SARS-CoV-2 Spike Protein Intensifies Cerebrovascular Complications in Diabetic hACE2 Mice through RAAS and TLR Signaling Activation
Abstract
:1. Introduction
2. Results
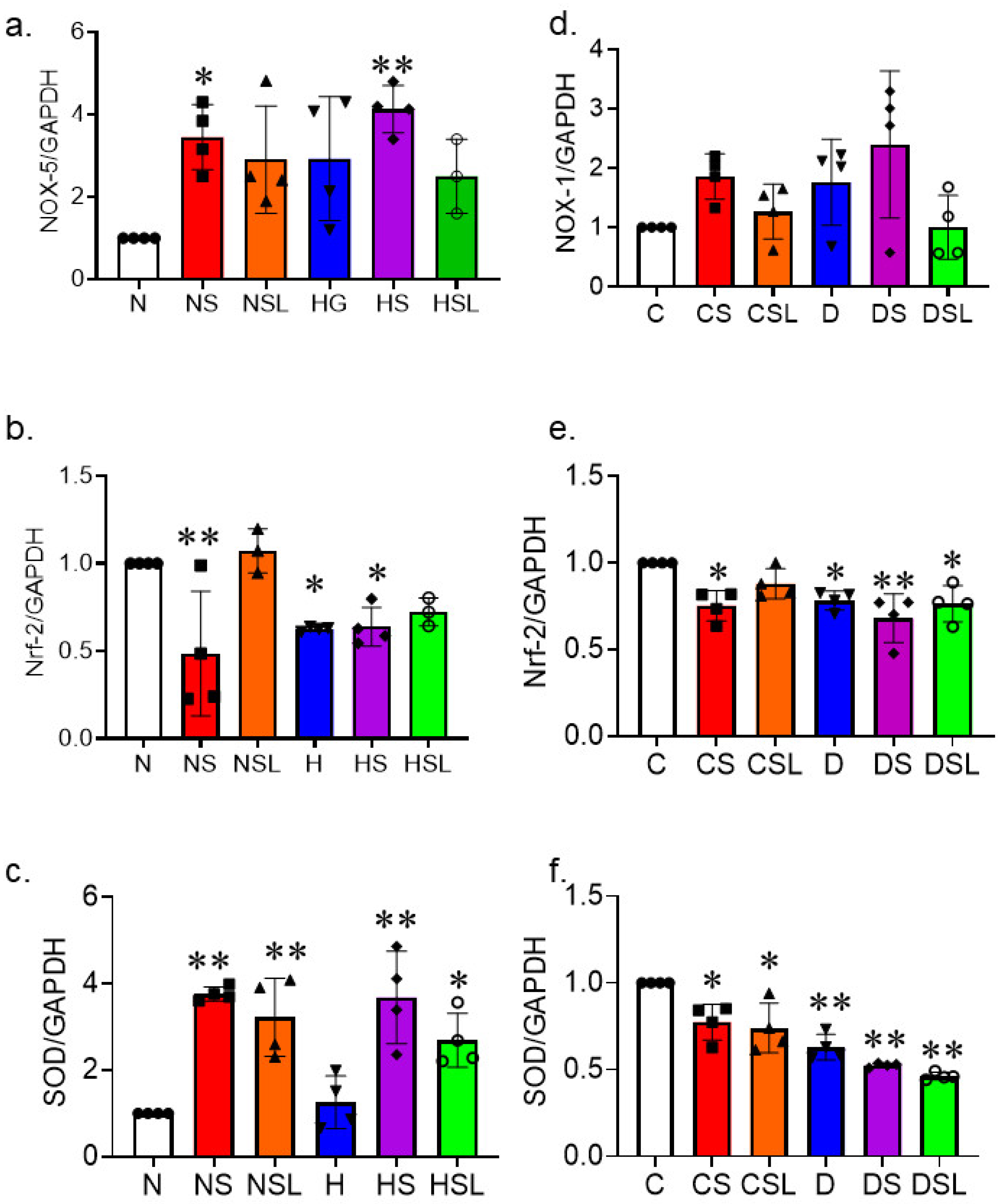
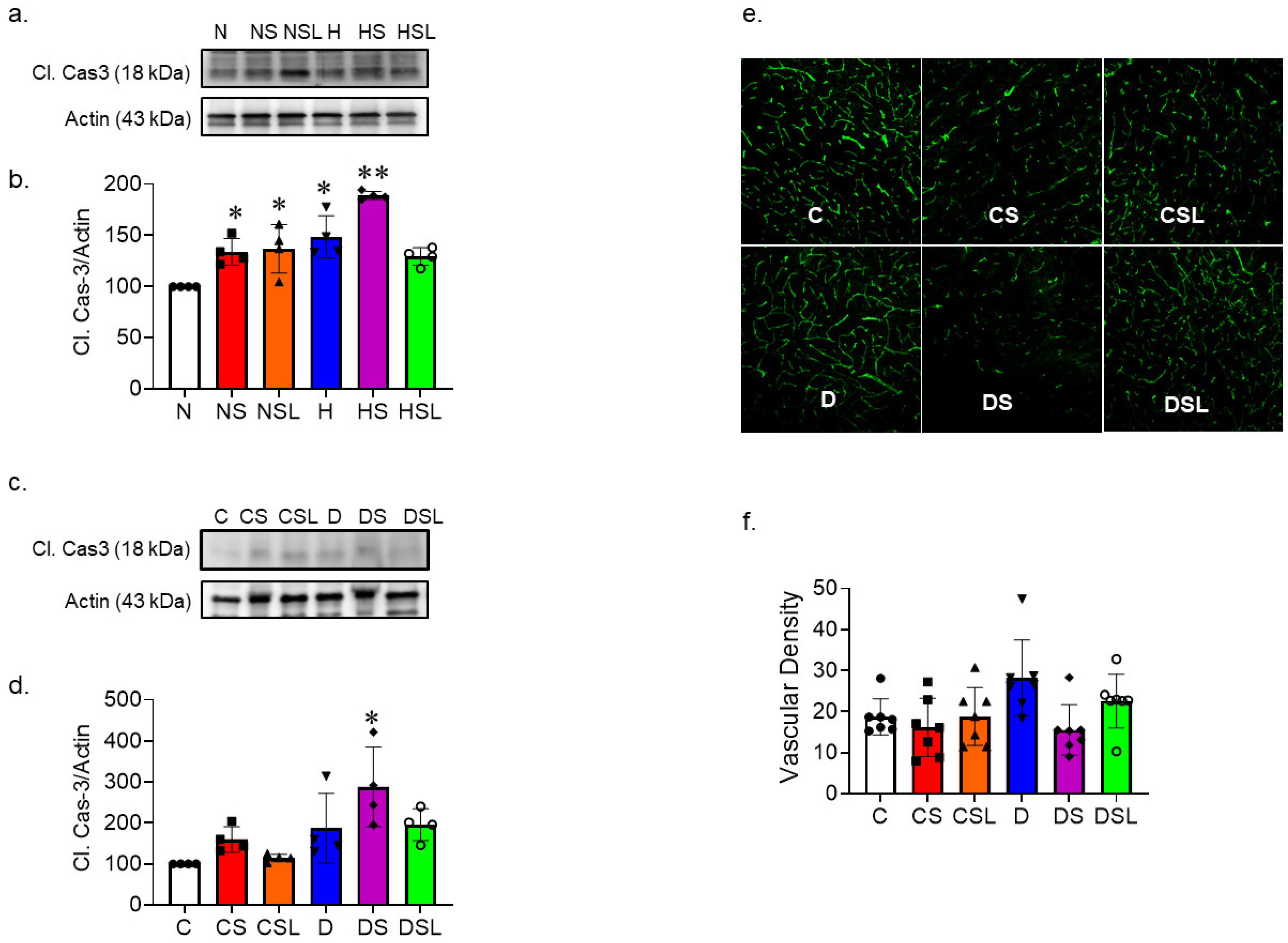
2.1. SARS-CoV-2 Spike Protein Aggravates Cerebrovascular Oxidative Stress in Diabetes
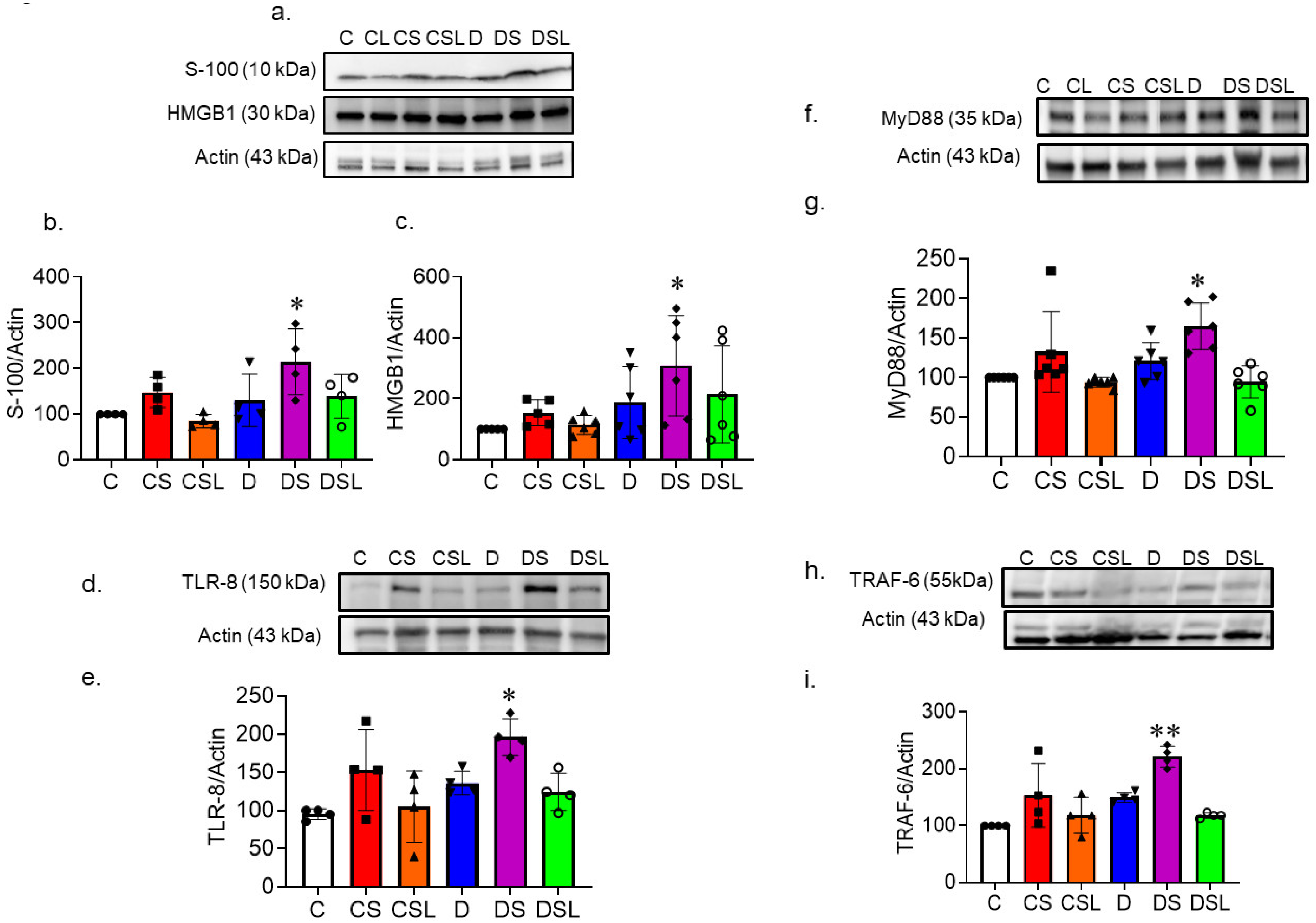
2.2. SARS-CoV-2 Spike Protein Increases Inflammation in Diabetic hACE2 Mice
2.3. SARS-CoV-2 Spike Protein Causes Increased Vascular Rarefaction in Diabetic hACE2 Mice
2.4. SARS-CoV-2 Spike Protein Causes Decreased Cerebral Blood Flow in Diabetic hACE2 Mice
2.5. SARS-CoV-2 Spike Protein Deteriorates Cognitive Function in Diabetic hACE2 Mice
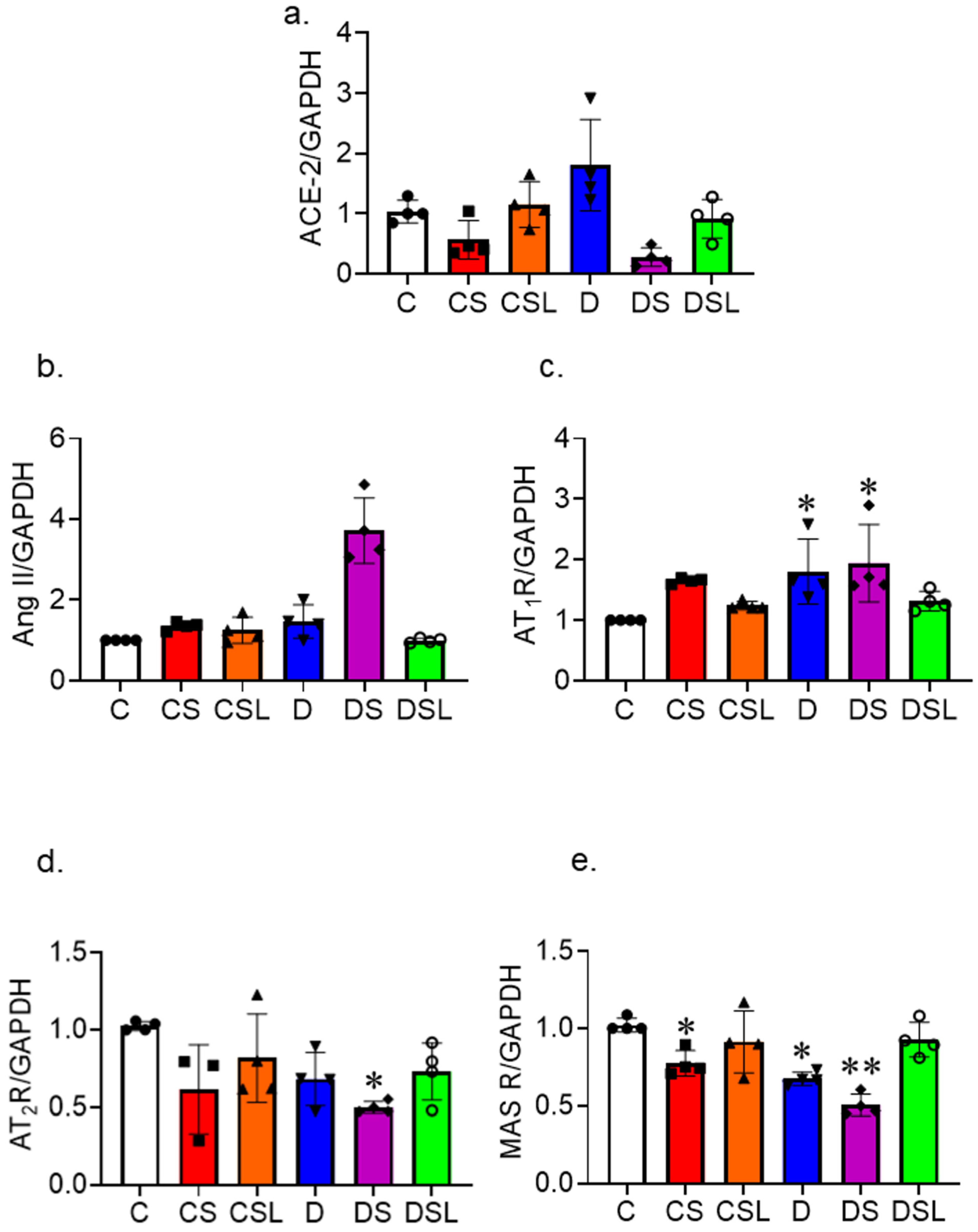
2.6. SARS-CoV-2 Spike Protein Disrupts the RAAS
2.7. SARS-CoV-2 Spike Protein Increases TLR Signaling in Diabetic hACE2 Mice
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Induction of Diabetes and Treatment
4.3. Assessment of Cognitive Functioning
4.4. Y-Maze
4.5. Assessment of Cerebral Blood Flow
4.6. Vascular Density Assessment
4.7. Cell Culture
4.8. Polymerase Chain Reaction
4.9. Immunoblotting
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- CDC. 2023. Available online: https://covid.cdc.gov/covid-data-tracker/#cases-deaths-testing-trends (accessed on 1 October 2023).
- Li, J.; Huang, D.Q.; Zou, B.; Yang, H.; Hui, W.Z.; Rui, F.; Yee, N.T.S.; Liu, C.; Nerurkar, S.N.; Kai, J.C.Y.; et al. Epidemiology of COVID-19: A systematic review and meta-analysis of clinical characteristics, risk factors, and outcomes. J. Med. Virol. 2021, 93, 1449–1458. [Google Scholar] [CrossRef] [PubMed]
- Wijeratne, T.; Crewther, S. COVID-19 and long-term neurological problems: Challenges ahead with Post-COVID-19 Neurological Syndrome. Aust. J. Gen. Pract. 2021, 50. [Google Scholar] [CrossRef] [PubMed]
- Wijeratne, T.; Crewther, S. Post-COVID 19 Neurological Syndrome (PCNS); a novel syndrome with challenges for the global neurology community. J. Neurol. Sci. 2020, 419, 117179. [Google Scholar] [CrossRef] [PubMed]
- Premraj, L.; Kannapadi, N.V.; Briggs, J.; Seal, S.M.; Battaglini, D.; Fanning, J.; Suen, J.; Robba, C.; Fraser, J.; Cho, S.M. Mid and long-term neurological and neuropsychiatric manifestations of post-COVID-19 syndrome: A meta-analysis. J. Neurol. Sci. 2022, 434, 120162. [Google Scholar] [CrossRef] [PubMed]
- Ejaz, H.; Alsrhani, A.; Zafar, A.; Javed, H.; Junaid, K.; Abdalla, A.E.; Abosalif, K.O.A.; Ahmed, Z.; Younas, S. COVID-19 and comorbidities: Deleterious impact on infected patients. J. Infect. Public Health 2020, 13, 1833–1839. [Google Scholar] [CrossRef]
- Mahamat-Saleh, Y.; Fiolet, T.; Rebeaud, M.E.; Mulot, M.; Guihur, A.; El Fatouhi, D.; Laouali, N.; Peiffer-Smadja, N.; Aune, D.; Severi, G. Diabetes, hypertension, body mass index, smoking and COVID-19-related mortality: A systematic review and meta-analysis of observational studies. BMJ Open 2021, 11, e052777. [Google Scholar] [CrossRef]
- Yu, B.; Li, C.; Sun, Y.; Wang, D.W. Insulin Treatment Is Associated with Increased Mortality in Patients with COVID-19 and Type 2 Diabetes. Cell Metab. 2021, 33, 65–77.e2. [Google Scholar] [CrossRef]
- Vinogradov, O.I.; Ogarkova, T.K.; Shamtieva, K.V.; Alexandrov, P.V.; Mushba, A.V.; Kanshina, D.S.; Yakovleva, D.V.; Surma, M.A.; Nikolaev, I.S.; Gorst, N.K. Predictors of Acute Encephalopathy in Patients with COVID-19. J. Clin. Med. 2021, 10, 4821. [Google Scholar] [CrossRef]
- Zheng, M.; Wang, X.; Guo, H.; Fan, Y.; Song, Z.; Lu, Z.; Wang, J.; Zheng, C.; Dong, L.; Ma, Y.; et al. The Cytokine Profiles and Immune Response Are Increased in COVID-19 Patients with Type 2 Diabetes Mellitus. J. Diabetes Res. 2021, 2021, 9526701. [Google Scholar] [CrossRef]
- Ma, Y.; Lu, D.; Bao, L.; Qu, Y.; Liu, J.; Qi, X.; Yu, L.; Zhang, X.; Qi, F.; Lv, Q.; et al. SARS-CoV-2 infection aggravates chronic comorbidities of cardiovascular diseases and diabetes in mice. Anim. Model. Exp. Med. 2021, 4, 2–15. [Google Scholar] [CrossRef]
- Mechi, A.; Al-Khalidi, A.; Al-Darraji, R.; Al-Dujaili, M.N.; Al-Buthabhak, K.; Alareedh, M.; Shaghee, F.; Nafakhi, H. Long-term persistent symptoms of COVID-19 infection in patients with diabetes mellitus. Int. J. Diabetes Dev. Ctries. 2022, 42, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Harrison, A.G.; Lin, T.; Wang, P. Mechanisms of SARS-CoV-2 Transmission and Pathogenesis. Trends Immunol. 2020, 41, 1100–1115. [Google Scholar] [CrossRef] [PubMed]
- Amruta, N.; Ismael, S.; Leist, S.R.; Gressett, T.E.; Srivastava, A.; Dinnon, K.H., 3rd; Engler-Chiurazzi, E.B.; Maness, N.J.; Qin, X.; Kolls, J.K.; et al. Mouse Adapted SARS-CoV-2 (MA10) Viral Infection Induces Neuroinflammation in Standard Laboratory Mice. Viruses 2022, 15, 114. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.H.; Chen, Q.; Gu, H.J.; Yang, G.; Wang, Y.X.; Huang, X.Y.; Liu, S.S.; Zhang, N.N.; Li, X.F.; Xiong, R.; et al. A Mouse Model of SARS-CoV-2 Infection and Pathogenesis. Cell Host Microbe 2020, 28, 124–133.e4. [Google Scholar] [CrossRef]
- Abdelsaid, M.; Coucha, M.; Hafez, S.; Yasir, A.; Johnson, M.H.; Ergul, A. Enhanced VEGF signalling mediates cerebral neovascularisation via downregulation of guidance protein ROBO4 in a rat model of diabetes. Diabetologia 2017, 60, 740–750. [Google Scholar] [CrossRef]
- Cagnazzo, F.; Arquizan, C.; Derraz, I.; Dargazanli, C.; Lefevre, P.H.; Riquelme, C.; Gaillard, N.; Mourand, I.; Gascou, G.; Bonafe, A.; et al. Neurological manifestations of patients infected with the SARS-CoV-2: A systematic review of the literature. J. Neurol. 2021, 268, 2656–2665. [Google Scholar] [CrossRef] [PubMed]
- Buetti, N.; Trimboli, P.; Mazzuchelli, T.; Lo Priore, E.; Balmelli, C.; Trkola, A.; Conti, M.; Martinetti, G.; Elzi, L.; Ceschi, A.; et al. Diabetes mellitus is a risk factor for prolonged SARS-CoV-2 viral shedding in lower respiratory tract samples of critically ill patients. Endocrine 2020, 70, 454–460. [Google Scholar] [CrossRef] [PubMed]
- El-Arif, G.; Farhat, A.; Khazaal, S.; Annweiler, C.; Kovacic, H.; Wu, Y.; Cao, Z.; Fajloun, Z.; Khattar, Z.A.; Sabatier, J.M. The Renin-Angiotensin System: A Key Role in SARS-CoV-2-Induced COVID-19. Molecules 2021, 26, 6945. [Google Scholar] [CrossRef] [PubMed]
- Fisher, L.; Polonsky, W.; Asuni, A.; Jolly, Y.; Hessler, D. The early impact of the COVID-19 pandemic on adults with type 1 or type 2 diabetes: A national cohort study. J. Diabetes Complicat. 2020, 34, 107748. [Google Scholar] [CrossRef]
- Najmeddin, F.; Solhjoo, M.; Ashraf, H.; Salehi, M.; Rasooli, F.; Ghoghaei, M.; Soleimani, A.; Bahreini, M. Effects of Renin-Angiotensin-Aldosterone Inhibitors on Early Outcomes of Hypertensive COVID-19 Patients: A Randomized Triple-Blind Clinical Trial. Am. J. Hypertens. 2021, 34, 1217–1226. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.L.; Block, J.P.; Rolka, D.B.; Pavkov, M.E.; Chevinsky, J.R.; Lekiachvili, A.; Carton, T.W.; Thacker, D.; Denson, J.L.; Paranjape, A.; et al. COVID-19 Outcomes Stratified by Control Status of Hypertension and Diabetes: Preliminary Findings from PCORnet, U.S. AJPM Focus 2022, 1, 100012. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Han, J.; Zhao, E.; Fang, J.; Wang, D.; Cheng, Y.; Shi, Y.; Wang, Z.; Yao, Z.; Lu, P.; et al. The effects of obesity and metabolic abnormalities on severe COVID-19-related outcomes after vaccination: A population-based study. Cell Metab. 2023, 35, 585–600.e5. [Google Scholar] [CrossRef] [PubMed]
- Richardson, S.; Hirsch, J.S.; Narasimhan, M.; Crawford, J.M.; McGinn, T.; Davidson, K.W.; The Northwell COVID-19 Research Consortium; Barnaby, D.P.; Becker, L.B.; Chelico, J.D.; et al. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA 2020, 323, 2052–2059. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, A.I.; Baskett, W.I.; Huang, W.; Shyu, D.; Myers, D.; Raju, M.; Lobanova, I.; Suri, M.F.K.; Naqvi, S.H.; French, B.R.; et al. Acute Ischemic Stroke and COVID-19: An Analysis of 27 676 Patients. Stroke 2021, 52, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Abdoli, S.; Silveira, M.; Doosti-Irani, M.; Fanti, P.; Miller-Bains, K.; Pavin, E.J.; Cardoso, E.B.; Vardanjani, L.R.; Noorian, K.; Hessler, D. Correction to: Cross-national comparison of psychosocial well-being and diabetes outcomes in adults with type 1 diabetes during the COVID-19 pandemic in US, Brazil, and Iran. Diabetol. Metab. Syndr. 2021, 13, 78. [Google Scholar] [CrossRef] [PubMed]
- Kazakou, P.; Lambadiari, V.; Ikonomidis, I.; Kountouri, A.; Panagopoulos, G.; Athanasopoulos, S.; Korompoki, E.; Kalomenidis, I.; Dimopoulos, M.A.; Mitrakou, A. Diabetes and COVID-19; A Bidirectional Interplay. Front. Endocrinol. 2022, 13, 780663. [Google Scholar] [CrossRef] [PubMed]
- Coucha, M.; Abdelsaid, M.; Ward, R.; Abdul, Y.; Ergul, A. Impact of Metabolic Diseases on Cerebral Circulation: Structural and Functional Consequences. Compr. Physiol. 2018, 8, 773–799. [Google Scholar] [CrossRef]
- Coucha, M.; Barrett, A.C.; Elgebaly, M.; Ergul, A.; Abdelsaid, M. Inhibition of Ephrin-B2 in brain pericytes decreases cerebral pathological neovascularization in diabetic rats. PLoS ONE 2019, 14, e0210523. [Google Scholar] [CrossRef]
- Ardellier, F.D.; Baloglu, S.; Sokolska, M.; Noblet, V.; Lersy, F.; Collange, O.; Ferre, J.C.; Maamar, A.; Carsin-Nicol, B.; Helms, J.; et al. Cerebral perfusion using ASL in patients with COVID-19 and neurological manifestations: A retrospective multicenter observational study. J. Neuroradiol. 2023, 50, 470–481. [Google Scholar] [CrossRef]
- Woo, M.S.; Malsy, J.; Pottgen, J.; Seddiq Zai, S.; Ufer, F.; Hadjilaou, A.; Schmiedel, S.; Addo, M.M.; Gerloff, C.; Heesen, C.; et al. Frequent neurocognitive deficits after recovery from mild COVID-19. Brain Commun. 2020, 2, fcaa205. [Google Scholar] [CrossRef]
- Delgado-Alonso, C.; Valles-Salgado, M.; Delgado-Alvarez, A.; Yus, M.; Gomez-Ruiz, N.; Jorquera, M.; Polidura, C.; Gil, M.J.; Marcos, A.; Matias-Guiu, J.; et al. Cognitive dysfunction associated with COVID-19: A comprehensive neuropsychological study. J. Psychiatr. Res. 2022, 150, 40–46. [Google Scholar] [CrossRef]
- Lykhmus, O.; Kalashnyk, O.; Koval, L.; Krynina, O.; Komisarenko, S.; Skok, M. Immunization with 674-685 fragment of SARS-CoV-2 spike protein induces neuroinflammation and impairs episodic memory of mice. Biochem. Biophys. Res. Commun. 2022, 622, 57–63. [Google Scholar] [CrossRef]
- Sonneville, R.; Dangayach, N.S.; Newcombe, V. Neurological complications of critically ill COVID-19 patients. Curr. Opin. Crit. Care 2023, 29, 61–67. [Google Scholar] [CrossRef]
- Abba, A.; Accorsi, C.; Agnes, P.; Alessi, E.; Amaudruz, P.; Annovi, A.; Desages, F.A.; Back, S.; Badia, C.; Bagger, J.; et al. The novel Mechanical Ventilator Milano for the COVID-19 pandemic. Phys. Fluids 2021, 33, 037122. [Google Scholar] [CrossRef]
- Beckman, D.; Bonillas, A.; Diniz, G.B.; Ott, S.; Roh, J.W.; Elizaldi, S.R.; Schmidt, B.A.; Sammak, R.L.; Van Rompay, K.K.A.; Iyer, S.S.; et al. SARS-CoV-2 infects neurons and induces neuroinflammation in a non-human primate model of COVID-19. Cell Rep. 2022, 41, 111573. [Google Scholar] [CrossRef]
- Rutkai, I.; Mayer, M.G.; Hellmers, L.M.; Ning, B.; Huang, Z.; Monjure, C.J.; Coyne, C.; Silvestri, R.; Golden, N.; Hensley, K.; et al. Neuropathology and virus in brain of SARS-CoV-2 infected non-human primates. Nat. Commun. 2022, 13, 1745. [Google Scholar] [CrossRef]
- Rom, S.; Zuluaga-Ramirez, V.; Gajghate, S.; Seliga, A.; Winfield, M.; Heldt, N.A.; Kolpakov, M.A.; Bashkirova, Y.V.; Sabri, A.K.; Persidsky, Y. Hyperglycemia-Driven Neuroinflammation Compromises BBB Leading to Memory Loss in Both Diabetes Mellitus (DM) Type 1 and Type 2 Mouse Models. Mol. Neurobiol. 2019, 56, 1883–1896. [Google Scholar] [CrossRef]
- Buicu, A.L.; Cernea, S.; Benedek, I.; Buicu, C.F.; Benedek, T. Systemic Inflammation and COVID-19 Mortality in Patients with Major Noncommunicable Diseases: Chronic Coronary Syndromes, Diabetes and Obesity. J. Clin. Med. 2021, 10, 1545. [Google Scholar] [CrossRef] [PubMed]
- Cariou, B.; Hadjadj, S.; Wargny, M.; Pichelin, M.; Al-Salameh, A.; Allix, I.; Amadou, C.; Arnault, G.; Baudoux, F.; Bauduceau, B.; et al. Phenotypic characteristics and prognosis of inpatients with COVID-19 and diabetes: The CORONADO study. Diabetologia 2020, 63, 1500–1515. [Google Scholar] [CrossRef] [PubMed]
- Roncon, L.; Zuin, M.; Rigatelli, G.; Zuliani, G. Diabetic patients with COVID-19 infection are at higher risk of ICU admission and poor short-term outcome. J. Clin. Virol. 2020, 127, 104354. [Google Scholar] [CrossRef] [PubMed]
- Elshafei, A.; Khidr, E.G.; El-Husseiny, A.A.; Gomaa, M.H. RAAS, ACE2 and COVID-19; a mechanistic review. Saudi J. Biol. Sci. 2021, 28, 6465–6470. [Google Scholar] [CrossRef]
- Busse, L.W.; Chow, J.H.; McCurdy, M.T.; Khanna, A.K. COVID-19 and the RAAS-a potential role for angiotensin II? Crit. Care 2020, 24, 136. [Google Scholar] [CrossRef]
- Srivastava, A.; Mussa, B.M. Effects of Varying Glucose Concentrations on ACE2’s Hypothalamic Expression and Its Potential Relation to COVID-19-Associated Neurological Dysfunction. Int. J. Mol. Sci. 2022, 23, 9645. [Google Scholar] [CrossRef] [PubMed]
- Kutz, A.; Conen, A.; Gregoriano, C.; Haubitz, S.; Koch, D.; Domenig, O.; Bernasconi, L.; Mueller, B.; Schuetz, P. Renin-angiotensin-aldosterone system peptide profiles in patients with COVID-19. Eur. J. Endocrinol. 2021, 184, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, R.M.; Young, M.K.; Petri, W.A.O.; Lyons, G.R.; Gilchrist, C.; Carey, R.M.; Petri, W.A., Jr. Repressed Ang 1-7 in COVID-19 Is Inversely Associated with Inflammation and Coagulation. mSphere 2022, 7, e0022022. [Google Scholar] [CrossRef] [PubMed]
- Bennion, D.M.; Haltigan, E.; Regenhardt, R.W.; Steckelings, U.M.; Sumners, C. Neuroprotective mechanisms of the ACE2-angiotensin-(1-7)-Mas axis in stroke. Curr. Hypertens. Rep. 2015, 17, 3. [Google Scholar] [CrossRef] [PubMed]
- Semenzato, L.; Botton, J.; Drouin, J.; Baricault, B.; Vabre, C.; Cuenot, F.; Penso, L.; Herlemont, P.; Sbidian, E.; Weill, A.; et al. Antihypertensive Drugs and COVID-19 Risk: A Cohort Study of 2 Million Hypertensive Patients. Hypertension 2021, 77, 833–842. [Google Scholar] [CrossRef]
- Hassib, M.; Hamilton, S.; Elkhouly, A.; Li, Y.; Kaplan, A.C. Renin-Angiotensin-Aldosterone System Inhibitors and COVID-19: A Meta-Analysis and Systematic Review. Cureus 2021, 13, e13124. [Google Scholar] [CrossRef]
- Jiang, X.; Li, H.; Liu, Y.; Bao, L.; Zhan, L.; Gao, H.; Deng, W.; Xue, J.; Liu, J.; Liu, X.; et al. The Effects of ATIR Blocker on the Severity of COVID-19 in Hypertensive Inpatients and Virulence of SARS-CoV-2 in Hypertensive hACE2 Transgenic Mice. J. Cardiovasc. Transl. Res. 2022, 15, 38–48. [Google Scholar] [CrossRef]
- Reddy, P.R.; Samavedam, S.; Aluru, N.; Boggu, R. Comparison of Severity of COVID-19 Infection among Patients Using RAAS Inhibitors and Non-RAAS Inhibitors. Indian J. Crit. Care Med. 2021, 25, 366–368. [Google Scholar] [CrossRef]
- Song, J.C.; White, C.M. Pharmacologic, pharmacokinetic, and therapeutic differences among angiotensin II receptor antagonists. Pharmacotherapy 2000, 20, 130–139. [Google Scholar] [CrossRef]
- Dutsch, A.; Schunkert, H. RAAS inhibition and beyond-cardiovascular medications in patients at risk of or affected by COVID-19. Herz 2023, 48, 206–211. [Google Scholar] [CrossRef]
- Chen, L.; Long, X.; Xu, Q.; Tan, J.; Wang, G.; Cao, Y.; Wei, J.; Luo, H.; Zhu, H.; Huang, L.; et al. Elevated serum levels of S100A8/A9 and HMGB1 at hospital admission are correlated with inferior clinical outcomes in COVID-19 patients. Cell Mol. Immunol. 2020, 17, 992–994. [Google Scholar] [CrossRef] [PubMed]
- Lim, R.R.; Vaidya, T.; Gadde, S.G.; Yadav, N.K.; Sethu, S.; Hainsworth, D.P.; Mohan, R.R.; Ghosh, A.; Chaurasia, S.S. Correlation between systemic S100A8 and S100A9 levels and severity of diabetic retinopathy in patients with type 2 diabetes mellitus. Diabetes Metab. Syndr. 2019, 13, 1581–1589. [Google Scholar] [CrossRef]
- Wu, H.; Chen, Z.; Xie, J.; Kang, L.N.; Wang, L.; Xu, B. High Mobility Group Box-1: A Missing Link between Diabetes and Its Complications. Mediat. Inflamm. 2016, 2016, 3896147. [Google Scholar] [CrossRef]
- Frank, M.G.; Nguyen, K.H.; Ball, J.B.; Hopkins, S.; Kelley, T.; Baratta, M.V.; Fleshner, M.; Maier, S.F. SARS-CoV-2 spike S1 subunit induces neuroinflammatory, microglial and behavioral sickness responses: Evidence of PAMP-like properties. Brain Behav. Immun. 2022, 100, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, S.; Bandyopadhyay, M. Molecular docking of SARS-CoV-2 Spike epitope sequences identifies heterodimeric peptide-protein complex formation with human Zo-1, TLR8 and brain specific glial proteins. Med. Hypotheses 2021, 157, 110706. [Google Scholar] [CrossRef]
- Salvi, V.; Nguyen, H.O.; Sozio, F.; Schioppa, T.; Gaudenzi, C.; Laffranchi, M.; Scapini, P.; Passari, M.; Barbazza, I.; Tiberio, L.; et al. SARS-CoV-2-associated ssRNAs activate inflammation and immunity via TLR7/8. JCI Insight 2021, 6, e150542. [Google Scholar] [CrossRef] [PubMed]
- Wallach, T.; Raden, M.; Hinkelmann, L.; Brehm, M.; Rabsch, D.; Weidling, H.; Kruger, C.; Kettenmann, H.; Backofen, R.; Lehnardt, S. Distinct SARS-CoV-2 RNA fragments activate Toll-like receptors 7 and 8 and induce cytokine release from human macrophages and microglia. Front. Immunol. 2022, 13, 1066456. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burnett, F.N.; Coucha, M.; Bolduc, D.R.; Hermanns, V.C.; Heath, S.P.; Abdelghani, M.; Macias-Moriarity, L.Z.; Abdelsaid, M. SARS-CoV-2 Spike Protein Intensifies Cerebrovascular Complications in Diabetic hACE2 Mice through RAAS and TLR Signaling Activation. Int. J. Mol. Sci. 2023, 24, 16394. https://doi.org/10.3390/ijms242216394
Burnett FN, Coucha M, Bolduc DR, Hermanns VC, Heath SP, Abdelghani M, Macias-Moriarity LZ, Abdelsaid M. SARS-CoV-2 Spike Protein Intensifies Cerebrovascular Complications in Diabetic hACE2 Mice through RAAS and TLR Signaling Activation. International Journal of Molecular Sciences. 2023; 24(22):16394. https://doi.org/10.3390/ijms242216394
Chicago/Turabian StyleBurnett, Faith N., Maha Coucha, Deanna R. Bolduc, Veronica C. Hermanns, Stan P. Heath, Maryam Abdelghani, Lilia Z. Macias-Moriarity, and Mohammed Abdelsaid. 2023. "SARS-CoV-2 Spike Protein Intensifies Cerebrovascular Complications in Diabetic hACE2 Mice through RAAS and TLR Signaling Activation" International Journal of Molecular Sciences 24, no. 22: 16394. https://doi.org/10.3390/ijms242216394
APA StyleBurnett, F. N., Coucha, M., Bolduc, D. R., Hermanns, V. C., Heath, S. P., Abdelghani, M., Macias-Moriarity, L. Z., & Abdelsaid, M. (2023). SARS-CoV-2 Spike Protein Intensifies Cerebrovascular Complications in Diabetic hACE2 Mice through RAAS and TLR Signaling Activation. International Journal of Molecular Sciences, 24(22), 16394. https://doi.org/10.3390/ijms242216394





