Ageing-Related Alterations in Renal Epithelial Glucose Transport
Abstract
:1. Introduction
2. Results
2.1. Laboratory Data
2.2. Blood Glucose Levels in Pyruvate Tolerance Test
2.3. Histology Study of Renal Tissue (H & E Staining, N = 4 in Each Group, Figure 2)

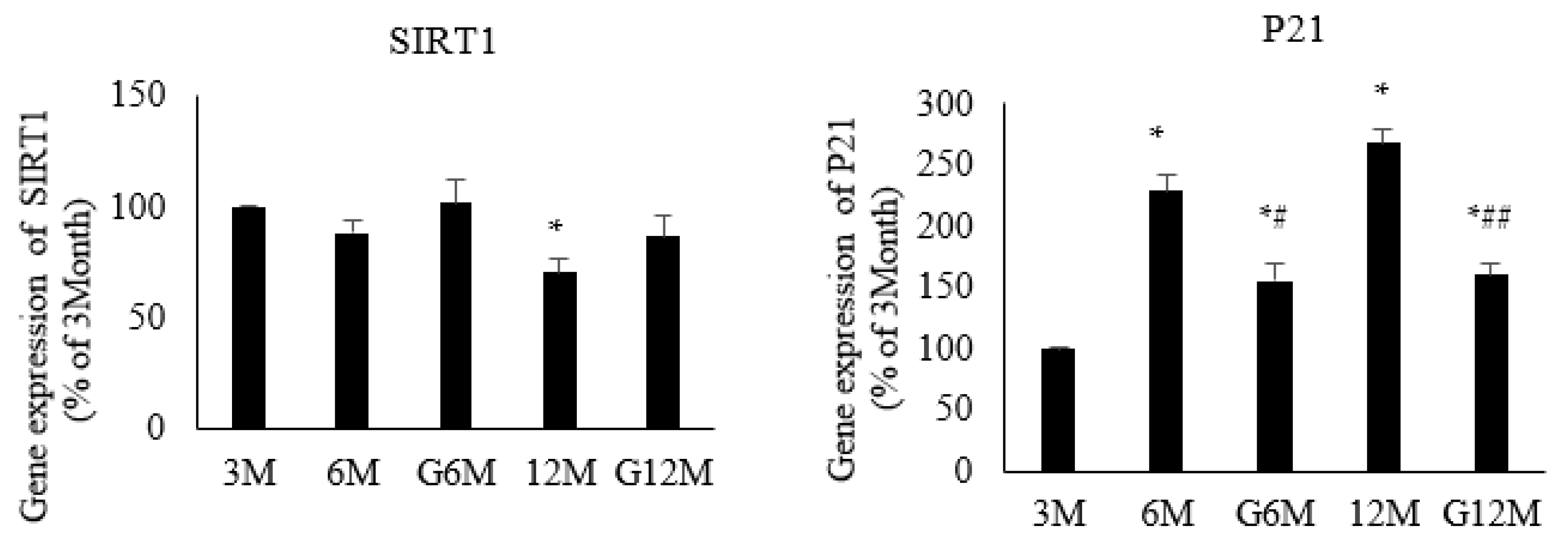
2.4. Gene Expression Analysis (N = 6 in Each Group, Figure 3)
2.5. Immunoblotting (N = 6 in Each Group, Figure 4)

3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Methods
4.3. Kidney Tissue
4.4. Pyruvate Tolerance Test
4.5. Gene Expression Analysis
4.5.1. RNA Isolation and cDNA Synthesis
4.5.2. Real-Time Polymerase Chain Reaction (PCR)
4.6. Hematoxylin and Eosin Staining
4.7. Protein Analysis (Immunoblotting)
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, J.; Huang, X.; Dou, L.; Yan, M.; Shen, T.; Tang, W.; Li, J. Aging and aging-related diseases: From molecular mechanisms to interventions and treatments. Signal Transduct. Target. Ther. 2022, 7, 391. [Google Scholar] [CrossRef]
- Franceschi, C.; Garagnani, P.; Morsiani, C.; Conte, M.; Santoro, A.; Grignolio, A.; Monti, D.; Capri, M.; Salvioli, S. The continuum of aging and age-related diseases: Common mechanisms but different rates. Front. Med. 2018, 5, 61. [Google Scholar] [CrossRef] [PubMed]
- Kubben, N.; Misteli, T. Shared molecular and cellular mechanisms of premature ageing and ageing-associated diseases. Nat. Rev. Mol. Cell Biol. 2017, 18, 595–609. [Google Scholar] [CrossRef]
- Wilding, J.P. The role of the kidneys in glucose homeostasis in type 2 diabetes: Clinical implications and therapeutic significance through sodium glucose co-transporter 2 inhibitors. Metabolism 2014, 63, 1228–1237. [Google Scholar] [CrossRef]
- Longo, M.; Bellastella, G.; Maiorino, M.I.; Meier, J.J.; Esposito, K.; Giugliano, D. Diabetes and ageing: From treatment goals to pharmacological therapy. Front. Endocrinol. 2019, 10, 45. [Google Scholar] [CrossRef]
- Brewer, R.A.; Gibbs, V.K.; Smith Jr, D.L. Targeting glucose metabolism for healthy aging. Nutr. Healthy Aging 2016, 4, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Chia, C.W.; Egan, J.M.; Ferrucci, L. Age-related changes in glucose metabolism, hyperglycemia, and cardiovascular risk. Circ. Res. 2018, 123, 886–904. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.G.; Halter, J.B. The pathophysiology of hyperglycemia in older adults: Clinical considerations. Diabetes Care. 2017, 40, 444–452. [Google Scholar] [CrossRef]
- Mather, A.; Pollock, C. Glucose handling by the kidney. Kidney Int. 2011, 79, S1–S6. [Google Scholar] [CrossRef]
- Triplitt, C.L. Understanding the kidneys’ role in blood glucose regulation. Am. J. Manag. Care 2012, 18, S11. [Google Scholar]
- Szablewski, L. Distribution of glucose transporters in renal diseases. J. Biomed. Sci. 2017, 24, 64. [Google Scholar] [CrossRef] [PubMed]
- Santer, R.; Calado, J. Familial renal glucosuria and SGLT2: From a mendelian trait to a therapeutic target. Clin. J. Am. Soc. Nephrol. 2010, 5, 133–141. [Google Scholar] [CrossRef] [PubMed]
- van Bommel, E.J.; Muskiet, M.H.; Tonneijck, L.; Kramer, M.H.; Nieuwdorp, M.; van Raalte, D.H. SGLT2 inhibition in the diabetic kidney—From mechanisms to clinical outcome. Clin. J. Am. Soc. Nephrol. 2017, 12, 700–710. [Google Scholar] [CrossRef]
- Yang, G.; An, S.S.; Ji, Y.; Zhang, W.; Pei, Y. Hydrogen sulfide signaling in oxidative stress and aging development. Oxidative Med. Cell. Longev. 2015, 2015, 357824. [Google Scholar] [CrossRef]
- Tabassum, R.; Jeong, N.Y.; Jung, J. Therapeutic importance of hydrogen sulfide in age-associated neurodegenerative diseases. Neural Regen. Res. 2020, 15, 653. [Google Scholar] [PubMed]
- Perridon, B.W.; Leuvenink, H.G.; Hillebrands, J.L.; van Goor, H.; Bos, E.M. The role of hydrogen sulfide in aging and age-related pathologies. Aging 2016, 8, 2264–2289. [Google Scholar] [CrossRef]
- Feng, J.; Lu, X.; Li, H.; Wang, S. The roles of hydrogen sulfide in renal physiology and disease states. Ren. Fail. 2022, 44, 1289–1308. [Google Scholar] [CrossRef]
- Kalra, S.; Sharma, S.K. Diabetes in the Elderly. Diabetes. Ther. 2018, 9, 493–500. [Google Scholar] [CrossRef]
- Szabo, C. Roles of hydrogen sulfide in the pathogenesis of diabetes mellitus and its complications. Antioxid. Redox Signal. 2012, 17, 68–80. [Google Scholar] [CrossRef]
- Pichette, J.; Gagnon, J. Implications of hydrogen sulfide in glucose regulation: How H2S can alter glucose homeostasis through metabolic hormones. Oxid. Med. Cell Longev. 2016, 2016, 3285074. [Google Scholar] [CrossRef]
- Sun, H.J.; Xiong, S.P.; Wang, Z.C.; Nie, X.W.; Bian, J.S. Hydrogen sulfide in diabetic complications revisited: The state of the art, challenges, and future directions. Antioxid. Redox Signal. 2023, 38, 18–44. [Google Scholar] [CrossRef] [PubMed]
- Hua, W.; Zhou, S.-L.; Gong, F.-Q. Biphasic regulation of hydrogen sulfide in inflammation. Chin. Med. J. 2013, 126, 1360–1363. [Google Scholar]
- Pan, Z.; Wang, J.; Xu, M.; Chen, S.; Li, X.; Sun, A.; Lou, N.; Ni, Y. Hydrogen sulfide protects against high glucose-induced lipid metabolic disturbances in 3T3-L1 adipocytes via the AMPK signaling pathway. Mol. Med. Rep. 2019, 20, 4119–4124. [Google Scholar] [CrossRef]
- Piragine, E.; Calderone, V. Pharmacological modulation of the hydrogen sulfide (H2S) system by dietary H2S-donors: A novel promising strategy in the prevention and treatment of type 2 diabetes mellitus. Phytother. Res. 2021, 35, 1817–1846. [Google Scholar] [CrossRef]
- Pichette, J.; Fynn-Sackey, N.; Gagnon, J. Hydrogen sulfide and sulfate prebiotic stimulates the secretion of GLP-1 and improves glycemia in male mice. Endocrinology 2017, 1, 3416–3425. [Google Scholar] [CrossRef]
- Bahadoran, Z.; Jeddi, S.; Mirmiran, P.; Kashfi, K.; Azizi, F.; Ghasemi, A. Association between serum hydrogen sulfide concentrations and dysglycemia: A population-based study. BMC Endocr. Disord. 2022, 22, 79. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Li, X.; Li, X.; Wei, X.; Wang, H. Hydrogen sulfide plays an important role in diabetic cardiomyopathy. Front. Cell Dev. Biol. 2021, 9, 627336. [Google Scholar] [CrossRef] [PubMed]
- Ng, H.Y.; Leung, F.F.; Kuo, W.H.; Lee, W.C.; Lee, C.T. Dapagliflozin and xanthine oxidase inhibitors improve insulin resistance and modulate renal glucose and urate transport in metabolic syndrome. Clin. Exp. Pharmacol. Physiol. 2021, 48, 1603–1612. [Google Scholar] [CrossRef]
- van der Aart-van der Beek, A.B.; de Boer, R.A.; Heerspink, H.J. Kidney and heart failure outcomes associated with SGLT2 inhibitor use. Nat. Rev. Nephrol. 2022, 18, 294–306. [Google Scholar] [CrossRef]
- Gerich, J.E. Role of the kidney in normal glucose homeostasis and in the hyperglycaemia of diabetes mellitus: Therapeutic implications. Diabetic Med. 2010, 27, 136–142. [Google Scholar] [CrossRef]
- Vallon, V. Molecular determinants of renal glucose reabsorption. Focus on “Glucose transport by human renal Na+/D-glucose cotransporters SGLT1 and SGLT2”. Am. J. Physiol. Cell Physiol. 2011, 300, C6–C8. [Google Scholar] [CrossRef]
- Vallon, V.; Thomson, S.C. Targeting renal glucose reabsorption to treat hyperglycemia: The pleiotropic effects of SGLT2 inhibition. Diabetologia 2017, 60, 215–225. [Google Scholar] [CrossRef]
- Fang, Y.; Gong, A.Y.; Haller, S.T.; Dworkin, L.D.; Liu, Z.; Gong, R. The ageing kidney: Molecular mechanisms and clinical implications. Ageing Res. Rev. 2020, 63, 101151. [Google Scholar] [CrossRef]
- O’Sullivan, E.D.; Hughes, J.; Ferenbach, D.A. Renal aging: Causes and consequences. J. Am. Soc. Nephrol. 2017, 28, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Papacocea, R.I.; Timofte, D.; Tanasescu, M.-D.; Balcangiu-Stroescu, A.-E.; Balan, D.G.; Tulin, A.; Stiru, O.; Vacaroiu, I.A.; Mihai, A.; Popa, C.C. Kidney aging process and the management of the elderly patient with renal impairment. Exp. Ther. Med. 2021, 21, 266. [Google Scholar] [CrossRef]
- Melk, A.; Schmidt, B.M.; Takeuchi, O.; Sawitzki, B.; Rayner, D.C.; Halloran, P.F. Expression of p16INK4a and other cell cycle regulator and senescence associated genes in aging human kidney. Kidney Int. 2004, 65, 510–520. [Google Scholar] [CrossRef] [PubMed]
- Sturmlechner, I.; Durik, M.; Sieben, C.J.; Baker, D.J.; Van Deursen, J.M. Cellular senescence in renal ageing and disease. Nat. Rev. Nephrol. 2017, 13, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Kuro-o, M. The klotho proteins in health and disease. Nat. Rev. Nephrol. 2019, 15, 27–44. [Google Scholar] [CrossRef]
- Sopjani, M.; Rinnerthaler, M.; Kruja, J.; Dermaku-Sopjani, M. Intracellular signaling of the aging suppressor protein Klotho. Curr. Mol. Med. 2015, 15, 27–37. [Google Scholar] [CrossRef]
- Buchanan, S.; Combet, E.; Stenvinkel, P.; Shiels, P.G. Klotho, aging, and the failing kidney. Front. Endocrinol. 2020, 27, 560. [Google Scholar] [CrossRef]
- Yang, H.; Fogo, A.B. Cell senescence in the aging kidney. J. Am. Soc. Nephrol. 2010, 21, 1436–1439. [Google Scholar] [CrossRef] [PubMed]
- Ngowi, E.E.; Sarfraz, M.; Afzal, A.; Khan, N.H.; Khattak, S.; Zhang, X.; Li, T.; Duan, S.F.; Ji, X.Y.; Wu, D.D. Roles of hydrogen sulfide donors in common kidney diseases. Front. Pharmacol. 2020, 11, 564281. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhao, H.; Guo, N. Protective effect of hydrogen sulfide on the kidney. Mol. Med. Rep. 2021, 24, 696. [Google Scholar] [CrossRef] [PubMed]
- Peleli, M.; Zampas, P.; Papapetropoulos, A. Hydrogen sulfide and the kidney: Physiological roles, contribution to pathophysiology, and therapeutic potential. Antioxid. Redox Signal. 2022, 36, 220–243. [Google Scholar] [CrossRef]
- Hou, C.L.; Wang, M.J.; Sun, C.; Huang, Y.; Jin, S.; Mu, X.P.; Chen, Y.; Zhu, Y.C. Protective effects of hydrogen sulfide in the ageing kidney. Oxid. Med. Cell Longev. 2016, 2016, 7570489. [Google Scholar] [CrossRef]
- Lee, H.J.; Feliers, D.; Barnes, J.L.; Oh, S.; Choudhury, G.G.; Diaz, V.; Galvan, V.; Strong, R.; Nelson, J.; Salmon, A. Hydrogen sulfide ameliorates aging-associated changes in the kidney. Geroscience 2018, 40, 163–176. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Z.; Ren, Y.; Wang, Y.; Fang, J.; Yue, H.; Ma, S.; Guan, F. Aging and age-related diseases: From mechanisms to therapeutic strategies. Biogerontology 2021, 22, 165–187. [Google Scholar] [CrossRef]
- Zhang, L.; Pitcher, L.E.; Prahalad, V.; Niedernhofer, L.J.; Robbins, P.D. Targeting cellular senescence with senotherapeutics: Senolytics and senomorphics. FEBS J. 2023, 290, 1362–1383. [Google Scholar] [CrossRef]
- Imaizumi, V.M.; Laurindo, L.F.; Manzan, B.; Guiguer, E.L.; Oshiiwa, M.; Otoboni, A.M.M.B.; Araujo, A.C.; Tofano, R.J.; Barbalho, S.M. Garlic: A systematic review of the effects on cardiovascular diseases. Crit. Rev. Food Sci. Nutr. 2022, 23, 1–23. [Google Scholar] [CrossRef]
- Lin, S.; Visram, F.; Liu, W.; Haig, A.; Jiang, J.; Mok, A.; Lian, D.; Wood, M.E.; Torregrossa, R.; Whiteman, M.; et al. GYY4137, a slow releasing hydrogen sulfide donor, ameliorates renal damage associated with chronic obstructive uropathy. J. Urol. 2016, 196, 1778–1787. [Google Scholar] [CrossRef]
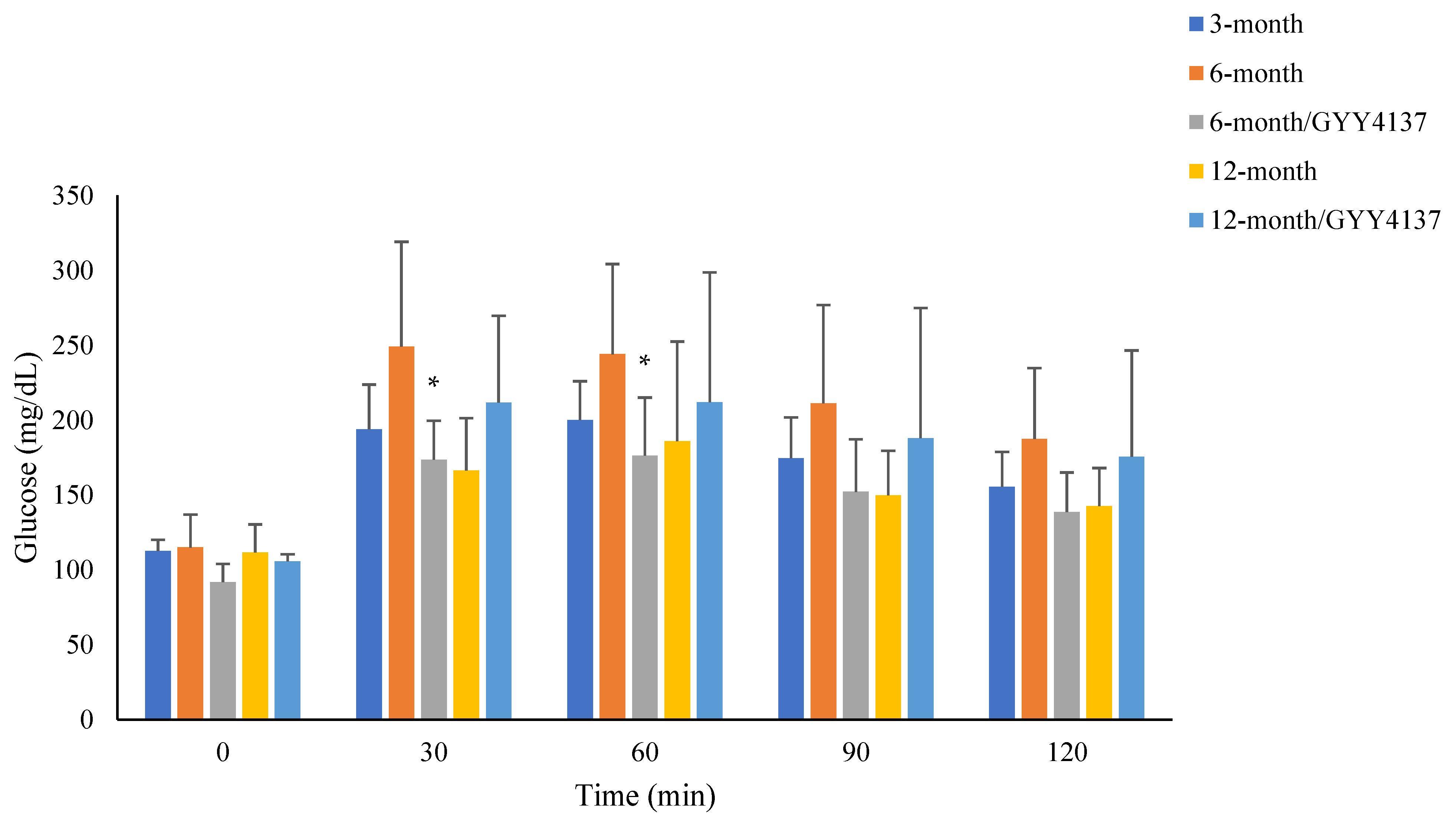
| 3-Month Group (N = 10) | 6-Month Group (N = 8) | 6-Month/ GYY4137 Group (N = 8) | 12-Month Group (N = 8) | 12-Month/ GYY4137 Group (N = 8) | |
|---|---|---|---|---|---|
| Body weight (g) | 592.3 ± 49.8 | 632.5 ± 65.5 | 595.5 ± 39.3 | 683.4 ± 70.4 * | 693.8 ± 60.2 * |
| 24 h urine, (mL) | 20.3 ± 3.1 | 17.4 ± 3.9 | 25.3 ± 8.7 | 20.7 ± 7.0 | 21.0 ± 4.4 |
| Blood glucose, (mg/dL) | 104.0 ± 9.7 | 111.8 ± 5.3 * | 108.3 ± 11.9 # | 118.7 ± 3.1 * | 103.0 ± 10.7 ## |
| Blood insulin, (μU/mL) | 2.5 ± 1.0 | 3.2 ± 1.9 | 3.3 ± 1.5 | 4.4 ± 1.1 * | 3.5 ± 1.0 * |
| HOMA-IR | 0.7 ± 0.2 | 0.6 ± 0.0 * | 0.7 ± 0.2 * | 1.2 ± 0.3 * | 0.9 ± 0.2 *## |
| Blood creatinine, (mg/dL) | 0.40 ± 0.04 | 0.47 ± 0.05 * | 0.44 ± 0.04 # | 0.49 ± 0.06 * | 0.45 ± 0.03 ## |
| Blood uric acid, (mg/dL) | 1.0 ± 0.5 | 0.9 ± 0.8 | 0.8 ± 0.6 | 1.0 ± 0.4 | 0.7 ± 0.4 |
| Creatinine clearance, (mg/min) | 3.6 ± 0.6 | 3.2 ± 0.3 | 3.4 ± 0.5 | 3.4 ± 0.6 | 3.7 ± 0.3 |
| Urine glucose excretion, (mg/24 h) | 53.2 ± 23.8 | 54.1 ± 29.4 | 50.8 ± 23.5 | 58.5 ± 23.5 | 52.3 ± 16.8 |
| Urine uric acid excretion, (mg/24 h) | 16.2 ± 3.8 | 15.2 ± 3.9 | 18.0 ± 2.3 | 23.9 ± 6.7 * | 24.8 ± 3.7 * |
| Urine protein excretion, (mg/24 h) | 58.7 ± 25.6 | 71.2 ± 16.0 * | 52.0 ± 11.9 | 87.1 ± 24.4 * | 52.5 ± 18.2 ## |
| Age | 3-M | 6-M | G6M | 12-M | G12M |
| Grade | 0 | 0 | 0 | 1 | 0-1 |
| Gene | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|
| β-actin | AGTACCCCATTGAACACGGC | TTTTCACGGTTGGCCTTAGG |
| P21 (Cdkn1a) | CACACAGGAGCAAAGTATG | TCAAAGTTCCACCGTTCT |
| Sirtuin 1 (SIRT1) | CCTCTGCCTCATCTACATT | TACTCGCCACCTAACCTA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, C.-T.; Ng, H.-Y.; Zhong, H.-R.; Wang, Y.; Liu, C.-H.; Lee, Y.-T. Ageing-Related Alterations in Renal Epithelial Glucose Transport. Int. J. Mol. Sci. 2023, 24, 16455. https://doi.org/10.3390/ijms242216455
Lee C-T, Ng H-Y, Zhong H-R, Wang Y, Liu C-H, Lee Y-T. Ageing-Related Alterations in Renal Epithelial Glucose Transport. International Journal of Molecular Sciences. 2023; 24(22):16455. https://doi.org/10.3390/ijms242216455
Chicago/Turabian StyleLee, Chien-Te, Hwee-Yeong Ng, Hua-Rong Zhong, Yi Wang, Chih-Han Liu, and Yuai-Ting Lee. 2023. "Ageing-Related Alterations in Renal Epithelial Glucose Transport" International Journal of Molecular Sciences 24, no. 22: 16455. https://doi.org/10.3390/ijms242216455
APA StyleLee, C.-T., Ng, H.-Y., Zhong, H.-R., Wang, Y., Liu, C.-H., & Lee, Y.-T. (2023). Ageing-Related Alterations in Renal Epithelial Glucose Transport. International Journal of Molecular Sciences, 24(22), 16455. https://doi.org/10.3390/ijms242216455






