IbINV Positively Regulates Resistance to Black Rot Disease Caused by Ceratocystis fimbriata in Sweet Potato
Abstract
1. Introduction
2. Results
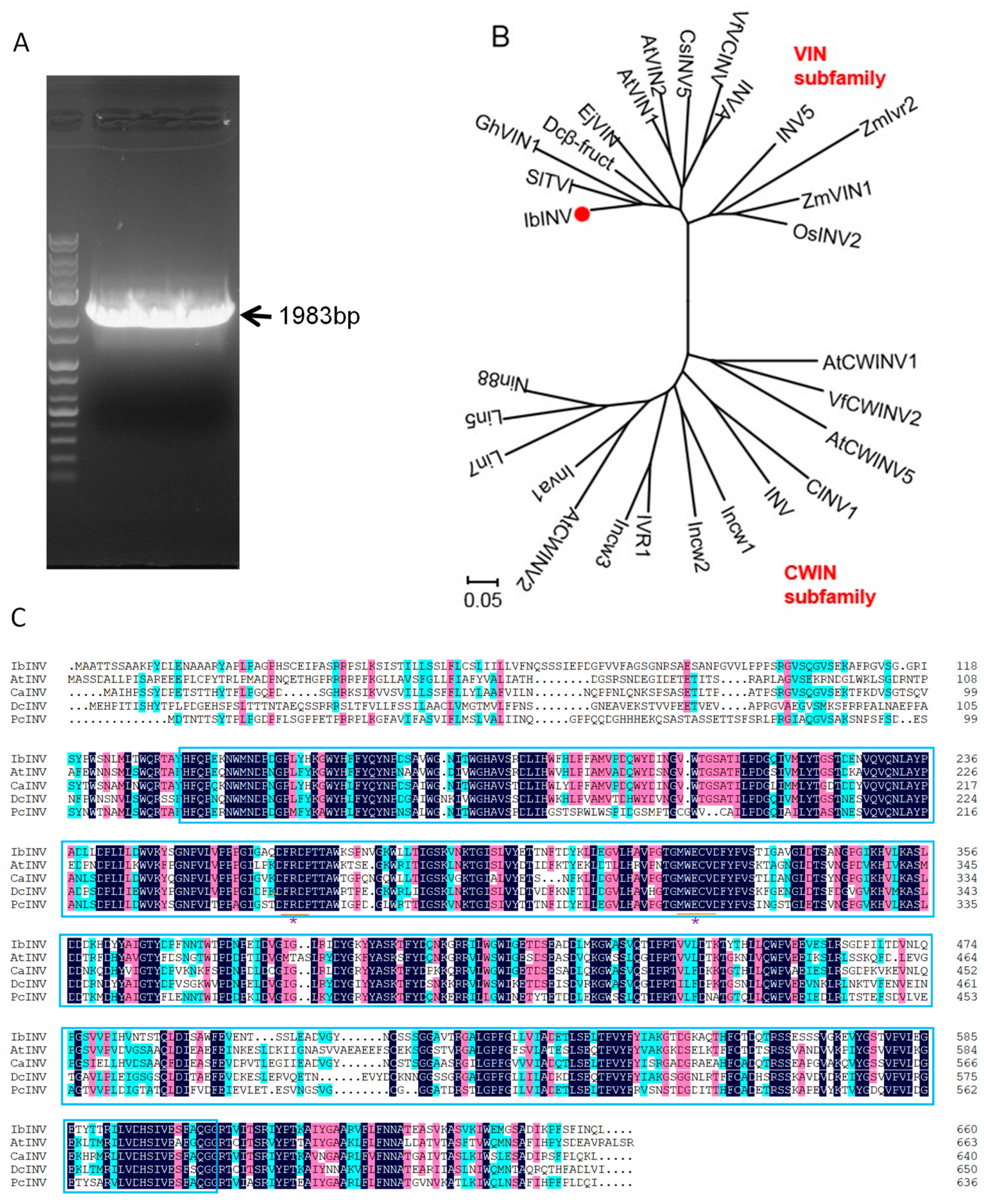
2.1. Cloning and Characterization of Sweet Potato IbINV Gene
2.2. Expression Patterns of IbINV in Various Tissues of Sweet Potato
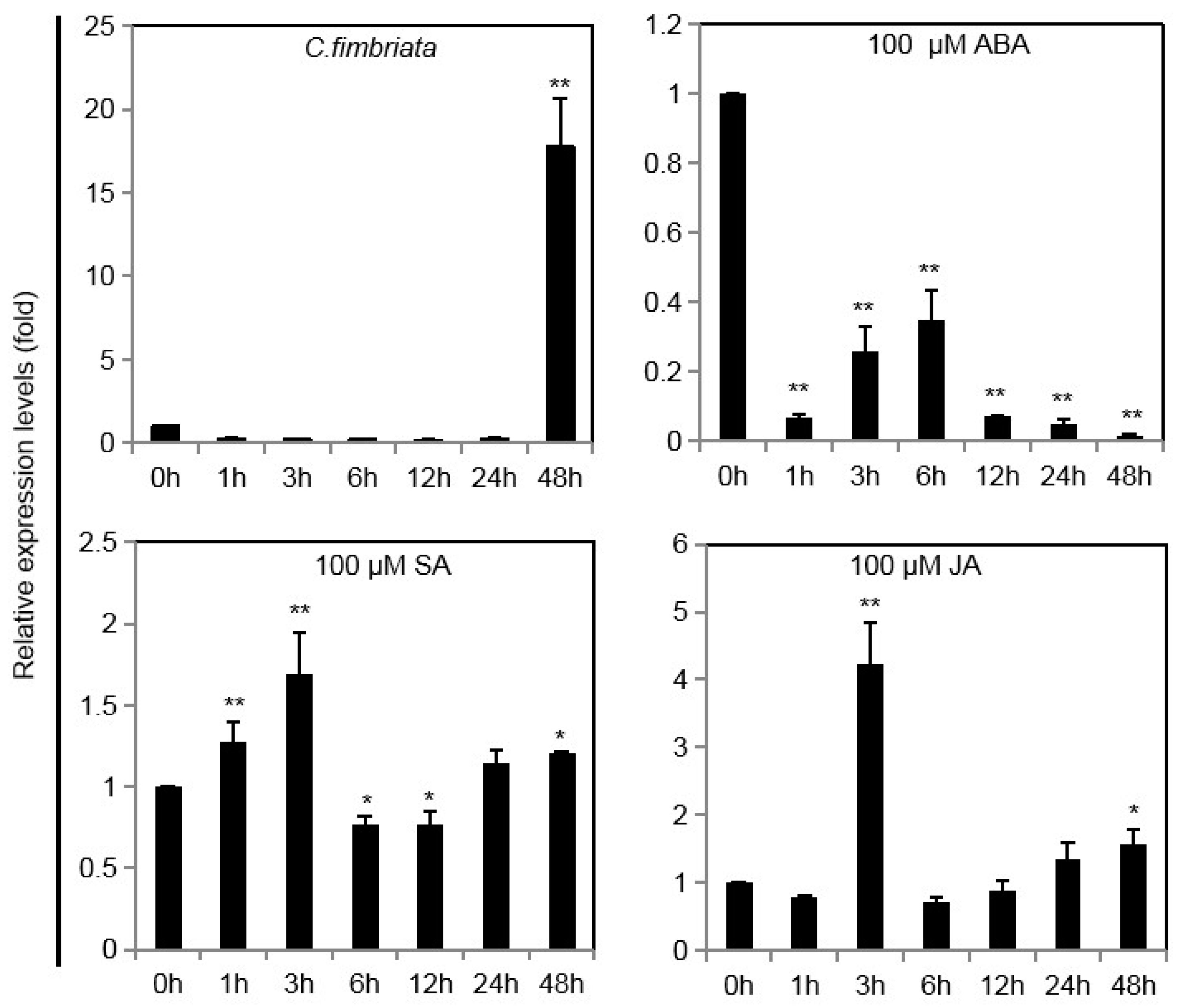
2.3. IbINV Expression Profiles under Various Stresses
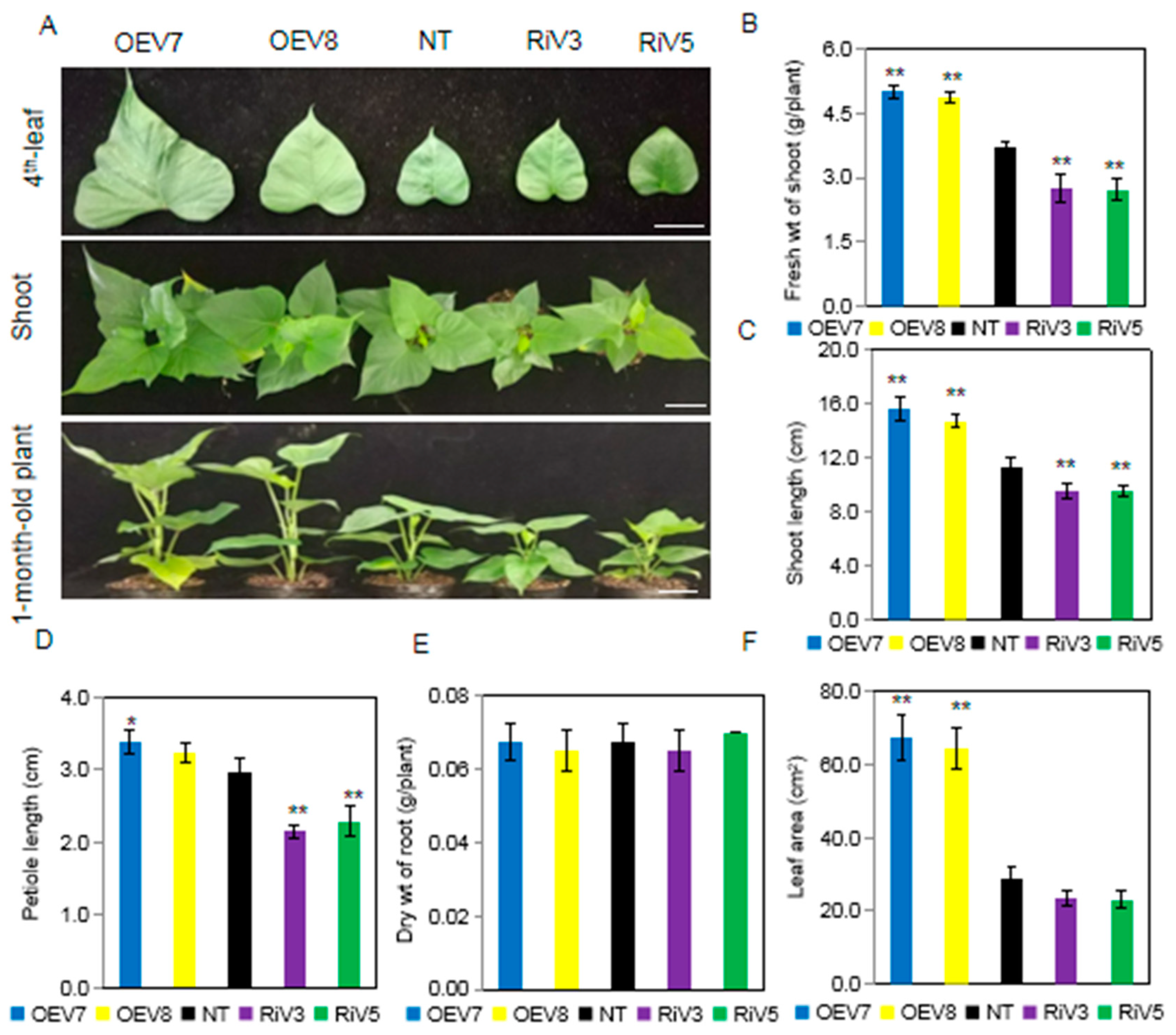
2.4. Generation of Transgenic Sweet Potato Plants with Modified IbINV Expression
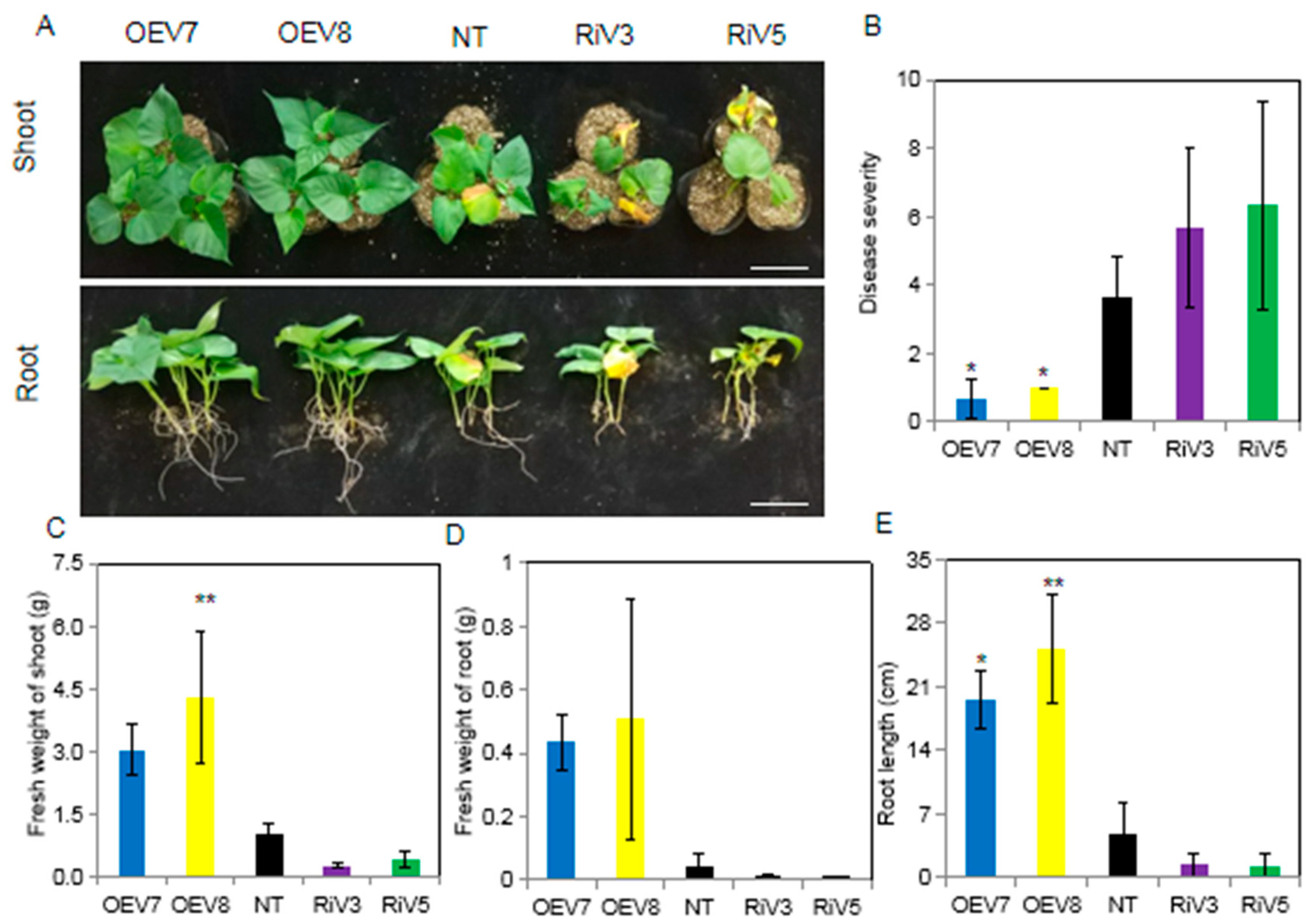
2.5. Resistance to Black Rot Disease of Transgenic Sweet Potato Plants
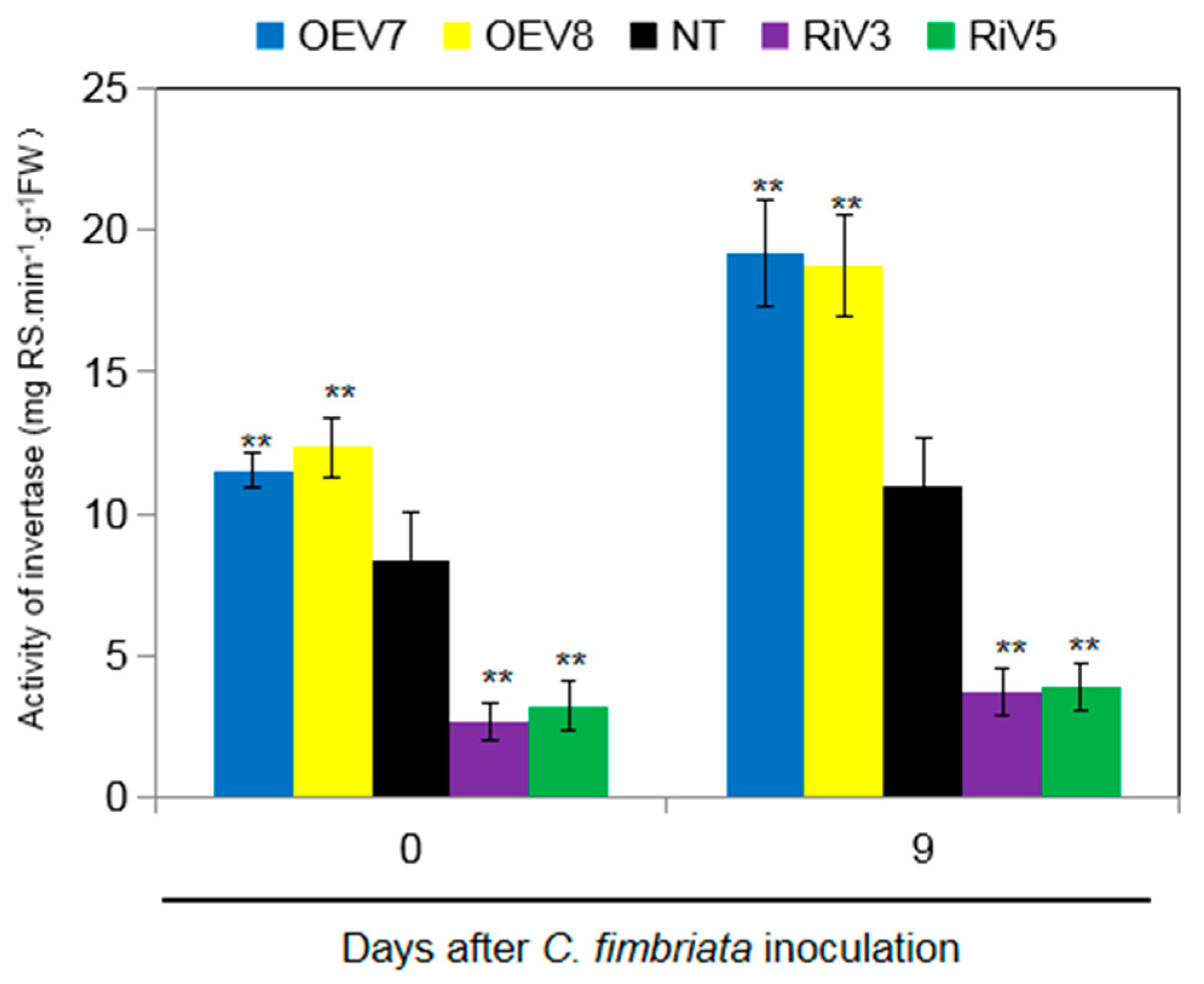
2.6. Invertase Activity in Transgenic Sweet Potato Plants
2.7. Sugar Content in Transgenic Sweet Potato Plants
3. Discussion
4. Materials and Methods
4.1. Plant and Fungal Strains
4.2. IbINV Cloning and Phylogenic Analysis
4.3. Recombinant Plasmid Construction
4.4. Sweet Potato Transformation for Generation of Transgenic Plants
4.5. C. fimbriata Inoculation and Stress Treatment
4.6. Gene Expression Analysis
4.7. Black Rot Disease Resistance Assay
4.8. Sugar Measurement
4.9. Analysis of Invertase Activity
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Ling, K.S.; Jackson, D.M.; Harrison, H.; Simmons, A.M.; Pesic-Vanesbroeck, Z. Field evaluation of yield effects on the u.s.a. heirloom sweetpotato cultivars infected by sweet potato leaf curl virus. Crop Prot. 2010, 29, 757–765. [Google Scholar] [CrossRef]
- Lamaro, G.P.; Tsehaye, Y.; Girma, A.; Vannini, A.; Fedeli, R.; Loppi, S. Essential mineral elements and potentially toxic elements in orange-fleshed sweet potato cultivated in northern Ethiopia. Biology 2023, 12, 266. [Google Scholar] [CrossRef] [PubMed]
- Kwak, S.S. Biotechnology of the sweetpotato: Ensuring global food and nutrition security in the face of climate change. Plant Cell Rep. 2019, 38, 1361–1363. [Google Scholar] [CrossRef] [PubMed]
- Shih, C.K.; Chen, C.M.; Hsiao, T.J.; Liu, C.W.; Li, S.C. White sweet potato as meal replacement for overweight white-collar workers: A randomized controlled trial. Nutrients 2019, 11, 165. [Google Scholar] [CrossRef]
- Herawati, E.R.N.; Santosa, U.; Sentana, S.; Ariani, D. Protective effects of anthocyanin extract from purple sweet potato (Ipomoea batatas L.) on blood MDA levels, liver and renal activity, and blood pressure of hyperglycemic rats. Prev. Nutr. Food Sci. 2020, 25, 375–379. [Google Scholar] [CrossRef]
- Padmaja, G. Uses and nutritional data of sweetpotato. In The Sweetpotato; Loebenstein, G., Thottappilly, G., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 189–234. [Google Scholar]
- Lamaro, G.P.; Tsehaye, Y.; Girma, A.; Vannini, A.; Fedeli, R.; Loppi, S. Evaluation of yield and nutraceutical traits of orange-fleshed sweet potato storage roots in two agro-climatic zones of northern Ethiopia. Plants 2023, 12, 1319. [Google Scholar] [CrossRef]
- Katayama, K.; Kobayashi, A.; Sakai, T.; Kuranouchi, T.; Kai, Y. Recent progress in sweetpotato breeding and cultivars for diverse applications in Japan. Breed. Sci. 2017, 67, 3–14. [Google Scholar] [CrossRef]
- Halsted, B.D. Some fungous diseases of the sweet potato. The black rot. New Jersey Agric. Exp. Stn. Bull. 1890, 76, 7–14. [Google Scholar]
- Dos Santos, A.F.; Ferreira, M.A.; Auer, C.G.; Buhrer, C.B.; Brito, N.M.; Scremin, R.M.; Mireski, M.C. First report of yerba mate wilt caused by Ceratocystis fimbriata in Brazil. Plant Dis. 2018, 11, 2381. [Google Scholar] [CrossRef]
- Xu, K.C.; Zhang, R.Q.; Lu, H.X.; Zhang, J.L.; Yang, J.; Huang, Q. First report of coffee canker disease caused by Ceratocystis fimbriata in China. Plant Dis. 2022, 6, 1756. [Google Scholar] [CrossRef]
- Xu, K.C.; Zhang, R.Q.; Li, J.; Li, X.; Yang, J.; Huang, Q. First report of rubber tree wilt caused by Ceratocystis fimbriata in China. Plant Dis. 2022, 6, 1762. [Google Scholar] [CrossRef]
- Scruggs, A.C.; Basaiah, T.; Adams, M.L.; Quwsada-Ocampo, L.M. Genetic diversity, fungicide sensitivity, and host resistance to Ceratocystis fimbriata infecting sweetpotato in North Carolina. Plant Dis. 2017, 101, 994–1001. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.W.; Pang, L.J.; Zhang, X.Q.; Lu, X.H.; Yin, L.Q.; Lu, G.Q.; Cheng, J.Y. Early discrimination and prediction of C. fimbriata- infected sweetpotatoes during the asymptomatic period using electronic nose. Foods 2022, 11, 1919. [Google Scholar] [CrossRef]
- Shi, Q.; Tan, X.D.; Mao, Z.F.; Tao, N.; Yi, Y.R. Study of ceratocystis fimbriata toxins determination from sweet potato by high performance thin-layer chromatography. Wei Sheng Yan Jiu = J. Hyg. Res. 2003, 32, 381–383. [Google Scholar]
- Xing, K.; Li, T.J.; Liu, Y.F.; Zhang, J.; Zhang, Y.; Shen, X.Q.; Li, X.Y.; Miao, X.M.; Feng, Z.Z.; Peng, X.; et al. Antifungal and eliciting properties of chitosan against Ceratocystis fimbriata in sweet potato. Food Chem. 2018, 268, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, T.J.; Xu, M.J.; Guo, J.H.; Zhang, C.M.; Feng, Z.Z.; Peng, X.; Li, Z.Y.; Xing, K.; Qin, S. Antifungal effect of volatile organic compounds produced by Pseudomonas chlororaphis subsp. aureofaciens SPS-41 on oxidative stress and mitochondrial dysfunction of Ceratocystis fimbriata. Pestic. Biochem. Physiol. 2021, 173, 104777. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, M.; Pan, S.Y.; Pan, C.; Li, Y.X.; Tian, J. Perillaldehyde controls postharvest black rot caused by Ceratocystis fimbriata in sweet potatoes. Front. Microbiol. 2018, 9, 1102. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, T.J.; Liu, Y.F.; Li, X.Y.; Zhang, C.M.; Feng, Z.Z.; Peng, X.; Li, Z.Y.; Qin, S.; Xing, K. Volatile organic compounds produced by Pseudomonas chlororaphis subsp. aureofaciens SPS-41 as biological fumigants to control Ceratocystis fimbriata in postharvest sweet potatoes. J. Agric. Food Chem. 2019, 67, 3702–3710. [Google Scholar] [CrossRef] [PubMed]
- Li, X.W.; Li, B.B.; Cai, S.R.; Zhang, Y.; Xu, M.J.; Zhang, C.M.; Yuan, B.; Xing, K.; Qin, S. Identification of rhizospheric actinomycete Streptomyces lavendulae SPS-33 and the inhibitory effect of its volatile organic compounds against Ceratocystis Fimbriata in postharvest sweet potato (Ipomoea Batatas (L.) Lam.). Microorganisms 2020, 8, 319. [Google Scholar] [CrossRef]
- Xu, M.J.; Guo, J.H.; Li, T.J.; Zhang, C.M.; Peng, X.; Xing, K.; Qin, S. Antibiotic effects of volatiles produced by Bacillus tequilensis XK29 against the black spot disease caused by Ceratocystis fimbriata in postharvest sweet potato. J. Agric. Food Chem. 2021, 69, 13045–13054. [Google Scholar] [CrossRef]
- Gong, Y.; Liu, J.Q.; Xu, M.J.; Zhang, C.M.; Gao, J.; Li, C.G.; Xing, K.; Qin, S. Antifungal volatile organic compounds from Streptomyces setonii WY228 control black spot disease of sweet potato. Appl. Environ. Microbiol. 2022, 88, 2317–2321. [Google Scholar] [CrossRef] [PubMed]
- Mohsin, S.M.; Hasanuzzaman, M.; Parvin, K.; Morokuma, M.; Fujita, M. Effect of tebuconazole and trifloxystrobin on Ceratocystis fimbriata to control black rot of sweet potato: Processes of reactive oxygen species generation and antioxidant defense responses. World J. Microbiol. Biotechnol. 2021, 37, 148. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhou, Y.D.; Laborda, P.; Wang, H.L.; Wang, R.; Chen, X.; Liu, F.Q.; Yang, D.J.; Wang, S.Y.; Shi, X.C.; et al. Mode of action and efficacy of quinolinic acid for the control of Ceratocystis fimbriata on sweet potato. Pest Manag. Sci. 2021, 77, 4564–4571. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.D.; Zou, D.S.; Xu, Z.H.; Chen, B.; Zhang, X.P.; Chen, F.L.; Zhang, M.Y. Combined effects of spent mushroom substrate and dicyandiamide on carbendazim dissipation in soils: Double-edged sword effects and potential risk controls. Environ. Pollut. 2023, 319, 120992. [Google Scholar] [CrossRef] [PubMed]
- Michelmore, R.; Coaker, G.; Bart, R.; Beattie, G.; Bent, A.; Bruce, T.; Cameron, D.; Dangl, J.; Dinesh-Kumar, S.; Edwards, R.; et al. Foundational and translational research opportunities to improve plant health. Mol. Plant Microb. Interact. 2017, 30, 515–516. [Google Scholar] [CrossRef] [PubMed]
- Muramoto, N.; Tanaka, T.; Shimamura, T.; Mitsukawa, N.; Hori, E.; Koda, K.; Otani, M.; Hirai, M.; Nakamura, K.; Imaeda, T. Transgenic sweet potato expressing thionin from barley gives resistance to black rot disease caused by Ceratocystis fimbriata in leaves and storage root. Plant Cell Rep. 2012, 31, 987–997. [Google Scholar] [CrossRef]
- Yan, L.; Wang, Y.N.; Zhang, H.; Zhang, Q.; Zhai, H.; Liu, Q.C.; He, S.Z. The plasma membrane-localized sucrose transporter IbSWEET10 contributes to the resistance of sweet potato to Fusarium oxysporum. Front. Plant Sci. 2017, 8, 197. [Google Scholar]
- Zhang, H.; Zhang, Q.; Zhai, H.; Gao, S.P.; Yang, L.; Wang, Z.; Xu, Y.T.; Huo, J.X.; Ren, Z.T.; Zhao, N.; et al. IbBBX24 promotes the jasmonic acid pathway and enhances Fusarium wilt resistance in sweet potato. Plant Cell. 2020, 32, 1102–1123. [Google Scholar] [CrossRef]
- Ferrieri, A.P.; Arce, C.C.M.; Machado, R.A.R.; Meza-Canales, I.D.; Lima, E.; Baldwin, I.T.; Erb, M. A Nicotiana attenuata cell wall invertase inhibitor (NaCWII) reduces growth and increases secondary metabolite biosynthesis in herbivore-attacked plants. New Phytol. 2015, 208, 519–530. [Google Scholar] [CrossRef]
- Xu, X.X.; Hu, Q.; Yang, W.N.; Jin, Y. The roles of cell wall invertase inhibitor in regulating chilling tolerance in tomato. BMC Plant Biol. 2017, 17, 195. [Google Scholar] [CrossRef]
- Sturm, A.; Chrispeels, M.T. cDNA cloning of carrot extracellular—Fructosidase and its expression in response to wounding and bacterial infection. Plant Cell. 1990, 2, 1107–1119. [Google Scholar]
- Ruan, Y.L.; Jin, Y.; Yang, Y.J.; Li, G.J.; Boyer, J.S. Sugar Input, Metabolism, and Signaling Mediated by Invertase: Roles in Development, Yield Potential, and Response to Drought and Heat. Mol. Plant. 2010, 3, 942–955. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Ni, D.A.; Ruan, Y.L. Posttranslational elevation of cell wall invertase activity by silencing its inhibitor in tomato delays leaf senescence and increases seed weight and fruit hexose Level. Plant Cell. 2009, 21, 2072–2089. [Google Scholar] [CrossRef]
- Yang, D.J.; Xie, Y.P.; Sun, H.J.; Bian, X.F.; Ke, Q.B.; Kim, H.S.; Ji, C.Y.; Jin, R.; Wang, W.B.; Zhang, C.L.; et al. IbINH positively regulates drought stress tolerance in sweetpotato. Plant Physiol. Biochem. 2020, 146, 403–410. [Google Scholar] [CrossRef]
- Wan, H.J.; Wu, L.M.; Yang, Y.J.; Zhou, G.Z.; Ruan, Y.L. Evolution of Sucrose Metabolism: The Dichotomy of Invertases and Beyond. Trends. Plant Sci. 2017, 2, 163–177. [Google Scholar] [CrossRef] [PubMed]
- Wobus, U.; Weber, H. Sugars as signal molecules in plant seed development. Biol. Chem. 1999, 380, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Murayama, S.; Handa, H. Genes for alkaline/neutral invertase in rice: Alkaline/neutral invertases are located in plant mitochondria and also in plastids. Planta 2007, 225, 1193–1203. [Google Scholar] [CrossRef]
- Verhaest, M.; Lammens, W.; Roy, K.L.; Coninck, B.D.; Ranter, C.J.D.; Laere, A.V.; Ende, W.V.D.; Rabijns, A. X-ray diffraction structure of a cell-wall invertase from Arabidopsis thaliana. Acta Crystallogr. D Biol. Crystallogr. 2006, 62, 1555–1563. [Google Scholar] [CrossRef]
- Alberto, F.; Bignon, C.; Sulzenbacher, G.; Henrissat, B.; Czjzek, M. The three-dimensional structure of invertase (β-fructosidase) from thermotoga maritima reveals a bimodular arrangement and an evolutionary relationship between retaining and inverting glycosidases. J. Biol. Chem. 2004, 279, 18903–18910. [Google Scholar] [CrossRef]
- Sturm, A. Invertases: Primary structures, functions and roles in plant development and sucrose partitioning. Plant Physiol. 1999, 121, 1–8. [Google Scholar] [CrossRef]
- Goetz, M.; Roitsch, T. The different pH optima and substrate specificities of extracellular and vacuolar invertases from plants are determined by a single amino-acid substitution. Plant J. 1999, 20, 707–711. [Google Scholar] [CrossRef] [PubMed]
- Herbers, K.; Sonnewald, U. Molecular determinants of sink strength. Curr. Opin. Plant Biol. 1998, 1, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.Q.; Lüscher, M.; Sturm, A. Antisense repression of vacuolar and cell wall invertase in transgenic carrot alters early plant development and sucrose partitioning. Plant Cell. 1999, 11, 177–189. [Google Scholar] [CrossRef]
- Wang, E.; Wang, J.; Zhu, X.; Hao, W.; Wang, L.; Li, Q.; Zhang, L.; He, W.; Lu, B.; Lin, H.; et al. Control of rice grain-filling and yield by a gene with a potential signature of domestication. Nat. Genet. 2008, 40, 1370–1374. [Google Scholar] [CrossRef]
- Zanor, M.I.; Osorio, S.; Nunes-Nesi, A.; Carrari, F.; Lohse, M.; Usadel, B.; Kühn, C.; Bleiss, W.; Giavalisco, P.; Willmitzer, L.; et al. RNA interference of LIN5 in tomato confirms its role in controlling Brix content, uncovers the influence of sugars on the levels of fruit hormones, and demonstrates the importance of sucrose cleavage for normal fruit development and fertility. Plant Physiol. 2009, 150, 1204–1218. [Google Scholar] [CrossRef]
- Wang, B. Molecular Cloning and Functional Analysis of Cytoplasmic Invertase Gene GmCInv in Soybean; Nanjing Agricultural University: Nanjing, China, 2014; pp. 37–38. [Google Scholar]
- Yu, X.Y.; Wang, X.F.; Zhang, W.Q.; Qian, T.T.; Tang, G.M.; Guo, Y.K.; Zheng, C.C. Antisense suppression of an acid invertase gene (MAI1) in muskmelon alters plant growth and fruit development. J. Exp. Bot. 2008, 59, 2969–2977. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Brugger, A.; Lamotte, O.; Vandelle, E.; Bourque, S.; Lecourieux, D.; Poinssot, B.; Wendehenne, D.; Pugin, A. Early signaling events induced by elicitors of plant defenses. Mol. Plant Microbe. Interact. 2006, 19, 711–724. [Google Scholar] [CrossRef]
- Alexandra, S.T.; Thierry, G. Sucrose and invertases, a part of the plant defense response to the biotic stresses. Front. Plant Sci. 2014, 5, 293. [Google Scholar]
- Bezrutczyk, M.; Yang, J.; Eom, J.S.; Prior, M.; Sosso, D.; Hartwig, T.; Szurek, B.; Oliva, R.; Vera-Cruz, C.; White, F.F.; et al. Sugar flux and signaling in plant-microbe interactions. Plant J. 2018, 93, 675–685. [Google Scholar] [CrossRef]
- Essmann, J.; Schmitz-Thom, I.; Schon, H.; Sonnewald, S.; Weis, E.; Scharte, J. RNA interference-mediated repression of cell wall invertase impairs defense in source leaves of tobacco. Plant Physiol. 2008, 147, 1288–1299. [Google Scholar] [CrossRef][Green Version]
- Kang, L.; Kim, H.S.; Kwon, Y.S.; Ke, Q.B.; Ji, C.Y.; Park, S.C.; Lee, H.S.; Deng, X.P.; Kwak, S.S. IbOr regulates photosynthesis under heat stress by stabilizing IbPsbP in sweetpotato. Front. Plant Sci. 2017, 8, 989. [Google Scholar] [CrossRef] [PubMed]
- Zuckerkandl, E.; Pauling, L. Evolutionary divergence and convergence in proteins. In Evolving Genes and Proteins; Bryson, V., Vogel, H.J., Eds.; Academic Press: New York, NY, USA, 1965; pp. 97–166. [Google Scholar]


| Primer Name | Sequence (5′-…-3′) | Application |
|---|---|---|
| IbINV_F | ATGGCCGCCACCACTTCTTCCG | IbINV isolation |
| IbINV_R | TTACAATTGATTGATGAAAGAG | IbINV isolation |
| IbINV_attb_F | AAAAAGCAGGCTGCATGGCCGCCACCACTTCT | attB-IbINV |
| IbINV_attb_R | AGAAAGCTGGGTCCAATTGATTGATGAAAGAG | attB-IbINV |
| IbINV-S_attb_F | AAAAAGCAGGCTGCATATCGCAAAGGGCACC | attB-IbINV-S |
| IbINV-S_attb_R | AGAAAGCTGGGTCCCAGATCTTCACCGACG | attB-IbINV-S |
| RTIbINV_F | GGGGCCGTTCGGACTTCT | RT-PCR for IbINV |
| RTIbINV_R | ACCGTGCTTCCATAAACCTCTT | RT-PCR for IbINV |
| Actin-F | AGCAGCATGAAGATTAAGGTTGTAGCAC | Reference gene |
| Actin-R | TGGAAAATTAGAAGCACTTCCTGTGAAC | Reference gene |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, D.; Bian, X.; Kim, H.S.; Jin, R.; Gao, F.; Chen, J.; Ma, J.; Tang, W.; Zhang, C.; Sun, H.; et al. IbINV Positively Regulates Resistance to Black Rot Disease Caused by Ceratocystis fimbriata in Sweet Potato. Int. J. Mol. Sci. 2023, 24, 16454. https://doi.org/10.3390/ijms242216454
Yang D, Bian X, Kim HS, Jin R, Gao F, Chen J, Ma J, Tang W, Zhang C, Sun H, et al. IbINV Positively Regulates Resistance to Black Rot Disease Caused by Ceratocystis fimbriata in Sweet Potato. International Journal of Molecular Sciences. 2023; 24(22):16454. https://doi.org/10.3390/ijms242216454
Chicago/Turabian StyleYang, Dongjing, Xiaofeng Bian, Ho Soo Kim, Rong Jin, Fangyuan Gao, Jingwei Chen, Jukui Ma, Wei Tang, Chengling Zhang, Houjun Sun, and et al. 2023. "IbINV Positively Regulates Resistance to Black Rot Disease Caused by Ceratocystis fimbriata in Sweet Potato" International Journal of Molecular Sciences 24, no. 22: 16454. https://doi.org/10.3390/ijms242216454
APA StyleYang, D., Bian, X., Kim, H. S., Jin, R., Gao, F., Chen, J., Ma, J., Tang, W., Zhang, C., Sun, H., Xie, Y., Li, Z., Kwak, S.-S., & Ma, D. (2023). IbINV Positively Regulates Resistance to Black Rot Disease Caused by Ceratocystis fimbriata in Sweet Potato. International Journal of Molecular Sciences, 24(22), 16454. https://doi.org/10.3390/ijms242216454







