Astrocytes: Lessons Learned from the Cuprizone Model
Abstract
1. Introduction
2. Glia Cells
3. Multiple Sclerosis Animal Models
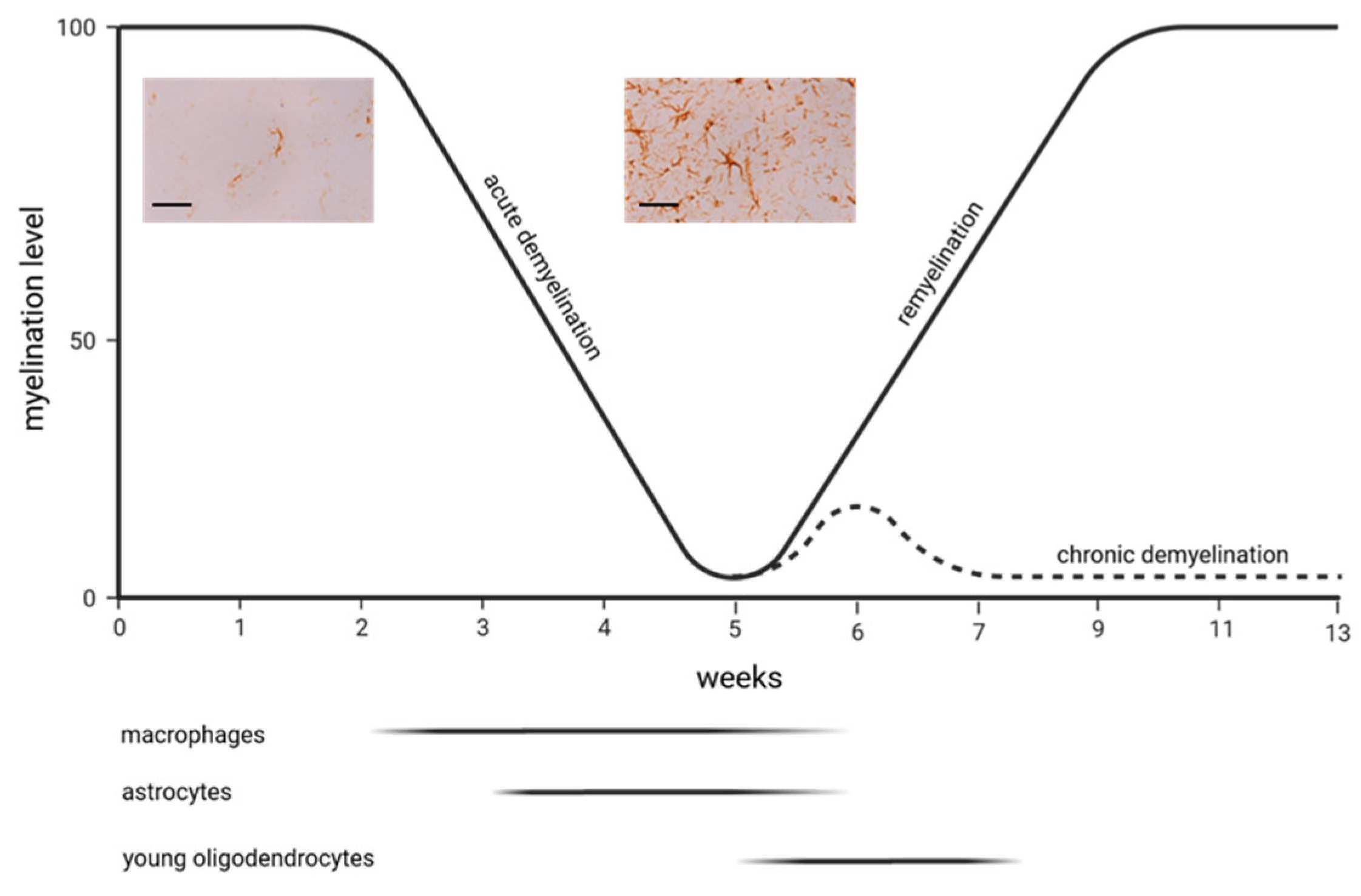
4. Astrocytes and the Cuprizone Model
5. Conclusions
| Citation | Main Finding(s) |
|---|---|
| [46] | Astrocyte proliferation via 3H-thymidine labelling |
| [48] | Astrocyte activation, demonstrated by IHC and ISH |
| [56] | IGF-1 is expressed by astrocytes and the receptor by oligodendrocytes |
| [92] | Altered astrocytic glutathione-S-transferase isoform expression during demyelination |
| [93] | Altered astrocytic glutathione-S-transferase isoform expression during remyelination |
| [47] | Astrogliosis promptly follows microgliosis during demyelination |
| [59] | Amelioration of cuprizone-induced pathology ameliorates the extent of astrocyte activation; IFN-γ overexpression, driven by the MBP reporter |
| [94] | Amelioration of cuprizone-induced pathology ameliorates the extent of astrocyte activation; MIP-1alpha deficiency |
| [60] | IL1β-deficient mice have lower IGF-1 levels during the remyelination phase |
| [95] | Astrocytes express MHC class I and II |
| [96] | Transgenic mice that overexpress PDGF-A in astrocytes have increased OPC numbers |
| [61] | Peripheral benzodiazepine receptor is expressed by astrocytes and microglia |
| [97] | Osteopontin is expressed by astrocytes and microglia |
| [98] | Notch1 is expressed by various cell types, including astrocytes, within remyelinating lesions |
| [84] | Lymphotoxin-alpha is expressed by astrocytes and exacerbates demyelination |
| [99] | Metallothionein-I and –II are expressed by astrocytes |
| [100] | Different pathologies, including axonal injury and astrocyte activation, are more pronounced in aged versus young mice during demyelination |
| [101] | Complement regulatory protein Crry overexpression in astrocytes protects against demyelination |
| [102] | The acyl-CoA synthetase, lipidosin, is expressed by astrocytes |
| [40] | Platelet-derived growth factor-A overexpression in astrocytes supports remyelination |
| [103] | Metallothionein I/II are expressed by astrocytes |
| [39] | Extent of astrocytosis differs between demyelinated white and grey matter areas |
| [104] | Cortical demyelination, but not astrogliosis per se, is associated with accelerated cortical spreading depression |
| [105] | Astrocyte progenitor cells accumulate in cuprizone lesions |
| [106] | ADAM12 is expressed by astrocytes |
| [107] | Increased numbers of astrocytes in vivo within the subventricular zone during demyelination, and numbers were decreased by intraventricular Noggin infusion |
| [108] | CXCL12 is expressed by astrocytes and microglia |
| [109] | Cx47 is expressed by astrocytes |
| [110] | Glial isoform of APP is expressed by astrocytes |
| [111] | C3a and C5a overexpression exacerbates demyelination and delays remyelination |
| [112] | IV-injected human-embryonic-stem-cell-derived neural precursor cells into mice express GFAP to a limited extent |
| [85] | IκB kinase 2 depletion in astrocytes ameliorates demyelination |
| [113] | Smad1, Smad5, and Smad8, intracellular effectors of the bone morphogenetic protein (BMP) family of proteins, are active in oligodendrocytes and a subset of astrocytes |
| [65] | TSPO is expressed by astrocytes and microglia |
| [114] | COX-1 is expressed by astrocytes and microglia |
| [86,115] | Galectin-1 and -3 are expressed by astrocytes and microglia; galectin-3-deficient mice show impaired remyelination |
| [116] | Serine palmitoyltransferase, the rate-limiting enzyme for ceramide de novo biosynthesis, is expressed by astrocytes |
| [117] | FABP7 is expressed by astrocytes |
| [118] | IGF1 is expressed by astrocytes |
| [119] | MMP3 and MMP9 are expressed by astrocytes |
| [52] | RXRβ is expressed by astrocytes |
| [120] | Carbonic Anhydrase II is expressed by astrocytes |
| [121] | Act1-deletion in astrocytes ameliorates demyelination |
| [122] | p65 is active in astrocytes |
| [87] | TNFR1 and TNFR2 are expressed by astrocytes and microglia; CXCL12 is expressed by astrocytes, which promotes OPC proliferation and differentiation |
| [72] | In contrast to microgliosis, astrocytosis persists during de- and remyelination. Astrocyte reaction is characterized, among others features, by early astrocyte proliferation and increased expression of GFAP, vimentin, and fibronectin. Furthermore, there is an elaboration of a dense network of processes |
| [73] | Astrocyte ablation results in impaired remyelination |
| [123] | IGF1 infusions can decrease astrocyte numbers during remyelination |
| [124] | Glutamate-aspartate transporter is expressed by astrocytes |
| [125] | Receptor protein tyrosine phosphatase β is expressed in astrocytes |
| [126] | IL6 is expressed by astrocytes |
| [88] | mGluR1, mGluR5, and BDNF are expressed by astrocytes; astrocyte-derived BDNF promotes recovery from cuprizone-induced demyelination |
| [77] | CXCL10 is mainly expressed by astrocytes |
| [127] | Oncostatin M receptor is expressed by astrocytes and microglia; OSMR deficiency aggravates demyelination; CNS-targeted OSM treatment ameliorates demyelination |
| [128] | Erk is especially activated in astrocytes and promotes demyelination |
| [129] | Transgenic mice that overexpress IL6 in astrocytes show reduced glia activation, axonal injury, and OPC recruitment |
| [82] | Transient Receptor Potential Ankyrin 1 (TRPA1) is expressed in astrocytes; TRPA1 deficiency significantly attenuated cuprizone-induced demyelination by reducing the apoptosis of mature oligodendrocytes |
| [130] | Activation of the astrocytic Nrf2/ARE system ameliorates demyelination |
| [131] | Transgenic mice that overexpress IL-17A in astrocytes show aggravation of demyelination |
| [132] | Transglutaminase 2 is expressed by astrocytes |
| [133] | Transgenic mice that overexpress IL6 in astrocytes show amelioration in the cuprizone-induced pathologies |
| [134] | S1P receptor 1 is expressed by astrocytes, and its modulation ameliorates demyelination |
| [135] | Astrocytes show NF-κB activation |
| [136] | Gli1 is expressed by astrocytes after chronic, but not acute, demyelination |
| [137] | Cuprizone induces astrocyte atrophy in the rat |
| [138] | Metallothionein I/II and Megalin are expressed by astrocytes |
| [139] | Sox10 converts astrocytes into oligodendrocyte-like cells |
| [140] | Transplanted astrocytes convert into oligodendrocyte-like cells |
| [35] | DDIT3 is expressed by oligodendrocytes and astrocytes |
| [141] | Sox2 converts astrocytes into oligodendrocyte-like cells |
| [91] | Astrocyte ablation augments remyelination after chronic demyelination |
| [63] | TSPO is expressed by microglia and astrocytes |
| [142] | Transferrin can be incorporated by all glial cells among astrocytes |
| [143] | Overexpression of GFAP reduces cuprizone-induced apoptosis, demyelination, and acute axonal damage |
| [144] | Mesenchymal stem cells reduce astogliosis and microgliosis |
| [145] | CD38 is expressed by astrocytes and microglia; CD38 ameliorates demyelination |
| [146] | Astrocytes express NRF2, HO-1, and PI3K; Ginkgolide K augments the expression of these proteins |
| [147] | Astrocytes express SOX2, CNTF, IGF2, and BDNF |
| [148] | Astrocyte-specific deletion of Transient receptor potential ankyrin 1 delays demyelination |
| [149] | PAR1 knock-out mice demonstrate skewing of reactive astrocyte signatures towards a pro-repair phenotype |
| [150] | Astrocytes express BDNF and GDNF; Ginkgolide B augments the expression of these proteins |
| [151] | Deletion of astrocytic Cav1.2 channels leads to reduced astrocytosis, microgliosis, ameliorates inflammation, and promotes remyelination |
| [152] | P2x7 receptors are expressed by astrocytes and microglia; demyelination is ameliorated in P2x7-deficient mice |
| [153] | Induced neural stem cells ameliorate astrocytosis |
| [154] | CD44 is expressed by astrocytes and microglia; Cd44 deficiency does not ameliorate cuprizone-induced pathology |
| [155] | Some astrocytes show oxidative damage to DNA (id est, 8-OHdG+) |
| [156] | SIRT1 is expressed by astrocytes, microglia, and mature oligodendrocytes |
| [157] | Mesenchymal stem cell transplantation ameliorates astrocytosis |
| [158] | AQP4 is expressed in astrocyte endfeet; polarized expression is reduced after demyelination |
| [159] | Astrocytes phagocytose myelin; astrocytes express BDNF, CTNF, Nestin, SOX2, Notch, and ß-catenin; expression profiles are regulated by ethyl pyruvate |
| [160] | Astrocytes express NgR1, SPARC, and Hevin; astrocytic NgR1 sublocalization alters during demyelination; |
| [161] | Astrocytes express C3; TIC knock-outs show reduced C3 expression |
| [162] | Aastrocyte participation in the tripartite synapse during demyelination |
| [163] | mGluR5 is expressed by astrocytes and orchestrates remyelination |
| [164] | EBI2 receptor is expressed in astrocytes and microglia |
| [165] | Astrocytes express TrkB, CTR1, ATP7A, and ATP7B; demyelination is ameliorated in mice lacking astrocytic TrkB expression |
| [166] | Fth deletion in Glast1/EAAT1-positive astrocytes inhibits remyelination |
| [167] | EAAT2 is expressed by astrocytes |
| [168] | Astrocytes express S100B, GFAP, vimentin, LCN2, and ALDH1L1 |
| [83] | LCN2 is expressed by astrocytes; oligodendrocyte loss is more severe in Lcn2-/- animals |
| [169] | Ongoing astrocytosis weeks after completion of remyelination |
| [170] | C3d, S100a10, Stat3, and Timp1 are expressed by astrocytes |
| [171] | C3d and S100a10 are expressed by astrocytes; Bu Shen Yi Sui capsules promote an A2 phenotype |
| [172] | Combined mesenchymal stem cell transplantation and astrocyte ablation support remyelination |
| [173] | Cuprizone intoxication induces astrocyte endfeet sweelings |
| [174] | Astrocytes express TRAP1; β-hydroxybutyrate downregulates the expression of this protein |
| [71] | High-resolution, single-nucleus RNA sequencing (snRNA-seq) analysis of gene expression changes across various brain cells, including astrocytes |
| [175] | Bone Marrow Mesenchymal Stem Cells reduce astrocytosis |
| [176] | Astrocytes express HNK-1-O-Man+ PTPRZ |
| [177] | A1 versus A2 astrocyte expression profiling |
| [178] | The AQP4 inhibitor TGN020 ameliorates astrocyte and microglia activation |
| [76] | Astrocytic transcriptome signature investigated via ribosomal tagging |
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hutchinson, N.A.; Koles, Z.J.; Smith, R.S. Conduction velocity in myelinated nerve fibres of Xenopus laevis. J. Physiol. 1970, 208, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Patani, R.; Hardingham, G.E.; Liddelow, S.A. Functional roles of reactive astrocytes in neuroinflammation and neurodegeneration. Nat. Rev. Neurol. 2023, 19, 395–409. [Google Scholar] [CrossRef]
- Valori, C.F.; Sulmona, C.; Brambilla, L.; Rossi, D. Astrocytes: Dissecting Their Diverse Roles in Amyotrophic Lateral Sclerosis and Frontotemporal Dementia. Cells 2023, 12, 1450. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Ortega, P.; Soria-Ortiz, M.B.; Rodríguez, V.M.; Vázquez-Martínez, E.O.; Díaz-Muñoz, M.; Reyes-Haro, D. Anorexia disrupts glutamate-glutamine homeostasis associated with astroglia in the prefrontal cortex of young female rats. Behav. Brain Res. 2022, 420, 113715. [Google Scholar] [CrossRef]
- Als, T.D.; Kurki, M.I.; Grove, J.; Voloudakis, G.; Therrien, K.; Tasanko, E.; Nielsen, T.T.; Naamanka, J.; Veerapen, K.; Levey, D.F.; et al. Depression pathophysiology, risk prediction of recurrence and comorbid psychiatric disorders using genome-wide analyses. Nat. Med. 2023, 29, 1832–1844. [Google Scholar] [CrossRef] [PubMed]
- Farsi, Z.; Nicolella, A.; Simmons, S.K.; Aryal, S.; Shepard, N.; Brenner, K.; Lin, S.; Herzog, L.; Moran, S.P.; Stalnaker, K.J.; et al. Brain-region-specific changes in neurons and glia and dysregulation of dopamine signaling in Grin2a mutant mice. Neuron 2023, 111, 3378–3396. [Google Scholar] [CrossRef]
- Yarom, Y.; Naparstek, Y.; Lev-Ram, V.; Holoshitz, J.; Ben-Nun, A.; Cohen, I.R. Immunospecific inhibition of nerve conduction by T lymphocytes reactive to basic protein of myelin. Nature 1983, 303, 246–247. [Google Scholar] [CrossRef] [PubMed]
- Franklin, R.J.; Ffrench-Constant, C. Remyelination in the CNS: From biology to therapy. Nat. Rev. Neurosci. 2008, 9, 839–855. [Google Scholar] [CrossRef]
- Friede, R.L. The relationship of body size, nerve cell size, axon length, and glial density in the cerebellum. Proc. Natl. Acad. Sci. USA 1963, 49, 187–193. [Google Scholar] [CrossRef]
- Schwartz, A.M.; Lapham, L.W.; van den Noort, S. Cytologic and cytochemical studies of neuroglia. IV. Experimentally induced protoplasmic astrocytosis in the Bergmann glia of cerebellum. Neurology 1966, 16, 1118–1126. [Google Scholar] [CrossRef]
- Rasmussen, K.E. A morphometric study of the Müller cell cytoplasm in the rat retina. J. Ultrastruct. Res. 1972, 39, 413–429. [Google Scholar] [CrossRef]
- Rhodes, R.H. A light microscopic study of the developing human neural retina. Am. J. Anat. 1979, 154, 195–209. [Google Scholar] [CrossRef] [PubMed]
- Wagner, H.J.; Pilgrim, C. A microprobe analysis of Gomori-positive glial cells in the rat arcuate nucleus. Histochemistry 1978, 55, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Colombo, J.A.; Reisin, H.D. Interlaminar astroglia of the cerebral cortex: A marker of the primate brain. Brain Res. 2004, 1006, 126–131. [Google Scholar] [CrossRef]
- Falcone, C.; McBride, E.L.; Hopkins, W.D.; Hof, P.R.; Manger, P.R.; Sherwood, C.C.; Noctor, S.C.; Martínez-Cerdeño, V. Redefining varicose projection astrocytes in primates. Glia 2022, 70, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Yates, D. Astrocytic cueing of neuronal migration. Nat. Rev. Neurosci. 2020, 21, 120. [Google Scholar] [CrossRef]
- Su, J.; Charalambakis, N.E.; Sabbagh, U.; Somaiya, R.D.; Monavarfeshani, A.; Guido, W.; Fox, M.A. Retinal inputs signal astrocytes to recruit interneurons into visual thalamus. Proc. Natl. Acad. Sci. USA 2020, 117, 2671–2682. [Google Scholar] [CrossRef]
- Fünfschilling, U.; Supplie, L.M.; Mahad, D.; Boretius, S.; Saab, A.S.; Edgar, J.; Brinkmann, B.G.; Kassmann, C.M.; Tzvetanova, I.D.; Möbius, W.; et al. Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature 2012, 485, 517–521. [Google Scholar] [CrossRef]
- MacVicar, B.A.; Choi, H.B. Astrocytes Provide Metabolic Support for Neuronal Synaptic Function in Response to Extracellular K(). Neurochem. Res. 2017, 42, 2588–2594. [Google Scholar] [CrossRef]
- Bonvento, G.; Bolaños, J.P. Astrocyte-neuron metabolic cooperation shapes brain activity. Cell Metab. 2021, 33, 1546–1564. [Google Scholar] [CrossRef]
- Roumes, H.; Jollé, C.; Blanc, J.; Benkhaled, I.; Chatain, C.P.; Massot, P.; Raffard, G.; Bouchaud, V.; Biran, M.; Pythoud, C.; et al. Lactate transporters in the rat barrel cortex sustain whisker-dependent BOLD fMRI signal and behavioral performance. Proc. Natl. Acad. Sci. USA 2021, 118, e2112466118. [Google Scholar] [CrossRef]
- Kress, B.T.; Iliff, J.J.; Xia, M.; Wang, M.; Wei, H.S.; Zeppenfeld, D.; Xie, L.; Kang, H.; Xu, Q.; Liew, J.A.; et al. Impairment of paravascular clearance pathways in the aging brain. Ann. Neurol. 2014, 76, 845–861. [Google Scholar] [CrossRef] [PubMed]
- Hablitz, L.M.; Plá, V.; Giannetto, M.; Vinitsky, H.S.; Stæger, F.F.; Metcalfe, T.; Nguyen, R.; Benrais, A.; Nedergaard, M. Circadian control of brain glymphatic and lymphatic fluid flow. Nat. Commun. 2020, 11, 4411. [Google Scholar] [CrossRef] [PubMed]
- Escartin, C.; Galea, E.; Lakatos, A.; O’Callaghan, J.P.; Petzold, G.C.; Serrano-Pozo, A.; Steinhäuser, C.; Volterra, A.; Carmignoto, G.; Agarwal, A.; et al. Reactive astrocyte nomenclature, definitions, and future directions. Nat. Neurosci. 2021, 24, 312–325. [Google Scholar] [CrossRef]
- Charabati, M.; Wheeler, M.A.; Weiner, H.L.; Quintana, F.J. Multiple sclerosis: Neuroimmune crosstalk and therapeutic targeting. Cell 2023, 186, 1309–1327. [Google Scholar] [CrossRef] [PubMed]
- Rawji, K.S.; Neumann, B.; Franklin, R.J.M. Glial aging and its impact on central nervous system myelin regeneration. Ann. N. Y. Acad. Sci. 2023, 1519, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Healy, L.M.; Stratton, J.A.; Kuhlmann, T.; Antel, J. The role of glial cells in multiple sclerosis disease progression. Nat. Rev. Neurol. 2022, 18, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Lau, L.W.; Keough, M.B.; Haylock-Jacobs, S.; Cua, R.; Döring, A.; Sloka, S.; Stirling, D.P.; Rivest, S.; Yong, V.W. Chondroitin sulfate proteoglycans in demyelinated lesions impair remyelination. Ann. Neurol. 2012, 72, 419–432. [Google Scholar] [CrossRef]
- Huang, J.K.; Jarjour, A.A.; Nait Oumesmar, B.; Kerninon, C.; Williams, A.; Krezel, W.; Kagechika, H.; Bauer, J.; Zhao, C.; Baron-Van Evercooren, A.; et al. Retinoid X receptor gamma signaling accelerates CNS remyelination. Nat. Neurosci. 2011, 14, 45–53. [Google Scholar] [CrossRef]
- Ghasemlou, N.; Jeong, S.Y.; Lacroix, S.; David, S. T cells contribute to lysophosphatidylcholine-induced macrophage activation and demyelination in the CNS. Glia 2007, 55, 294–302. [Google Scholar] [CrossRef]
- Buschmann, J.P.; Berger, K.; Awad, H.; Clarner, T.; Beyer, C.; Kipp, M. Inflammatory response and chemokine expression in the white matter corpus callosum and gray matter cortex region during cuprizone-induced demyelination. J. Mol. Neurosci. 2012, 48, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Voss, E.V.; Škuljec, J.; Gudi, V.; Skripuletz, T.; Pul, R.; Trebst, C.; Stangel, M. Characterisation of microglia during de- and remyelination: Can they create a repair promoting environment? Neurobiol. Dis. 2012, 45, 519–528. [Google Scholar] [CrossRef]
- Blakemore, W.F. Observations on oligodendrocyte degeneration, the resolution of status spongiosus and remyelination in cuprizone intoxication in mice. J. Neurocytol. 1972, 1, 413–426. [Google Scholar] [CrossRef] [PubMed]
- Jhelum, P.; Santos-Nogueira, E.; Teo, W.; Haumont, A.; Lenoël, I.; Stys, P.K.; David, S. Ferroptosis Mediates Cuprizone-Induced Loss of Oligodendrocytes and Demyelination. J. Neurosci. 2020, 40, 9327–9341. [Google Scholar] [CrossRef]
- Fischbach, F.; Nedelcu, J.; Leopold, P.; Zhan, J.; Clarner, T.; Nellessen, L.; Beißel, C.; van Heuvel, Y.; Goswami, A.; Weis, J.; et al. Cuprizone-induced graded oligodendrocyte vulnerability is regulated by the transcription factor DNA damage-inducible transcript 3. Glia 2019, 67, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Pasquini, L.A.; Calatayud, C.A.; Bertone Uña, A.L.; Millet, V.; Pasquini, J.M.; Soto, E.F. The neurotoxic effect of cuprizone on oligodendrocytes depends on the presence of pro-inflammatory cytokines secreted by microglia. Neurochem. Res. 2007, 32, 279–292. [Google Scholar] [CrossRef]
- Taraboletti, A.; Walker, T.; Avila, R.; Huang, H.; Caporoso, J.; Manandhar, E.; Leeper, T.C.; Modarelli, D.A.; Medicetty, S.; Shriver, L.P. Cuprizone Intoxication Induces Cell Intrinsic Alterations in Oligodendrocyte Metabolism Independent of Copper Chelation. Biochemistry 2017, 56, 1518–1528. [Google Scholar] [CrossRef]
- Morgan, M.L.; Teo, W.; Hernandez, Y.; Brideau, C.; Cummins, K.; Kuipers, H.F.; Stys, P.K. Cuprizone-induced Demyelination in Mouse Brain is not due to Depletion of Copper. ASN Neuro 2022, 14, 17590914221126367. [Google Scholar] [CrossRef]
- Gudi, V.; Moharregh-Khiabani, D.; Skripuletz, T.; Koutsoudaki, P.N.; Kotsiari, A.; Skuljec, J.; Trebst, C.; Stangel, M. Regional differences between grey and white matter in cuprizone induced demyelination. Brain Res. 2009, 1283, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Vana, A.C.; Flint, N.C.; Harwood, N.E.; Le, T.Q.; Fruttiger, M.; Armstrong, R.C. Platelet-derived growth factor promotes repair of chronically demyelinated white matter. J. Neuropathol. Exp. Neurol. 2007, 66, 975–988. [Google Scholar] [CrossRef]
- Fessel, J. Abnormal oligodendrocyte function in schizophrenia explains the long latent interval in some patients. Transl. Psychiatry 2022, 12, 120. [Google Scholar] [CrossRef]
- Gouvêa-Junqueira, D.; Falvella, A.C.B.; Antunes, A.; Seabra, G.; Brandão-Teles, C.; Martins-de-Souza, D.; Crunfli, F. Novel Treatment Strategies Targeting Myelin and Oligodendrocyte Dysfunction in Schizophrenia. Front. Psychiatry 2020, 11, 379. [Google Scholar] [CrossRef]
- Behrangi, N.; Heinig, L.; Frintrop, L.; Santrau, E.; Kurth, J.; Krause, B.; Atanasova, D.; Clarner, T.; Fragoulis, A.; Joksch, M.; et al. Siponimod ameliorates metabolic oligodendrocyte injury via the sphingosine-1 phosphate receptor 5. Proc. Natl. Acad. Sci. USA 2022, 119, e2204509119. [Google Scholar] [CrossRef]
- Gudi, V.; Gingele, S.; Skripuletz, T.; Stangel, M. Glial response during cuprizone-induced de- and remyelination in the CNS: Lessons learned. Front. Cell. Neurosci. 2014, 8, 73. [Google Scholar] [CrossRef] [PubMed]
- Ludwin, S.K. An autoradiographic study of cellular proliferation in remyelination of the central nervous system. Am. J. Pathol. 1979, 95, 683–696. [Google Scholar] [PubMed]
- Hu, V.W.; Black, G.E.; Torres-Duarte, A.; Abramson, F.P. 3H-thymidine is a defective tool with which to measure rates of DNA synthesis. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2002, 16, 1456–1457. [Google Scholar] [CrossRef]
- Hiremath, M.M.; Saito, Y.; Knapp, G.W.; Ting, J.P.; Suzuki, K.; Matsushima, G.K. Microglial/macrophage accumulation during cuprizone-induced demyelination in C57BL/6 mice. J. Neuroimmunol. 1998, 92, 38–49. [Google Scholar] [CrossRef]
- Mackenzie, A. Immunohistochemical demonstration of glial fibrillary acidic protein in scrapie. J. Comp. Pathol. 1983, 93, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Norkute, A.; Hieble, A.; Braun, A.; Johann, S.; Clarner, T.; Baumgartner, W.; Beyer, C.; Kipp, M. Cuprizone treatment induces demyelination and astrocytosis in the mouse hippocampus. J. Neurosci. Res. 2009, 87, 1343–1355. [Google Scholar] [CrossRef]
- Koutsoudaki, P.N.; Skripuletz, T.; Gudi, V.; Moharregh-Khiabani, D.; Hildebrandt, H.; Trebst, C.; Stangel, M. Demyelination of the hippocampus is prominent in the cuprizone model. Neurosci. Lett. 2009, 451, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Skripuletz, T.; Bussmann, J.H.; Gudi, V.; Koutsoudaki, P.N.; Pul, R.; Moharregh-Khiabani, D.; Lindner, M.; Stangel, M. Cerebellar cortical demyelination in the murine cuprizone model. Brain Pathol. 2010, 20, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Steelman, A.J.; Thompson, J.P.; Li, J. Demyelination and remyelination in anatomically distinct regions of the corpus callosum following cuprizone intoxication. Neurosci. Res. 2012, 72, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.; Awad, H.; Slowik, A.; Beyer, C.; Kipp, M.; Clarner, T. Regional heterogeneity of cuprizone-induced demyelination: Topographical aspects of the midline of the corpus callosum. J. Mol. Neurosci. 2013, 49, 80–88. [Google Scholar] [CrossRef]
- Wagenknecht, N.; Becker, B.; Scheld, M.; Beyer, C.; Clarner, T.; Hochstrasser, T.; Kipp, M. Thalamus Degeneration and Inflammation in Two Distinct Multiple Sclerosis Animal Models. J. Mol. Neurosci. 2016, 60, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Sen, M.K.; Almuslehi, M.S.M.; Coorssen, J.R.; Mahns, D.A.; Shortland, P.J. Behavioural and histological changes in cuprizone-fed mice. Brain Behav. Immun. 2020, 87, 508–523. [Google Scholar] [CrossRef]
- Komoly, S.; Hudson, L.D.; Webster, H.D.; Bondy, C.A. Insulin-like growth factor I gene expression is induced in astrocytes during experimental demyelination. Proc. Natl. Acad. Sci. USA 1992, 89, 1894–1898. [Google Scholar] [CrossRef] [PubMed]
- Cannella, B.; Pitt, D.; Capello, E.; Raine, C.S. Insulin-like growth factor-1 fails to enhance central nervous system myelin repair during autoimmune demyelination. Am. J. Pathol. 2000, 157, 933–943. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lovett-Racke, A.E.; Bittner, P.; Cross, A.H.; Carlino, J.A.; Racke, M.K. Regulation of experimental autoimmune encephalomyelitis with insulin-like growth factor (IGF-1) and IGF-1/IGF-binding protein-3 complex (IGF-1/IGFBP3). J. Clin. Investig. 1998, 101, 1797–1804. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Gillig, T.A.; Ye, P.; D’Ercole, A.J.; Matsushima, G.K.; Popko, B. Interferon-gamma protects against cuprizone-induced demyelination. Mol. Cell. Neurosci. 2000, 16, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Mason, J.L.; Suzuki, K.; Chaplin, D.D.; Matsushima, G.K. Interleukin-1beta promotes repair of the CNS. J. Neurosci. 2001, 21, 7046–7052. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.K.; Baidoo, K.; Verina, T.; Guilarte, T.R. Peripheral benzodiazepine receptor imaging in CNS demyelination: Functional implications of anatomical and cellular localization. Brain 2004, 127 Pt 6, 1379–1392. [Google Scholar] [CrossRef] [PubMed]
- Wickstrøm, T.; Clarke, A.; Gausemel, I.; Horn, E.; Jørgensen, K.; Khan, I.; Mantzilas, D.; Rajanayagam, T.; in ’t Veld, D.J.; Trigg, W. The development of an automated and GMP compliant FASTlab™ Synthesis of [(18) F]GE-180; a radiotracer for imaging translocator protein (TSPO). J. Label. Compd. Radiopharm. 2014, 57, 42–48. [Google Scholar] [CrossRef]
- Nack, A.; Brendel, M.; Nedelcu, J.; Daerr, M.; Nyamoya, S.; Beyer, C.; Focke, C.; Deussing, M.; Hoornaert, C.; Ponsaerts, P.; et al. Expression of Translocator Protein and [18F]-GE180 Ligand Uptake in Multiple Sclerosis Animal Models. Cells 2019, 8, 94. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.K.; Guilarte, T.R. Imaging the peripheral benzodiazepine receptor response in central nervous system demyelination and remyelination. Toxicol. Sci. 2006, 91, 532–539. [Google Scholar] [CrossRef]
- Mattner, F.; Bandin, D.L.; Staykova, M.; Berghofer, P.; Gregoire, M.C.; Ballantyne, P.; Quinlivan, M.; Fordham, S.; Pham, T.; Willenborg, D.O.; et al. Evaluation of [¹²³I]-CLINDE as a potent SPECT radiotracer to assess the degree of astroglia activation in cuprizone-induced neuroinflammation. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 1516–1528. [Google Scholar] [CrossRef] [PubMed]
- Zinnhardt, B.; Belloy, M.; Fricke, I.B.; Orije, J.; Guglielmetti, C.; Hermann, S.; Wagner, S.; Schäfers, M.; Van der Linden, A.; Jacobs, A.H. Molecular Imaging of Immune Cell Dynamics During De- and Remyelination in the Cuprizone Model of Multiple Sclerosis by [(18)F]DPA-714 PET and MRI. Theranostics 2019, 9, 1523–1537. [Google Scholar] [CrossRef]
- Unterrainer, M.; Mahler, C.; Vomacka, L.; Lindner, S.; Havla, J.; Brendel, M.; Böning, G.; Ertl-Wagner, B.; Kümpfel, T.; Milenkovic, V.M.; et al. TSPO PET with [(18)F]GE-180 sensitively detects focal neuroinflammation in patients with relapsing-remitting multiple sclerosis. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1423–1431. [Google Scholar] [CrossRef]
- Vomacka, L.; Albert, N.L.; Lindner, S.; Unterrainer, M.; Mahler, C.; Brendel, M.; Ermoschkin, L.; Gosewisch, A.; Brunegraf, A.; Buckley, C.; et al. TSPO imaging using the novel PET ligand [(18)F]GE-180: Quantification approaches in patients with multiple sclerosis. EJNMMI Res. 2017, 7, 89. [Google Scholar] [CrossRef]
- Nutma, E.; Fancy, N.; Weinert, M.; Tsartsalis, S.; Marzin, M.C.; Muirhead, R.C.J.; Falk, I.; Breur, M.; de Bruin, J.; Hollaus, D.; et al. Translocator protein is a marker of activated microglia in rodent models but not human neurodegenerative diseases. Nat. Commun. 2023, 14, 5247. [Google Scholar] [CrossRef] [PubMed]
- Bush, T.G.; Savidge, T.C.; Freeman, T.C.; Cox, H.J.; Campbell, E.A.; Mucke, L.; Johnson, M.H.; Sofroniew, M.V. Fulminant jejuno-ileitis following ablation of enteric glia in adult transgenic mice. Cell 1998, 93, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Zhou, Y.; Cai, Z.; Terekhova, M.; Swain, A.; Andhey, P.S.; Guimaraes, R.M.; Ulezko Antonova, A.; Qiu, T.; Sviben, S.; et al. Transcriptomic atlas and interaction networks of brain cells in mouse CNS demyelination and remyelination. Cell Rep. 2023, 42, 112293. [Google Scholar] [CrossRef] [PubMed]
- Hibbits, N.; Yoshino, J.; Le, T.Q.; Armstrong, R.C. Astrogliosis during acute and chronic cuprizone demyelination and implications for remyelination. ASN Neuro 2012, 4, 393–408. [Google Scholar] [CrossRef] [PubMed]
- Skripuletz, T.; Hackstette, D.; Bauer, K.; Gudi, V.; Pul, R.; Voss, E.; Berger, K.; Kipp, M.; Baumgärtner, W.; Stangel, M. Astrocytes regulate myelin clearance through recruitment of microglia during cuprizone-induced demyelination. Brain 2013, 136 Pt 1, 147–167. [Google Scholar] [CrossRef] [PubMed]
- Höflich, K.M.; Beyer, C.; Clarner, T.; Schmitz, C.; Nyamoya, S.; Kipp, M.; Hochstrasser, T. Acute axonal damage in three different murine models of multiple sclerosis: A comparative approach. Brain Res. 2016, 1650, 125–133. [Google Scholar] [CrossRef]
- Hochstrasser, T.; Rühling, S.; Hecher, K.; Fabisch, K.H.; Chrzanowski, U.; Brendel, M.; Eckenweber, F.; Sacher, C.; Schmitz, C.; Kipp, M. Stereological Investigation of Regional Brain Volumes after Acute and Chronic Cuprizone-Induced Demyelination. Cells 2019, 8, 1024. [Google Scholar] [CrossRef] [PubMed]
- Schröder, L.J.; Mulenge, F.; Pavlou, A.; Skripuletz, T.; Stangel, M.; Gudi, V.; Kalinke, U. Dynamics of reactive astrocytes fosters tissue regeneration after cuprizone-induced demyelination. Glia 2023, 71, 2573–2590. [Google Scholar] [CrossRef] [PubMed]
- Clarner, T.; Janssen, K.; Nellessen, L.; Stangel, M.; Skripuletz, T.; Krauspe, B.; Hess, F.M.; Denecke, B.; Beutner, C.; Linnartz-Gerlach, B.; et al. CXCL10 triggers early microglial activation in the cuprizone model. J. Immunol. 2015, 194, 3400–3413. [Google Scholar] [CrossRef]
- Casper, K.B.; McCarthy, K.D. GFAP-positive progenitor cells produce neurons and oligodendrocytes throughout the CNS. Mol. Cell. Neurosci. 2006, 31, 676–684. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.; Barragan, J.; Bashiruddin, S.; Smith, C.J.; Tyrrell, C.; Parsons, M.J.; Doris, R.; Kucenas, S.; Downes, G.B.; Velez, C.M.; et al. Gfap-positive radial glial cells are an essential progenitor population for later-born neurons and glia in the zebrafish spinal cord. Glia 2016, 64, 1170–1189. [Google Scholar] [CrossRef]
- Lennon, V.A.; Kryzer, T.J.; Pittock, S.J.; Verkman, A.S.; Hinson, S.R. IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J. Exp. Med. 2005, 202, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Hinson, S.R.; Pittock, S.J.; Lucchinetti, C.F.; Roemer, S.F.; Fryer, J.P.; Kryzer, T.J.; Lennon, V.A. Pathogenic potential of IgG binding to water channel extracellular domain in neuromyelitis optica. Neurology 2007, 69, 2221–2231. [Google Scholar] [CrossRef]
- Sághy, É.; Sipos, É.; Ács, P.; Bölcskei, K.; Pohóczky, K.; Kemény, Á.; Sándor, Z.; Szőke, É.; Sétáló, G., Jr.; Komoly, S.; et al. TRPA1 deficiency is protective in cuprizone-induced demyelination-A new target against oligodendrocyte apoptosis. Glia 2016, 64, 2166–2180. [Google Scholar] [CrossRef]
- Gasterich, N.; Bohn, A.; Sesterhenn, A.; Nebelo, F.; Fein, L.; Kaddatz, H.; Nyamoya, S.; Kant, S.; Kipp, M.; Weiskirchen, R.; et al. Lipocalin 2 attenuates oligodendrocyte loss and immune cell infiltration in mouse models for multiple sclerosis. Glia 2022, 70, 2188–2206. [Google Scholar] [CrossRef]
- Plant, S.R.; Arnett, H.A.; Ting, J.P. Astroglial-derived lymphotoxin-alpha exacerbates inflammation and demyelination, but not remyelination. Glia 2005, 49, 1–14. [Google Scholar] [CrossRef]
- Raasch, J.; Zeller, N.; van Loo, G.; Merkler, D.; Mildner, A.; Erny, D.; Knobeloch, K.P.; Bethea, J.R.; Waisman, A.; Knust, M.; et al. IkappaB kinase 2 determines oligodendrocyte loss by non-cell-autonomous activation of NF-kappaB in the central nervous system. Brain 2011, 134 Pt 4, 1184–1198. [Google Scholar] [CrossRef]
- Pasquini, L.A.; Millet, V.; Hoyos, H.C.; Giannoni, J.P.; Croci, D.O.; Marder, M.; Liu, F.T.; Rabinovich, G.A.; Pasquini, J.M. Galectin-3 drives oligodendrocyte differentiation to control myelin integrity and function. Cell Death Differ. 2011, 18, 1746–1756. [Google Scholar] [CrossRef]
- Patel, J.R.; Williams, J.L.; Muccigrosso, M.M.; Liu, L.; Sun, T.; Rubin, J.B.; Klein, R.S. Astrocyte TNFR2 is required for CXCL12-mediated regulation of oligodendrocyte progenitor proliferation and differentiation within the adult CNS. Acta Neuropathol. 2012, 124, 847–860. [Google Scholar] [CrossRef] [PubMed]
- Fulmer, C.G.; VonDran, M.W.; Stillman, A.A.; Huang, Y.; Hempstead, B.L.; Dreyfus, C.F. Astrocyte-derived BDNF supports myelin protein synthesis after cuprizone-induced demyelination. J. Neurosci. 2014, 34, 8186–8196. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.; Piaton, G.; Lubetzki, C. Astrocytes--friends or foes in multiple sclerosis? Glia 2007, 55, 1300–1312. [Google Scholar] [CrossRef] [PubMed]
- Correale, J.; Farez, M.F. The Role of Astrocytes in Multiple Sclerosis Progression. Front. Neurol. 2015, 6, 180. [Google Scholar] [CrossRef]
- Madadi, S.; Pasbakhsh, P.; Tahmasebi, F.; Mortezaee, K.; Khanehzad, M.; Boroujeni, F.B.; Noorzehi, G.; Kashani, I.R. Astrocyte ablation induced by La-aminoadipate (L-AAA) potentiates remyelination in a cuprizone demyelinating mouse model. Metab. Brain Dis. 2019, 34, 593–603. [Google Scholar] [CrossRef]
- Cammer, W.; Zhang, H. Atypical localization of the oligodendrocytic isoform (PI) of glutathione-S-transferase in astrocytes during cuprizone intoxication. J. Neurosci. Res. 1993, 36, 183–190. [Google Scholar] [CrossRef]
- Tansey, F.A.; Zhang, H.; Cammer, W. Rapid upregulation of the Pi isoform of glutathione-S-transferase in mouse brains after withdrawal of the neurotoxicant, cuprizone. Mol. Chem. Neuropathol. 1997, 31, 161–170. [Google Scholar] [CrossRef]
- McMahon, E.J.; Cook, D.N.; Suzuki, K.; Matsushima, G.K. Absence of macrophage-inflammatory protein-1alpha delays central nervous system demyelination in the presence of an intact blood-brain barrier. J. Immunol. 2001, 167, 2964–2971. [Google Scholar] [CrossRef]
- Arnett, H.A.; Wang, Y.; Matsushima, G.K.; Suzuki, K.; Ting, J.P. Functional genomic analysis of remyelination reveals importance of inflammation in oligodendrocyte regeneration. J. Neurosci. 2003, 23, 9824–9832. [Google Scholar] [CrossRef]
- Woodruff, R.H.; Fruttiger, M.; Richardson, W.D.; Franklin, R.J. Platelet-derived growth factor regulates oligodendrocyte progenitor numbers in adult CNS and their response following CNS demyelination. Mol. Cell. Neurosci. 2004, 25, 252–262. [Google Scholar] [CrossRef]
- Selvaraju, R.; Bernasconi, L.; Losberger, C.; Graber, P.; Kadi, L.; Avellana-Adalid, V.; Picard-Riera, N.; Baron Van Evercooren, A.; Cirillo, R.; Kosco-Vilbois, M.; et al. Osteopontin is upregulated during in vivo demyelination and remyelination and enhances myelin formation in vitro. Mol. Cell. Neurosci. 2004, 25, 707–721. [Google Scholar] [CrossRef] [PubMed]
- Stidworthy, M.F.; Genoud, S.; Li, W.W.; Leone, D.P.; Mantei, N.; Suter, U.; Franklin, R.J. Notch1 and Jagged1 are expressed after CNS demyelination, but are not a major rate-determining factor during remyelination. Brain 2004, 127 Pt 9, 1928–1941. [Google Scholar] [CrossRef] [PubMed]
- Zatta, P.; Raso, M.; Zambenedetti, P.; Wittkowski, W.; Messori, L.; Piccioli, F.; Mauri, P.L.; Beltramini, M. Copper and zinc dismetabolism in the mouse brain upon chronic cuprizone treatment. Cell. Mol. Life Sci. CMLS 2005, 62, 1502–1513. [Google Scholar] [CrossRef] [PubMed]
- Irvine, K.A.; Blakemore, W.F. Age increases axon loss associated with primary demyelination in cuprizone-induced demyelination in C57BL/6 mice. J. Neuroimmunol. 2006, 175, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Briggs, D.T.; Martin, C.B.; Ingersoll, S.A.; Barnum, S.R.; Martin, B.K. Astrocyte-specific expression of a soluble form of the murine complement control protein Crry confers demyelination protection in the cuprizone model. Glia 2007, 55, 1405–1415. [Google Scholar] [CrossRef]
- Song, S.Y.; Kato, C.; Adachi, E.; Moriya-Sato, A.; Inagawa-Ogashiwa, M.; Umeda, R.; Hashimoto, N. Expression of an acyl-CoA synthetase, lipidosin, in astrocytes of the murine brain and its up-regulation during remyelination following cuprizone-induced demyelination. J. Neurosci. Res. 2007, 85, 3586–3597. [Google Scholar] [CrossRef]
- Biancotti, J.C.; Kumar, S.; de Vellis, J. Activation of inflammatory response by a combination of growth factors in cuprizone-induced demyelinated brain leads to myelin repair. Neurochem. Res. 2008, 33, 2615–2628. [Google Scholar] [CrossRef]
- Merkler, D.; Klinker, F.; Jürgens, T.; Glaser, R.; Paulus, W.; Brinkmann, B.G.; Sereda, M.W.; Stadelmann-Nessler, C.; Guedes, R.C.; Brück, W.; et al. Propagation of spreading depression inversely correlates with cortical myelin content. Ann. Neurol. 2009, 66, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Doucette, J.R.; Jiao, R.; Nazarali, A.J. Age-related and cuprizone-induced changes in myelin and transcription factor gene expression and in oligodendrocyte cell densities in the rostral corpus callosum of mice. Cell. Mol. Neurobiol. 2010, 30, 607–629. [Google Scholar] [CrossRef] [PubMed]
- Baertling, F.; Kokozidou, M.; Pufe, T.; Clarner, T.; Windoffer, R.; Wruck, C.J.; Brandenburg, L.O.; Beyer, C.; Kipp, M. ADAM12 is expressed by astrocytes during experimental demyelination. Brain Res. 2010, 1326, 1–14. [Google Scholar] [CrossRef]
- Cate, H.S.; Sabo, J.K.; Merlo, D.; Kemper, D.; Aumann, T.D.; Robinson, J.; Merson, T.D.; Emery, B.; Perreau, V.M.; Kilpatrick, T.J. Modulation of bone morphogenic protein signalling alters numbers of astrocytes and oligodendroglia in the subventricular zone during cuprizone-induced demyelination. J. Neurochem. 2010, 115, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.R.; McCandless, E.E.; Dorsey, D.; Klein, R.S. CXCR4 promotes differentiation of oligodendrocyte progenitors and remyelination. Proc. Natl. Acad. Sci. USA 2010, 107, 11062–11067. [Google Scholar] [CrossRef]
- Parenti, R.; Cicirata, F.; Zappalà, A.; Catania, A.; La Delia, F.; Cicirata, V.; Tress, O.; Willecke, K. Dynamic expression of Cx47 in mouse brain development and in the cuprizone model of myelin plasticity. Glia 2010, 58, 1594–1609. [Google Scholar] [CrossRef]
- Clarner, T.; Buschmann, J.P.; Beyer, C.; Kipp, M. Glial amyloid precursor protein expression is restricted to astrocytes in an experimental toxic model of multiple sclerosis. J. Mol. Neurosci. 2011, 43, 268–274. [Google Scholar] [CrossRef]
- Ingersoll, S.A.; Martin, C.B.; Barnum, S.R.; Martin, B.K. CNS-specific expression of C3a and C5a exacerbate demyelination severity in the cuprizone model. Mol. Immunol. 2010, 48, 219–230. [Google Scholar] [CrossRef]
- Crocker, S.J.; Bajpai, R.; Moore, C.S.; Frausto, R.F.; Brown, G.D.; Pagarigan, R.R.; Whitton, J.L.; Terskikh, A.V. Intravenous administration of human embryonic stem cell-derived neural precursor cells attenuates cuprizone-induced central nervous system (CNS) demyelination. Neuropathol. Appl. Neurobiol. 2011, 37, 643–653. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hegarty, S.V.; O’Keeffe, G.W.; Sullivan, A.M. BMP-Smad 1/5/8 signalling in the development of the nervous system. Prog. Neurobiol. 2013, 109, 28–41. [Google Scholar] [CrossRef]
- Palumbo, S.; Toscano, C.D.; Parente, L.; Weigert, R.; Bosetti, F. Time-dependent changes in the brain arachidonic acid cascade during cuprizone-induced demyelination and remyelination. Prostaglandins Leukot. Essent. Fat. Acids 2011, 85, 29–35. [Google Scholar] [CrossRef][Green Version]
- Hoyos, H.C.; Marder, M.; Ulrich, R.; Gudi, V.; Stangel, M.; Rabinovich, G.A.; Pasquini, L.A.; Pasquini, J.M. The Role of Galectin-3: From Oligodendroglial Differentiation and Myelination to Demyelination and Remyelination Processes in a Cuprizone-Induced Demyelination Model. Adv. Exp. Med. Biol. 2016, 949, 311–332. [Google Scholar] [CrossRef]
- Kim, S.; Steelman, A.J.; Zhang, Y.; Kinney, H.C.; Li, J. Aberrant upregulation of astroglial ceramide potentiates oligodendrocyte injury. Brain Pathol. 2012, 22, 41–57. [Google Scholar] [CrossRef]
- Kipp, M.; Gingele, S.; Pott, F.; Clarner, T.; van der Valk, P.; Denecke, B.; Gan, L.; Siffrin, V.; Zipp, F.; Dreher, W.; et al. BLBP-expression in astrocytes during experimental demyelination and in human multiple sclerosis lesions. Brain Behav. Immun. 2011, 25, 1554–1568. [Google Scholar] [CrossRef]
- Gudi, V.; Škuljec, J.; Yildiz, Ö.; Frichert, K.; Skripuletz, T.; Moharregh-Khiabani, D.; Voss, E.; Wissel, K.; Wolter, S.; Stangel, M. Spatial and temporal profiles of growth factor expression during CNS demyelination reveal the dynamics of repair priming. PLoS ONE 2011, 6, e22623. [Google Scholar] [CrossRef] [PubMed]
- Skuljec, J.; Gudi, V.; Ulrich, R.; Frichert, K.; Yildiz, O.; Pul, R.; Voss, E.V.; Wissel, K.; Baumgärtner, W.; Stangel, M. Matrix metalloproteinases and their tissue inhibitors in cuprizone-induced demyelination and remyelination of brain white and gray matter. J. Neuropathol. Exp. Neurol. 2011, 70, 758–769. [Google Scholar] [CrossRef]
- Silvestroff, L.; Bartucci, S.; Pasquini, J.; Franco, P. Cuprizone-induced demyelination in the rat cerebral cortex and thyroid hormone effects on cortical remyelination. Exp. Neurol. 2012, 235, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Kang, Z.; Liu, L.; Spangler, R.; Spear, C.; Wang, C.; Gulen, M.F.; Veenstra, M.; Ouyang, W.; Ransohoff, R.M.; Li, X. IL-17-induced Act1-mediated signaling is critical for cuprizone-induced demyelination. J. Neurosci. 2012, 32, 8284–8292. [Google Scholar] [CrossRef]
- Brück, W.; Pförtner, R.; Pham, T.; Zhang, J.; Hayardeny, L.; Piryatinsky, V.; Hanisch, U.K.; Regen, T.; van Rossum, D.; Brakelmann, L.; et al. Reduced astrocytic NF-κB activation by laquinimod protects from cuprizone-induced demyelination. Acta Neuropathol. 2012, 124, 411–424. [Google Scholar] [CrossRef] [PubMed]
- Sabo, J.K.; Aumann, T.D.; Kilpatrick, T.J.; Cate, H.S. Investigation of sequential growth factor delivery during cuprizone challenge in mice aimed to enhance oligodendrogliogenesis and myelin repair. PLoS ONE 2013, 8, e63415. [Google Scholar] [CrossRef]
- Azami Tameh, A.; Clarner, T.; Beyer, C.; Atlasi, M.A.; Hassanzadeh, G.; Naderian, H. Regional regulation of glutamate signaling during cuprizone-induced demyelination in the brain. Ann. Anat. 2013, 195, 415–423. [Google Scholar] [CrossRef]
- Kanekiyo, K.; Inamori, K.; Kitazume, S.; Sato, K.; Maeda, J.; Higuchi, M.; Kizuka, Y.; Korekane, H.; Matsuo, I.; Honke, K.; et al. Loss of branched O-mannosyl glycans in astrocytes accelerates remyelination. J. Neurosci. 2013, 33, 10037–10047. [Google Scholar] [CrossRef] [PubMed]
- Tezuka, T.; Tamura, M.; Kondo, M.A.; Sakaue, M.; Okada, K.; Takemoto, K.; Fukunari, A.; Miwa, K.; Ohzeki, H.; Kano, S.; et al. Cuprizone short-term exposure: Astrocytic IL-6 activation and behavioral changes relevant to psychosis. Neurobiol. Dis. 2013, 59, 63–68. [Google Scholar] [CrossRef]
- Janssens, K.; Maheshwari, A.; Van den Haute, C.; Baekelandt, V.; Stinissen, P.; Hendriks, J.J.; Slaets, H.; Hellings, N. Oncostatin M protects against demyelination by inducing a protective microglial phenotype. Glia 2015, 63, 1729–1737. [Google Scholar] [CrossRef]
- Okazaki, R.; Doi, T.; Hayakawa, K.; Morioka, K.; Imamura, O.; Takishima, K.; Hamanoue, M.; Sawada, Y.; Nagao, M.; Tanaka, S.; et al. The crucial role of Erk2 in demyelinating inflammation in the central nervous system. J. Neuroinflammation 2016, 13, 235. [Google Scholar] [CrossRef] [PubMed]
- Petković, F.; Campbell, I.L.; Gonzalez, B.; Castellano, B. Astrocyte-targeted production of interleukin-6 reduces astroglial and microglial activation in the cuprizone demyelination model: Implications for myelin clearance and oligodendrocyte maturation. Glia 2016, 64, 2104–2119. [Google Scholar] [CrossRef] [PubMed]
- Draheim, T.; Liessem, A.; Scheld, M.; Wilms, F.; Weißflog, M.; Denecke, B.; Kensler, T.W.; Zendedel, A.; Beyer, C.; Kipp, M.; et al. Activation of the astrocytic Nrf2/ARE system ameliorates the formation of demyelinating lesions in a multiple sclerosis animal model. Glia 2016, 64, 2219–2230. [Google Scholar] [CrossRef]
- Zimmermann, J.; Emrich, M.; Krauthausen, M.; Saxe, S.; Nitsch, L.; Heneka, M.T.; Campbell, I.L.; Müller, M. IL-17A Promotes Granulocyte Infiltration, Myelin Loss, Microglia Activation, and Behavioral Deficits During Cuprizone-Induced Demyelination. Mol. Neurobiol. 2018, 55, 946–957. [Google Scholar] [CrossRef] [PubMed]
- Espitia Pinzon, N.; Sanz-Morello, B.; Brevé, J.J.; Bol, J.G.; Drukarch, B.; Bauer, J.; Baron, W.; van Dam, A.M. Astrocyte-derived tissue Transglutaminase affects fibronectin deposition, but not aggregation, during cuprizone-induced demyelination. Sci. Rep. 2017, 7, 40995. [Google Scholar] [CrossRef]
- Petković, F.; Campbell, I.L.; Gonzalez, B.; Castellano, B. Reduced cuprizone-induced cerebellar demyelination in mice with astrocyte-targeted production of IL-6 is associated with chronically activated, but less responsive microglia. J. Neuroimmunol. 2017, 310, 97–102. [Google Scholar] [CrossRef]
- Kim, S.; Bielawski, J.; Yang, H.; Kong, Y.; Zhou, B.; Li, J. Functional antagonism of sphingosine-1-phosphate receptor 1 prevents cuprizone-induced demyelination. Glia 2018, 66, 654–669. [Google Scholar] [CrossRef]
- Sanadgol, N.; Golab, F.; Mostafaie, A.; Mehdizadeh, M.; Khalseh, R.; Mahmoudi, M.; Abdollahi, M.; Vakilzadeh, G.; Taghizadeh, G.; Sharifzadeh, M. Low, but not high, dose triptolide controls neuroinflammation and improves behavioral deficits in toxic model of multiple sclerosis by dampening of NF-κB activation and acceleration of intrinsic myelin repair. Toxicol. Appl. Pharmacol. 2018, 342, 86–98. [Google Scholar] [CrossRef]
- Sanchez, M.A.; Sullivan, G.M.; Armstrong, R.C. Genetic detection of Sonic hedgehog (Shh) expression and cellular response in the progression of acute through chronic demyelination and remyelination. Neurobiol. Dis. 2018, 115, 145–156. [Google Scholar] [CrossRef]
- Omotoso, G.O.; Ukwubile, I.I.; Arietarhire, L.; Sulaimon, F.; Gbadamosi, I.T. Kolaviron protects the brain in cuprizone-induced model of experimental multiple sclerosis via enhancement of intrinsic antioxidant mechanisms: Possible therapeutic applications? Pathophysiology 2018, 25, 299–306. [Google Scholar] [CrossRef]
- Jakovac, H.; Grubić Kezele, T.; Radošević-Stašić, B. Expression Profiles of Metallothionein I/II and Megalin in Cuprizone Model of De- and Remyelination. Neuroscience 2018, 388, 69–86. [Google Scholar] [CrossRef]
- Mokhtarzadeh Khanghahi, A.; Satarian, L.; Deng, W.; Baharvand, H.; Javan, M. In vivo conversion of astrocytes into oligodendrocyte lineage cells with transcription factor Sox10; Promise for myelin repair in multiple sclerosis. PLoS ONE 2018, 13, e0203785. [Google Scholar] [CrossRef]
- Zare, L.; Baharvand, H.; Javan, M. In vivo conversion of astrocytes to oligodendrocyte lineage cells using chemicals: Targeting gliosis for myelin repair. Regen. Med. 2018, 13, 803–819. [Google Scholar] [CrossRef] [PubMed]
- Farhangi, S.; Dehghan, S.; Totonchi, M.; Javan, M. In vivo conversion of astrocytes to oligodendrocyte lineage cells in adult mice demyelinated brains by Sox2. Mult. Scler. Relat. Disord. 2019, 28, 263–272. [Google Scholar] [CrossRef]
- Carden, T.R.; Correale, J.; Pasquini, J.M.; Pérez, M.J. Transferrin Enhances Microglial Phagocytic Capacity. Mol. Neurobiol. 2019, 56, 6324–6340. [Google Scholar] [CrossRef]
- Kramann, N.; Menken, L.; Pförtner, R.; Schmid, S.N.; Stadelmann, C.; Wegner, C.; Brück, W. Glial fibrillary acidic protein expression alters astrocytic chemokine release and protects mice from cuprizone-induced demyelination. Glia 2019, 67, 1308–1319. [Google Scholar] [CrossRef]
- Barati, S.; Kashani, I.R.; Tahmasebi, F.; Mehrabi, S.; Joghataei, M.T. Effect of mesenchymal stem cells on glial cells population in cuprizone induced demyelination model. Neuropeptides 2019, 75, 75–84. [Google Scholar] [CrossRef]
- Roboon, J.; Hattori, T.; Ishii, H.; Takarada-Iemata, M.; Le, T.M.; Shiraishi, Y.; Ozaki, N.; Yamamoto, Y.; Sugawara, A.; Okamoto, H.; et al. Deletion of CD38 Suppresses Glial Activation and Neuroinflammation in a Mouse Model of Demyelination. Front. Cell. Neurosci. 2019, 13, 258. [Google Scholar] [CrossRef]
- Li, Q.Y.; Miao, Q.; Sui, R.X.; Cao, L.; Ma, C.G.; Xiao, B.G.; Xiao, W.; Yu, W.B. Ginkgolide K supports remyelination via induction of astrocytic IGF/PI3K/Nrf2 axis. Int. Immunopharmacol. 2019, 75, 105819. [Google Scholar] [CrossRef]
- An, J.; Yin, J.J.; He, Y.; Sui, R.X.; Miao, Q.; Wang, Q.; Yu, J.Z.; Yu, J.W.; Shi, F.D.; Ma, C.G.; et al. Temporal and Spatial Dynamics of Astroglial Reaction and Immune Response in Cuprizone-Induced Demyelination. Neurotox. Res. 2020, 37, 587–601. [Google Scholar] [CrossRef]
- Kriszta, G.; Nemes, B.; Sándor, Z.; Ács, P.; Komoly, S.; Berente, Z.; Bölcskei, K.; Pintér, E. Investigation of Cuprizone-Induced Demyelination in mGFAP-Driven Conditional Transient Receptor Potential Ankyrin 1 (TRPA1) Receptor Knockout Mice. Cells 2019, 9, 81. [Google Scholar] [CrossRef]
- Yoon, H.; Choi, C.I.; Triplet, E.M.; Langley, M.R.; Kleppe, L.S.; Kim, H.N.; Simon, W.L.; Scarisbrick, I.A. Blocking the Thrombin Receptor Promotes Repair of Demyelinated Lesions in the Adult Brain. J. Neurosci. 2020, 40, 1483–1500. [Google Scholar] [CrossRef]
- Yin, J.J.; He, Y.; An, J.; Miao, Q.; Sui, R.X.; Wang, Q.; Yu, J.Z.; Xiao, B.G.; Ma, C.G. Dynamic Balance of Microglia and Astrocytes Involved in the Remyelinating Effect of Ginkgolide B. Front. Cell. Neurosci. 2019, 13, 572. [Google Scholar] [CrossRef]
- Zamora, N.N.; Cheli, V.T.; Santiago González, D.A.; Wan, R.; Paez, P.M. Deletion of Voltage-Gated Calcium Channels in Astrocytes during Demyelination Reduces Brain Inflammation and Promotes Myelin Regeneration in Mice. J. Neurosci. 2020, 40, 3332–3347. [Google Scholar] [CrossRef] [PubMed]
- Bernal-Chico, A.; Manterola, A.; Cipriani, R.; Katona, I.; Matute, C.; Mato, S. P2x7 receptors control demyelination and inflammation in the cuprizone model. Brain Behav. Immun. Health 2020, 4, 100062. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, G.M.; Knutsen, A.K.; Peruzzotti-Jametti, L.; Korotcov, A.; Bosomtwi, A.; Dardzinski, B.J.; Bernstock, J.D.; Rizzi, S.; Edenhofer, F.; Pluchino, S.; et al. Transplantation of induced neural stem cells (iNSCs) into chronically demyelinated corpus callosum ameliorates motor deficits. Acta Neuropathol. Commun. 2020, 8, 84. [Google Scholar] [CrossRef] [PubMed]
- Reinbach, C.; Stadler, M.S.; Pröbstl, N.; Chrzanowski, U.; Schmitz, C.; Kipp, M.; Hochstrasser, T. CD44 expression in the cuprizone model. Brain Res. 2020, 1745, 146950. [Google Scholar] [CrossRef]
- Luo, M.; Deng, M.; Yu, Z.; Zhang, Y.; Xu, S.; Hu, S.; Xu, H. Differential Susceptibility and Vulnerability of Brain Cells in C57BL/6 Mouse to Mitochondrial Dysfunction Induced by Short-Term Cuprizone Exposure. Front. Neuroanat. 2020, 14, 30. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, N.; Zhang, R.; Jin, L.; Petridis, A.K.; Loers, G.; Zheng, X.; Wang, Z.; Siebert, H.C. Cuprizone-Induced Demyelination in Mouse Hippocampus Is Alleviated by Ketogenic Diet. J. Agric. Food Chem. 2020, 68, 11215–11228. [Google Scholar] [CrossRef]
- Tahmasebi, F.; Pasbakhsh, P.; Barati, S.; Madadi, S.; Kashani, I.R. The effect of microglial ablation and mesenchymal stem cell transplantation on a cuprizone-induced demyelination model. J. Cell. Physiol. 2021, 236, 3552–3564. [Google Scholar] [CrossRef]
- Rohr, S.O.; Greiner, T.; Joost, S.; Amor, S.; Valk, P.V.; Schmitz, C.; Kipp, M. Aquaporin-4 Expression during Toxic and Autoimmune Demyelination. Cells 2020, 9, 2187. [Google Scholar] [CrossRef]
- He, Y.; An, J.; Yin, J.J.; Miao, Q.; Sui, R.X.; Han, Q.X.; Ding, Z.B.; Huang, J.J.; Ma, C.G.; Xiao, B.G. Ethyl Pyruvate-Derived Transdifferentiation of Astrocytes to Oligodendrogenesis in Cuprizone-Induced Demyelinating Model. Neurotherapeutics 2021, 18, 488–502. [Google Scholar] [CrossRef]
- Espírito-Santo, S.; Coutinho, V.G.; Dezonne, R.S.; Stipursky, J.; Dos Santos-Rodrigues, A.; Batista, C.; Paes-de-Carvalho, R.; Fuss, B.; Gomes, F.C.A. Astrocytes as a target for Nogo-A and implications for synapse formation in vitro and in a model of acute demyelination. Glia 2021, 69, 1429–1443. [Google Scholar] [CrossRef]
- Marzan, D.E.; Brügger-Verdon, V.; West, B.L.; Liddelow, S.; Samanta, J.; Salzer, J.L. Activated microglia drive demyelination via CSF1R signaling. Glia 2021, 69, 1583–1604. [Google Scholar] [CrossRef]
- Baltan, S.; Jawaid, S.S.; Chomyk, A.M.; Kidd, G.J.; Chen, J.; Battapady, H.D.; Chan, R.; Dutta, R.; Trapp, B.D. Neuronal hibernation following hippocampal demyelination. Acta Neuropathol. Commun. 2021, 9, 34. [Google Scholar] [CrossRef]
- Saitta, K.S.; Lercher, L.D.; Sainato, D.M.; Patel, A.; Huang, Y.; McAuliffe, G.; Dreyfus, C.F. CHPG enhances BDNF and myelination in cuprizone-treated mice through astrocytic metabotropic glutamate receptor 5. Glia 2021, 69, 1950–1965. [Google Scholar] [CrossRef] [PubMed]
- Klejbor, I.; Shimshek, D.R.; Klimaszewska-Łata, J.; Velasco-Estevez, M.; Moryś, J.; Karaszewski, B.; Szutowicz, A.; Rutkowska, A. EBI2 is expressed in glial cells in multiple sclerosis lesions, and its knock-out modulates remyelination in the cuprizone model. Eur. J. Neurosci. 2021, 54, 5173–5188. [Google Scholar] [CrossRef] [PubMed]
- Colombo, E.; Triolo, D.; Bassani, C.; Bedogni, F.; Di Dario, M.; Dina, G.; Fredrickx, E.; Fermo, I.; Martinelli, V.; Newcombe, J.; et al. Dysregulated copper transport in multiple sclerosis may cause demyelination via astrocytes. Proc. Natl. Acad. Sci. USA 2021, 118, e2025804118. [Google Scholar] [CrossRef] [PubMed]
- Cheli, V.T.; Santiago González, D.A.; Wan, Q.; Denaroso, G.; Wan, R.; Rosenblum, S.L.; Paez, P.M. H-ferritin expression in astrocytes is necessary for proper oligodendrocyte development and myelination. Glia 2021, 69, 2981–2998. [Google Scholar] [CrossRef]
- An, J.; He, Y.; Yin, J.J.; Ding, Z.B.; Han, Q.X.; Chen, Y.Y.; Wang, Q.; Chai, Z.; Yu, J.Z.; Song, L.J.; et al. Temporal and spatial evolution of various functional neurons during demyelination induced by cuprizone. J. Neurophysiol. 2021, 126, 1756–1771. [Google Scholar] [CrossRef]
- Castillo-Rodriguez, M.L.A.; Gingele, S.; Schröder, L.J.; Möllenkamp, T.; Stangel, M.; Skripuletz, T.; Gudi, V. Astroglial and oligodendroglial markers in the cuprizone animal model for de- and remyelination. Histochem. Cell Biol. 2022, 158, 15–38. [Google Scholar] [CrossRef]
- Kawabe, Y.; Tanaka, T.; Isonishi, A.; Nakahara, K.; Tatsumi, K.; Wanaka, A. Characterization of Glial Populations in the Aging and Remyelinating Mouse Corpus Callosum. Neurochem. Res. 2022, 47, 2826–2838. [Google Scholar] [CrossRef]
- Silva Oliveira Junior, M.; Schira-Heinen, J.; Reiche, L.; Han, S.; de Amorim, V.C.M.; Lewen, I.; Gruchot, J.; Göttle, P.; Akkermann, R.; Azim, K.; et al. Myelin repair is fostered by the corticosteroid medrysone specifically acting on astroglial subpopulations. EBioMedicine 2022, 83, 104204. [Google Scholar] [CrossRef]
- Zha, Z.; Liu, Y.J.; Liu, S.S.; Zhang, N.; Li, J.L.; Qi, F.; Jin, L.Y.; Xue, B.; Yang, T.; Fan, Y.P.; et al. Bu Shen Yi Sui Capsule Promotes Myelin Repair by Modulating the Transformation of A1/A2 Reactive Astrocytes In Vivo and In Vitro. Oxidative Med. Cell. Longev. 2022, 2022, 3800004. [Google Scholar] [CrossRef] [PubMed]
- Madadi, S.; Shiri, E.; Pasbakhsh, P.; Tahmasebi, F.; Kazemzadeh, S.; Zibara, K.; Kashani, I.R. Combination Therapy of Mesenchymal Stem Cell Transplantation and Astrocyte Ablation Improve Remyelination in a Cuprizone-Induced Demyelination Mouse Model. Mol. Neurobiol. 2022, 59, 7278–7292. [Google Scholar] [CrossRef]
- Fallier-Becker, P.; Bonzheim, I.; Pfeiffer, F. Cuprizone feeding induces swollen astrocyte endfeet. Pflug. Arch. 2022, 474, 1275–1283. [Google Scholar] [CrossRef]
- Sun, W.; Wen, M.; Liu, M.; Wang, Q.; Liu, Q.; Li, L.; Siebert, H.C.; Loers, G.; Zhang, R.; Zhang, N. Effect of β-hydroxybutyrate on behavioral alterations, molecular and morphological changes in CNS of multiple sclerosis mouse model. Front. Aging Neurosci. 2022, 14, 1075161. [Google Scholar] [CrossRef]
- Lei, C.; Chen, H.; Chen, K. Effects of Bone Marrow Mesenchymal Stem Cells on Myelin Repair and Emotional Changes of a Cuprizone-Induced Demyelination Model. J. Integr. Neurosci. 2023, 22, 40. [Google Scholar] [CrossRef]
- Takahashi, K.; Kanekiyo, K.; Sakuda, K.; Muto, Y.; Iguchi, M.; Matsuda, N.; Hashimoto, Y.; Kanai, K.; Ogawa, H.; Hirase, H.; et al. Brain-specific glycosylation of protein tyrosine phosphatase receptor type Z (PTPRZ) marks a demyelination-associated astrocyte subtype. J. Neurochem. 2023, 166, 547–559. [Google Scholar] [CrossRef]
- Frankle, L.; Riley, A.; Tomor, R.; Lee, H.; Jarzembak, K.; Benedict, O.; Sternbach, S.; Shelestak, J.; McDonough, J.; Clements, R. Changes to Astrocyte-associated Protein Expression at Different Timepoints of Cuprizone Treatment. BioRxiv 2023. [Google Scholar] [CrossRef]
- Mohamadi, Y.; Borhani-Haghighi, M. TGN020 application against aquaporin 4 improved multiple sclerosis by inhibiting astrocytes, microglia, and NLRP3 inflammasome in a cuprizone mouse model. J. Chem. Neuroanat. 2023, 132, 102306. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kipp, M. Astrocytes: Lessons Learned from the Cuprizone Model. Int. J. Mol. Sci. 2023, 24, 16420. https://doi.org/10.3390/ijms242216420
Kipp M. Astrocytes: Lessons Learned from the Cuprizone Model. International Journal of Molecular Sciences. 2023; 24(22):16420. https://doi.org/10.3390/ijms242216420
Chicago/Turabian StyleKipp, Markus. 2023. "Astrocytes: Lessons Learned from the Cuprizone Model" International Journal of Molecular Sciences 24, no. 22: 16420. https://doi.org/10.3390/ijms242216420
APA StyleKipp, M. (2023). Astrocytes: Lessons Learned from the Cuprizone Model. International Journal of Molecular Sciences, 24(22), 16420. https://doi.org/10.3390/ijms242216420






