Ethanol Extract of Radix Asteris Suppresses Osteoclast Differentiation and Alleviates Osteoporosis
Abstract
:1. Introduction
2. Results
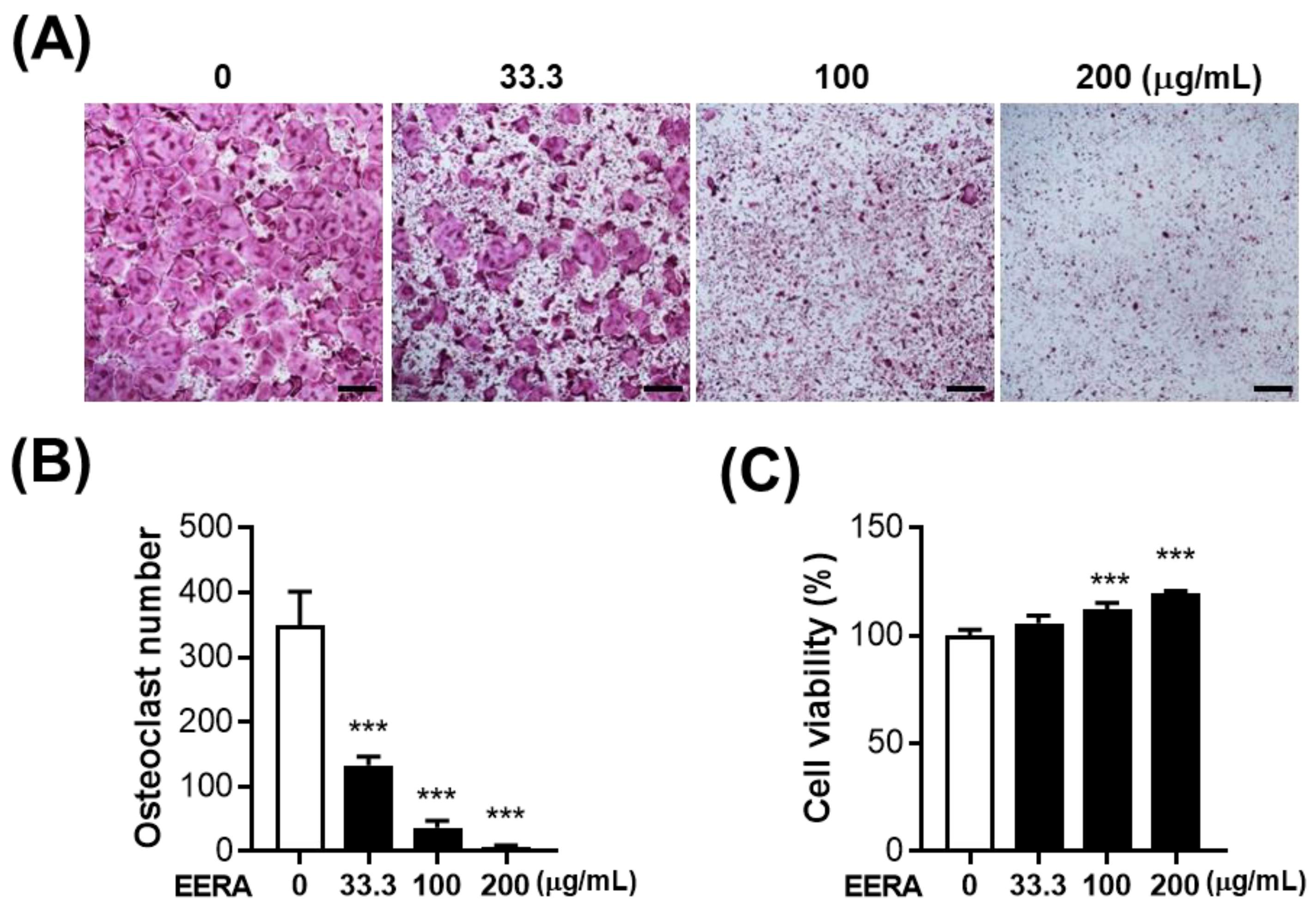
2.1. EERA Suppresses Osteoclast Differentiation in an Osteoclast Precursor–Osteocyte Coculture System
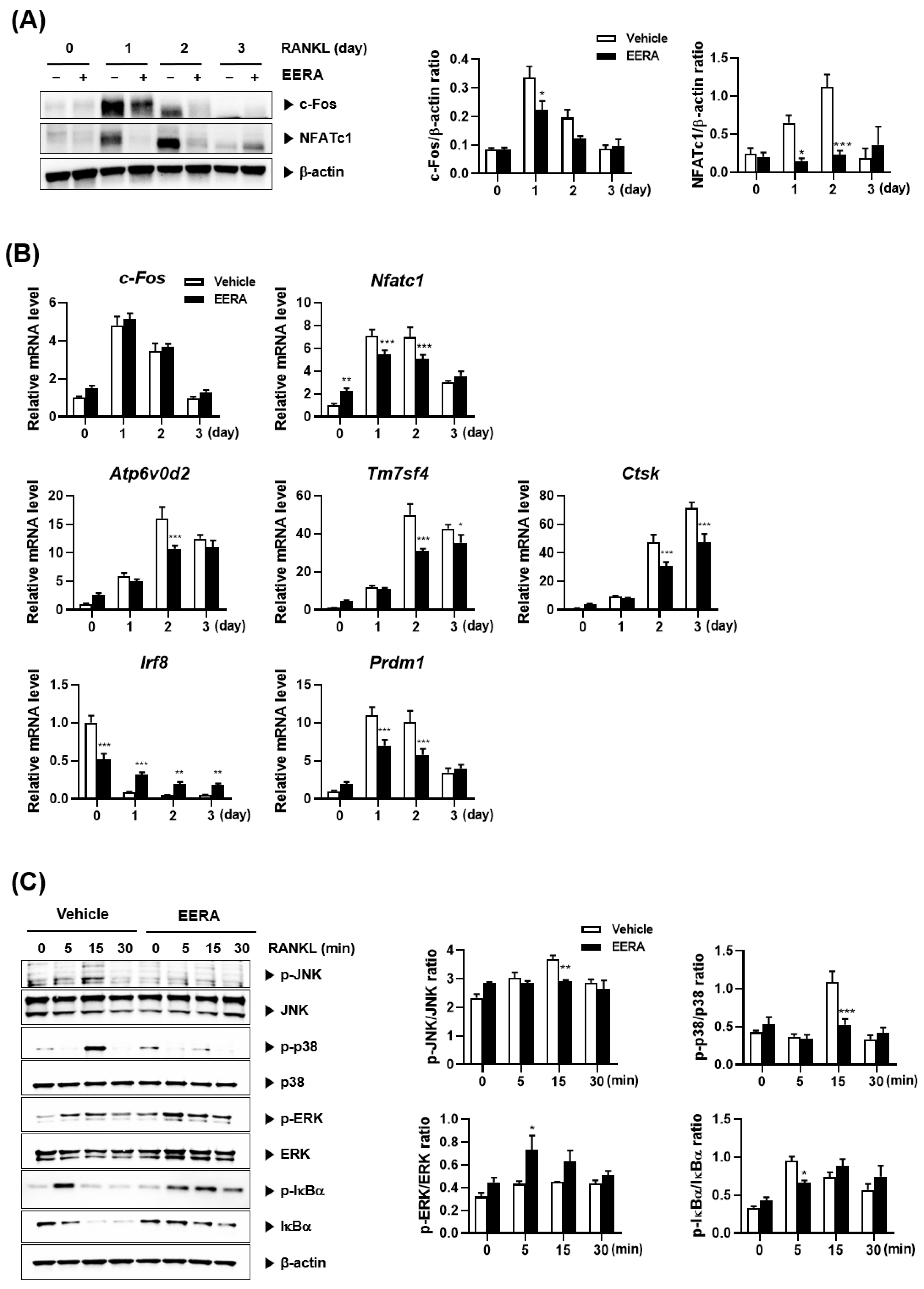
2.2. EERA Inhibits RANKL-Driven Osteoclastogenesis
2.3. EERA Modulates RANKL-Driven Signaling Pathway
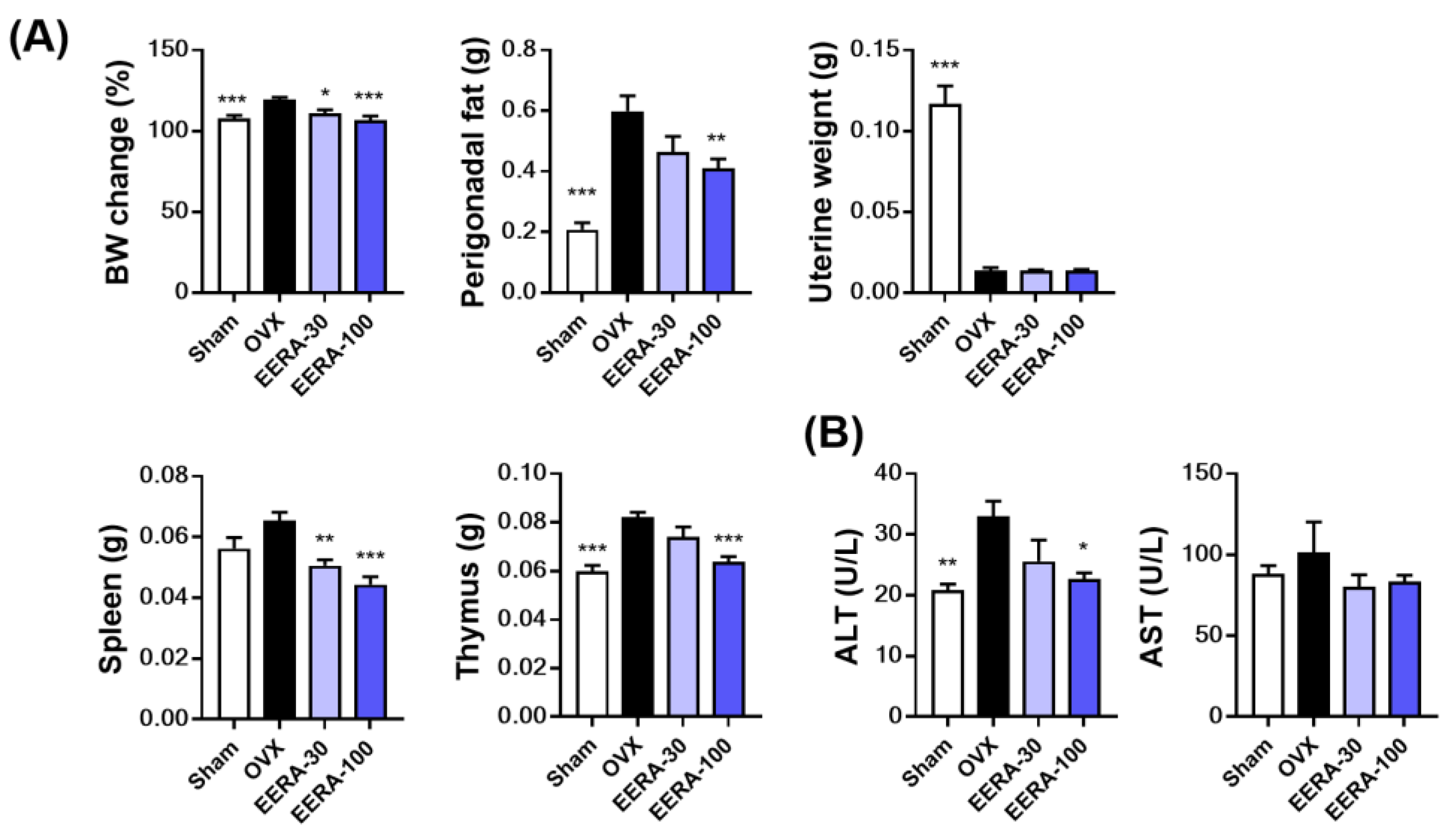
2.4. EERA Administration Attenuates Bone Loss and Fat Accumulation in OVX Mice
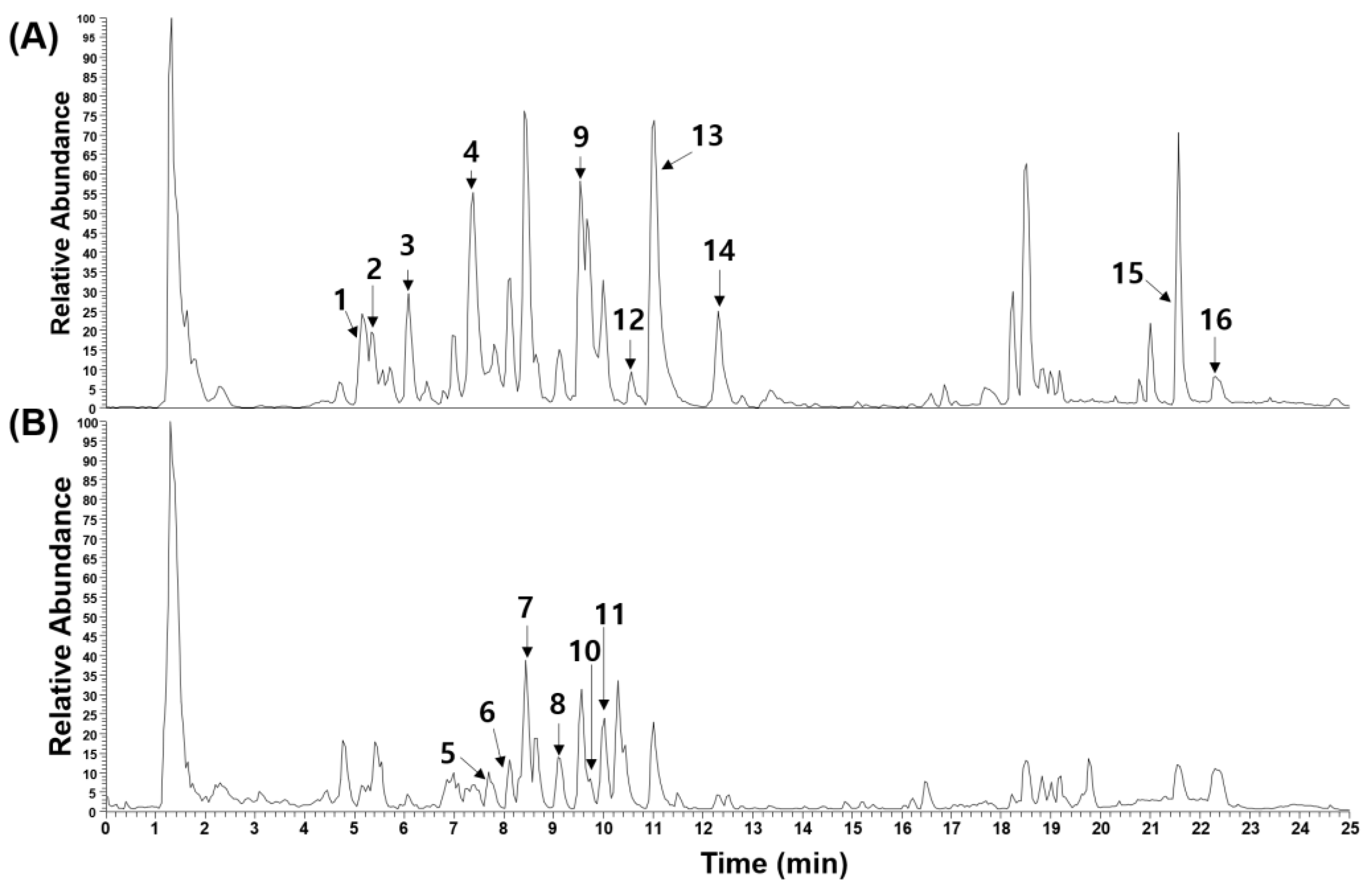
2.5. Phytochemical Characterization of EERA
| Peak No. | R.T. (Min) | Ion Mode | Error (ppm) | Formula | Expected Mass (m/z) | Measured Mass (m/z) | MS/MS Fragments (m/z) | Identification | Reference |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 5.15 | 0.142 | C16H18O9 | 353.0878 | 353.0879 | 191.0553, 179.0338, 161.0234, 135.0437 | Chlorogenic acid * | [43] | |
| 2 | 5.43 | 2.564 | C17H20O9 | 367.1012 | 367.1033 | 193.0498, 173.0445, 134.0360 | One of feruloylquinic acid | [14] | |
| 3 | 6.18 | 3.109 | C17H20O9 | 367.1012 | 367.1035 | 193.0497, 173.0444, 137.0230 | One of feruloylquinic acid | [14] | |
| 4 | 7.39 | 0.342 | C25H24O12 | 515.1195 | 515.1193 | 191.0554, 179.0340, 161.0233, 135.0440, 111.0437 | 3,4-Dicaffeoylquinic acid * | [43] | |
| 5 | 7.69 | 0.368 | C25H32ClN5O7 | 550.2063 | 550.2061 | 465.1531, 447.1421, 318.0839, 300.0741 | Astin E | [43] | |
| 6 | 8.16 | 0.544 | C25H33N5O8 | 532.2408 | 532.2399 | 235.1075, 179.0841, 131.0491, 106.0655 | Iso-asterinin A | [44] | |
| 7 | 8.53 | 0.732 | C25H33N5O8 | 532.2408 | 532.2398 | 235.1075, 131.0492, 106.0656 | Asterinin A | [44] | |
| 8 | 9.09 | 0.649 | C25H33Cl2N5O7 | 586.1830 | 586.1826 | 558.2021, 251.0350, 131.0492, 106.0656 | Astin A | [43] | |
| 9 | 9.53 | 0.135 | C15H10O7 | 301.0354 | 301.0353 | 301.0355, 178.9977, 151.0025 | Quercetin * | [43] | |
| 10 | 9.67 | 0.339 | C25H33N5O7 | 516.2453 | 516.2451 | 338.1709, 235.1075, 193.0969, 179.0815 | Astin J | [43] | |
| 11 | 9.97 | 0.871 | C25H33Cl2N5O7 | 570.1881 | 570.1876 | 485.1148, 163.0389, 131.0491, 106.0654 | Astin C | [43] | |
| 12 | 10.61 | 0.051 | C67H108O34 | 1455.6637 | 1455.6639 | 1323.6244, 781.4380, 673.2198 | Astersaponin A | [43] | |
| 13 | 11.03 | 0.450 | C15H10O6 | 285.0405 | 285.0406 | 257.0446, 151.0024 | Kaempferol * | [43] | |
| 14 | 12.31 | 3.491 | C18H34O5 | 329.2326 | 329.2334 | 229.1442, 211.1334 | Pinellic acid | [45] | |
| 15 | 21.57 | 0.033 | C25H24O12 | 279.2329 | 279.2330 | 279.2330 | Linoleic acid * | [46] | |
| 16 | 22.41 | 0.095 | C10H8O4 | 281.2486 | 281.2487 | 281.2487 | Oleic acid * | [46] |
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. EERA Preparation
4.3. BMM Culture and Cytotoxicity Assay
4.4. Osteoclast Differentiation Assay
4.5. Real-Time PCR Analysis
4.6. Western Blot Analysis
4.7. Analysis of RANKL Protein Expression
4.8. Animal Experiments
4.9. μ-CT Analysis
4.10. UHPLC-MS/MS Analysis
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feng, X.; Teitelbaum, S.L. Osteoclasts: New Insights. Bone Res. 2013, 1, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Ono, T.; Nakashima, T. Recent advances in osteoclast biology. Histochem. Cell Biol. 2018, 149, 325–341. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Nakamura, K.; Takahasi, N.; Suda, T. Role of RANKL in physiological and pathological bone resorption and therapeutics targeting the RANKL-RANK signaling system. Immunol. Rev. 2005, 208, 30–49. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, T.; Hayashi, M.; Fukunaga, T.; Kurata, K.; Oh-Hora, M.; Feng, J.Q.; Bonewald, L.F.; Kodama, T.; Wutz, A.; Wagner, E.F.; et al. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat. Med. 2011, 17, 1231–1234. [Google Scholar] [CrossRef]
- Xiong, J.; Piemontese, M.; Onal, M.; Campbell, J.; Goellner, J.J.; Dusevich, V.; Bonewald, L.; Manolagas, S.C.; O’Brien, C.A. Osteocytes, not Osteoblasts or Lining Cells, are the Main Source of the RANKL Required for Osteoclast Formation in Remodeling Bone. PLoS ONE 2015, 10, e0138189. [Google Scholar] [CrossRef]
- Gohda, J.; Akiyama, T.; Koga, T.; Takayanagi, H.; Tanaka, S.; Inoue, J. RANK-mediated amplification of TRAF6 signaling leads to NFATc1 induction during osteoclastogenesis. EMBO J. 2005, 24, 790–799. [Google Scholar] [CrossRef]
- Kobayashi, N.; Kadono, Y.; Naito, A.; Matsumoto, K.; Yamamoto, T.; Tanaka, S.; Inoue, J. Segregation of TRAF6-mediated signaling pathways clarifies its role in osteoclastogenesis. EMBO J. 2001, 20, 1271–1280. [Google Scholar] [CrossRef]
- Takayanagi, H.; Kim, S.; Koga, T.; Nishina, H.; Isshiki, M.; Yoshida, H.; Saiura, A.; Isobe, M.; Yokochi, T.; Inoue, J.; et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev. Cell 2002, 3, 889–901. [Google Scholar] [CrossRef]
- Matsuo, K.; Galson, D.L.; Zhao, C.; Peng, L.; Laplace, C.; Wang, K.Z.; Bachler, M.A.; Amano, H.; Aburatani, H.; Ishikawa, H.; et al. Nuclear factor of activated T-cells (NFAT) rescues osteoclastogenesis in precursors lacking c-Fos. J. Biol. Chem. 2004, 279, 26475–26480. [Google Scholar] [CrossRef]
- Nishikawa, K.; Nakashima, T.; Hayashi, M.; Fukunaga, T.; Kato, S.; Kodama, T.; Takahashi, S.; Calame, K.; Takayanagi, H. Blimp1-mediated repression of negative regulators is required for osteoclast differentiation. Proc. Natl. Acad. Sci. USA 2010, 107, 3117–3122. [Google Scholar] [CrossRef]
- Putnam, S.E.; Scutt, A.M.; Bicknell, K.; Priestley, C.M.; Williamson, E.M. Natural products as alternative treatments for metabolic bone disorders and for maintenance of bone health. Phytother. Res. 2007, 21, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Che, C.T.; Wong, M.S.; Lam, C.W. Natural Products from Chinese Medicines with Potential Benefits to Bone Health. Molecules 2016, 21, 239. [Google Scholar] [CrossRef] [PubMed]
- Li, K.J.; Liu, Y.Y.; Wang, D.; Yan, P.Z.; Lu, D.C.; Zhao, D.S. Radix Asteris: Traditional Usage, Phytochemistry and Pharmacology of An Important Traditional Chinese Medicine. Molecules 2022, 27, 5388. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Cheng, S.; Xiang, J.; Yu, B.; Zhang, M.; Zhang, C.; Xu, X. Expectorant, antitussive, anti-inflammatory activities and compositional analysis of Aster tataricus. J. Ethnopharmacol. 2015, 164, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wu, H.; Li, Y.; Liu, J.; Jia, Z.; Xu, W.; Xiao, H.; Wang, W. Aster tataricus attenuates asthma efficiently by simultaneously inhibiting tracheal ring contraction and inflammation. Biomed. Pharmacother. 2020, 130, 110616. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Dong, J.; Liu, J.; Xu, W.; Wei, Z.; Li, Y.; Wu, H.; Xiao, H. Network Pharmacology-Based Investigation of Protective Mechanism of Aster tataricus on Lipopolysaccharide-Induced Acute Lung Injury. Int. J. Mol. Sci. 2019, 20, 543. [Google Scholar] [CrossRef]
- Wu, H.; Chen, Y.; Huang, B.; Yu, Y.; Zhao, S.; Liu, J.; Jia, Z.; Xiao, H. Aster tataricus alleviates constipation by antagonizing the binding of acetylcholine to muscarinic receptor and inhibiting Ca2+ influx. Biomed. Pharmacother. 2021, 133, 111005. [Google Scholar] [CrossRef]
- Rho, J.; Seo, C.S.; Park, H.S.; Jeong, H.Y.; Moon, O.S.; Seo, Y.W.; Son, H.Y.; Won, Y.S.; Kwun, H.J. Asteris Radix et Rhizoma suppresses testosterone-induced benign prostatic hyperplasia in rats by regulating apoptosis and inflammation. J. Ethnopharmacol. 2020, 255, 112779. [Google Scholar] [CrossRef]
- Du, H.; Zhang, M.; Yao, K.; Hu, Z. Protective effect of Aster tataricus extract on retinal damage on the virtue of its antioxidant and anti-inflammatory effect in diabetic rat. Biomed. Pharmacother. 2017, 89, 617–622. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, Y.K.; Harris, S.; Ahuja, S.S.; Bonewald, L.F. MLO-Y4 osteocyte-like cells support osteoclast formation and activation. J. Bone Min. Res. 2002, 17, 2068–2079. [Google Scholar] [CrossRef]
- Gu, D.R.; Yang, H.; Kim, S.C.; Hwang, Y.H.; Ha, H. Water Extract of Piper longum Linn Ameliorates Ovariectomy-Induced Bone Loss by Inhibiting Osteoclast Differentiation. Nutrients 2022, 14, 3667. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Rho, J.; Jeong, D.; Sul, J.Y.; Kim, T.; Kim, N.; Kang, J.S.; Miyamoto, T.; Suda, T.; Lee, S.K.; et al. v-ATPase V0 subunit d2-deficient mice exhibit impaired osteoclast fusion and increased bone formation. Nat. Med. 2006, 12, 1403–1409. [Google Scholar] [CrossRef] [PubMed]
- Yagi, M.; Miyamoto, T.; Sawatani, Y.; Iwamoto, K.; Hosogane, N.; Fujita, N.; Morita, K.; Ninomiya, K.; Suzuki, T.; Miyamoto, K.; et al. DC-STAMP is essential for cell-cell fusion in osteoclasts and foreign body giant cells. J. Exp. Med. 2005, 202, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Lee, S.H.; Ha Kim, J.; Choi, Y.; Kim, N. NFATc1 induces osteoclast fusion via up-regulation of Atp6v0d2 and the dendritic cell-specific transmembrane protein (DC-STAMP). Mol. Endocrinol. 2008, 22, 176–185. [Google Scholar] [CrossRef]
- Lotinun, S.; Kiviranta, R.; Matsubara, T.; Alzate, J.A.; Neff, L.; Lüth, A.; Koskivirta, I.; Kleuser, B.; Vacher, J.; Vuorio, E.; et al. Osteoclast-specific cathepsin K deletion stimulates S1P-dependent bone formation. J. Clin. Investig. 2013, 123, 666–681. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Takami, M.; Yamada, A.; Wang, X.; Koga, T.; Hu, X.; Tamura, T.; Ozato, K.; Choi, Y.; Ivashkiv, L.B.; et al. Interferon regulatory factor-8 regulates bone metabolism by suppressing osteoclastogenesis. Nat. Med. 2009, 15, 1066–1071. [Google Scholar] [CrossRef]
- Choi, E.B.; Agidigbi, T.S.; Kang, I.S.; Kim, C. ERK Inhibition Increases RANKL-Induced Osteoclast Differentiation in RAW 264.7 Cells by Stimulating AMPK Activation and RANK Expression and Inhibiting Anti-Osteoclastogenic Factor Expression. Int. J. Mol. Sci. 2022, 23, 13512. [Google Scholar] [CrossRef]
- Ikeda, F.; Nishimura, R.; Matsubara, T.; Tanaka, S.; Inoue, J.; Reddy, S.V.; Hata, K.; Yamashita, K.; Hiraga, T.; Watanabe, T.; et al. Critical roles of c-Jun signaling in regulation of NFAT family and RANKL-regulated osteoclast differentiation. J. Clin. Investig. 2004, 114, 475–484. [Google Scholar] [CrossRef]
- Huang, H.; Chang, E.J.; Ryu, J.; Lee, Z.H.; Lee, Y.; Kim, H.H. Induction of c-Fos and NFATc1 during RANKL-stimulated osteoclast differentiation is mediated by the p38 signaling pathway. Biochem. Biophys. Res. Commun. 2006, 351, 99–105. [Google Scholar] [CrossRef]
- Yamashita, T.; Yao, Z.; Li, F.; Zhang, Q.; Badell, I.R.; Schwarz, E.M.; Takeshita, S.; Wagner, E.F.; Noda, M.; Matsuo, K.; et al. NF-kappaB p50 and p52 regulate receptor activator of NF-kappaB ligand (RANKL) and tumor necrosis factor-induced osteoclast precursor differentiation by activating c-Fos and NFATc1. J. Biol. Chem. 2007, 282, 18245–18253. [Google Scholar] [CrossRef]
- Rogers, N.H.; Perfield, J.W., 2nd; Strissel, K.J.; Obin, M.S.; Greenberg, A.S. Reduced energy expenditure and increased inflammation are early events in the development of ovariectomy-induced obesity. Endocrinology 2009, 150, 2161–2168. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, A.M.; Srivastava, K.; Sharan, K.; Yadav, D.; Maurya, R.; Singh, D. Daidzein prevents the increase in CD4+CD28null T cells and B lymphopoesis in ovariectomized mice: A key mechanism for anti-osteoclastogenic effect. PLoS ONE 2011, 6, e21216. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.P.; Lin, S.J.; Wan, W.B.; Zuo, H.L.; Yao, F.F.; Ruan, H.B.; Xu, J.; Song, W.; Zhou, Y.C.; Wen, S.Y.; et al. Chlorogenic Acid Prevents Osteoporosis by Shp2/PI3K/Akt Pathway in Ovariectomized Rats. PLoS ONE 2016, 11, e0166751. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Lee, J.Y.; Kwon, Y.I.; Jang, H.D. Anti-Osteoclastic Activity of Artemisia capillaris Thunb. Extract Depends upon Attenuation of Osteoclast Differentiation and Bone Resorption-Associated Acidification Due to Chlorogenic Acid, Hyperoside, and Scoparone. Int. J. Mol. Sci. 2017, 18, 322. [Google Scholar] [CrossRef]
- Peng, S.G.; Pang, Y.L.; Zhu, Q.; Kang, J.H.; Liu, M.X.; Wang, Z. Chlorogenic Acid Functions as a Novel Agonist of PPARγ2 during the Differentiation of Mouse 3T3-L1 Preadipocytes. Biomed. Res. Int. 2018, 2018, 8594767. [Google Scholar] [CrossRef]
- Cho, A.-S.; Jeon, S.-M.; Kim, M.-J.; Yeo, J.; Seo, K.-I.; Choi, M.-S.; Lee, M.-K. Chlorogenic acid exhibits anti-obesity property and improves lipid metabolism in high-fat diet-induced-obese mice. Food Chem. Toxicol. 2010, 48, 937–943. [Google Scholar] [CrossRef]
- Yang, Y.J.; Lu, L.J.; Wang, J.J.; Ma, S.Y.; Xu, B.L.; Lin, R.; Chen, Q.S.; Ma, Z.G.; Mo, Y.L.; Wang, D.T. Tubson-2 decoction ameliorates rheumatoid arthritis complicated with osteoporosis in CIA rats involving isochlorogenic acid A regulating IL-17/MAPK pathway. Phytomedicine 2023, 116, 154875. [Google Scholar] [CrossRef]
- Sung, Y.Y.; Kim, D.S.; Kim, H.K. Akebia quinata extract exerts anti-obesity and hypolipidemic effects in high-fat diet-fed mice and 3T3-L1 adipocytes. J. Ethnopharmacol. 2015, 168, 17–24. [Google Scholar] [CrossRef]
- Yuan, Z.; Min, J.; Zhao, Y.; Cheng, Q.; Wang, K.; Lin, S.; Luo, J.; Liu, H. Quercetin rescued TNF-alpha-induced impairments in bone marrow-derived mesenchymal stem cell osteogenesis and improved osteoporosis in rats. Am. J. Transl. Res. 2018, 10, 4313–4321. [Google Scholar]
- Zhou, Y.; Wu, Y.; Ma, W.; Jiang, X.; Takemra, A.; Uemura, M.; Xia, L.; Lin, K.; Xu, Y. The effect of quercetin delivery system on osteogenesis and angiogenesis under osteoporotic conditions. J. Mater. Chem. B 2017, 5, 612–625. [Google Scholar] [CrossRef]
- Nowak, B.; Matuszewska, A.; Nikodem, A.; Filipiak, J.; Landwójtowicz, M.; Sadanowicz, E.; Jędrzejuk, D.; Rzeszutko, M.; Zduniak, K.; Piasecki, T.; et al. Oral administration of kaempferol inhibits bone loss in rat model of ovariectomy-induced osteopenia. Pharmacol. Rep. 2017, 69, 1113–1119. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wu, J.; Xu, B.; Yuan, Z.; Leng, Y.; Min, J.; Lan, X.; Luo, J. Kaempferol promotes bone formation in part via the mTOR signaling pathway. Mol. Med. Rep. 2019, 20, 5197–5207. [Google Scholar] [CrossRef] [PubMed]
- Tori, M.; Murata, J.; Nakashima, K.; Sono, M. The structure of linoleic acid ester of trans-lachnophyllol isolated from Aster tataricus. Spectroscopy 2001, 15, 719878. [Google Scholar] [CrossRef]
- Zhao, D.X.; Hu, B.Q.; Zhang, M.; Zhang, C.F.; Xu, X.H. Simultaneous separation and determination of phenolic acids, pentapeptides, and triterpenoid saponins in the root of Aster tataricus by high-performance liquid chromatography coupled with electrospray ionization quadrupole time-of-flight mass spectrometry. J. Sep. Sci. 2015, 38, 571–575. [Google Scholar] [CrossRef]
- Sun, Y.; Li, L.; Liao, M.; Su, M.; Wan, C.; Zhang, L.; Zhang, H. A systematic data acquisition and mining strategy for chemical profiling of Aster tataricus rhizoma (Ziwan) by UHPLC-Q-TOF-MS and the corresponding anti-depressive activity screening. J. Pharm. Biomed. Anal. 2018, 154, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Bartella, L.; Mazzotti, F.; Talarico, I.R.; Santoro, I.; Di Donna, L. Paper Spray Tandem Mass Spectrometry for Assessing Oleic, Linoleic and Linolenic Acid Content in Edible Vegetable Oils. Separations 2023, 10, 26. [Google Scholar] [CrossRef]
- Xiong, J.; Onal, M.; Jilka, R.L.; Weinstein, R.S.; Manolagas, S.C.; O’Brien, C.A. Matrix-embedded cells control osteoclast formation. Nat. Med. 2011, 17, 1235–1241. [Google Scholar] [CrossRef]
- Kato, Y.; Windle, J.J.; Koop, B.A.; Mundy, G.R.; Bonewald, L.F. Establishment of an osteocyte-like cell line, MLO-Y4. J. Bone Min. Res. 1997, 12, 2014–2023. [Google Scholar] [CrossRef]
- Takayanagi, H.; Kim, S.; Matsuo, K.; Suzuki, H.; Suzuki, T.; Sato, K.; Yokochi, T.; Oda, H.; Nakamura, K.; Ida, N.; et al. RANKL maintains bone homeostasis through c-Fos-dependent induction of interferon-beta. Nature 2002, 416, 744–749. [Google Scholar] [CrossRef]
- Polyzos, S.A.; Anastasilakis, A.D.; Efstathiadou, Z.A.; Yavropoulou, M.P.; Makras, P. Postmenopausal osteoporosis coexisting with other metabolic diseases: Treatment considerations. Maturitas 2021, 147, 19–25. [Google Scholar] [CrossRef]
- Black, D.M.; Rosen, C.J. Clinical Practice. Postmenopausal Osteoporosis. N. Engl. J. Med. 2016, 374, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Komori, T. Animal models for osteoporosis. Eur. J. Pharmacol. 2015, 759, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Pacifici, R. T cells: Critical bone regulators in health and disease. Bone 2010, 47, 461–471. [Google Scholar] [CrossRef]
- Peng, W.J.; Xin, R.H.; Luo, Y.J.; Liang, G.; Ren, L.H.; Liu, Y.; Wang, G.B.; Zheng, J.F. Evaluation of the acute and subchronic toxicity of Aster tataricus L. F. Afr. J. Tradit. Complement. Altern. Med. 2016, 13, 38–53. [Google Scholar] [CrossRef]
- Wang, L.; Li, M.-D.; Cao, P.-P.; Zhang, C.-F.; Huang, F.; Xu, X.-H.; Liu, B.-L.; Zhang, M. Astin B, a cyclic pentapeptide from Aster tataricus, induces apoptosis and autophagy in human hepatic L-02 cells. Chem. Biol. Interact. 2014, 223, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Cao, P.; Zhang, C.; Xu, X.; Zhang, M. Screening and analyzing potential hepatotoxic compounds in the ethanol extract of Asteris Radix by HPLC/DAD/ESI-MSn technique. J. Pharm. Biomed. Anal. 2012, 67–68, 51–62. [Google Scholar] [CrossRef]
- Jang, S.A.; Hwang, Y.H.; Yang, H.; Ryuk, J.A.; Kim, T.; Ha, H. Water Extract of Mentha arvensis L. Attenuates Estrogen Deficiency-Induced Bone Loss by Inhibiting Osteoclast Differentiation. Front. Pharmacol. 2021, 12, 719602. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.-J.; Yang, H.; Kim, S.C.; Gu, D.R.; Ryuk, J.A.; Jang, S.-A.; Ha, H. Ethanol Extract of Radix Asteris Suppresses Osteoclast Differentiation and Alleviates Osteoporosis. Int. J. Mol. Sci. 2023, 24, 16526. https://doi.org/10.3390/ijms242216526
Lee S-J, Yang H, Kim SC, Gu DR, Ryuk JA, Jang S-A, Ha H. Ethanol Extract of Radix Asteris Suppresses Osteoclast Differentiation and Alleviates Osteoporosis. International Journal of Molecular Sciences. 2023; 24(22):16526. https://doi.org/10.3390/ijms242216526
Chicago/Turabian StyleLee, Sung-Ju, Hyun Yang, Seong Cheol Kim, Dong Ryun Gu, Jin Ah Ryuk, Seon-A Jang, and Hyunil Ha. 2023. "Ethanol Extract of Radix Asteris Suppresses Osteoclast Differentiation and Alleviates Osteoporosis" International Journal of Molecular Sciences 24, no. 22: 16526. https://doi.org/10.3390/ijms242216526
APA StyleLee, S.-J., Yang, H., Kim, S. C., Gu, D. R., Ryuk, J. A., Jang, S.-A., & Ha, H. (2023). Ethanol Extract of Radix Asteris Suppresses Osteoclast Differentiation and Alleviates Osteoporosis. International Journal of Molecular Sciences, 24(22), 16526. https://doi.org/10.3390/ijms242216526





