Tumor Necrosis Factor-Alpha Induces Proangiogenic Profiling of Cardiosphere-Derived Cell Secretome and Increases Its Ability to Stimulate Angiogenic Properties of Endothelial Cells
Abstract
:1. Introduction
2. Results
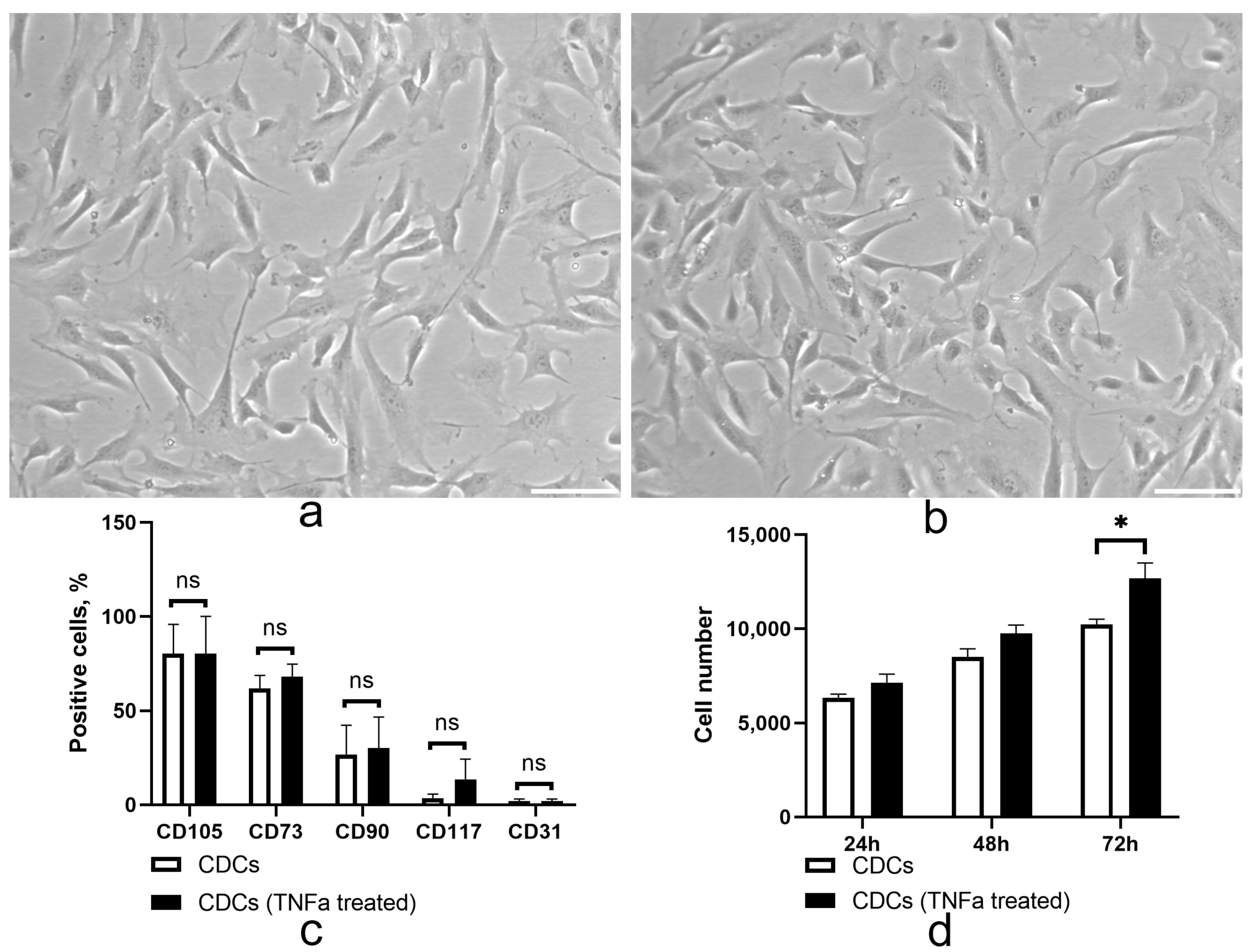
2.1. TNF Treatment Did Not Alter the Immunophenotype but May Enhance the Proliferative Properties of Cardiosphere-Derived Cells
2.2. TNFa Upregulates Angiogenic Proteins in Cardiosphere-Derived Cells
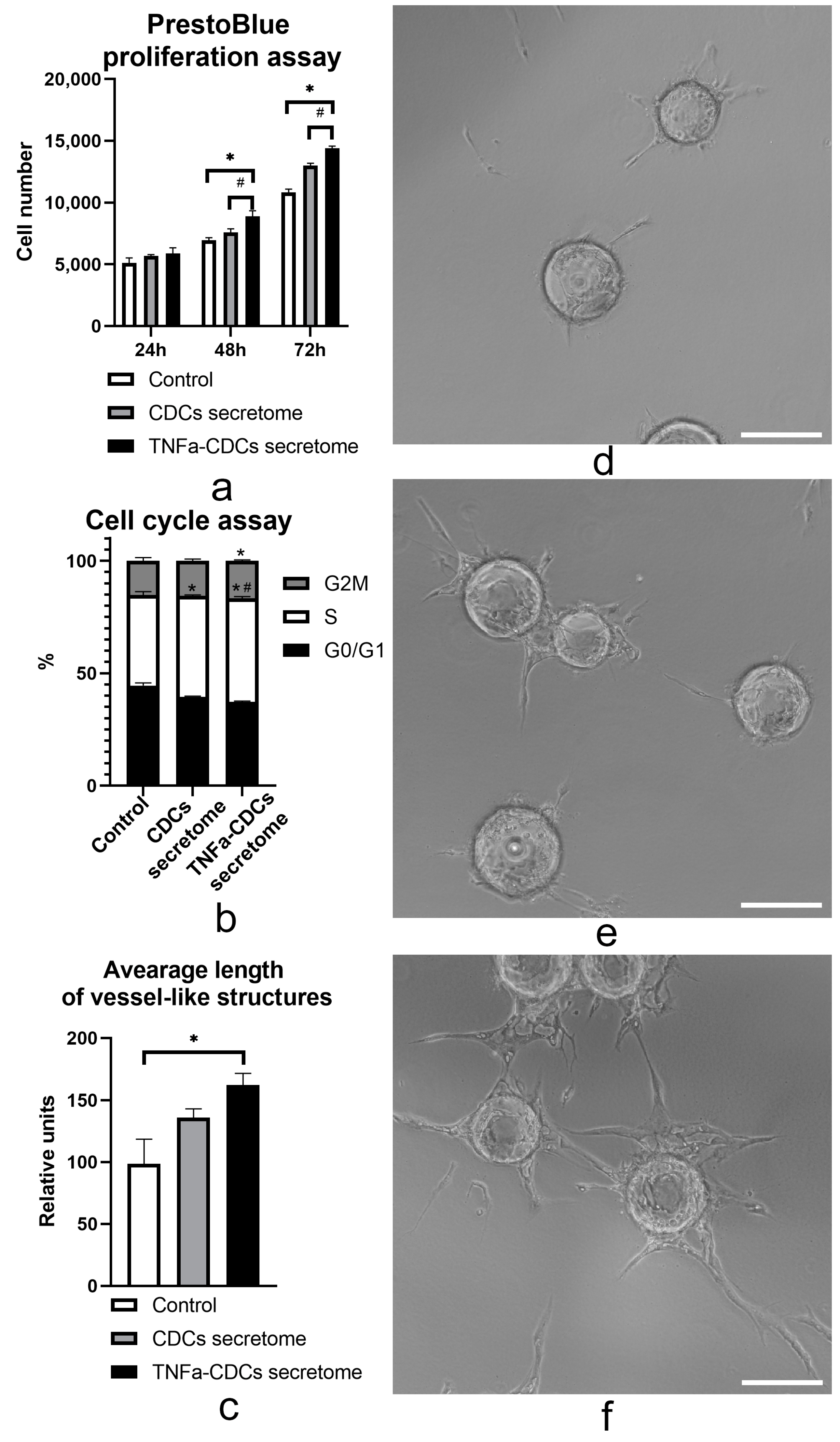
2.3. The TNFa-Induced Secretome In Vitro Stimulates Proliferation and Proangiogenic Properties of Endothelial Cells
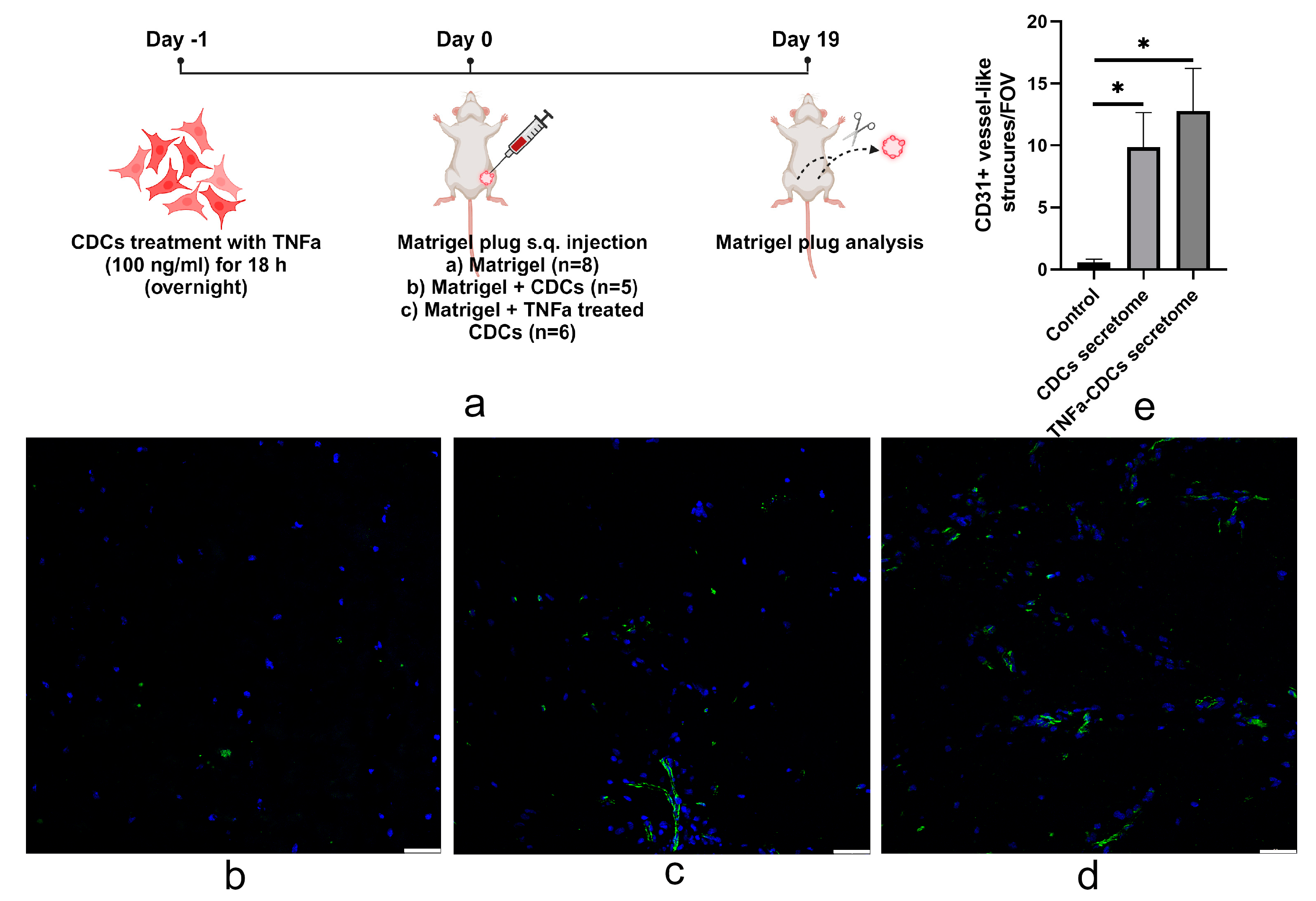
2.4. TNFa-Pretreated Cardiosphere-Derived Cells Stimulate Vascularization of Matrigel after Transplantation
3. Discussion
4. Materials and Methods
4.1. Cardiosphere, Cardiosphere-Derived Cell Isolation, and TNFa Treatments
4.2. Flow Cytometry
4.3. PrestoBlue Assay
4.4. Cell Cycle Assay
4.5. Magpix Analysis
4.6. Fibrin Gel Bead Angiogenesis Assay
4.7. Matrigel Plug Assay
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fritz, J.; Belovari, K.; Ulmer, H.; Zaruba, M.-M.; Messner, M.; Ungericht, M.; Siebert, U.; Ruschitzka, F.; Bauer, A.; Poelzl, G. Aetiology, Ejection Fraction and Mortality in Chronic Heart Failure: A Mediation Analysis. Heart 2023. [Google Scholar] [CrossRef]
- Cotter, G.; Moshkovitz, Y.; Milovanov, O.; Salah, A.; Blatt, A.; Krakover, R.; Vered, Z.; Kaluski, E. Acute Heart Failure: A Novel Approach to Its Pathogenesis and Treatment. Eur. J. Heart Fail. 2002, 4, 227–234. [Google Scholar] [CrossRef]
- Kogan, P.S.; Wirth, F.; Tomar, A.; Darr, J.; Teperino, R.; Lahm, H.; Dreßen, M.; Puluca, N.; Zhang, Z.; Neb, I.; et al. Uncovering the Molecular Identity of Cardiosphere-Derived Cells (CDCs) by Single-Cell RNA Sequencing. Basic Res. Cardiol. 2022, 117, 11. [Google Scholar] [CrossRef]
- Dergilev, K.V.; Vasilets, I.D.; Tsokolaeva, Z.I.; Zubkova, E.S.; Parfenova, E.V. Perspectives of Cell Therapy for Myocardial Infarction and Heart Failure Based on Cardiosphere Cells. Ter. Arkh. 2020, 92, 111–120. [Google Scholar] [CrossRef]
- Günter, J.; Wolint, P.; Bopp, A.; Steiger, J.; Cambria, E.; Hoerstrup, S.P.; Emmert, M.Y. Microtissues in Cardiovascular Medicine: Regenerative Potential Based on a 3D Microenvironment. Stem Cells Int. 2016, 2016, 9098523. [Google Scholar] [CrossRef]
- Dergilev, K.V.; Vasilets, Y.D.; Tsokolaeva, Z.I.; Parfenova, E.V. Transforming Growth Factor Beta-1 (TGF-Β1) Regulates Assembly of Cardiac Spheroids. Bull. Exp. Biol. Med. 2021, 170, 550–554. [Google Scholar] [CrossRef]
- Vasilets, Y.D.; Dergilev, K.V.; Tsokolaeva, Z.I.; Parfenova, E.V. Culturing of Cardiac Cells in 3D Spheroids Modulates Their Expression Profile and Increases Secretion of Proangiogenic Growth Factors. Bull. Exp. Biol. Med. 2022, 173, 235–239. [Google Scholar] [CrossRef]
- Cheng, K.; Malliaras, K.; Smith, R.R.; Shen, D.; Sun, B.; Blusztajn, A.; Xie, Y.; Ibrahim, A.; Aminzadeh, M.A.; Liu, W.; et al. Human Cardiosphere-Derived Cells from Advanced Heart Failure Patients Exhibit Augmented Functional Potency in Myocardial Repair. JACC Heart Fail. 2014, 2, 49–61. [Google Scholar] [CrossRef]
- Smith, R.R.; Barile, L.; Cho, H.C.; Leppo, M.K.; Hare, J.M.; Messina, E.; Giacomello, A.; Abraham, M.R.; Marbán, E. Regenerative Potential of Cardiosphere-Derived Cells Expanded from Percutaneous Endomyocardial Biopsy Specimens. Circulation 2007, 115, 896–908. [Google Scholar] [CrossRef]
- Gallet, R.; Dawkins, J.; Valle, J.; Simsolo, E.; De Couto, G.; Middleton, R.; Tseliou, E.; Luthringer, D.; Kreke, M.; Smith, R.R.; et al. Exosomes Secreted by Cardiosphere- Derived Cells Reduce Scarring, Attenuate Adverse Remodelling, and Improve Function in Acute and Chronic Porcine Myocardial Infarction. Eur. Heart J. 2017, 38, 201–211. [Google Scholar] [CrossRef]
- Li, T.S.; Cheng, K.; Malliaras, K.; Smith, R.R.; Zhang, Y.; Sun, B.; Matsushita, N.; Blusztajn, A.; Terrovitis, J.; Kusuoka, H.; et al. Direct Comparison of Different Stem Cell Types and Subpopulations Reveals Superior Paracrine Potency and Myocardial Repair Efficacy with Cardiosphere-Derived Cells. J. Am. Coll. Cardiol. 2012, 59, 942–953. [Google Scholar] [CrossRef]
- Namazi, H.; Namazi, I.; Ghiasi, P.; Ansari, H.; Rajabi, S.; Hajizadeh-Saffar, E.; Aghdami, N.; Mohit, E. Exosomes Secreted by Normoxic and Hypoxic Cardiosphere-Derived Cells Have Anti-Apoptotic Effect. Iran. J. Pharm. Res. 2018, 17, 377–385. [Google Scholar]
- Lin, Y.N.; Mesquita, T.; Sanchez, L.; Chen, Y.H.; Liu, W.; Li, C.; Rogers, R.; Wang, Y.; Li, X.; Wu, D.; et al. Extracellular Vesicles from Immortalized Cardiosphere-Derived Cells Attenuate Arrhythmogenic Cardiomyopathy in Desmoglein-2 Mutant Mice. Eur. Heart J. 2021, 42, 3558–3571. [Google Scholar] [CrossRef]
- Ishigami, S.; Ohtsuki, S.; Tarui, S.; Ousaka, D.; Eitoku, T.; Kondo, M.; Okuyama, M.; Kobayashi, J.; Baba, K.; Arai, S.; et al. Intracoronary autologous cardiac progenitor cell transfer in patients with hypoplastic left heart syndrome: The TICAP prospective phase 1 controlled trial. Circ. Res. 2015, 116, 653–664. [Google Scholar] [CrossRef]
- Boncler, M.; Różalski, M.; Krajewska, U.; Podsędek, A.; Watala, C. Comparison of PrestoBlue and MTT assays of cellular viability in the assessment of anti-proliferative effects of plant extracts on human endothelial cells. J. Pharmacol. Toxicol. Methods 2014, 69, 9–16. [Google Scholar] [CrossRef]
- Najar, M.; Raicevic, G.; Kazan, H.F.; de Bruyn, C.; Bron, D.; Toungouz, M.; Lagneaux, L. Immune-Related Antigens, Surface Molecules and Regulatory Factors in Human-Derived Mesenchymal Stromal Cells: The Expression and Impact of Inflammatory Priming. Stem Cell Rev. Rep. 2012, 8, 1188–1198. [Google Scholar] [CrossRef]
- Gilles, S.; Zahler, S.; Welsch, U.; Sommerhoff, C.P.; Becker, B.F. Release of TNFAlpha during Myocardial Reperfusion Depends on Oxidative Stress and Is Prevented by Mast Cell Stabilizers. Cardiovasc. Res. 2003, 60, 608–616. [Google Scholar] [CrossRef]
- Huang, S.W.; Chen, J.; Ouyang, B.; Yang, C.H.; Chen, M.Y.; Guan, X.D. Immunotherapy Improves Immune Homeostasis and Increases Survival Rate of Septic Patients. Chin. J. Traumatol. 2009, 12, 344–349. [Google Scholar] [CrossRef]
- Reil, J.C.; Gilles, S.; Zahler, S.; Brandl, A.; Drexler, H.; Hültner, L.; Matrisian, L.M.; Welsch, U.; Becker, B.F. Insights from Knock-out Models Concerning Postischemic Release of TNFalpha from Isolated Mouse Hearts. J. Mol. Cell. Cardiol. 2007, 42, 133–141. [Google Scholar] [CrossRef]
- Kleinbongard, P.; Böse, D.; Baars, T.; Möhlenkamp, S.; Konorza, T.; Schöner, S.; Elter- Schulz, M.; Eggebrecht, H.; Degen, H.; Haude, M.; et al. Vasoconstrictor Potential of Coronary Aspirate from Patients Undergoing Stenting of Saphenous Vein Aortocoronary Bypass Grafts and Its Pharmacological Attenuation. Circ. Res. 2011, 108, 344–352. [Google Scholar] [CrossRef]
- Boczkowski, J.; Philipp, I.; Tedgui, A.; Bernard, C.; Merval, R.; Desmonts, J.M.; Aubier, M. Effects of Inhibition of Nitric Oxide Synthesis on TNFa Serum Levels in E. coli Endotoxemic Rats. Life Sci. 1995, 57, PL147–PL152. [Google Scholar] [CrossRef]
- Thielmann, M.; Dörge, H.; Martin, C.; Belosjorow, S.; Schwanke, U.; Van De Sand, A.; Konietzka, I.; Büchert, A.; Krüger, A.; Schulz, R.; et al. Myocardial Dysfunction with Coronary Microembolization: Signal Transduction through a Sequence of Nitric Oxide, Tumor Necrosis Factor-Alpha, and Sphingosine. Circ. Res. 2002, 90, 807–813. [Google Scholar] [CrossRef]
- Berthonneche, C.; Sulpice, T.; Boucher, F.; Gouraud, L.; De Leiris, J.; O’Connor, S.E.; Herbert, J.M.; Janiak, P. New Insights into the Pathological Role of TNFAlpha in Early Cardiac Dysfunction and Subsequent Heart Failure after Infarction in Rats. Am. J. Physiol. Heart Circ. Physiol. 2004, 287, H340–H350. [Google Scholar] [CrossRef]
- Moro, C.; Jouan, M.G.; Rakotovao, A.; Toufektsian, M.C.; Ormezzano, O.; Nagy, N.; Tosaki, A.; De Leiris, J.; Boucher, F. Delayed Expression of Cytokines after Reperfused Myocardial Infarction: Possible Trigger for Cardiac Dysfunction and Ventricular Remodeling. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H3014–H3019. [Google Scholar] [CrossRef]
- Torre-Amione, G. Immune Activation in Chronic Heart Failure. Am. J. Cardiol. 2005, 95, 3–8. [Google Scholar] [CrossRef]
- Deswal, A.; Petersen, N.J.; Feldman, A.M.; Young, J.B.; White, B.G.; Mann, D.L. Cytokines and Cytokine Receptors in Advanced Heart Failure: An Analysis of the Cytokine Database from the Vesnarinone Trial (VEST). Circulation 2001, 103, 2055–2059. [Google Scholar] [CrossRef]
- Rodríguez-Reyna, T.S.; Arrieta, O.; Castillo-Martínez, L.; Orea-Tejeda, A.; Guevara, P.; Rebollar, V.; Granados, J. Tumour Necrosis Factor Alpha and Troponin T as Predictors of Poor Prognosis in Patients with Stable Heart Failure. Clin. Investig. Med. 2005, 28, 23–29. [Google Scholar]
- Steele, H.; Cheng, J.; Willicut, A.; Dell, G.; Breckenridge, J.; Culberson, E.; Ghastine, A.; Tardif, V.; Herro, R. TNF Superfamily Control of Tissue Remodeling and Fibrosis. Front. Immunol. 2023, 14, 1219907. [Google Scholar] [CrossRef]
- Cabal-Hierro, L.; Lazo, P.S. Signal Transduction by Tumor Necrosis Factor Receptors. Cell. Signal. 2012, 24, 1297–1305. [Google Scholar] [CrossRef]
- Wajant, H.; Pfizenmaier, K.; Scheurich, P. Tumor Necrosis Factor Signaling. Cell Death Differ. 2003, 10, 45–65. [Google Scholar] [CrossRef]
- Straface, E.; Malorni, W.; Pietraforte, D. Sex Differences in Redox Biology: A Mandatory New Point of View Approaching Human Inflammatory Diseases. Antioxid. Redox Signal. 2017, 26, 44–45. [Google Scholar] [CrossRef]
- MacEwan, D.J. TNF Receptor Subtype Signalling: Differences and Cellular Consequences. Cell. Signal. 2002, 14, 477–492. [Google Scholar] [CrossRef]
- Zubkova, E.S.; Beloglazova, I.B.; Makarevich, P.I.; Boldyreva, M.A.; Sukhareva, O.Y.; Shestakova, M.V.; Dergilev, K.V.; Parfyonova, Y.V.; Menshikov, M.Y. Regulation of Adipose Tissue Stem Cells Angiogenic Potential by Tumor Necrosis Factor-Alpha. J. Cell. Biochem. 2016, 117, 180–196. [Google Scholar] [CrossRef]
- Nör, J.E.; Christensen, J.; Mooney, D.J.; Polverini, P.J. Vascular Endothelial Growth Factor (VEGF)-Mediated Angiogenesis Is Associated with Enhanced Endothelial Cell Survival and Induction of Bcl-2 Expression. Am. J. Pathol. 1999, 154, 375–384. [Google Scholar] [CrossRef]
- Zachary, I. VEGF Signalling: Integration and Multi-Tasking in Endothelial Cell Biology. Biochem. Soc. Trans. 2003, 31, 1171–1177. [Google Scholar] [CrossRef]
- Suffee, N.; Richard, B.; Hlawaty, H.; Oudar, O.; Charnaux, N.; Sutton, A. Angiogenic Properties of the Chemokine RANTES/CCL5. Biochem. Soc. Trans. 2011, 39, 1649–1653. [Google Scholar] [CrossRef]
- Bussolino, F.; Wang, J.M.; Defilippi, P.; Turrini, F.; Sanavio, F.; Edgell, C.J.S.; Aglietta, M.; Arese, P.; Mantovani, A. Granulocyte- and Granulocyte-Macrophage-Colony Stimulating Factors Induce Human Endothelial Cells to Migrate and Proliferate. Nature 1989, 337, 471–473. [Google Scholar] [CrossRef]
- Valdembri, D.; Serini, G.; Vacca, A.; Ribatti, D.; Bussolino, F. In vivo activation of JAK2/STAT-3 pathway during angiogenesis induced by GM-CSF. FASEB J. 2001, 16, 225–257. [Google Scholar] [CrossRef]
- Shintani, S.; Ishikawa, T.; Nonaka, T.; Li, C.; Nakashiro, K.; Wong, D.T.; Hamakawa, H. Growth-regulated oncogene-1 expression is associated with angiogenesis and lymph node metastasis in human oral cancer. Oncology 2004, 66, 316–322. [Google Scholar] [CrossRef]
- Petzelbauer, P.; Watson, C.A.; Pfau, S.E.; Pober, J.S. IL-8 and angiogenesis: Evidence that human endothelial cells lack receptors and do not respond to IL-8 In Vitro. Cytokine 1995, 7, 267–272. [Google Scholar] [CrossRef]
- Belperio, J.A.; Keane, M.P.; Arenberg, D.A.; Addison, C.L.; Ehlert, J.E.; Burdick, M.D.; Strieter, R.M. CXC chemokines in angiogenesis. J. Leukoc. Biol. 2000, 68, 1–8. [Google Scholar] [CrossRef]
- Noronha Nc, N.D.C.; Mizukami, A.; Caliári-Oliveira, C.; Cominal, J.G.; Rocha, J.L.M.; Covas, D.T.; Swiech, K.; Malmegrim, K.C.R. Priming Approaches to Improve the Efficacy of Mesenchymal Stromal Cell-Based Therapies. Stem Cell Res. Ther. 2019, 10, 131. [Google Scholar] [CrossRef]
- Miceli, V.; Bulati, M.; Iannolo, G.; Zito, G.; Gallo, A.; Conaldi, P.G. Therapeutic Properties of Mesenchymal Stromal/Stem Cells: The Need of Cell Priming for Cell-Free Therapies in Regenerative Medicine. Int. J. Mol. Sci. 2021, 22, 763. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dergilev, K.; Zubkova, E.; Guseva, A.; Tsokolaeva, Z.; Goltseva, Y.; Beloglazova, I.; Ratner, E.; Andreev, A.; Partigulov, S.; Lepilin, M.; et al. Tumor Necrosis Factor-Alpha Induces Proangiogenic Profiling of Cardiosphere-Derived Cell Secretome and Increases Its Ability to Stimulate Angiogenic Properties of Endothelial Cells. Int. J. Mol. Sci. 2023, 24, 16575. https://doi.org/10.3390/ijms242316575
Dergilev K, Zubkova E, Guseva A, Tsokolaeva Z, Goltseva Y, Beloglazova I, Ratner E, Andreev A, Partigulov S, Lepilin M, et al. Tumor Necrosis Factor-Alpha Induces Proangiogenic Profiling of Cardiosphere-Derived Cell Secretome and Increases Its Ability to Stimulate Angiogenic Properties of Endothelial Cells. International Journal of Molecular Sciences. 2023; 24(23):16575. https://doi.org/10.3390/ijms242316575
Chicago/Turabian StyleDergilev, Konstantin, Ekaterina Zubkova, Alika Guseva, Zoya Tsokolaeva, Yulia Goltseva, Irina Beloglazova, Elizaveta Ratner, Alexander Andreev, Stanislav Partigulov, Mikhail Lepilin, and et al. 2023. "Tumor Necrosis Factor-Alpha Induces Proangiogenic Profiling of Cardiosphere-Derived Cell Secretome and Increases Its Ability to Stimulate Angiogenic Properties of Endothelial Cells" International Journal of Molecular Sciences 24, no. 23: 16575. https://doi.org/10.3390/ijms242316575
APA StyleDergilev, K., Zubkova, E., Guseva, A., Tsokolaeva, Z., Goltseva, Y., Beloglazova, I., Ratner, E., Andreev, A., Partigulov, S., Lepilin, M., Menshikov, M., & Parfyonova, Y. (2023). Tumor Necrosis Factor-Alpha Induces Proangiogenic Profiling of Cardiosphere-Derived Cell Secretome and Increases Its Ability to Stimulate Angiogenic Properties of Endothelial Cells. International Journal of Molecular Sciences, 24(23), 16575. https://doi.org/10.3390/ijms242316575






