Smooth Muscle Actin as a Criterion for Gravisensitivity of Stomach and Jejunum in Laboratory Rodents
Abstract
:1. Introduction
2. Results
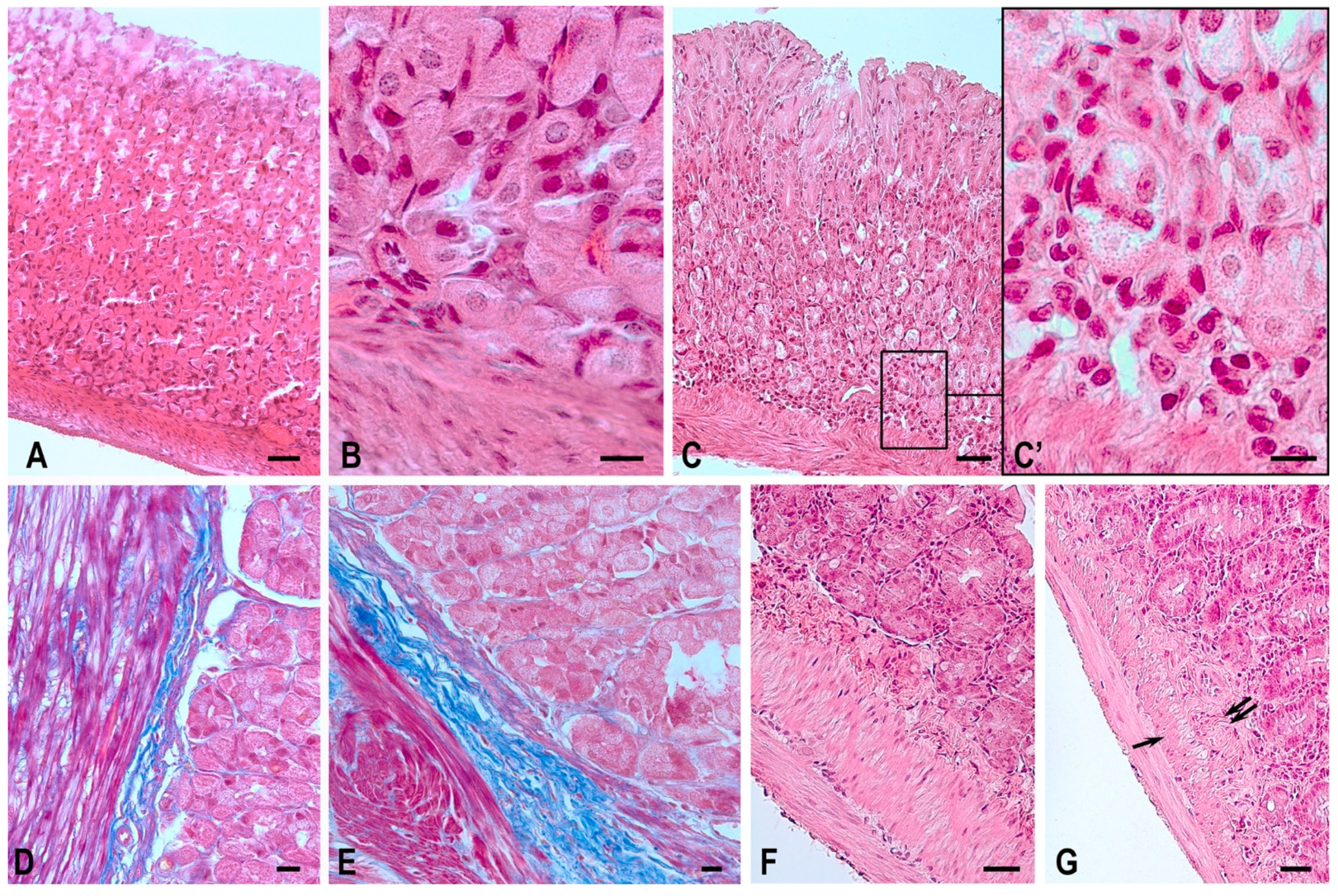
2.1. Routine Microscopy
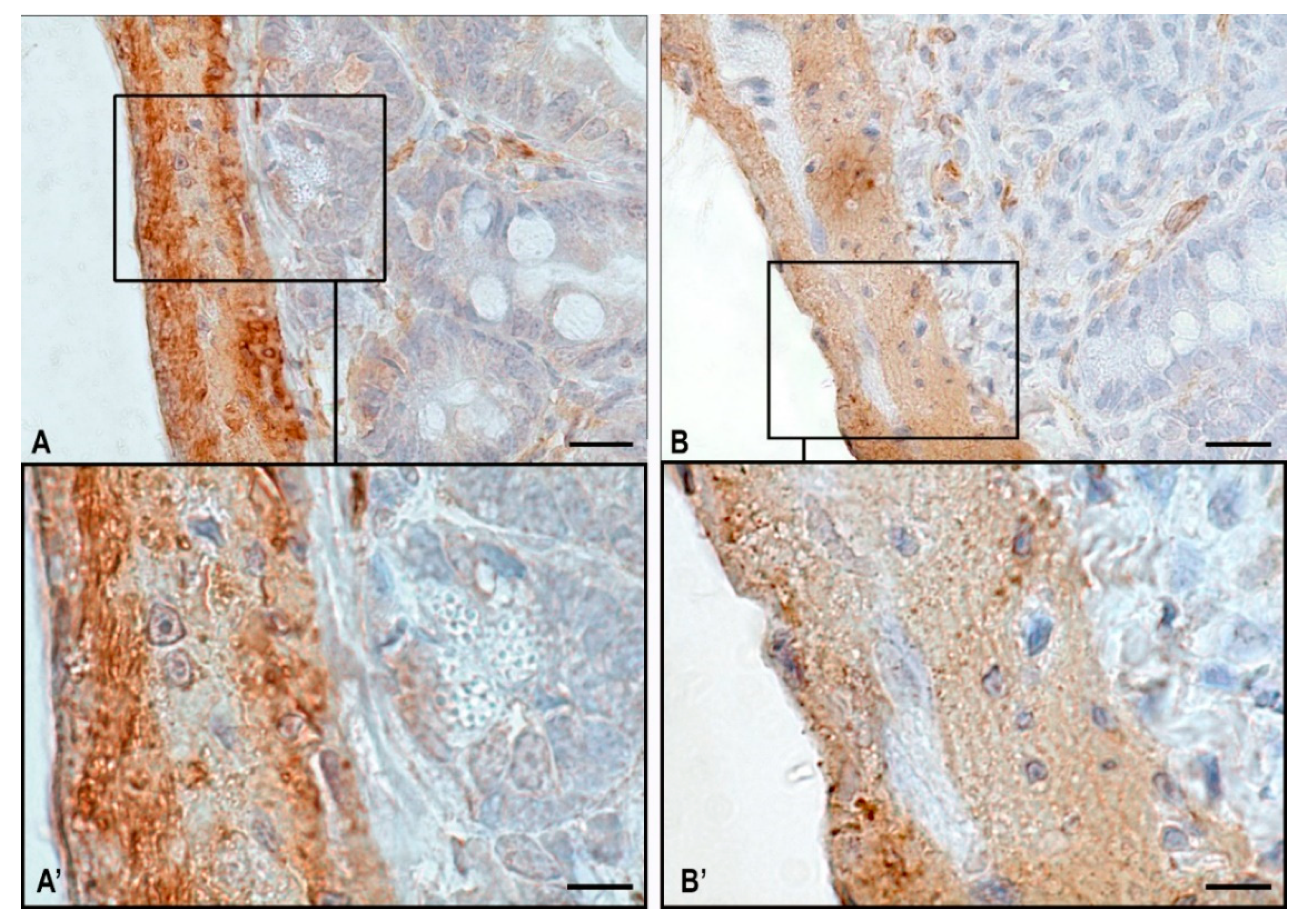
2.2. Content of α-SMA in the Stomach
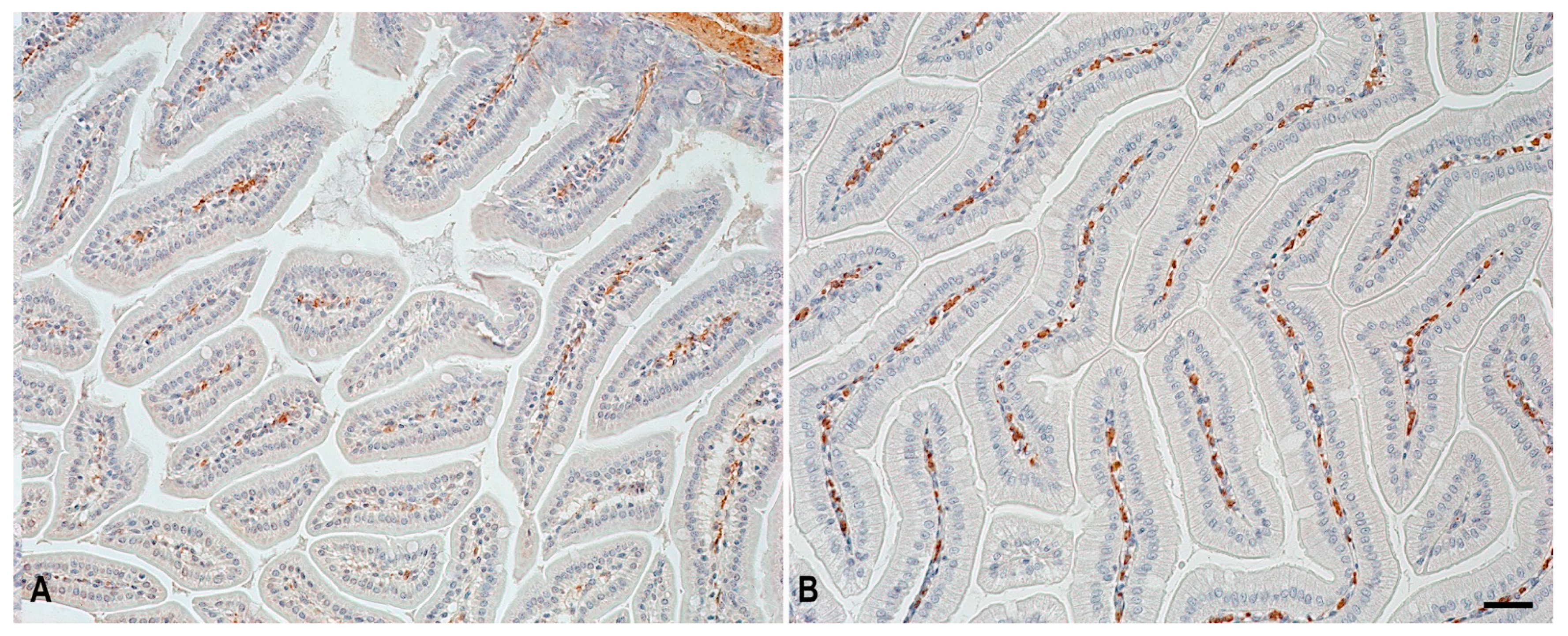
2.3. α-SMA Expression in Structural Components of the Jejunum
3. Discussion
4. Materials and Methods
4.1. Experimental Design
4.2. Histoprocessing
4.3. Tissue Probe Staining
4.4. Image Acquisition
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chai, S.; Guo, S.; Yang, J.; Lu, S.; Yue, Y.; Li, H.; Sun, P.; Zhang, T.; Xu, B.; Sun, H.; et al. Inflammation is involved in response of gastric mucosal epithelial cells under simulated microgravity by integrated transcriptomic analysis. Am. J. Transl. Res. 2021, 13, 9195–9207. [Google Scholar] [PubMed]
- Buravkova, L.; Larina, I.; Andreeva, E.; Grigoriev, A. Microgravity Effects on the Matrisome. Cells 2021, 10, 2226. [Google Scholar] [CrossRef] [PubMed]
- Andreev-Andrievskiy, A.; Dolgov, O.; Alberts, J.; Popova, A.; Lagereva, E.; Anokhin, K.; Vinogradova, O. Mice display learning and behavioral deficits after a 30-day spaceflight on Bion-M1 satellite. Behav. Brain Res. 2022, 419, 113682. [Google Scholar] [CrossRef] [PubMed]
- Prasad, B.; Grimm, D.; Strauch, S.M.; Erzinger, G.S.; Corydon, T.J.; Lebert, M.; Magnusson, N.E.; Infanger, M.; Richter, P.; Kruger, M. Influence of Microgravity on Apoptosis in Cells, Tissues, and Other Systems In Vivo and In Vitro. Int. J. Mol. Sci. 2020, 21, 9373. [Google Scholar] [CrossRef] [PubMed]
- Tyrina, E.A.; Andreeva, E.R.; Buravkova, L.B. Simulated microgravity affects stroma-dependent ex vivo myelopoiesis. Tissue Cell 2023, 80, 101987. [Google Scholar] [CrossRef] [PubMed]
- Shishkin, N.; Kitov, V.; Sayenko, D.; Tomilovskaya, E. Sensory organization of postural control after long term space flight. Front. Neural. Circuits 2023, 17, 1135434. [Google Scholar] [CrossRef]
- Buckey, J.C.; Thamer, S.; Lan, M. Bone loss and kidney stone risk in weightlessness. Curr. Opin. Nephrol. Hypertens. 2023, 32, 172–176. [Google Scholar] [CrossRef]
- Yang, J.Q.; Jiang, N.; Li, Z.P.; Guo, S.; Chen, Z.Y.; Li, B.B.; Chai, S.B.; Lu, S.Y.; Yan, H.F.; Sun, P.M.; et al. The effects of microgravity on the digestive system and the new insights it brings to the life sciences. Life Sci. Space Res. 2020, 27, 74–82. [Google Scholar] [CrossRef]
- Afonin, B.V. The hemodynamic mechanism determining the emergence of a hypersecretory state of the stomach in microgravity conditions. Aerosp. Environ. Med. 2013, 47, 10–11. [Google Scholar]
- Cromer, W.E.; Zawieja, D.C. Acute exposure to space flight results in evidence of reduced lymph Transport, tissue fluid Shifts, and immune alterations in the rat gastrointestinal system. Life Sci. Space Res. 2018, 17, 74–82. [Google Scholar] [CrossRef]
- Hand, A.R.; Dagdeviren, D.; Larson, N.A.; Haxhi, C.; Mednieks, M.I. Effects of spaceflight on the mouse submandibular gland. Arch. Oral. Biol. 2020, 110, 104621. [Google Scholar] [CrossRef] [PubMed]
- Laurens, C.; Simon, C.; Vernikos, J.; Gauquelin-Koch, G.; Blanc, S.; Bergouignan, A. Revisiting the Role of Exercise Countermeasure on the Regulation of Energy Balance During Space Flight. Front. Physiol. 2019, 10, 321. [Google Scholar] [CrossRef] [PubMed]
- Atiakshin, D.A.; Bykov, E.G. Morphological changes in the stomach wall of Mongolian gerbils after a 12-day orbital flight on the spacecraft “Photon-M3”. Aerosp. Environ. Med. 2012, 46, 26–33. [Google Scholar]
- Weisbrodt, N.W.; Booth, F.W.; Lai, M.; Thomason, D.B. mRNA Levels in Smooth Muscle. In Final Reports of the U.S. Experiments Flown on the Soviet Biosatellite Cosmos 2044; NASA Technical Memorandum 108802; Experiment K-7-11—Part II; NASA Ames Research Center: Mountain View, CA, USA, 1994; pp. 363–371. [Google Scholar]
- Dabertrand, F.; Porte, Y.; Macrez, N.; Morel, J.L. Spaceflight regulates ryanodine receptor subtype 1 in portal vein myocytes in the opposite way of hypertension. J. Appl. Physiol. 2012, 112, 471–480. [Google Scholar] [CrossRef]
- Liu, H.; Liang, M.; Deng, Y.; Li, Y. Simulated Microgravity Alters P-Glycoprotein Efflux Function and Expression via the Wnt/β-Catenin Signaling Pathway in Rat Intestine and Brain. Int. J. Mol. Sci. 2023, 24, 5438. [Google Scholar] [CrossRef]
- Vinken, M. Hepatology in space: Effects of spaceflight and simulated microgravity on the liver. Liver Int. 2022, 42, 2599–2606. [Google Scholar] [CrossRef]
- Lu, S.Y.; Guo, S.; Chai, S.B.; Yang, J.Q.; Yue, Y.; Li, H.; Yan, H.F.; Zhang, T.; Sun, P.M.; Sun, H.W.; et al. Proteomic analysis of the effects of simulated microgravity in human gastric mucosal cells. Life Sci. Space Res. 2022, 32, 26–37. [Google Scholar] [CrossRef]
- Borsdorf, M.; Bol, M.; Siebert, T. Influence of layer separation on the determination of stomach smooth muscle properties. Pflug. Arch. 2021, 473, 911–920. [Google Scholar] [CrossRef]
- Donadon, M.; Santoro, M.M. The origin and mechanisms of smooth muscle cell development in vertebrates. Development 2021, 148, dev19738. [Google Scholar] [CrossRef]
- Samoilenko, T.V.; Shishkina, V.V.; Antakova, L.N.; Atyakshin, D.A. Smooth muscle tissue is a promising target of translational research in space biomedicine. Aerosp. Environ. Med. 2022, 56, 5–15. [Google Scholar] [CrossRef]
- Tang, D.D. The Dynamic Actin Cytoskeleton in Smooth Muscle. Adv. Pharmacol. 2018, 81, 1–38. [Google Scholar] [CrossRef] [PubMed]
- Pastula, A.; Marcinkiewicz, J. Cellular Interactions in the Intestinal Stem Cell Niche. Arch. Immunol. Ther. Exp. 2019, 67, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Jerome, J.A.; Wenzel, S.E.; Trejo Bittar, H.E. Digital Imaging Analysis Reveals Reduced Alveolar alpha-Smooth Muscle Actin Expression in Severe Asthma. Appl. Immunohistochem. Mol. Morphol. 2021, 29, 506–512. [Google Scholar] [CrossRef] [PubMed]
- Shishkina, V.; Kostin, A.; Volodkin, A.; Samoilova, V.; Buchwalow, I.; Tiemann, M.; Atiakshin, D. The Remodeling of Dermal Collagen Fibrous Structures in Mice under Zero Gravity: The Role of Mast Cells. Int. J. Mol. Sci. 2023, 24, 1939. [Google Scholar] [CrossRef] [PubMed]
- Smirnov, K.V.; Lizko, N.N. Problems of space gastroenterology and microenvironment. Nahrung 1987, 31, 563–566. [Google Scholar] [CrossRef] [PubMed]
- Afonin, B.V.; Noskov, V.B.; Poliakov, V.V. The state of the digestive system organs during long space flight. Fiziol. Cheloveka 2003, 29, 53–57. [Google Scholar] [PubMed]
- Afonin, B.V.; Goncharova, N.P.; Sedova, E.A. Skin electrical activity of the stomach and intestine during long-term antiorthostatic hypokinesia. Fiziol. Cheloveka 2007, 33, 100–104. [Google Scholar] [CrossRef]
- Afonin, B.V. Analysis of possible causes activation a stomach and pancreas excretory and incretory function after completion of space flight on the international space station. Fiziol. Cheloveka 2013, 39, 62–70. [Google Scholar]
- Harm, D.L.; Sandoz, G.R.; Stern, R.M. Changes in gastric myoelectric activity during space flight. Dig. Dis. Sci. 2002, 47, 1737–1745. [Google Scholar] [CrossRef]
- Grigoriev, A.I.; Huntoon, C.L.; Morukov, B.V.; Lane, H.W.; Larina, I.M.; Smith, S.M. Endocrine, renal, and circulatory influences on fluid and electrolyte homeostasis during weightlessness: A joint Russian-U.S. project. J. Gravit. Physiol. 1996, 3, 83–86. [Google Scholar]
- Atiakshin, D.; Kostin, A.; Shishkina, V.; Burtseva, A.; Buravleva, A.; Volodkin, A.; Elieh-Ali-Komi, D.; Buchwalow, I.; Tiemann, M. Space-Flight- and Microgravity-Dependent Alteration of Mast Cell Population and Protease Expression in Digestive Organs of Mongolian Gerbils. Int. J. Mol. Sci. 2023, 24, 13604. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Olseen, A.; Xie, Y.; Alexandru, C.; Outland, A.; Herrera, A.F.; Syder, A.J.; Wykosky, J.; Perrino, B.A. Mfge8 attenuates human gastric antrum smooth muscle contractions. J. Muscle. Res. Cell Motil. 2021, 42, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Fedeli, V.; Cucina, A.; Dinicola, S.; Fabrizi, G.; Catizone, A.; Gesualdi, L.; Ceccarelli, S.; Harrath, A.H.; Alwasel, S.H.; Ricci, G.; et al. Microgravity Modifies the Phenotype of Fibroblast and Promotes Remodeling of the Fibroblast-Keratinocyte Interaction in a 3D Co-Culture Model. Int. J. Mol. Sci. 2022, 23, 2163. [Google Scholar] [CrossRef] [PubMed]
- Sapudom, J.; ElGindi, M.; Arnoux, M.; Drou, N.; Garcia-Sabate, A.; Teo, J.C.M. Fibroblast Differentiation and Matrix Remodeling Impaired under Simulated Microgravity in 3D Cell Culture Model. Int. J. Mol. Sci. 2021, 22, 11911. [Google Scholar] [CrossRef]
- Kang, H.; Fan, Y.; Sun, A.; Jia, X.; Deng, X. Simulated microgravity exposure modulates the phenotype of cultured vascular smooth muscle cells. Cell Biochem. Biophys. 2013, 66, 121–130. [Google Scholar] [CrossRef]
- Mao, Q.W.; Zhang, L.F.; Zhang, L.N.; Ma, J. Ultrastructural changes of arterial wall from different body parts of rats during simulated weightlessness. Space Med. Med. Eng. 1999, 12, 249–253. [Google Scholar]
- Sychev, V.N.; Ilyin, E.A.; Yarmanova, E.N.; Rakov, D.V.; Ushakov, I.B.; Kirilin, A.N.; Orlov, O.I.; Grigoriev, A.I. The BION-M1 project: Overview and first results. Aviakosm Ekolog. Med. 2014, 48, 7–14. [Google Scholar]
- Andreev-Andrievsky, A.A.; Shenkman, B.S.; Popova, A.S.; Dolguikh, O.N.; Anokhin, K.V.; Soldatov, P.E.; Ilyin, E.A.; Sychev, V.N. Experimental studies with mice on the program of the biosatellite BION-M1 mission. Aviakosm Ekolog. Med. 2014, 48, 14–27. [Google Scholar]
- Andreev-Andrievskiy, A.; Popova, A.; Boyle, R.; Alberts, J.; Shenkman, B.; Vinogradova, O.; Dolgov, O.; Anokhin, K.; Tsvirkun, D.; Soldatov, P.; et al. Mice in Bion-M 1 space mission: Training and selection. PLoS ONE 2014, 9, e104830. [Google Scholar] [CrossRef]
- Ilyin, E.A. Biological experiments in flights of unmanned space craft Foton-M2 and Foton-M3. Aviakosm Ekolog. Med. 2013, 47, 47–54. [Google Scholar]
- Krivonosov, Y.S.; Gulimova, V.I.; Buzmakov, A.V.; Zolotov, D.A.; Cedola, A.; Bukreeva, I.; Asadchikov, V.E.; Saveliev, S.V. Micro-CT Study of Mongolian Gerbil Humeral Bone After Prolonged Spaceflight Based on a New Algorithm for Delimitation of Long-Bone Regions. Front. Physiol. 2021, 12, 752893. [Google Scholar] [CrossRef] [PubMed]
- Markina, E.A.; Bobyleva, P.I.; Alekseeva, O.Y.; Andrianova, I.V.; Andreeva, E.R.; Buravkova, L.B. Characteristics of Bone Marrow Progenitor Cells of C57BL/6N Mice after 30-Day Hindlimb Suspension and 12-Hour Readaptation to Support Loading. Cell Tissue Biol. 2020, 14, 99–101. [Google Scholar] [CrossRef]
- Buchwalow, I.B.; Böcker, W. Immunohistochemistry: Basics and Methods; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2010. [Google Scholar]

| Groups of Animals | Mucosa | Muscularis Externa | ||||
|---|---|---|---|---|---|---|
| Analyzed Area, mm2 (M ± m) | Area of α-SMA Expression, mm2 (M ± m) | % α-SMA | Analyzed Area, mm2 (M ± m) | Area of α-SMA Expression, mm2 (M ± m) | % α-SMA | |
| The stomach | ||||||
| VC-SF, n = 8 | 3.86 ± 0.04 | 0.19 ± 0.01 | 4.87 | 3.12 ± 0.07 | 2.74 ± 0.06 | 87.78 |
| SF, n = 5 | 3.88 ± 0.09 | 0.20 ± 0.01 | 5.28 * | 3.10 ± 0.06 | 2.32 ± 0.02 * | 74.91 * |
| VC-RSF, n = 8 | 3.73 ± 0.02 | 0.20 ± 0.002 | 5.45 | 3.29 ± 0.02 | 2.81 ± 0.09 | 85.32 |
| RSF, n = 5 | 3.72 ± 0.02 | 0.21 ± 0.002 | 5.59 | 2.94 ± 0.05 * | 2.35 ± 0.04 * | 79.79 |
| The jejunum | ||||||
| VC-SF, n = 8 | 3.71 ± 0.02 | 0.17 ± 0.002 | 4.50 | 0.57 ± 0.02 | 0.49 ± 0.01 | 85.92 |
| SF, n = 5 | 3.78 ± 0.05 | 0.20 ± 0.01 * | 5.17 * | 0.58 ± 0.02 | 0.43 ± 0.001 * | 73.50 * |
| VC-RSF, n = 8 | 3.73 ± 0.03 | 0.16 ± 0.01 | 4.42 | 0.60 ± 0.03 | 0.51 ± 0.03 | 84.81 |
| RSF, n = 5 | 3.61 ± 0.03 | 0.19 ± 0.002 * | 5.22 * | 0.57 ± 0.03 | 0.46 ± 0.03 | 80.89 |
| Groups of Animals | Mucosa | Muscularis Externa | ||||
|---|---|---|---|---|---|---|
| Analyzed Area, mm2 (M ± m) | Area of α-SMA Expression, mm2 (M ± m) | % α-SMA | Analyzed Area, mm2 (M ± m) | Area of α-SMA Expression, mm2 (M ± m) | % α-SMA | |
| The stomach | ||||||
| Vivarium control, n = 12 | 3.76 ± 0.03 | 0.21 ± 0.002 | 5.67 | 3.59 ± 0.02 | 3.24 ± 0.02 | 90.16 |
| Space flight, n = 12 | 3.55 ± 0.02 | 0.20 ± 0.002 | 5.52 | 3.42 ± 0.03 | 2.59 ± 0.04 * | 75.80 * |
| Synchronous ground experiment, n = 11 | 3.54 ± 0.02 | 0.19 ± 0.001 | 5.46 * | 3.57 ± 0.02 | 3.08 ± 0.02 | 86.23 |
| The jejunum | ||||||
| Vivarium control, n = 12 | 3.68 ± 0.02 | 0.26 ± 0.003 | 7.16 | 1.51 ± 0.02 | 1.35 ± 0.01 | 88.97 |
| Space flight, n = 12 | 3.68 ± 0.02 | 0.31 ± 0.002 | 8.48 | 1.49 ± 0.01 | 1.13 ± 0.01 | 75.71 * |
| Synchronous ground experiment, n = 11 | 3.65 ± 0.01 | 0.26 ± 0.005 | 7.12 * | 1.49 ± 0.02 | 1.24 ± 0.02 | 83.29 |
| Groups of Animals | Mucosa | Muscularis Externa | ||||
|---|---|---|---|---|---|---|
| Analyzed Area, mm2 (M ± m) | Area of α-SMA Expression, mm2 (M ± m) | % α-SMA | Analyzed Area, mm2 (M ± m) | Area of α-SMA Expression, mm2 (M ± m) | % α-SMA | |
| The stomach | ||||||
| control group, n = 6 | 3.76 ± 0.03 | 0.21 ± 0.003 | 5.55 | 3.01 ± 0.04 | 2.66 ± 0.04 | 88.51 |
| group anti-orthostatic suspension, n = 6 | 3.64 ± 0.06 | 0.16 ± 0.001 * | 4.45 | 3.49 ± 0.06 | 2.97 ± 0.04 * | 85 * |
| readaptation group, n = 6 | 3.76 ± 0.06 | 0.21 ± 0.004 | 5.7 | 3.09 ± 0.03 | 2.63 ± 0.04 | 85.08 * |
| The jejunum | ||||||
| control group, n = 6 | 3.70 ± 0.04 | 0.17 ± 0.005 | 4.61 | 0.58 ± 0.007 | 0.49 ± 0.005 | 86.04 |
| group anti-orthostatic suspension, n = 6 | 3.73 ± 0.05 | 0.15 ± 0.004 | 3.96 * | 0.57 ± 0.007 | 0.51 ± 0.007 * | 89.44 * |
| readaptation group, n = 6 | 3.73 ± 0.06 | 0.17 ± 0.004 | 4.53 | 0.57 ± 0.004 | 0.50 ± 0.005 | 86.59 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samoilenko, T.; Shishkina, V.; Antakova, L.; Goryushkina, Y.; Kostin, A.; Buchwalow, I.; Tiemann, M.; Atiakshin, D. Smooth Muscle Actin as a Criterion for Gravisensitivity of Stomach and Jejunum in Laboratory Rodents. Int. J. Mol. Sci. 2023, 24, 16539. https://doi.org/10.3390/ijms242216539
Samoilenko T, Shishkina V, Antakova L, Goryushkina Y, Kostin A, Buchwalow I, Tiemann M, Atiakshin D. Smooth Muscle Actin as a Criterion for Gravisensitivity of Stomach and Jejunum in Laboratory Rodents. International Journal of Molecular Sciences. 2023; 24(22):16539. https://doi.org/10.3390/ijms242216539
Chicago/Turabian StyleSamoilenko, Tatyana, Viktoriya Shishkina, Lyubov Antakova, Yelena Goryushkina, Andrey Kostin, Igor Buchwalow, Markus Tiemann, and Dmitrii Atiakshin. 2023. "Smooth Muscle Actin as a Criterion for Gravisensitivity of Stomach and Jejunum in Laboratory Rodents" International Journal of Molecular Sciences 24, no. 22: 16539. https://doi.org/10.3390/ijms242216539
APA StyleSamoilenko, T., Shishkina, V., Antakova, L., Goryushkina, Y., Kostin, A., Buchwalow, I., Tiemann, M., & Atiakshin, D. (2023). Smooth Muscle Actin as a Criterion for Gravisensitivity of Stomach and Jejunum in Laboratory Rodents. International Journal of Molecular Sciences, 24(22), 16539. https://doi.org/10.3390/ijms242216539






