Purinerg System
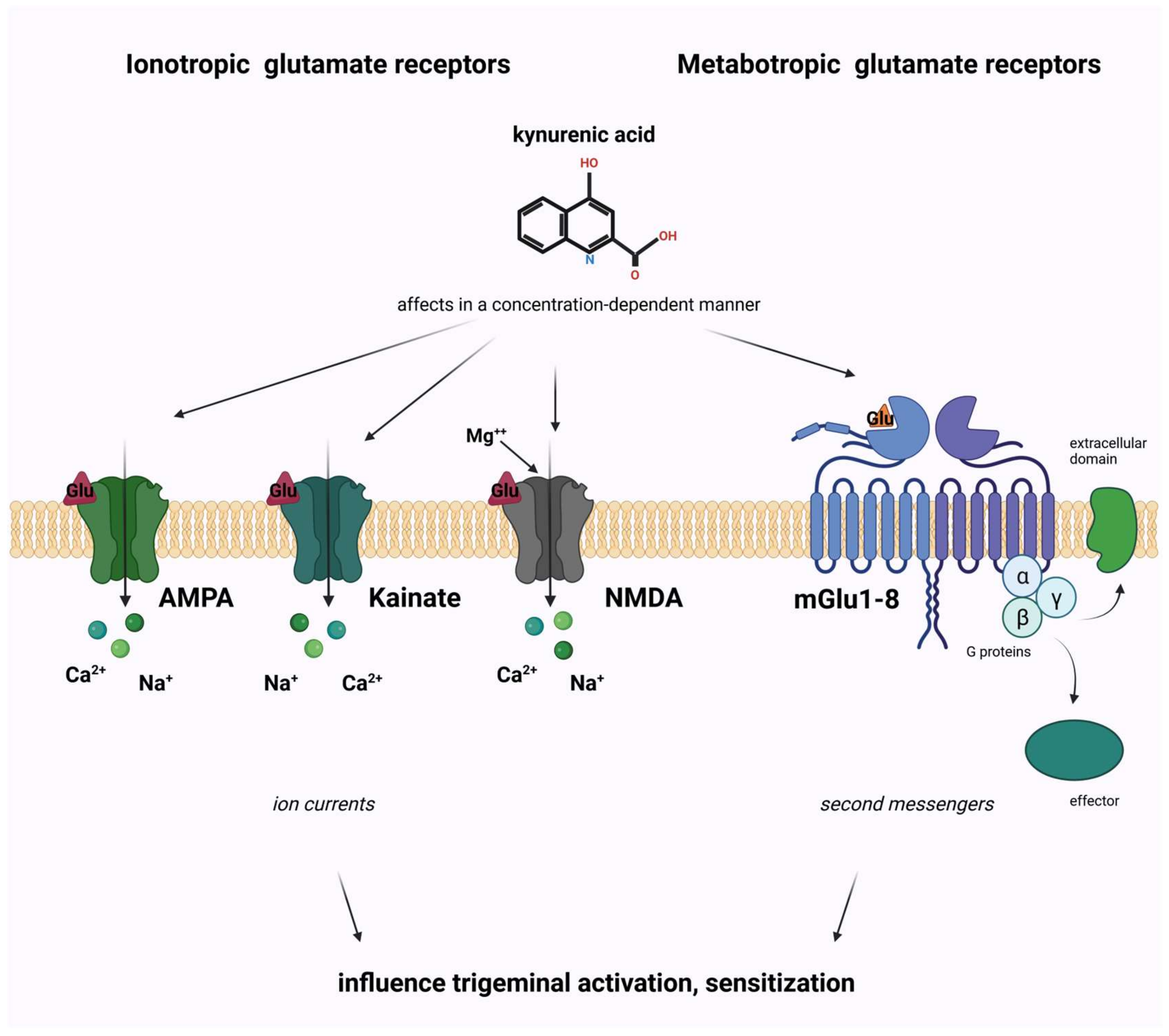
4. The Interplay of Glutamate and the Kynurenine Pathway in Migraine
4.1. Glutamate and Its Receptors
4.2. The Kynurenine Pathway
4.3. The Role of Kynurenine Pathway in Migraine Pathomechanism Connected to Glutamate Receptors
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 3-HA | 3-hydroxyanthranilic acid |
| 3-HK | 3-hydroxy-L-kynurenine |
| AA | arachidonic acid |
| ADP | adenosine diphosphate |
| AMPA | α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid |
| ANA | anthranilic acid |
| ASICs | acid-sensing ion channels |
| ATP | adenosine 5-triphosphate |
| BKCa | large-conductance calcium-activated potassium |
| cAMP | cyclic adenosine monophosphate |
| CFA | Complete Freund’s Adjuvant |
| cGMP | cyclic guanosine monophosphate |
| CGRP | calcitonin gene-related peptide |
| CNS | central nervous system |
| CSD | cortical spreading depression |
| FHM | familial hemiplegic migraine |
| IDO | indoleamine 2,3-dioxygenase |
| KA1 | N-(2-N,N-dimethylaminoethyl)-4-oxo-1H-quinoline-2-carboxamide hydrochloride |
| KA2 | N-(2-N-pyrrolidinylethyl)-4-oxo-1H-quinoline-2-carboxamide hydrochloride |
| KATII | kynurenine aminotransferase II |
| KATP | ATP-sensitive potassium |
| KMO | kynurenine 3-monooxygenase |
| KYNU | L-kynurenine hydrolase |
| LGICs | ligand-dependent ion channels |
| L-KYN | L-kynurenine |
| NAD+ | nicotinamide adenine dinucleotide |
| NKA | neurokinin A |
| NMDA | N-methyl-D-aspartate |
| NMDAR | N-methyl-D-aspartate receptor |
| nNOS | neuronal nitric oxide synthase |
| NO | nitric oxide |
| NTG | nitroglycerin |
| PACAP | pituitary adenylate cyclase-activating polypeptide |
| PAG | periaqueductal grey |
| PGI2 | prostaglandin I2 |
| PKA | protein kinase A |
| PNS | peripheral nervous system |
| PPADS | pyridoxal phosphate-6-azo(benzene-2,4-disulfonic acid) |
| QUIN | quinolinic acid |
| SP | substance P |
| TALK | TWIK-related alkaline pH-activated K+ channel |
| TASK | TWIK-related acid-sensitive K+ channel |
| TDO | tryptophan 2,3-dioxygenase |
| TG | trigeminal ganglion |
| THIK | tandem pore domain halothane-inhibited K+ channel |
| TNC | caudal trigeminal nucleus |
| TNP-ATP | 2′,3′-O-(2,4,6-trinitrophenol)adenosine-5′-triphosphate |
| TREK | TWIK-related K+ channel |
| TRESK | TWIK-related spinal cord K+ channel |
| TRPV1 | transient receptor potential vanilloid 1 |
| TVS | trigeminovascular system |
| TWIK | tandem of P domains in a weak inward rectifying K+ channel |
| UDP | uridine-diphosphate |
| UTP | uridine-triphosphate |
| VGICs | voltage-gated ion channels |
References
- Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd ed. Cephalalgia 2013, 33, 629–808. [Google Scholar] [CrossRef]
- Humphries, E.S.A.; Dart, C. Neuronal and Cardiovascular Potassium Channels as Therapeutic Drug Targets: Promise and Pitfalls. SLAS Discov. Adv. Sci. Drug Discov. 2015, 20, 1055–1073. [Google Scholar] [CrossRef]
- Al-Karagholi, M.A.-M. Involvement of Potassium Channel Signalling in Migraine Pathophysiology. Pharmaceuticals 2023, 16, 438. [Google Scholar] [CrossRef]
- Wang, J.; Ou, S.-W.; Wang, Y.-J. Distribution and function of voltage-gated sodium channels in the nervous system. Channels 2017, 11, 534–554. [Google Scholar] [CrossRef]
- Südhof, T.C. Calcium Control of Neurotransmitter Release. Cold Spring Harb. Perspect. Biol. 2011, 4, a011353. [Google Scholar] [CrossRef]
- Longden, T.A.; Hill-Eubanks, D.C.; Nelson, M.T. Ion channel networks in the control of cerebral blood flow. J. Cereb. Blood Flow Metab. 2015, 36, 492–512. [Google Scholar] [CrossRef]
- Yan, J.; Dussor, G. Ion Channels and Migraine. Headache J. Head Face Pain 2014, 54, 619–639. [Google Scholar] [CrossRef]
- Fejes, A.; Pardutz, A.; Toldi, J.; Vecsei, L. Kynurenine Metabolites and Migraine: Experimental Studies and Therapeutic Perspectives. Curr. Neuropharmacol. 2011, 9, 376–387. [Google Scholar] [CrossRef]
- GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1211–1259. [Google Scholar] [CrossRef]
- Goadsby, P.J.; Holland, P.R.; Martins-Oliveira, M.; Hoffmann, J.; Schankin, C.; Akerman, S. Pathophysiology of Migraine: A Disorder of Sensory Processing. Physiol. Rev. 2017, 97, 553–622. [Google Scholar] [CrossRef]
- Edvinsson, L. Tracing neural connections to pain pathways with relevance to primary headaches. Cephalalgia 2011, 31, 737–747. [Google Scholar] [CrossRef]
- Spekker, E.; Tanaka, M.; Szabó, Á.; Vécsei, L. Neurogenic Inflammation: The Participant in Migraine and Recent Advancements in Translational Research. Biomedicines 2021, 10, 76. [Google Scholar] [CrossRef]
- Cross, S.A. Pathophysiology of Pain. Mayo Clin. Proc. 1994, 69, 375–383. [Google Scholar] [CrossRef]
- Noseda, R.; Burstein, R. Migraine pathophysiology: Anatomy of the trigeminovascular pathway and associated neurological symptoms, cortical spreading depression, sensitization, and modulation of pain. Pain 2013, 154, S44–S53. [Google Scholar] [CrossRef]
- Dodick, D.W. A Phase-by-Phase Review of Migraine Pathophysiology. Headache J. Head Face Pain 2018, 58, 4–16. [Google Scholar] [CrossRef]
- Pietrobon, D.; Moskowitz, M.A. Chaos and commotion in the wake of cortical spreading depression and spreading depolarizations. Nat. Rev. Neurosci. 2014, 15, 379–393. [Google Scholar] [CrossRef]
- Zhang, X.; Levy, D.; Noseda, R.; Kainz, V.; Jakubowski, M.; Burstein, R. Activation of Meningeal Nociceptors by Cortical Spreading Depression: Implications for Migraine with Aura. J. Neursci. 2010, 30, 8807–8814. [Google Scholar] [CrossRef]
- Gursoy-Ozdemir, Y.; Qiu, J.; Matsuoka, N.; Bolay, H.; Bermpohl, D.; Jin, H.; Wang, X.; Rosenberg, G.A.; Lo, E.H.; Moskowitz, M.A. Cortical spreading depression activates and upregulates MMP-9. J. Clin. Investig. 2004, 113, 1447–1455. [Google Scholar] [CrossRef]
- Pietrobon, D.; Moskowitz, M.A. Pathophysiology of Migraine. Annu. Rev. Physiol. 2013, 75, 365–391. [Google Scholar] [CrossRef]
- Cohen, C.F.; Roh, J.; Lee, S.H.; Park, C.-K.; Berta, T. Targeting Nociceptive Neurons and Transient Receptor Potential Channels for the Treatment of Migraine. Int. J. Mol. Sci. 2023, 24, 7897. [Google Scholar] [CrossRef]
- Zhang, X.-C.; Strassman, A.M.; Burstein, R.; Levy, D. Sensitization and Activation of Intracranial Meningeal Nociceptors by Mast Cell Mediators. Experiment 2007, 322, 806–812. [Google Scholar] [CrossRef]
- Levy, D. Endogenous Mechanisms Underlying the Activation and Sensitization of Meningeal Nociceptors: The Role of Immuno-Vascular Interactions and Cortical Spreading Depression. Curr. Pain Headache Rep. 2012, 16, 270–277. [Google Scholar] [CrossRef]
- Liu, Y.; Broman, J.; Zhang, M.; Edvinsson, L. Brainstem and Thalamic Projections from a Craniovascular Sensory Nervous Centre in the Rostral Cervical Spinal Dorsal Horn of Rats. Cephalalgia 2009, 29, 935–948. [Google Scholar] [CrossRef]
- Weiller, C.; May, A.; Limmroth, V.; Jüptner, M.; Kaube, H.; Schayck, R.; Coenen, H.; Dlener, H. Brain stem activation in spontaneous human migraine attacks. Nat. Med. 1995, 1, 658–660. [Google Scholar] [CrossRef]
- Akerman, S.; Holland, P.R.; Goadsby, P.J. Diencephalic and brainstem mechanisms in migraine. Nat. Rev. Neurosci. 2011, 12, 570–584. [Google Scholar] [CrossRef]
- Settle, M. The Hypothalamus. Neonatal Netw. 2000, 19, 9–14. [Google Scholar] [CrossRef]
- Goadsby, P.J.; Holland, P.R. Pathophysiology of Migraine. Neurol. Clin. 2019, 37, 651–671. [Google Scholar] [CrossRef]
- Strassman, A.M.; Raymond, S.A.; Burstein, R. Sensitization of meningeal sensory neurons and the origin of headaches. Nature 1996, 384, 560–564. [Google Scholar] [CrossRef]
- Kullmann, D.M. The neuronal channelopathies. Brain 2002, 125, 1177–1195. [Google Scholar] [CrossRef]
- Carrera, P.; Stenirri, S.; Ferrari, M.; Battistini, S. Familial hemiplegic migraine: A ion channel disorder. Brain Res. Bull. 2001, 56, 239–241. [Google Scholar] [CrossRef]
- Uchitel, O.D.; Inchauspe, C.G.; Di Guilmi, M.N. Calcium channels and synaptic transmission in familial hemiplegic migraine type 1 animal models. Biophys. Rev. 2013, 6, 15–26. [Google Scholar] [CrossRef]
- Pietrobon, D. Ion channels in migraine disorders. Curr. Opin. Physiol. 2018, 2, 98–108. [Google Scholar] [CrossRef]
- Sutherland, H.G.; Albury, C.L.; Griffiths, L.R. Advances in genetics of migraine. J. Headache Pain 2019, 20, 72. [Google Scholar] [CrossRef]
- Barker, B.S.; Young, G.T.; Soubrane, C.H.; Stephens, G.J.; Stevens, E.B.; Patel, M.K. Chapter 2—Ion Channels. In Conn’s Translational Neuroscience; Michael Conn, P., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 11–43. [Google Scholar] [CrossRef]
- De Lera Ruiz, M.; Kraus, R.L. Voltage-Gated Sodium Channels: Structure, Function, Pharmacology, and Clinical Indications. J. Med. Chem. 2015, 58, 7093–7118. [Google Scholar] [CrossRef]
- Alexander, S.P.; Peters, J.A.; Kelly, E.; Marrion, N.V.; Faccenda, E.; Harding, S.D.; Pawson, A.J.; Sharman, J.L.; Southan, C.; Davies, J.A.; et al. The Concise Guide to Pharmacology 2017/18: Ligand-gated ion channels. Br. J. Pharmacol. 2017, 174, S130–S159. [Google Scholar] [CrossRef]
- Al-Karagholi, M.A.; Hansen, J.M.; Severinsen, J.; Jansen-Olesen, I.; Ashina, M. The KATP channel in migraine pathophysiology: A novel therapeutic target for migraine. J. Headache Pain 2017, 18, 90. [Google Scholar] [CrossRef]
- Al-Karagholi, M.A.-M.; Gram, C.; Nielsen, C.A.W.; Ashina, M. Targeting BKCa Channels in Migraine: Rationale and Perspectives. CNS Drugs 2020, 34, 325–335. [Google Scholar] [CrossRef]
- Kokoti, L.; Al-Karagholi, M.A.-M.; Ashina, M. Latest Insights into the Pathophysiology of Migraine: The ATP-Sensitive Potassium Channels. Curr. Pain Headache Rep. 2020, 24, 77. [Google Scholar] [CrossRef]
- Gozalov, A.; Jansen-Olesen, I.; Klaerke, D.; Olesen, J. Role of BKCa Channels in Cephalic Vasodilation Induced by CGRP, NO and Transcranial Electrical Stimulation in the Rat. Cephalalgia 2007, 27, 1120–1127. [Google Scholar] [CrossRef]
- Gozalov, A.; Jansen-Olesen, I.; Klaerke, D.; Olesen, J. Role of KATPChannels in Cephalic Vasodilatation Induced by Calcitonin Gene-Related Peptide, Nitric Oxide, and Transcranial Electrical Stimulation in the Rat. Headache J. Head Face Pain 2008, 48, 1202–1213. [Google Scholar] [CrossRef]
- Bruch, L.; Rubel, S.; Kästner, A.; Gellert, K.; Gollasch, M.; Witt, C. Pituitary adenylate cyclase activating peptides relax human pulmonary arteries by opening of KATP and KCa channels. Thorax 1998, 53, 586–587. [Google Scholar] [CrossRef][Green Version]
- Christensen, S.L.; Munro, G.; Petersen, S.; Shabir, A.; Jansen-Olesen, I.; Kristensen, D.M.; Olesen, J. ATP sensitive potassium (KATP) channel inhibition: A promising new drug target for migraine. Cephalalgia 2020, 40, 650–664. [Google Scholar] [CrossRef]
- Al-Hassany, L.; Boucherie, D.M.; Creeney, H.; van Drie, R.W.A.; Farham, F.; Favaretto, S.; Gollion, C.; Grangeon, L.; Lyons, H.; Marschollek, K.; et al. Future targets for migraine treatment beyond CGRP. J. Headache Pain 2023, 24, 76. [Google Scholar] [CrossRef]
- Guo, S.; Jansen-Olesen, I.; Olesen, J.; Christensen, S.L. Role of PACAP in migraine: An alternative to CGRP? Neurobiol. Dis. 2023, 176, 105946. [Google Scholar] [CrossRef]
- Aguilar-Bryan, L.; Bryan, J. Molecular Biology of Adenosine Triphosphate-Sensitive Potassium Channels. Endocr. Rev. 1999, 20, 101–135. [Google Scholar] [CrossRef]
- Yamada, K.; Inagaki, N. Neuroprotection by KATP channels. J. Mol. Cell Cardiol. 2005, 38, 945–949. [Google Scholar] [CrossRef]
- Ashina, M.; Hansen, J.M.; Olesen, J. Pearls and pitfalls in human pharmacological models of migraine: 30 years’ experience. Cephalalgia 2013, 33, 540–553. [Google Scholar] [CrossRef]
- Jansen-Olesen, I.; Gulbenkian, S.; Engel, U.; e Sá, M.C.; Edvinsson, L. Peptidergic and non-peptidergic innervation and vasomotor responses of human lenticulostriate and posterior cerebral arteries. Peptides 2004, 25, 2105–2114. [Google Scholar] [CrossRef]
- Miyoshi, H.; Nakaya, Y. Calcitonin gene-related peptide activates the K+ channels of vascular smooth muscle cells via adenylate cyclase. Basic Res. Cardiol. 1995, 90, 332–336. [Google Scholar] [CrossRef]
- Chalovich, J.M.; Eisenberg, E. Inhibition of actomyosin ATPase activity by troponin-tropomyosin without blocking the binding of myosin to actin. J. Biol. Chem. 1982, 257, 2432–2437. [Google Scholar] [CrossRef]
- Ray, C.J.; Marshall, J.M. The cellular mechanisms by which adenosine evokes release of nitric oxide from rat aortic endothelium. J. Physiol. 2005, 570, 85–96. [Google Scholar] [CrossRef]
- Clement, A.; Guo, S.; Jansen-Olesen, I.; Christensen, S.L. ATP-Sensitive Potassium Channels in Migraine: Translational Findings and Therapeutic Potential. Cells 2022, 11, 2406. [Google Scholar] [CrossRef]
- Hempelmann, R.G.; Seebeck, J.; Kruse, M.-L.; Ziegler, A.; Mehdorn, H. Role of potassium channels in the relaxation induced by the nitric oxide (NO) donor DEA/NO in the isolated rat basilar artery. Neurosci. Lett. 2001, 313, 21–24. [Google Scholar] [CrossRef]
- Al-Karagholi, M.A.-M.; Hansen, J.M.; Guo, S.; Olesen, J.; Ashina, M. Opening of ATP-sensitive potassium channels causes migraine attacks: A new target for the treatment of migraine. Brain 2019, 142, 2644–2654. [Google Scholar] [CrossRef]
- Al-Karagholi, M.A.-M.; Ghanizada, H.; Nielsen, C.A.W.; Hougaard, A.; Ashina, M. Opening of ATP sensitive potassium channels causes migraine attacks with aura. Brain 2021, 144, 2322–2332. [Google Scholar] [CrossRef]
- Clement, A.; Christensen, S.L.; Jansen-Olesen, I.; Olesen, J.; Guo, S. The ATP sensitive potassium channel (KATP) is a novel target for migraine drug development. Front. Mol. Neurosci. 2023, 16, 1182515. [Google Scholar] [CrossRef]
- Ghatta, S.; Nimmagadda, D.; Xu, X.; O’Rourke, S.T. Large-conductance, calcium-activated potassium channels: Structural and functional implications. Pharmacol. Ther. 2006, 110, 103–116. [Google Scholar] [CrossRef]
- Adelman, J.P.; Shen, K.-Z.; Kavanaugh, M.P.; Warren, R.A.; Wu, Y.-N.; Lagrutta, A.; Bond, C.T.; North, R.A. Calcium-activated potassium channels expressed from cloned complementary DNAs. Neuron 1992, 9, 209–216. [Google Scholar] [CrossRef]
- Wulf-Johansson, H.; Amrutkar, D.; Hay-Schmidt, A.; Poulsen, A.; Klaerke, D.; Olesen, J.; Jansen-Olesen, I. Localization of large conductance calcium-activated potassium channels and their effect on calcitonin gene-related peptide release in the rat trigemino–neuronal pathway. Neuroscience 2010, 167, 1091–1102. [Google Scholar] [CrossRef]
- Schubert, R.; Nelson, M.T. Protein kinases: Tuners of the BKCa channel in smooth muscle. Trends Pharmacol. Sci. 2001, 22, 505–512. [Google Scholar] [CrossRef]
- Storer, R.; Immke, D.; Yin, R.; Goadsby, P. Large Conductance Calcium-Activated Potassium Channels (BKCa) Modulate Trigeminovascular Nociceptive Transmission. Cephalalgia 2009, 29, 1242–1258. [Google Scholar] [CrossRef]
- Mistry, D.K.; Garland, C.J. Nitric oxide (NO)-induced activation of large conductance Ca2+-dependent K+ channels (BKCa) in smooth muscle cells isolated from the rat mesenteric artery. Br. J. Pharmacol. 1998, 124, 1131–1140. [Google Scholar] [CrossRef]
- Al-Karagholi, M.A.-M.; Ghanizada, H.; Nielsen, C.A.W.; Skandarioon, C.; Snellman, J.; Lopez, C.L.; Hansen, J.M.; Ashina, M. Opening of BKCa channels alters cerebral hemodynamic and causes headache in healthy volunteers. Cephalalgia 2020, 40, 1145–1154. [Google Scholar] [CrossRef]
- Al-Karagholi, M.A.-M.; Ghanizada, H.; Nielsen, C.A.W.; Skandarioon, C.; Snellman, J.; Lopez-Lopez, C.; Hansen, J.M.; Ashina, M. Opening of BKCa channels causes migraine attacks: A new downstream target for the treatment of migraine. Pain 2021, 162, 2512–2520. [Google Scholar] [CrossRef]
- Koide, M.; Syed, A.U.; Braas, K.M.; May, V.; Wellman, G.C. Pituitary Adenylate Cyclase Activating Polypeptide (PACAP) Dilates Cerebellar Arteries Through Activation of Large-Conductance Ca2+-Activated (BK) and ATP-Sensitive (KATP) K+ Channels. J. Mol. Neurosci. 2014, 54, 443–450. [Google Scholar] [CrossRef][Green Version]
- Enyedi, P.; Czirják, G. Molecular Background of Leak K+Currents: Two-Pore Domain Potassium Channels. Physiol. Rev. 2010, 90, 559–605. [Google Scholar] [CrossRef]
- Hervieu, G.; Cluderay, J.; Gray, C.; Green, P.; Ranson, J.; Randall, A.; Meadows, H. Distribution and expression of TREK-1, a two-pore-domain potassium channel, in the adult rat CNS. Neuroscience 2001, 103, 899–919. [Google Scholar] [CrossRef]
- Talley, E.M.; Solórzano, G.; Lei, Q.; Kim, D.; Bayliss, D.A. CNS Distribution of Members of the Two-Pore-Domain (KCNK) Potassium Channel Family. J. Neurosci. 2001, 21, 7491–7505. [Google Scholar] [CrossRef]
- Prado, P.; Landra-Willm, A.; Verkest, C.; Ribera, A.; Chassot, A.-A.; Baron, A.; Sandoz, G. TREK channel activation suppresses migraine pain phenotype. iScience 2021, 24, 102961. [Google Scholar] [CrossRef]
- Andres-Bilbe, A.; Castellanos, A.; Pujol-Coma, A.; Callejo, G.; Comes, N.; Gasull, X. The Background K+ Channel TRESK in Sensory Physiology and Pain. Int. J. Mol. Sci. 2020, 21, 5206. [Google Scholar] [CrossRef]
- Pettingill, P.; Weir, G.A.; Wei, T.; Wu, Y.; Flower, G.; Lalic, T.; Handel, A.; Duggal, G.; Chintawar, S.; Cheung, J.; et al. A causal role for TRESK loss of function in migraine mechanisms. Brain 2019, 142, 3852–3867. [Google Scholar] [CrossRef]
- Lafrenière, R.G.; Cader, M.Z.; Poulin, J.-F.; Andres-Enguix, I.; Simoneau, M.; Gupta, N.; Boisvert, K.; Lafrenière, F.; McLaughlan, S.; Dubé, M.-P.; et al. A dominant-negative mutation in the TRESK potassium channel is linked to familial migraine with aura. Nat. Med. 2010, 16, 1157–1160. [Google Scholar] [CrossRef]
- Royal, P.; Andres-Bilbe, A.; Prado, P.Á.; Verkest, C.; Wdziekonski, B.; Schaub, S.; Baron, A.; Lesage, F.; Gasull, X.; Levitz, J.; et al. Migraine-Associated TRESK Mutations Increase Neuronal Excitability through Alternative Translation Initiation and Inhibition of TREK. Neuron 2019, 101, 232–245. [Google Scholar] [CrossRef]
- Imbrici, P.; Nematian-Ardestani, E.; Hasan, S.; Pessia, M.; Tucker, S.J.; D’adamo, M.C. Altered functional properties of a missense variant in the TRESK K+ channel (KCNK18) associated with migraine and intellectual disability. Eur. J. Physiol. 2020, 472, 923–930. [Google Scholar] [CrossRef]
- Wemmie, J.A.; Taugher, R.J.; Kreple, C.J. Acid-sensing ion channels in pain and disease. Nat. Rev. Neurosci. 2013, 14, 461–471. [Google Scholar] [CrossRef]
- Gründer, S.; Chen, X. Structure, function, and pharmacology of acid-sensing ion channels (ASICs): Focus on ASIC1a. Int. J. Physiol. Pathophysiol. Pharmacol. 2010, 2, 73–94. [Google Scholar]
- Wemmie, J.A.; Askwith, C.C.; Lamani, E.; Cassell, M.D.; Freeman, J.H.; Welsh, M.J. Acid-Sensing Ion Channel 1 Is Localized in Brain Regions with High Synaptic Density and Contributes to Fear Conditioning. J. Neurosci. 2003, 23, 5496–5502. [Google Scholar] [CrossRef]
- Dussor, G. ASICs as therapeutic targets for migraine. Neuropharmacology 2015, 94, 64–71. [Google Scholar] [CrossRef]
- Mamet, J.; Lazdunski, M.; Voilley, N. How Nerve Growth Factor Drives Physiological and Inflammatory Expressions of Acid-sensing Ion Channel 3 in Sensory Neurons. J. Biol. Chem. 2003, 278, 48907–48913. [Google Scholar] [CrossRef]
- Waldmann, R.; Bassilana, F.; de Weille, J.; Champigny, G.; Heurteaux, C.; Lazdunski, M. Molecular Cloning of a Non-inactivating Proton-gated Na+ Channel Specific for Sensory Neurons. J. Biol. Chem. 1997, 272, 20975–20978. [Google Scholar] [CrossRef]
- Lingueglia, E. Acid-sensing Ion Channels in Sensory Perception. J. Biol. Chem. 2007, 282, 17325–17329. [Google Scholar] [CrossRef]
- Yan, J.; Wei, X.; Bs, C.B.; Edelmayer, R.M.; Dussor, G. pH-Evoked Dural Afferent Signaling Is Mediated by ASIC3 and Is Sensitized by Mast Cell Mediators. Headache J. Head Face Pain 2013, 53, 1250–1261. [Google Scholar] [CrossRef]
- Page, A.J.; Brierley, S.M.; Martin, C.M.; Price, M.P.; Symonds, E.; Butler, R.; Wemmie, J.A.; Blackshaw, L.A. Different contributions of ASIC channels 1a, 2, and 3 in gastrointestinal mechanosensory function. Gut 2005, 54, 1408–1415. [Google Scholar] [CrossRef]
- Yagi, J.; Wenk, H.N.; Naves, L.A.; McCleskey, E.W. Sustained Currents Through ASIC3 Ion Channels at the Modest pH Changes That Occur During Myocardial Ischemia. Circ. Res. 2006, 99, 501–509. [Google Scholar] [CrossRef]
- Deval, E.; Noël, J.; Gasull, X.; Delaunay, A.; Alloui, A.; Friend, V.; Eschalier, A.; Lazdunski, M.; Lingueglia, E. Acid-Sensing Ion Channels in Postoperative Pain. J. Neurosci. 2011, 31, 6059–6066. [Google Scholar] [CrossRef]
- Durham, P.L.; Masterson, C.G. Two mechanisms involved in trigeminal CGRP release: Implications for migraine treatment. Headache J. Head Face Pain 2012, 53, 67–80. [Google Scholar] [CrossRef]
- Holton, C.M.; Strother, L.C.; Dripps, I.; Pradhan, A.A.; Goadsby, P.J.; Holland, P.R. Acid-sensing ion channel 3 blockade inhibits durovascular and nitric oxide-mediated trigeminal pain. Br. J. Pharmacol. 2020, 177, 2478–2486. [Google Scholar] [CrossRef]
- Holland, P.R.; Akerman, S.; Andreou, A.P.; Karsan, N.; Wemmie, J.A.; Goadsby, P.J. Acid-sensing ion channel 1: A novel therapeutic target for migraine with aura. Ann. Neurol. 2012, 72, 559–563. [Google Scholar] [CrossRef]
- Piilgaard, H.; Lauritzen, M. Persistent Increase in Oxygen Consumption and Impaired Neurovascular Coupling after Spreading Depression in Rat Neocortex. J. Cereb. Blood Flow Metab. 2009, 29, 1517–1527. [Google Scholar] [CrossRef]
- Kuroda, Y. Physiological roles of adenosine derivatives which are released during neurotransmission in mammalian brain. J. Physiol. 1978, 74, 463–470. [Google Scholar]
- Pasquini, S.; Contri, C.; Borea, P.A.; Vincenzi, F.; Varani, K. Adenosine and Inflammation: Here, There and Everywhere. Int. J. Mol. Sci. 2021, 22, 7685. [Google Scholar] [CrossRef]
- Seiffge, D.; Kremer, E. Inlfuence of ADP, blood flow velocity, and vessel diameter on the laser-induced thrombus formation. Thromb. Res. 1986, 42, 331–341. [Google Scholar] [CrossRef]
- Petroianu, G.A.; Aloum, L.; Adem, A. Neuropathic pain: Mechanisms and therapeutic strategies. Front. Cell Dev. Biol. 2023, 11, 1072629. [Google Scholar] [CrossRef]
- Dunwiddie, T.V.; Fredholm, B.B. Adenosine A1 receptors inhibit adenylate cyclase activity and neurotransmitter release and hyperpolarize pyramidal neurons in rat hippocampus. J. Pharmacol. Exp. Ther. 1989, 249, 31–37. [Google Scholar]
- Shryock, J.C.; Snowdy, S.; Baraldi, P.G.; Cacciari, B.; Spalluto, G.; Monopoli, A.; Ongini, E.; Baker, S.P.; Belardinelli, L. A 2A-Adenosine Receptor Reserve for Coronary Vasodilation. Circulation 1998, 98, 711–718. [Google Scholar] [CrossRef]
- Chen, M.; Gu, J.G. A P2X Receptor-Mediated Nociceptive Afferent Pathway to Lamina I of the Spinal Cord. Mol. Pain 2005, 1, 4. [Google Scholar] [CrossRef]
- Cockayne, D.A.; Hamilton, S.G.; Zhu, Q.-M.; Dunn, P.M.; Zhong, Y.; Novakovic, S.; Malmberg, A.B.; Cain, G.; Berson, A.; Kassotakis, L.; et al. Urinary bladder hyporeflexia and reduced pain-related behaviour in P2X3-deficient mice. Nature 2000, 407, 1011–1015. [Google Scholar] [CrossRef]
- Honore, P.; Mikusa, J.; Bianchi, B.; McDonald, H.; Cartmell, J.; Faltynek, C.; Jarvis, M.F. TNP-ATP, a potent P2X3 receptor antagonist, blocks acetic acid-induced abdominal constriction in mice: Comparison with reference analgesics. Pain 2002, 96, 99–105. [Google Scholar] [CrossRef]
- Fabbretti, E.; D’Arco, M.; Fabbro, A.; Simonetti, M.; Nistri, A.; Giniatullin, R. Delayed Upregulation of ATP P2X3 Receptors of Trigeminal Sensory Neurons by Calcitonin Gene-Related Peptide. J. Neurosci. 2006, 26, 6163–6171. [Google Scholar] [CrossRef]
- Zhao, J.; Harrison, S.; Levy, D. Meningeal P2X7 signaling mediates migraine-related intracranial mechanical hypersensitivity. J. Neurosci. 2023, 43, 5975–5985. [Google Scholar] [CrossRef]
- Bohár, Z.; Nagy-Grócz, G.; Fejes-Szabó, A.; Tar, L.; László, A.M.; Büki, A.; Szabadi, N.; Vraukó, V.; Vécsei, L.; Párdutz, Á. Diverse effects of Brilliant Blue G administration in models of trigeminal activation in the rat. J. Neural Transm. 2015, 122, 1621–1631. [Google Scholar] [CrossRef]
- Ceruti, S.; Fumagalli, M.; Villa, G.; Verderio, C.; Abbracchio, M.P. Purinoceptor-mediated calcium signaling in primary neuron-glia trigeminal cultures. Cell Calcium 2008, 43, 576–590. [Google Scholar] [CrossRef]
- Gerevich, Z.; Illes, P. P2Y receptors and pain transmission. Purinergic Signal. 2004, 1, 3–10. [Google Scholar] [CrossRef]
- Gerevich, Z.; Müller, C.; Illes, P. Metabotropic P2Y1 receptors inhibit P2X3 receptor-channels in rat dorsal root ganglion neurons. Eur. J. Pharmacol. 2005, 521, 34–38. [Google Scholar] [CrossRef]
- Okada, M.; Nakagawa, T.; Minami, M.; Satoh, M. Analgesic Effects of Intrathecal Administration of P2Y Nucleotide Receptor Agonists UTP and UDP in Normal and Neuropathic Pain Model Rats. Experiment 2002, 303, 66–73. [Google Scholar] [CrossRef]
- Di Clemente, L.; Coppola, G.; Magis, D.; Gérardy, P.-Y.; Fumal, A.; De Pasqua, V.; Di Piero, V.; Schoenen, J. Nitroglycerin sensitises in healthy subjects CNS structures involved in migraine pathophysiology: Evidence from a study of nociceptive blink reflexes and visual evoked potentials. Pain 2009, 144, 156–161. [Google Scholar] [CrossRef]
- Tassorelli, C.; Joseph, S.A. Systemic nitroglycerin induces Fos immunoreactivity in brainstem and forebrain structures of the rat. Brain Res. 1995, 682, 167–181. [Google Scholar] [CrossRef]
- Gölöncsér, F.; Baranyi, M.; Iring, A.; Hricisák, L.; Otrokocsi, L.; Benyó, Z.; Sperlágh, B. Involvement of P2Y12 receptors in a nitroglycerin-induced model of migraine in male mice. Br. J. Pharmacol. 2021, 178, 4626–4645. [Google Scholar] [CrossRef]
- Szalardy, L.; Klivenyi, P.; Zadori, D.; Fulop, F.; Toldi, J.; Vecsei, L. Mitochondrial disturbances, tryptophan metabolites and neurodegeneration: Medicinal chemistry aspects. Curr. Med. Chem. 2012, 19, 1899–1920. [Google Scholar] [CrossRef]
- Egerton, A.; Grace, A.A.; Stone, J.; Bossong, M.G.; Sand, M.; McGuire, P. Glutamate in schizophrenia: Neurodevelopmental perspectives and drug development. Schizophr. Res. 2020, 223, 59–70. [Google Scholar] [CrossRef]
- Henter, I.D.; Park, L.T.; Zarate, C.A. Novel Glutamatergic Modulators for the Treatment of Mood Disorders: Current Status. CNS Drugs 2021, 35, 527–543. [Google Scholar] [CrossRef]
- Vecsei, L.; Majlath, Z.; Balog, A.; Tajti, J. Drug targets of migraine and neuropathy: Treatment of hyperexcitability. CNS Neurol. Disord. Drug Targets 2015, 14, 664–676. [Google Scholar] [CrossRef]
- Martínez, F.; Castillo, J.; Rodríguez, J.R.; Leira, R.; Noya, M. Neuroexcitatory Amino Acid Levels in Plasma and Cerebrospinal Fluid During Migraine Attacks. Cephalalgia 1993, 13, 89–93. [Google Scholar] [CrossRef]
- Olney, J.W.; Sharpe, L.G. Brain Lesions in an Infant Rhesus Monkey Treated with Monosodium Glutamate. Science 1969, 166, 386–388. [Google Scholar] [CrossRef]
- Choi, D.W. Glutamate neurotoxicity in cortical cell culture is calcium dependent. Neurosci. Lett. 1985, 58, 293–297. [Google Scholar] [CrossRef]
- Koga, K.; Li, S.; Zhuo, M. Metabotropic Glutamate Receptor Dependent Cortical Plasticity in Chronic Pain. Curr. Neuropharmacol. 2016, 14, 427–434. [Google Scholar] [CrossRef]
- Birch, P.J.; Grossman, C.J.; Hayes, A.G. Kynurenic acid antagonises responses to NMDA via an action at the strychnine-insensitive glycine receptor. Eur. J. Pharmacol. 1988, 154, 85–87. [Google Scholar] [CrossRef]
- Kessler, M.; Terramani, T.; Lynch, G.; Baudry, M. A Glycine Site Associated with N-Methyl-d-Aspartic Acid Receptors: Characterization and Identification of a New Class of Antagonists. J. Neurochem. 1989, 52, 1319–1328. [Google Scholar] [CrossRef]
- Prescott, C.; Weeks, A.M.; Staley, K.J.; Partin, K.M. Kynurenic acid has a dual action on AMPA receptor responses. Neurosci. Lett. 2006, 402, 108–112. [Google Scholar] [CrossRef]
- Rózsa, Á.; Robotka, H.; Vécsei, L.; Toldi, J. The Janus-face kynurenic acid. J. Neural Transm. 2008, 115, 1087–1091. [Google Scholar] [CrossRef]
- Kemp, J.A.; Foster, A.C.; Leeson, P.D.; Priestley, T.; Tridgett, R.; Iversen, L.L.; Woodruff, G.N. 7-Chlorokynurenic acid is a selective antagonist at the glycine modulatory site of the N-methyl-D-aspartate receptor complex. Proc. Natl. Acad. Sci. USA 1988, 85, 6547–6550. [Google Scholar] [CrossRef]
- Sas, K.; Robotka, H.; Rózsa, E.; Ágoston, M.; Szénási, G.; Gigler, G.; Marosi, M.; Kis, Z.; Farkas, T.; Vécsei, L.; et al. Kynurenine diminishes the ischemia-induced histological and electrophysiological deficits in the rat hippocampus. Neurobiol. Dis. 2008, 32, 302–308. [Google Scholar] [CrossRef]
- Fejes-Szabó, A.; Bohár, Z.; Vámos, E.; Nagy-Grócz, G.; Tar, L.; Veres, G.; Zádori, D.; Szentirmai, M.; Tajti, J.; Szatmári, I.; et al. Pre-treatment with new kynurenic acid amide dose-dependently prevents the nitroglycerine-induced neuronal activation and sensitization in cervical part of trigemino-cervical complex. J. Neural Transm. 2014, 121, 725–738. [Google Scholar] [CrossRef]
- Vamos, E.; Pardutz, A.; Fejes, A.; Tajti, J.; Toldi, J.; Vecsei, L. Modulatory effects of probenecid on the nitroglycerin-induced changes in the rat caudal trigeminal nucleus. Eur. J. Pharmacol. 2009, 621, 33–37. [Google Scholar] [CrossRef]
- Vámos, E.; Párdutz, Á.; Varga, H.; Bohár, Z.; Tajti, J.; Fülöp, F.; Toldi, J.; Vécsei, L. l-kynurenine combined with probenecid and the novel synthetic kynurenic acid derivative attenuate nitroglycerin-induced nNOS in the rat caudal trigeminal nucleus. Neuropharmacology 2009, 57, 425–429. [Google Scholar] [CrossRef]
- Nagy-Grócz, G.; Tar, L.; Bohár, Z.; Fejes-Szabó, A.; Laborc, K.F.; Spekker, E.; Vécsei, L.; Párdutz, Á. The modulatory effect of anandamide on nitroglycerin-induced sensitization in the trigeminal system of the rat. Cephalalgia 2015, 36, 849–861. [Google Scholar] [CrossRef]
- Nagy-Grócz, G.; Laborc, K.F.; Veres, G.; Bajtai, A.; Bohár, Z.; Zádori, D.; Fejes-Szabó, A.; Spekker, E.; Vécsei, L.; Párdutz, Á. The Effect of Systemic Nitroglycerin Administration on the Kynurenine Pathway in the Rat. Front. Neurol. 2017, 8, 278. [Google Scholar] [CrossRef]
- Lukács, M.; Warfvinge, K.; Kruse, L.S.; Tajti, J.; Fülöp, F.; Toldi, J.; Vécsei, L.; Edvinsson, L. KYNA analogue SZR72 modifies CFA-induced dural inflammation- regarding expression of pERK1/2 and IL-1β in the rat trigeminal ganglion. J. Headache Pain 2016, 17, 64. [Google Scholar] [CrossRef]
- Spekker, E.; Laborc, K.F.; Bohár, Z.; Nagy-Grócz, G.; Fejes-Szabó, A.; Szűcs, M.; Vécsei, L.; Párdutz, Á. Effect of dural inflammatory soup application on activation and sensitization markers in the caudal trigeminal nucleus of the rat and the modulatory effects of sumatriptan and kynurenic acid. J. Headache Pain 2021, 22, 17. [Google Scholar] [CrossRef]
- Cseh, E.K.; Veres, G.; Körtési, T.; Polyák, H.; Nánási, N.; Tajti, J.; Párdutz, Á.; Klivényi, P.; Vécsei, L.; Zádori, D. Neurotransmitter and tryptophan metabolite concentration changes in the complete Freund’s adjuvant model of orofacial pain. J. Headache Pain 2020, 21, 35. [Google Scholar] [CrossRef]
- Fejes-Szabo, A.; Bohar, Z.; Nagy-Grocz, G.; Vamos, E.; Tar, L.; Podor, B.; Tajti, J.; Toldi, J.; Vecsei, L.; Pardutz, Á. Effect of probenecid on the pain-related behaviour and morphological markers in orofacial formalin test of the rat. CNS Neurol. Disord. Drug Targets 2015, 14, 350–359. [Google Scholar] [CrossRef]
- Veres, G.; Fejes-Szabó, A.; Zádori, D.; Nagy-Grócz, G.; László, A.M.; Bajtai, A.; Mándity, I.; Szentirmai, M.; Bohár, Z.; Laborc, K.; et al. A comparative assessment of two kynurenic acid analogs in the formalin model of trigeminal activation: A behavioral, immunohistochemical and pharmacokinetic study. J. Neural Transm. 2016, 124, 99–112. [Google Scholar] [CrossRef]
- Greco, R.; Demartini, C.; Zanaboni, A.M.; Redavide, E.; Pampalone, S.; Toldi, J.; Fülöp, F.; Blandini, F.; Nappi, G.; Sandrini, G.; et al. Effects of kynurenic acid analogue 1 (KYNA-A1) in nitroglycerin-induced hyperalgesia: Targets and anti-migraine mechanisms. Cephalalgia 2016, 37, 1272–1284. [Google Scholar] [CrossRef]
- Knyihár-Csillik, E.; Chadaide, Z.; Okuno, E.; Krisztin-Péva, B.; Toldi, J.; Varga, C.; Molnár, A.; Csillik, B.; Vécsei, L. Kynurenine aminotransferase in the supratentorial dura mater of the rat: Effect of stimulation of the trigeminal ganglion. Exp. Neurol. 2004, 186, 242–247. [Google Scholar] [CrossRef]
- Knapp, L.; Szita, B.; Kocsis, K.; Vécsei, L.; Toldi, J. Nitroglycerin enhances the propagation of cortical spreading depression: Comparative studies with sumatriptan and novel kynurenic acid analogues. Drug Des. Dev. Ther. 2016, 11, 27–34. [Google Scholar] [CrossRef]
- Körtési, T.; Tuka, B.; Tajti, J.; Bagoly, T.; Fülöp, F.; Helyes, Z.; Vécsei, L. Kynurenic Acid Inhibits the Electrical Stimulation Induced Elevated Pituitary Adenylate Cyclase-Activating Polypeptide Expression in the TNC. Front. Neurol. 2018, 8, 745. [Google Scholar] [CrossRef]
- Curto, M.; Lionetto, L.; Negro, A.; Capi, M.; Fazio, F.; Giamberardino, M.A.; Simmaco, M.; Nicoletti, F.; Martelletti, P. Altered kynurenine pathway metabolites in serum of chronic migraine patients. J. Headache Pain 2015, 17, 47. [Google Scholar] [CrossRef]
- Curto, M.; Lionetto, L.; Negro, A.; Capi, M.; Perugino, F.; Fazio, F.; Giamberardino, M.A.; Simmaco, M.; Nicoletti, F.; Martelletti, P. Altered serum levels of kynurenine metabolites in patients affected by cluster headache. J. Headache Pain 2015, 17, 27. [Google Scholar] [CrossRef]
- Tuka, B.; Nyári, A.; Cseh, E.K.; Körtési, T.; Veréb, D.; Tömösi, F.; Kecskeméti, G.; Janáky, T.; Tajti, J.; Vécsei, L. Clinical relevance of depressed kynurenine pathway in episodic migraine patients: Potential prognostic markers in the peripheral plasma during the interictal period. J. Headache Pain 2021, 22, 60. [Google Scholar] [CrossRef]
- Tuka, B.; Körtési, T.; Nánási, N.; Tömösi, F.; Janáky, T.; Veréb, D.; Szok, D.; Tajti, J.; Vécsei, L. Cluster headache and kynurenines. J. Headache Pain 2023, 24, 35. [Google Scholar] [CrossRef]
- Curto, M.; Lionetto, L.; Fazio, F.; Mitsikostas, D.-D.; Martelletti, P. Fathoming the kynurenine pathway in migraine: Why understanding the enzymatic cascades is still critically important. Intern. Emerg. Med. 2015, 10, 413–421. [Google Scholar] [CrossRef]
- Sato, K.; Kiyama, H.; Park, H.T.; Tohyama, M. AMPA, KA and NMDA receptors are expressed in the rat DRG neurones. NeuroReport 1993, 4, 1263–1265. [Google Scholar] [CrossRef]
- Mecs, L.; Tuboly, G.; Nagy, E.; Benedek, G.; Horvath, G. The Peripheral Antinociceptive Effects of Endomorphin-1 and Kynurenic Acid in the Rat Inflamed Joint Model. Obstet. Anesthesia Dig. 2009, 109, 1297–1304. [Google Scholar] [CrossRef]
- Zhang, Y.-Q.; Ji, G.-C.; Wu, G.-C.; Zhao, Z.-Q. Kynurenic acid enhances electroacupuncture analgesia in normal and carrageenan-injected rats. Brain Res. 2003, 966, 300–307. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spekker, E.; Nagy-Grócz, G.; Vécsei, L. Ion Channel Disturbances in Migraine Headache: Exploring the Potential Role of the Kynurenine System in the Context of the Trigeminovascular System. Int. J. Mol. Sci. 2023, 24, 16574. https://doi.org/10.3390/ijms242316574
Spekker E, Nagy-Grócz G, Vécsei L. Ion Channel Disturbances in Migraine Headache: Exploring the Potential Role of the Kynurenine System in the Context of the Trigeminovascular System. International Journal of Molecular Sciences. 2023; 24(23):16574. https://doi.org/10.3390/ijms242316574
Chicago/Turabian StyleSpekker, Eleonóra, Gábor Nagy-Grócz, and László Vécsei. 2023. "Ion Channel Disturbances in Migraine Headache: Exploring the Potential Role of the Kynurenine System in the Context of the Trigeminovascular System" International Journal of Molecular Sciences 24, no. 23: 16574. https://doi.org/10.3390/ijms242316574
APA StyleSpekker, E., Nagy-Grócz, G., & Vécsei, L. (2023). Ion Channel Disturbances in Migraine Headache: Exploring the Potential Role of the Kynurenine System in the Context of the Trigeminovascular System. International Journal of Molecular Sciences, 24(23), 16574. https://doi.org/10.3390/ijms242316574







