Unique miRomics Expression Profiles in Tannerella forsythia-Infected Mandibles during Periodontitis Using Machine Learning
Abstract
:1. Introduction
2. Results
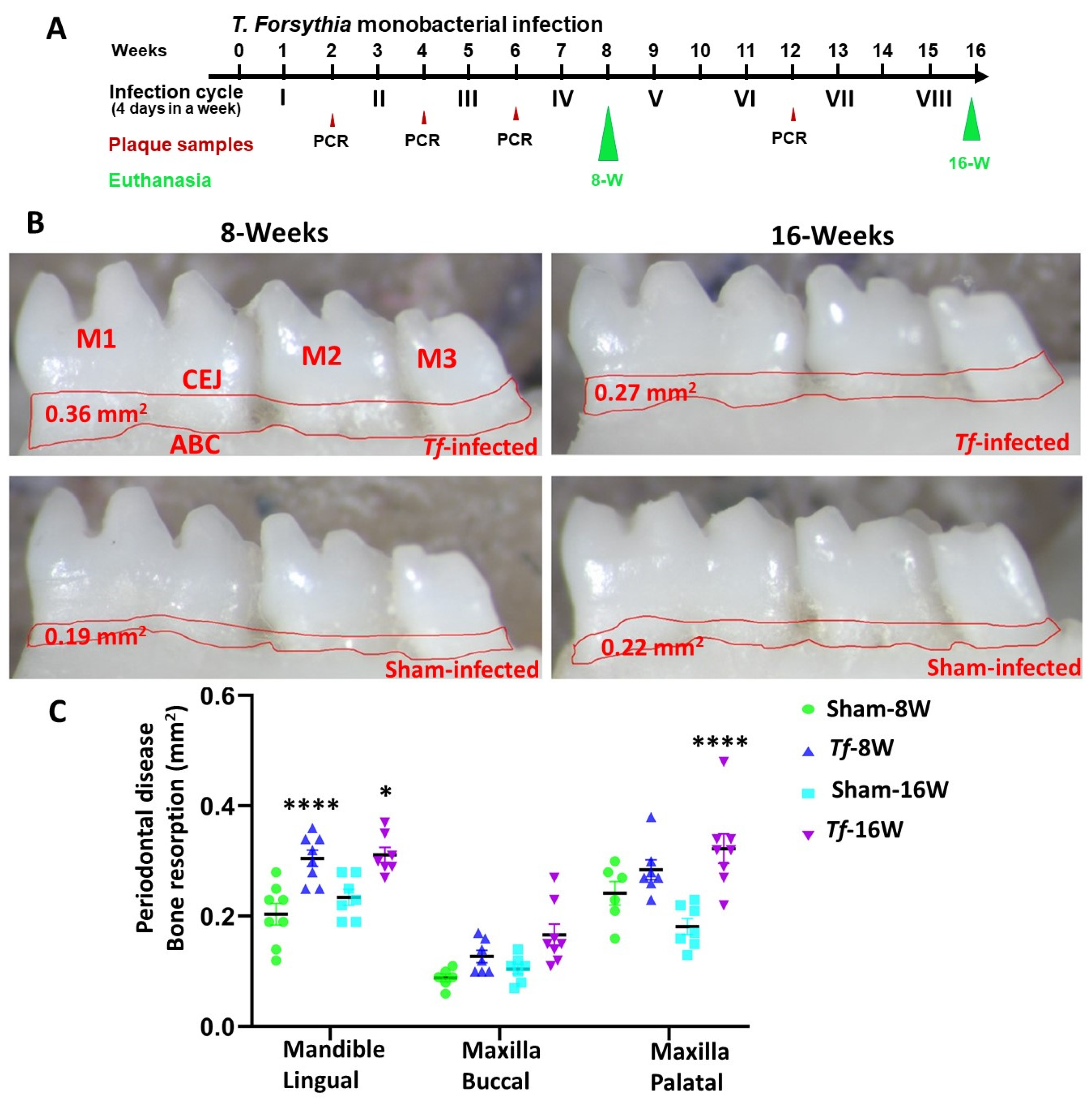
2.1. Chronic Infection of T. forsythia Effectively Colonized in Mice Gingival Surface
2.2. Higher Alveolar Bone Resorption (ABR) and Bacterial Dissemination to Distal Organs
2.3. NanoString Analysis of miRNAs in T. forsythia-Infected Mandibles
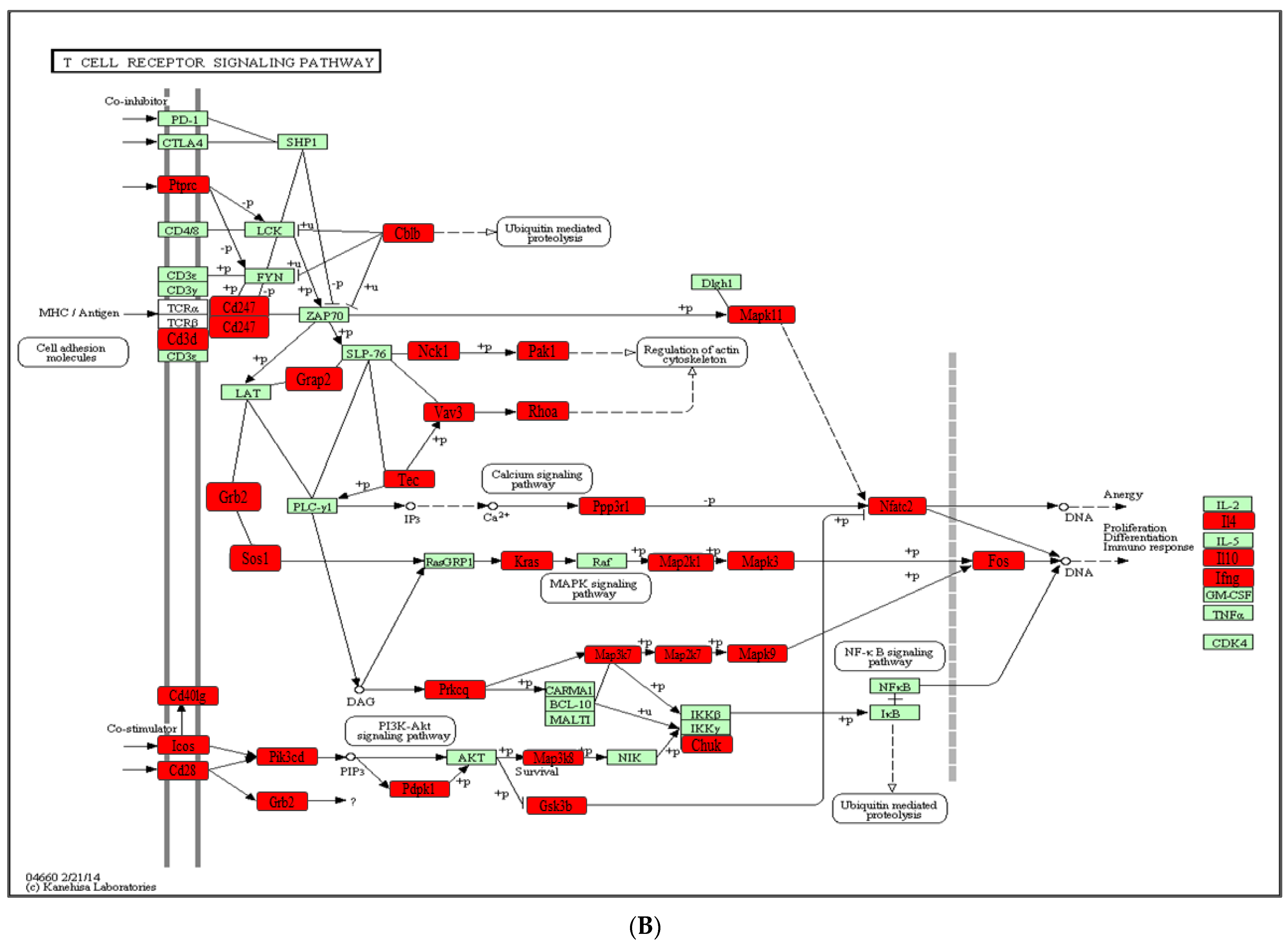
2.4. Functional Pathways Analysis of DE miRNAs
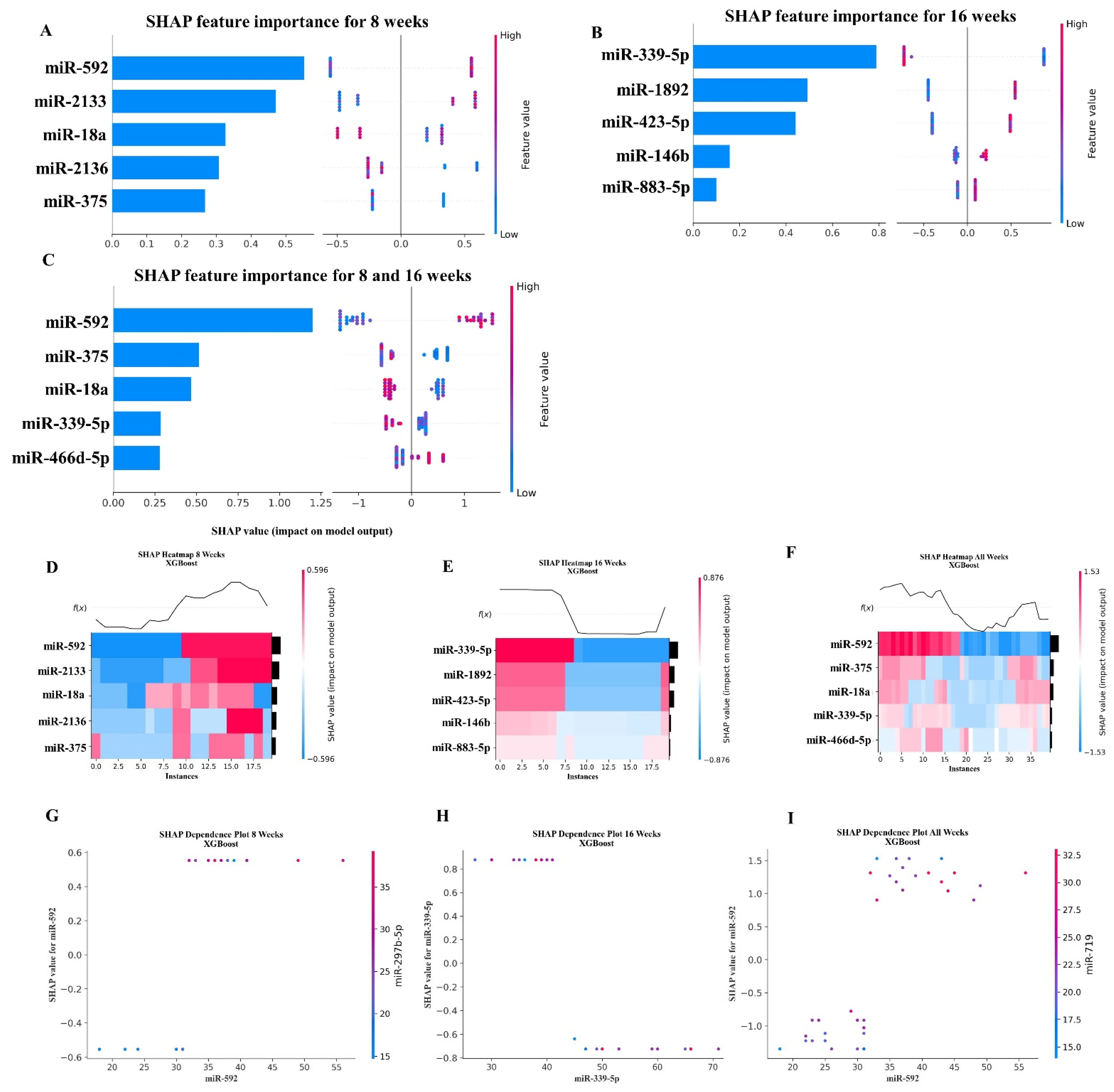
2.5. XGBoost Analysis of miRNA Copies
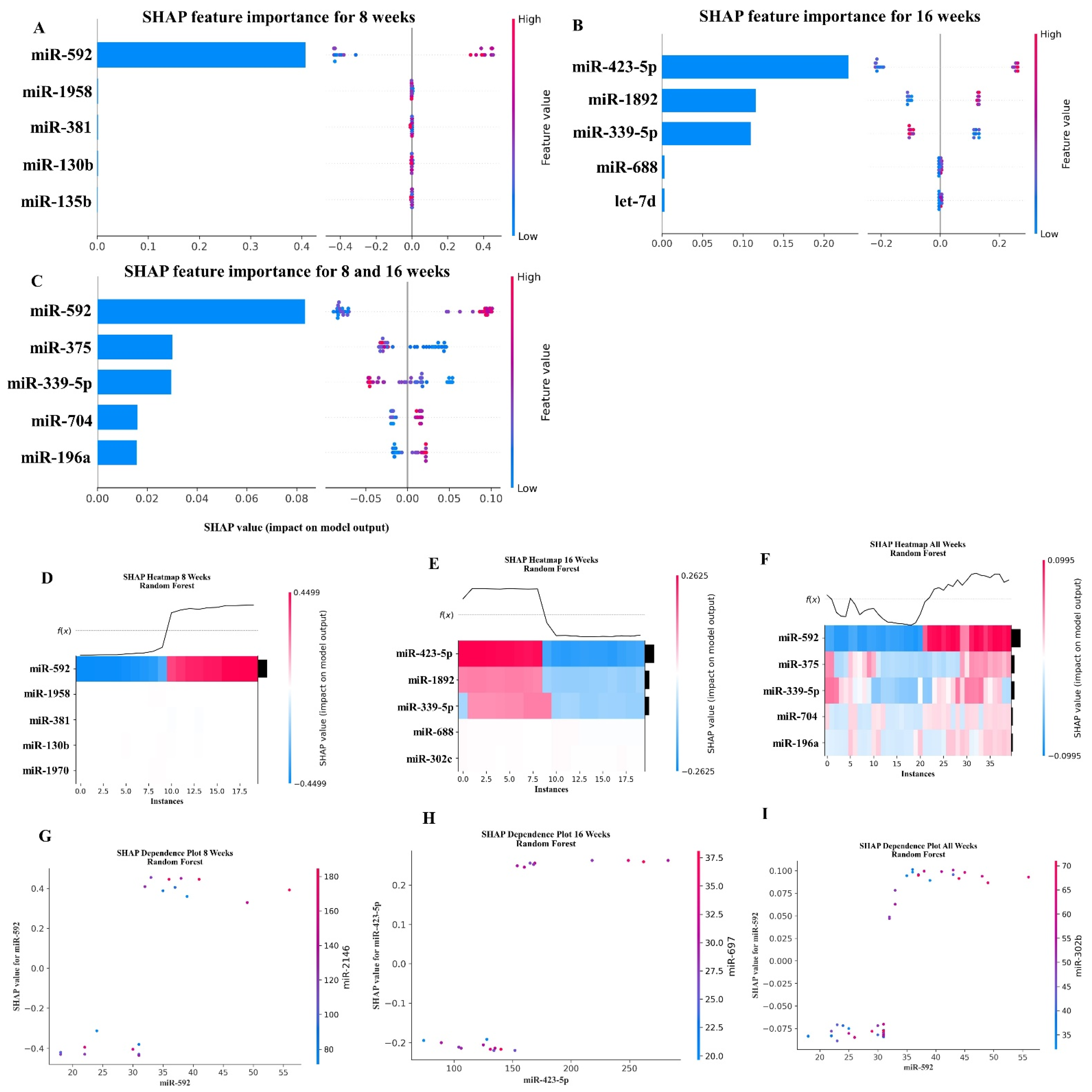
2.6. Random Forest Machine Learning Model
3. Discussion
4. Materials and Methods
4.1. Induction of Periodontitis in C57BL/6J Mouse Using Intraoral Infection
4.2. Gingival Plaque Sample Analysis and Bacterial Dissemination to Distal Organs
4.3. Alveolar Bone Resorption (ABR) Measurements of Mandibles and Maxilla
4.4. NanoString nCounter miRNA Panel and Data Analysis
4.5. Bioinformatic Analysis
4.6. Machine Learning Model Analysis
4.7. Random Forest Machine Learning Model
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. Metazoan MicroRNAs. Cell 2018, 173, 20–51. [Google Scholar] [CrossRef] [PubMed]
- Leng, Q.; Chen, L.; Lv, Y. RNA-based scaffolds for bone regeneration: Application and mechanisms of mRNA, miRNA and siRNA. Theranostics 2020, 10, 3190–3205. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Hannon, G.J. MicroRNAs: Small RNAs with a big role in gene regulation. Nat. Rev. Genet. 2004, 5, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, J.L.; Sullivan, J.C. Circulating cell-free micro-RNA as biomarkers: From myocardial infarction to hypertension. Clin. Sci. 2022, 136, 1341–1346. [Google Scholar] [CrossRef]
- Tam, S.; de Borja, R.; Tsao, M.S.; McPherson, J.D. Robust global microRNA expression profiling using next-generation sequencing technologies. Lab. Investig. 2014, 94, 350–358. [Google Scholar] [CrossRef]
- Hajishengallis, G. Periodontitis: From microbial immune subversion to systemic inflammation. Nat. Rev. Immunol. 2015, 15, 30–44. [Google Scholar] [CrossRef]
- Curtis, M.A.; Diaz, P.I.; Van Dyke, T.E. The role of the microbiota in periodontal disease. Periodontol. 2000 2020, 83, 14–25. [Google Scholar] [CrossRef]
- Socransky, S.S.; Haffajee, A.D.; Cugini, M.A.; Smith, C.; Kent, R.L., Jr. Microbial complexes in subgingival plaque. J. Clin. Periodontol. 1998, 25, 134–144. [Google Scholar] [CrossRef]
- Mishima, E.; Sharma, A. Tannerella forsythia invasion in oral epithelial cells requires phosphoinositide 3-kinase activation and clathrin-mediated endocytosis. Microbiology 2011, 157, 2382–2391. [Google Scholar] [CrossRef]
- Chukkapalli, S.S.; Rivera-Kweh, M.F.; Velsko, I.M.; Chen, H.; Zheng, D.; Bhattacharyya, I.; Gangula, P.R.; Lucas, A.R.; Kesavalu, L. Chronic oral infection with major periodontal bacteria Tannerella forsythia modulates systemic atherosclerosis risk factors and inflammatory markers. Pathog. Dis. 2015, 73, ftv009. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Inagaki, S.; Sigurdson, W.; Kuramitsu, H.K. Synergy between Tannerella forsythia and Fusobacterium nucleatum in biofilm formation. Oral Microbiol. Immunol. 2005, 20, 39–42. [Google Scholar] [CrossRef] [PubMed]
- Honap, T.P.; Monroe, C.R.; Johnson, S.J.; Jacobson, D.K.; Abin, C.A.; Austin, R.M.; Sandberg, P.; Levine, M.; Sankaranarayanan, K.; Lewis, C.M., Jr. Oral metagenomes from Native American Ancestors reveal distinct microbial lineages in the pre-contact era. Am. J. Biol. Anthropol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Su, L.; Duan, X.; Chen, X.; Hays, A.; Upadhyayula, S.; Shivde, J.; Wang, H.; Li, Y.; Huang, D.; et al. MicroRNA-21 down-regulates inflammation and inhibits periodontitis. Mol. Immunol. 2018, 101, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.F.; Shu, R.; Jiang, S.Y.; Liu, D.L.; Zhang, X.L. Comparison of microRNA profiles of human periodontal diseased and healthy gingival tissues. Int. J. Oral Sci. 2011, 3, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Mahendra, J.; Mahendra, L.; Fageeh, H.N.; Fageeh, H.I.; Ibraheem, W.; Abdulkarim, H.H.; Kanakamedala, A.; Prakash, P.; Srinivasan, S.; Balaji, T.M.; et al. miRNA-146a and miRNA-126 as Potential Biomarkers in Patients with Coronary Artery Disease and Generalized Periodontitis. Materials 2021, 14, 4692. [Google Scholar] [CrossRef]
- Motedayyen, H.; Ghotloo, S.; Saffari, M.; Sattari, M.; Amid, R. Evaluation of MicroRNA-146a and Its Targets in Gingival Tissues of Patients With Chronic Periodontitis. J. Periodontol. 2015, 86, 1380–1385. [Google Scholar] [CrossRef]
- Amaral, S.A.; Pereira, T.S.F.; Brito, J.A.R.; Cortelli, S.C.; Cortelli, J.R.; Gomez, R.S.; Costa, F.O.; Miranda Cota, L.O. Comparison of miRNA expression profiles in individuals with chronic or aggressive periodontitis. Oral Dis. 2019, 25, 561–568. [Google Scholar] [CrossRef]
- Nik Mohamed Kamal, N.N.S.; Awang, R.A.R.; Mohamad, S.; Shahidan, W.N.S. Plasma- and Saliva Exosome Profile Reveals a Distinct MicroRNA Signature in Chronic Periodontitis. Front. Physiol. 2020, 11, 587381. [Google Scholar] [CrossRef]
- Aravindraja, C.; Kashef, M.R.; Vekariya, K.M.; Ghanta, R.K.; Karanth, S.; Chan, E.K.L.; Kesavalu, L. Global Noncoding microRNA Profiling in Mice Infected with Partial Human Mouth Microbes (PAHMM) Using an Ecological Time-Sequential Polybacterial Periodontal Infection (ETSPPI) Model Reveals Sex-Specific Differential microRNA Expression. Int. J. Mol. Sci. 2022, 23, 5107. [Google Scholar] [CrossRef]
- Aravindraja, C.; Vekariya, K.M.; Botello-Escalante, R.; Rahaman, S.O.; Chan, E.K.L.; Kesavalu, L. Specific microRNA Signature Kinetics in Porphyromonas gingivalis-Induced Periodontitis. Int. J. Mol. Sci. 2023, 24, 2327. [Google Scholar] [CrossRef] [PubMed]
- Aravindraja, C.; Jeepipalli, S.; Vekariya, K.M.; Botello-Escalante, R.; Chan, E.K.; Kesavalu, L. Oral Spirochete Treponema denticola Intraoral Infection Reveals Unique miR-133a, miR-486, miR-126-3p, miR-126-5p miRNA Expression Kinetics during Periodontitis. Int. J. Mol. Sci. 2023, 24, 12105. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.H.; Yang, J.C.; Jeng, J.C.; Chen, Y.C.; Lu, T.H.; Tzeng, S.L.; Wu, Y.C.; Wu, C.J.; Rau, C.S. Circulating microRNA signatures in mice exposed to lipoteichoic acid. J. Biomed. Sci. 2013, 20, 2. [Google Scholar] [CrossRef] [PubMed]
- Law, Y.Y.; Lee, W.F.; Hsu, C.J.; Lin, Y.Y.; Tsai, C.H.; Huang, C.C.; Wu, M.H.; Tang, C.H.; Liu, J.F. miR-let-7c-5p and miR-149-5p inhibit proinflammatory cytokine production in osteoarthritis and rheumatoid arthritis synovial fibroblasts. Aging (Albany NY) 2021, 13, 17227–17236. [Google Scholar] [CrossRef]
- Coppola, A.; Romito, A.; Borel, C.; Gehrig, C.; Gagnebin, M.; Falconnet, E.; Izzo, A.; Altucci, L.; Banfi, S.; Antonarakis, S.E.; et al. Cardiomyogenesis is controlled by the miR-99a/let-7c cluster and epigenetic modifications. Stem Cell Res. 2014, 12, 323–337. [Google Scholar] [CrossRef] [PubMed]
- Costantini, E.; Sinjari, B.; Di Giovanni, P.; Aielli, L.; Caputi, S.; Muraro, R.; Murmura, G.; Reale, M. TNFα, IL-6, miR-103a-3p, miR-423-5p, miR-23a-3p, miR-15a-5p and miR-223-3p in the crevicular fluid of periodontopathic patients correlate with each other and at different stages of the disease. Sci. Rep. 2023, 13, 126. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, A.R.; Brambila, M.F.; Martinez, G.; Chapa, G.; Nares, S. Dysregulation of human miRNAs and increased prevalence of HHV miRNAs in obese periodontitis subjects. J. Clin. Periodontol. 2019, 46, 51–61. [Google Scholar] [CrossRef]
- Ghiam, S.; Eslahchi, C.; Shahpasand, K.; Habibi-Rezaei, M.; Gharaghani, S. Exploring the role of non-coding RNAs as potential candidate biomarkers in the cross-talk between diabetes mellitus and Alzheimer’s disease. Front. Aging Neurosci. 2022, 14, 955461. [Google Scholar] [CrossRef]
- Perri, R.; Nares, S.; Zhang, S.; Barros, S.P.; Offenbacher, S. MicroRNA modulation in obesity and periodontitis. J. Dent. Res. 2012, 91, 33–38. [Google Scholar] [CrossRef]
- Li, T.; Cao, H.; Zhuang, J.; Wan, J.; Guan, M.; Yu, B.; Li, X.; Zhang, W. Identification of miR-130a, miR-27b and miR-210 as serum biomarkers for atherosclerosis obliterans. Clin. Chim. Acta 2011, 412, 66–70. [Google Scholar] [CrossRef]
- Kang, L.; Li, N.; Wang, L. The Expression of miR-23a and miR-146a in the Saliva of Patients with Periodontitis and Its Clinical Significance. BioMed Res. Int. 2021, 2021, 5135278. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Feng, J.; Wang, W. Expression of miR-155 and miR-146a in the saliva of patients with periodontitis and its clinical value. Am. J. Transl. Res. 2021, 13, 6670–6677. [Google Scholar] [PubMed]
- Ghotloo, S.; Motedayyen, H.; Amani, D.; Saffari, M.; Sattari, M. Assessment of microRNA-146a in generalized aggressive periodontitis and its association with disease severity. J. Periodontal Res. 2019, 54, 27–32. [Google Scholar] [CrossRef]
- Singh, Y.; Kaul, V.; Mehra, A.; Chatterjee, S.; Tousif, S.; Dwivedi, V.P.; Suar, M.; Van Kaer, L.; Bishai, W.R.; Das, G. Mycobacterium tuberculosis controls microRNA-99b (miR-99b) expression in infected murine dendritic cells to modulate host immunity. J. Biol. Chem. 2013, 288, 5056–5061. [Google Scholar] [CrossRef] [PubMed]
- Venugopal, P.; Koshy, T.; Lavu, V.; Ranga Rao, S.; Ramasamy, S.; Hariharan, S.; Venkatesan, V. Differential expression of microRNAs let-7a, miR-125b, miR-100, and miR-21 and interaction with NF-kB pathway genes in periodontitis pathogenesis. J. Cell. Physiol. 2018, 233, 5877–5884. [Google Scholar] [CrossRef] [PubMed]
- Aavik, E.; Lumivuori, H.; Leppanen, O.; Wirth, T.; Hakkinen, S.K.; Brasen, J.H.; Beschorner, U.; Zeller, T.; Braspenning, M.; van Criekinge, W.; et al. Global DNA methylation analysis of human atherosclerotic plaques reveals extensive genomic hypomethylation and reactivation at imprinted locus 14q32 involving induction of a miRNA cluster. Eur. Heart J. 2015, 36, 993–1000. [Google Scholar] [CrossRef]
- Yang, Y.; Ago, T.; Zhai, P.; Abdellatif, M.; Sadoshima, J. Thioredoxin 1 negatively regulates angiotensin II-induced cardiac hypertrophy through upregulation of miR-98/let-7. Circ. Res. 2011, 108, 305–313. [Google Scholar] [CrossRef]
- Kazemian, S.; Ahmadi, R.; Ferns, G.A.; Rafiei, A.; Azadegan-Dehkordi, F.; Khaledifar, A.; Mohammad-Rezaei, M.; Bagheri, N. Correlation of miR-24-3p and miR-595 expression with CCL3, CCL4, IL1-β, TNFαIP3, and NF-κBIα genes in PBMCs of patients with coronary artery disease. EXCLI J. 2022, 21, 1184–1195. [Google Scholar] [CrossRef]
- Gupta, A.K.; Kumar, M. An integrative approach toward identification and analysis of therapeutic targets involved in HPV pathogenesis with a focus on carcinomas. Cancer Biomark. 2023, 36, 31–52. [Google Scholar] [CrossRef]
- Lin, S.C.; Liu, C.J.; Lin, J.A.; Chiang, W.F.; Hung, P.S.; Chang, K.W. miR-24 up-regulation in oral carcinoma: Positive association from clinical and in vitro analysis. Oral Oncol. 2010, 46, 204–208. [Google Scholar] [CrossRef]
- Ursu, S.; Majid, S.; Garger, C.; de Semir, D.; Bezrookove, V.; Desprez, P.Y.; McAllister, S.; Soroceanu, L.; Nosrati, M.; Yimam, K.; et al. Novel tumor suppressor role of miRNA-876 in cholangiocarcinoma. Oncogenesis 2019, 8, 42. [Google Scholar] [CrossRef] [PubMed]
- Expression of Concern. Cell. Microbiol. 2020, 22, e13259. [CrossRef]
- Chen, J.; Tang, Z.; Chen, Z.; Wei, Y.; Liang, H.; Zhang, X.; Gao, Z.; Zhu, H. MicroRNA-218-5p regulates inflammation response via targeting TLR4 in atherosclerosis. BMC Cardiovasc. Disord. 2023, 23, 122. [Google Scholar] [CrossRef]
- Liao, H.; Zhang, Z.; Liu, Z.; Lin, W.; Huang, J.; Huang, Y. Inhibited microRNA-218-5p attenuates synovial inflammation and cartilage injury in rats with knee osteoarthritis by promoting sclerostin. Life Sci. 2021, 267, 118893. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Tang, H.; Li, X.Y.; Deng, M.F.; Wei, N.; Wang, X.; Zhou, Y.F.; Wang, D.Q.; Fu, P.; Wang, J.Z.; et al. Targeting the HDAC2/HNF-4A/miR-101b/AMPK Pathway Rescues Tauopathy and Dendritic Abnormalities in Alzheimer’s Disease. Mol. Ther. 2017, 25, 752–764. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Li, M.; Ban, J.; Li, Z. miR-23b mediates TNF-α-Inhibited Osteogenic Differentiation of Human Periodontal Ligament Stem Cells by Targeting Runx2. Int. J. Med. Sci. 2021, 18, 3674–3683. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.H.; Li, L.C.; Yang, S.F.; Tsai, C.F.; Chuang, Y.T.; Chu, H.J.; Ueng, K.C. MicroRNA Let-7a, -7e and -133a Attenuate Hypoxia-Induced Atrial Fibrosis via Targeting Collagen Expression and the JNK Pathway in HL1 Cardiomyocytes. Int. J. Mol. Sci. 2022, 23, 9636. [Google Scholar] [CrossRef]
- Uttamani, J.R.; Naqvi, A.R.; Estepa, A.M.V.; Kulkarni, V.; Brambila, M.F.; Martinez, G.; Chapa, G.; Wu, C.D.; Li, W.; Rivas-Tumanyan, S.; et al. Downregulation of miRNA-26 in chronic periodontitis interferes with innate immune responses and cell migration by targeting phospholipase C beta 1. J. Clin. Periodontol. 2023, 50, 102–113. [Google Scholar] [CrossRef]
- Siow, M.Y.; Ng, L.P.; Vincent-Chong, V.K.; Jamaludin, M.; Abraham, M.T.; Abdul Rahman, Z.A.; Kallarakkal, T.G.; Yang, Y.H.; Cheong, S.C.; Zain, R.B. Dysregulation of miR-31 and miR-375 expression is associated with clinical outcomes in oral carcinoma. Oral Dis. 2014, 20, 345–351. [Google Scholar] [CrossRef]
- Mitani, Y.; Roberts, D.B.; Fatani, H.; Weber, R.S.; Kies, M.S.; Lippman, S.M.; El-Naggar, A.K. MicroRNA profiling of salivary adenoid cystic carcinoma: Association of miR-17-92 upregulation with poor outcome. PLoS ONE 2013, 8, e66778. [Google Scholar] [CrossRef]
- Krongbaramee, T.; Zhu, M.; Qian, Q.; Zhang, Z.; Eliason, S.; Shu, Y.; Qian, F.; Akkouch, A.; Su, D.; Amendt, B.A.; et al. Plasmid encoding microRNA-200c ameliorates periodontitis and systemic inflammation in obese mice. Mol. Ther. Nucleic Acids 2021, 23, 1204–1216. [Google Scholar] [CrossRef]
- Kalea, A.Z.; Hoteit, R.; Suvan, J.; Lovering, R.C.; Palmen, J.; Cooper, J.A.; Khodiyar, V.K.; Harrington, Z.; Humphries, S.E.; D’Aiuto, F. Upregulation of gingival tissue miR-200b in obese periodontitis subjects. J. Dent. Res. 2015, 94, 59S–69S. [Google Scholar] [CrossRef]
- Hu, R.P.; Lu, Y.Y.; Zhang, X.J. MiR-34b-5p knockdown attenuates bleomycin-induced pulmonary fibrosis by targeting tissue inhibitor of metalloproteinase 3 (TIMP3). Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 2273–2279. [Google Scholar] [CrossRef]
- Stoecklin-Wasmer, C.; Guarnieri, P.; Celenti, R.; Demmer, R.T.; Kebschull, M.; Papapanou, P.N. MicroRNAs and their target genes in gingival tissues. J. Dent. Res. 2012, 91, 934–940. [Google Scholar] [CrossRef]
- Li, Q.; Yao, Y.; Eades, G.; Liu, Z.; Zhang, Y.; Zhou, Q. Downregulation of miR-140 promotes cancer stem cell formation in basal-like early stage breast cancer. Oncogene 2014, 33, 2589–2600. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhao, Y.; Sun, X.H.; Ge, J.; Zhang, B.; Wang, X.; Cao, X.C. Down-regulation of miR-129-5p via the Twist1-Snail feedback loop stimulates the epithelial-mesenchymal transition and is associated with poor prognosis in breast cancer. Oncotarget 2015, 6, 34423–34436. [Google Scholar] [CrossRef] [PubMed]
- Rong, W.; Rome, C.; Yao, S. Increased Expression of miR-7a-5p and miR-592 during Expansion of Rat Dental Pulp Stem Cells and Their Implication in Osteogenic Differentiation. Cells Tissues Organs 2022, 211, 41–56. [Google Scholar] [CrossRef] [PubMed]
- Baltan, S.; Sandau, U.S.; Brunet, S.; Bastian, C.; Tripathi, A.; Nguyen, H.; Liu, H.; Saugstad, J.A.; Zarnegarnia, Y.; Dutta, R. Identification of miRNAs That Mediate Protective Functions of Anti-Cancer Drugs During White Matter Ischemic Injury. ASN Neuro 2021, 13, 17590914211042220. [Google Scholar] [CrossRef]
- Howard, S.; Richardson, S.; Benyeogor, I.; Omosun, Y.; Dye, K.; Medhavi, F.; Lundy, S.; Adebayo, O.; Igietseme, J.U.; Eko, F.O. Differential miRNA Profiles Correlate With Disparate Immunity Outcomes Associated With Vaccine Immunization and Chlamydial Infection. Front. Immunol. 2021, 12, 625318. [Google Scholar] [CrossRef]
- Hu, D.L.; Liu, Y.Q.; Chen, F.K.; Sheng, Y.H.; Yang, R.; Kong, X.Q.; Cao, K.J.; Zhang, J.S.; Qian, L.M. Differential expression of microRNAs in cardiac myocytes compared to undifferentiated P19 cells. Int. J. Mol. Med. 2011, 28, 59–64. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhao, Z.N.; Cheng, J.T.; Zhang, B.; Xu, J.; Huang, F.; Zhao, R.N.; Chen, Y.J. Ibandronate promotes osteogenic differentiation of periodontal ligament stem cells by regulating the expression of microRNAs. Biochem. Biophys. Res. Commun. 2011, 404, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Naito, S.; Sequeira-Gross, T.; Petersen, J.; Detlef, I.; Sachse, M.; Zeller, T.; Reichenspurner, H.; Girdauskas, E. Circulating microRNAs in the prediction of BAV aortopathy: Do the expression patterns correlate between blood and aortic tissue? Rev. Cardiovasc. Med. 2022, 23, 47. [Google Scholar] [CrossRef] [PubMed]
- Na, J.; Jin, H.; Wang, X.; Huang, K.; Sun, S.; Li, Q.; Zhang, W. The crosstalk of HDAC3, microRNA-18a and ADRB3 in the progression of heart failure. Cell Biosci. 2021, 11, 31. [Google Scholar] [CrossRef]
- van Almen, G.C.; Verhesen, W.; van Leeuwen, R.E.; van de Vrie, M.; Eurlings, C.; Schellings, M.W.; Swinnen, M.; Cleutjens, J.P.; van Zandvoort, M.A.; Heymans, S.; et al. MicroRNA-18 and microRNA-19 regulate CTGF and TSP-1 expression in age-related heart failure. Aging Cell 2011, 10, 769–779. [Google Scholar] [CrossRef] [PubMed]
- Rusu-Nastase, E.G.; Lupan, A.M.; Marinescu, C.I.; Neculachi, C.A.; Preda, M.B.; Burlacu, A. MiR-29a Increase in Aging May Function as a Compensatory Mechanism Against Cardiac Fibrosis Through SERPINH1 Downregulation. Front. Cardiovasc. Med. 2021, 8, 810241. [Google Scholar] [CrossRef]
- Yuan, L.; Tang, C.; Li, D.; Yang, Z. MicroRNA-18a Expression in Female Coronary Heart Disease and Regulatory Mechanism on Endothelial Cell by Targeting Estrogen Receptor. J. Cardiovasc. Pharmacol. 2018, 72, 277–284. [Google Scholar] [CrossRef]
- Zhu, D.; Zhu, Z.; Qi, H. NEAT1/microRNA 339-5p/SPI1 Axis Feedback Loop Contributes to Osteogenic Differentiation in Acute Suppurative Osteomyelitis in Children. J. Inflamm. Res. 2023, 16, 2675–2687. [Google Scholar] [CrossRef]
- Vallelunga, A.; Iannitti, T.; Capece, S.; Somma, G.; Russillo, M.C.; Foubert-Samier, A.; Laurens, B.; Sibon, I.; Meissner, W.G.; Barone, P.; et al. Serum miR-96-5P and miR-339-5P Are Potential Biomarkers for Multiple System Atrophy and Parkinson’s Disease. Front. Aging Neurosci. 2021, 13, 632891. [Google Scholar] [CrossRef]
- Bi, X.; Zhang, Y.; Yu, Y.; Yuan, J.; Xu, S.; Liu, F.; Ye, J.; Liu, P. MiRNA-339-5p promotes isoproterenol-induced cardiomyocyte hypertrophy by targeting VCP to activate the mTOR signaling. Cell Biol. Int. 2022, 46, 288–299. [Google Scholar] [CrossRef]
- Witvrouwen, I.; Gevaert, A.B.; Possemiers, N.; Beckers, P.J.; Vorlat, A.; Heidbuchel, H.; Van Laere, S.J.; Van Craenenbroeck, A.H.; Van Craenenbroeck, E.M. Circulating microRNA as predictors for exercise response in heart failure with reduced ejection fraction. Eur. J. Prev. Cardiol. 2021, 28, 1673–1681. [Google Scholar] [CrossRef]
- Saddic, L.A.; Chang, T.W.; Sigurdsson, M.I.; Heydarpour, M.; Raby, B.A.; Shernan, S.K.; Aranki, S.F.; Body, S.C.; Muehlschlegel, J.D. Integrated microRNA and mRNA responses to acute human left ventricular ischemia. Physiol. Genom. 2015, 47, 455–462. [Google Scholar] [CrossRef]
- Dai, T.; Zhao, X.; Li, Y.; Yu, L.; Li, Y.; Zhou, X.; Gong, Q. miR-423 Promotes Breast Cancer Invasion by Activating NF-κB Signaling. OncoTargets Ther. 2020, 13, 5467–5478. [Google Scholar] [CrossRef] [PubMed]
- Ongoz Dede, F.; Gokmenoglu, C.; Turkmen, E.; Bozkurt Dogan, S.; Ayhan, B.S.; Yildirim, K. Six miRNA expressions in the saliva of smokers and non-smokers with periodontal disease. J. Periodontal Res. 2023, 58, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Al-Rawi, N.H.; Al-Marzooq, F.; Al-Nuaimi, A.S.; Hachim, M.Y.; Hamoudi, R. Salivary microRNA 155, 146a/b and 203: A pilot study for potentially non-invasive diagnostic biomarkers of periodontitis and diabetes mellitus. PLoS ONE 2020, 15, e0237004. [Google Scholar] [CrossRef]
- Ye, Q.; Huang, Z.; Lu, W.; Yan, F.; Zeng, W.; Xie, J.; Zhong, W. Identification of the common differentially expressed genes and pathogenesis between neuropathic pain and aging. Front. Neurosci. 2022, 16, 994575. [Google Scholar] [CrossRef] [PubMed]
- Sugino, Y.; Kugawa, F. Effect of miR-433-3p and miR-883b-5p on murine CYP 3A family enzymes in AML12 cells. Pharmazie 2018, 73, 519–525. [Google Scholar] [CrossRef]
- Zhang, C.; Yao, J.; Liu, C.; Yang, K.; Zhang, W.; Sun, D.; Gu, W. The Role of Thyroid Hormone Synthesis Gene-Related miRNAs Profiling in Structural and Functional Changes of The Thyroid Gland Induced by Excess Iodine. Biol. Trace Elem. Res. 2023. [Google Scholar] [CrossRef]
- Yehya, N.; Yerrapureddy, A.; Tobias, J.; Margulies, S.S. MicroRNA modulate alveolar epithelial response to cyclic stretch. BMC Genom. 2012, 13, 154. [Google Scholar] [CrossRef]
- Ding, S.; Qu, Y.; Yang, S.; Zhao, Y.; Xu, G. Novel miR-1958 Promotes Mycobacterium tuberculosis Survival in RAW264.7 Cells by Inhibiting Autophagy Via Atg5. J. Microbiol. Biotechnol. 2019, 29, 989–998. [Google Scholar] [CrossRef]
- Fujimori, K.; Yoneda, T.; Tomofuji, T.; Ekuni, D.; Azuma, T.; Maruyama, T.; Mizuno, H.; Sugiura, Y.; Morita, M. Detection of Salivary miRNAs Reflecting Chronic Periodontitis: A Pilot Study. Molecules 2019, 24, 1034. [Google Scholar] [CrossRef]
- Zhang, B.; Lin, T.; He, H. Comparative analysis of blood and saliva expression profiles in chronic and refractory periodontitis patients. BMC Oral Health 2015, 15, 166. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Huang, J.; Yan, H.; Li, X.; Ding, C.; Wang, Q.; Lu, Z. Protective effect of microRNA-381 against inflammatory damage of endothelial cells during coronary heart disease by targeting CXCR4. Mol. Med. Rep. 2020, 21, 1439–1448. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhao, S.; Cheng, L.; Lin, Z.; Zeng, M.; Ruan, Z.; Sun, B.; Luo, Z.; Tang, Y.; Long, H. Mg2+-mediated autophagy-dependent polarization of macrophages mediates the osteogenesis of bone marrow stromal stem cells by interfering with macrophage-derived exosomes containing miR-381. J. Orthop. Res. 2022, 40, 1563–1576. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Liu, R.; Ju, H.; Huang, M.; Li, Z.; Li, W.; Wang, Y. microRNA-130b-3p Attenuates Septic Cardiomyopathy by Regulating the AMPK/mTOR Signaling Pathways and Directly Targeting ACSL4 against Ferroptosis. Int. J. Biol. Sci. 2023, 19, 4223–4241. [Google Scholar] [CrossRef]
- Ro, W.B.; Kang, M.H.; Song, D.W.; Kim, H.S.; Lee, G.W.; Park, H.M. Identification and Characterization of Circulating MicroRNAs as Novel Biomarkers in Dogs With Heart Diseases. Front. Vet. Sci. 2021, 8, 729929. [Google Scholar] [CrossRef] [PubMed]
- Rangel-Zuniga, O.A.; Vals-Delgado, C.; Alcala-Diaz, J.F.; Quintana-Navarro, G.M.; Krylova, Y.; Leon-Acuna, A.; Luque, R.M.; Gomez-Delgado, F.; Delgado-Lista, J.; Ordovas, J.M.; et al. A set of miRNAs predicts T2DM remission in patients with coronary heart disease: From the CORDIOPREV study. Mol. Ther. Nucleic Acids 2021, 23, 255–263. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, S.; Dong, T.; Yang, J.; Xie, Y.; Wu, Y.; Kang, K.; Hu, S.; Gou, D.; Wei, Y. Profiling of differentially expressed microRNAs in arrhythmogenic right ventricular cardiomyopathy. Sci. Rep. 2016, 6, 28101. [Google Scholar] [CrossRef]
- Mao, Z.; Liu, G.; Xiao, G.Y.; Zhao, C.; Zou, Y.C. CircCDR1as Suppresses Bone Microvascular Endothelial Cell Activity and Angiogenesis Through Targeting miR-135b/ FIH-1 Axis. Orthop. Surg. 2021, 13, 573–582. [Google Scholar] [CrossRef]
- Yu, Z.; Zhao, S.; Wang, L.; Wang, J.; Zhou, J. miRNA-339-5p Plays an Important Role in Invasion and Migration of Pancreatic Cancer Cells. Med. Sci. Monit. 2019, 25, 7509–7517. [Google Scholar] [CrossRef]
- Zhou, J.; Nie, H.; Liu, P.; Wang, Z.; Yao, B.; Yang, L. Down-regulation of miR-339 promotes differentiation of BMSCs and alleviates osteoporosis by targeting DLX5. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 29–36. [Google Scholar] [CrossRef]
- Wang, C.; Huang, Y.; Zhang, J.; Fang, Y. MiRNA-339-5p suppresses the malignant development of gastric cancer via targeting ALKBH1. Exp. Mol. Pathol. 2020, 115, 104449. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Rui, X.; Wei, W.; Shao, S.; Zhu, Y. Prognostic value of miR-339-5p in patients with prostate cancer and its effects on tumor progression. Exp. Ther. Med. 2021, 21, 390. [Google Scholar] [CrossRef] [PubMed]
- Chun, N.; Coca, S.G.; He, J.C. A protective role for microRNA-688 in acute kidney injury. J. Clin. Investig. 2018, 128, 5216–5218. [Google Scholar] [CrossRef] [PubMed]
- Park, C.Y.; Jeker, L.T.; Carver-Moore, K.; Oh, A.; Liu, H.J.; Cameron, R.; Richards, H.; Li, Z.; Adler, D.; Yoshinaga, Y.; et al. A resource for the conditional ablation of microRNAs in the mouse. Cell Rep. 2012, 1, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Zhai, R.; Hua, B.; Bao, L.; Wang, D.; Li, Y.; Yao, W.; Fan, H.; Hao, C. miR-let-7d attenuates EMT by targeting HMGA2 in silica-induced pulmonary fibrosis. RSC Adv. 2019, 9, 19355–19364. [Google Scholar] [CrossRef]
- Xie, T.; Liang, J.; Guo, R.; Liu, N.; Noble, P.W.; Jiang, D. Comprehensive microRNA analysis in bleomycin-induced pulmonary fibrosis identifies multiple sites of molecular regulation. Physiol. Genom. 2011, 43, 479–487. [Google Scholar] [CrossRef]
- Luan, X.; Zhou, X.; Fallah, P.; Pandya, M.; Lyu, H.; Foyle, D.; Burch, D.; Diekwisch, T.G.H. MicroRNAs: Harbingers and shapers of periodontal inflammation. Semin. Cell Dev. Biol. 2022, 124, 85–98. [Google Scholar] [CrossRef]
- Nisha, K.J.; Janam, P.; Harshakumar, K. Identification of a novel salivary biomarker miR-143-3p for periodontal diagnosis: A proof of concept study. J. Periodontol. 2019, 90, 1149–1159. [Google Scholar] [CrossRef]
- Nahid, M.A.; Rivera, M.; Lucas, A.; Chan, E.K.; Kesavalu, L. Polymicrobial infection with periodontal pathogens specifically enhances microRNA miR-146a in ApoE−/− mice during experimental periodontal disease. Infect. Immun. 2011, 79, 1597–1605. [Google Scholar] [CrossRef]
- Mico-Martinez, P.; Alminana-Pastor, P.J.; Alpiste-Illueca, F.; Lopez-Roldan, A. MicroRNAs and periodontal disease: A qualitative systematic review of human studies. J. Periodontal Implant. Sci. 2021, 51, 386–397. [Google Scholar] [CrossRef]
- Yu, W.; Gu, Q.; Wu, D.; Zhang, W.; Li, G.; Lin, L.; Lowe, J.M.; Hu, S.; Li, T.W.; Zhou, Z.; et al. Identification of potentially functional circRNAs and prediction of circRNA-miRNA-mRNA regulatory network in periodontitis: Bridging the gap between bioinformatics and clinical needs. J. Periodontal Res. 2022, 57, 594–614. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, W.; Anniwaer, A.; Li, B.; Chen, Y.; Yu, Z.; Yu, X. The Role of MicroRNAs in Predicting the Neurological Outcome of Patients with Subarachnoid Hemorrhage: A Meta-analysis. Cell. Mol. Neurobiol. 2023, 43, 2883–2893. [Google Scholar] [CrossRef] [PubMed]
- Kesavalu, L.; Holt, S.C.; Ebersole, J.L. In vitro environmental regulation of Porphyromonas gingivalis growth and virulence. Oral Microbiol. Immunol. 2003, 18, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Rivera, M.F.; Lee, J.Y.; Aneja, M.; Goswami, V.; Liu, L.; Velsko, I.M.; Chukkapalli, S.S.; Bhattacharyya, I.; Chen, H.; Lucas, A.R.; et al. Polymicrobial infection with major periodontal pathogens induced periodontal disease and aortic atherosclerosis in hyperlipidemic ApoEnull mice. PLoS ONE 2013, 8, e57178. [Google Scholar] [CrossRef]
- Kesavalu, L.; Sathishkumar, S.; Bakthavatchalu, V.; Matthews, C.; Dawson, D.; Steffen, M.; Ebersole, J.L. Rat model of polymicrobial infection, immunity, and alveolar bone resorption in periodontal disease. Infect. Immun. 2007, 75, 1704–1712. [Google Scholar] [CrossRef]
- Chukkapalli, S.; Rivera-Kweh, M.; Gehlot, P.; Velsko, I.; Bhattacharyya, I.; Calise, S.J.; Satoh, M.; Chan, E.K.; Holoshitz, J.; Kesavalu, L. Periodontal bacterial colonization in synovial tissues exacerbates collagen-induced arthritis in B10.RIII mice. Arthritis Res. Ther. 2016, 18, 161. [Google Scholar] [CrossRef]
- Aravindraja, C.; Sakthivel, R.; Liu, X.; Goodwin, M.; Veena, P.; Godovikova, V.; Fenno, J.C.; Levites, Y.; Golde, T.E.; Kesavalu, L. Intracerebral but Not Peripheral Infection of Live Porphyromonas gingivalis Exacerbates Alzheimer’s Disease like Amyloid Pathology in APP-TgCRND8 Mice. Int. J. Mol. Sci. 2022, 23, 3328. [Google Scholar] [CrossRef]
- Kesavalu, L.; Ebersole, J.L.; Machen, R.L.; Holt, S.C. Porphyromonas gingivalis virulence in mice: Induction of immunity to bacterial components. Infect. Immun. 1992, 60, 1455–1464. [Google Scholar] [CrossRef]
- Prokopec, S.D.; Watson, J.D.; Waggott, D.M.; Smith, A.B.; Wu, A.H.; Okey, A.B.; Pohjanvirta, R.; Boutros, P.C. Systematic evaluation of medium-throughput mRNA abundance platforms. RNA 2013, 19, 51–62. [Google Scholar] [CrossRef]
- Veldman-Jones, M.H.; Brant, R.; Rooney, C.; Geh, C.; Emery, H.; Harbron, C.G.; Wappett, M.; Sharpe, A.; Dymond, M.; Barrett, J.C.; et al. Evaluating Robustness and Sensitivity of the NanoString Technologies nCounter Platform to Enable Multiplexed Gene Expression Analysis of Clinical Samples. Cancer Res. 2015, 75, 2587–2593. [Google Scholar] [CrossRef]
- Cioce, M.; Rutigliano, D.; Puglielli, A.; Fazio, V.M. Butein-instigated miR-186-5p-dependent modulation of TWIST1 affects resistance to cisplatin and bioenergetics of Malignant Pleural Mesothelioma cells. Cancer Drug Resist. 2022, 5, 814–828. [Google Scholar] [CrossRef] [PubMed]
- Vlachos, I.S.; Zagganas, K.; Paraskevopoulou, M.D.; Georgakilas, G.; Karagkouni, D.; Vergoulis, T.; Dalamagas, T.; Hatzigeorgiou, A.G. DIANA-miRPath v3.0: Deciphering microRNA function with experimental support. Nucleic Acids Res. 2015, 43, W460–W466. [Google Scholar] [CrossRef]
- Huang, H.Y.; Lin, Y.C.; Cui, S.; Huang, Y.; Tang, Y.; Xu, J.; Bao, J.; Li, Y.; Wen, J.; Zuo, H.; et al. miRTarBase update 2022: An informative resource for experimentally validated miRNA-target interactions. Nucleic Acids Res. 2022, 50, D222–D230. [Google Scholar] [CrossRef] [PubMed]
- Gillies, S.O. Shapely: Manipulation and Analysis of Geometric Objects. 2007. Available online: https://github.com/Toblerity/Shapely (accessed on 11 August 2023).
- Chen, T.; Guestrin, C. XGBoost: A Scalable Tree Boosting System. In Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, San Francisco, CA, USA, 13–17 August 2016; ACM: New York, NY, USA, 2016; pp. 785–794. [Google Scholar]
- Du, G.; Ren, C.; Wang, J.; Ma, J. The Clinical Value of Blood miR-654-5p, miR-126, miR-10b, and miR-144 in the Diagnosis of Colorectal Cancer. Comput. Math. Methods Med. 2022, 2022, 8225966. [Google Scholar] [CrossRef]
- Pradhan, U.K.; Sharma, N.K.; Kumar, P.; Kumar, A.; Gupta, S.; Shankar, R. miRbiom: Machine-learning on Bayesian causal nets of RBP-miRNA interactions successfully predicts miRNA profiles. PLoS ONE 2021, 16, e0258550. [Google Scholar] [CrossRef]
- Gholizadeh, M.; Lapczuk-Romanska, J.; Post, M.; Komaniecka, N.; Mazlooman, S.R.; Kaderali, L.; Drozdzik, M. A Mixture Method for Robust Detection HCV Early Diagnosis Biomarker with ML Approach and Molecular Docking. Int. J. Mol. Sci. 2023, 24, 7207. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.S.; Su, C.; Tellez, M.; Albandar, J.M.; Rao, R.; Iyer, V.; Shi, E.; Wu, H. Developing and testing a prediction model for periodontal disease using machine learning and big electronic dental record data. Front. Artif. Intell. 2022, 5, 979525. [Google Scholar] [CrossRef]
- Chi, H.; Chen, H.; Wang, R.; Zhang, J.; Jiang, L.; Zhang, S.; Jiang, C.; Huang, J.; Quan, X.; Liu, Y.; et al. Proposing new early detection indicators for pancreatic cancer: Combining machine learning and neural networks for serum miRNA-based diagnostic model. Front. Oncol. 2023, 13, 1244578. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Dai, A.; Tran, R.; Wang, J. Text mining-based identification of promising miRNA biomarkers for diabetes mellitus. Front. Endocrinol. 2023, 14, 1195145. [Google Scholar] [CrossRef]
- Li, L.Z.; Zhang, C.Z.; Liu, L.L.; Yi, C.; Lu, S.X.; Zhou, X.; Zhang, Z.J.; Peng, Y.H.; Yang, Y.Z.; Yun, J.P. miR-720 inhibits tumor invasion and migration in breast cancer by targeting TWIST1. Carcinogenesis 2014, 35, 469–478. [Google Scholar] [CrossRef]
- Iwaki, J.; Kikuchi, K.; Mizuguchi, Y.; Kawahigashi, Y.; Yoshida, H.; Uchida, E.; Takizawa, T. MiR-376c down-regulation accelerates EGF-dependent migration by targeting GRB2 in the HuCCT1 human intrahepatic cholangiocarcinoma cell line. PLoS ONE 2013, 8, e69496. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Yuan, M.H.; Wu, H.T.; Chen, W.J.; Zhang, M.L.; Ye, Q.Q.; Liu, J.; Zhang, G.J. MicroRNA-488 inhibits proliferation and motility of tumor cells via downregulating FSCN1, modulated by Notch3 in breast carcinomas. Cell Death Dis. 2020, 11, 912. [Google Scholar] [CrossRef]
- Che, Q.; Wang, W.; Duan, P.; Fang, F.; Liu, C.; Zhou, T.; Li, H.; Xiong, C.; Zhao, K. Downregulation of miR-322 promotes apoptosis of GC-2 cell by targeting Ddx3x. Reprod. Biol. Endocrinol. 2019, 17, 63. [Google Scholar] [CrossRef] [PubMed]
- Musilova, K.; Devan, J.; Cerna, K.; Seda, V.; Pavlasova, G.; Sharma, S.; Oppelt, J.; Pytlik, R.; Prochazka, V.; Prouzova, Z.; et al. miR-150 downregulation contributes to the high-grade transformation of follicular lymphoma by upregulating FOXP1 levels. Blood 2018, 132, 2389–2400. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.L.; Chen, Y.L.; Ge, Z.Z.; Qu, Y.Y.; Cao, Y.; Kang, Z.X. Downregulation of serum exosomal miR-150-5p is associated with poor prognosis in patients with colorectal cancer. Cancer Biomark. 2019, 26, 69–77. [Google Scholar] [CrossRef]
- Winkler, I.; Bitter, C.; Winkler, S.; Weichenhan, D.; Thavamani, A.; Hengstler, J.G.; Borkham-Kamphorst, E.; Kohlbacher, O.; Plass, C.; Geffers, R.; et al. Identification of Pparγ-modulated miRNA hubs that target the fibrotic tumor microenvironment. Proc. Natl. Acad. Sci. USA 2020, 117, 454–463. [Google Scholar] [CrossRef]
- Ran, J.; Li, Y.; Liu, L.; Zhu, Y.; Ni, Y.; Huang, H.; Liu, Z.; Miao, Z.; Zhang, L. Apelin enhances biological functions in lung cancer A549 cells by downregulating exosomal miR-15a-5p. Carcinogenesis 2021, 42, 243–253. [Google Scholar] [CrossRef]


| Group/Bacteria/Weeks | Positive Gingival Plaque Samples (n = 10) | |||
|---|---|---|---|---|
| 8-Week-Time Point | 16-Week-Time Point | |||
| 2 Weeks | 4 Weeks | 6 Weeks | 12 Weeks | |
| Group I/T. forsythia ATCC 43037 (8 weeks) | 5/10 | 8/10 | --- | --- |
| Group II/sham infection (8 weeks) | 0/10 | NC | --- | --- |
| Group III/T. forsythia ATCC 43037 (16 weeks) | 1/10 | 7/10 | NC | 10/10 |
| Group IV/sham infection (16 weeks) | 0/10 | NC | NC | 0/10 |
| Weeks/Infection/Sex | Upregulated miRNAs (p < 0.05) | Downregulated miRNAs (p < 0.05) |
|---|---|---|
| 8 Weeks/T. forsythia-infected vs. 8 Weeks/sham-infected (n = 10) | 0 | 67 (miR-375, miR-200c, miR-200b, miR-141, miR-34b-5p) |
| 8 Weeks/T. forsythia-infected Female vs. Male (n = 5) | 15 | 21 |
| 16 Weeks/T. forsythia-infected vs. 16 Weeks/sham-infected (n = 10) | 16 (miR-1902, miR-let-7c, miR-146a, miR-423-5p) | 32 (miR-2135, miR-720, miR-376c, miR-488, miR-322) |
| 16 Weeks/T. forsythia-infected Female vs. Male (n = 5) | 1 | 10 |
| 8 Weeks/T. forsythia-infected vs. 16 Weeks/T. forsythia-infected | 5 | 8 |
| Upregulated miRNAs in a 16-Week T. forsythia Infection | ||||
|---|---|---|---|---|
| miRNAs | Fold Change | p-Value | Reported Functions | Number of Target Genes |
| miR-1902 | 1.67 | 0.00313 | Overexpressed in mouse serum and whole blood after intraperitoneal injection of lipoteichoic acid [23]. | -- |
| mmu-let-7c | 1.39 | 0.0045 | Interfere with critical inflammatory cytokine production (IL-1β, IL-6, and TNF) in human osteoarthritis and rheumatoid arthritis [24]. Playing a role in cardiomyogenesis promotion activity [25]. | 1782 (e.g., Fyco1, ADH4, Socs1, Steap4, S100pbp) |
| miR-423-5p | 1.32 | 4.7 × 10−7 | Upregulated expression in severe periodontal disease [26]. Higher expression in obese periodontitis subjects [27]. Identified as new candidate biomarker in the cross-talk between diabetes mellitus and Alzheimer’s disease [28]. | 8 (e.g., Smtnl2, Rnf114, Prune, Mcl1, Pura) |
| miR-210 | 1.31 | 0.02324 | Upregulated in periodontal disease and obesity-suffering subjects [29]. Elevated in the muscle samples of peripheral artery diseases and atherosclerosis obliterans [30]. Frequently elevated in multiple cancers such as HCC, prostate cancer, colorectal cancer and gastric cancer. | 427 (e.g., Fyco1, Arl8b, Tpm3, Gcnt4, P2rx7) |
| miR-146a | 1.3 | 0.01937 | Overexpressed in saliva of patients with periodontitis and its expression is increased with the deterioration of periodontitis in the patients [31,32]. Elevated levels are associated with reduction in proinflammatory cytokines in aggressive periodontitis [33]. | 360 (e.g., Srrm2, Cpt1a, Calu, BC030336, Maff) |
| miR-99b | 1.28 | 0.00041 | Upregulated in M. tuberculosis infected murine dendritic cells [34]. | 4 (e.g., Hnrnpu, Tcf7l2, Trim71, Ctnnd1) |
| mmu-let-7a | 1.26 | 0.00595 | Significantly upregulated in chronic periodontitis patients and interact with NF-κB pathway genes [35]. | 1038 (e.g., Fyco1, S100pbp, Foxo1, Snx5, Igf1, Cxxc5, Rxra) |
| miR-127 | 1.25 | 0.04454 | Upregulated in human atherosclerotic plaques with similar expression of RTL1 [36]. | 4 (e.g., Prx, Kpna2, Atf4, Hsp90ab1) |
| miR-98 | 1.24 | 0.04528 | Cardiac hypertrophy can be inhibited by upregulation of thioredoxin 1 which elevates the levels of miR-98 [37]. | 586 (e.g., Srrm2, Vps26b, Vps54, Cep97, Snx25, Prdm1) |
| miR-24 | 1.23 | 0.00075 | Upregulated in inflamed gingival biopsies [27]. Decreased levels reported in coronary artery diseases [38]. Potential therapeutic target candidate for Human papillomavirus-mediated carcinoma [39]. Elevated levels in oral squamous cell carcinoma [40]. | 359 (e.g., Fyco1, Mxd1, Flcn, Cblb, Bcl2l1, Cltc) |
| miR-876-3p | 1.23 | 0.01747 | Reduces tumor cell growth and suppressor at its elevated levels [41]. | --- |
| miR-218 | 1.19 | 0.01142 | Decreased expression is associated with protective effect in periodontitis [42]. Reduced cardiomyocyte hypertrophy. Reduced expression is a clinical marker for atherosclerosis [43]. Inhibition of miR-218 results in the attenuation of synovial inflammation and cartilage injury in the knee osteoarthritis rat model [44]. | 1139 (e.g., Epg5, Dmxl1, Cxcr4, Socs3, Rassf3, Acly, Fyco1) |
| miR-101b | 1.17 | 0.04246 | Major mediator of tauopathy and dendritic abnormalities in Alzheimer’s disease progression [45]. | 230 (e.g., Cd55, Brd8, Dhx9, Tcp1, Cltc, Fos, Tes) |
| miR-23b | 1.14 | 0.00697 | miR-23b mediates TNF-inhibited osteogenic differentiation of human periodontal ligament stem cells [46]. | 784 (e.g., Sla, Fbxo5, Nus1, Pvr, Chd1, Igf1, Tle4) |
| mmu-let-7e | 1.14 | 0.01353 | Inhibits the expression of collagen and post-transcriptional repression in HL1 cardiomyocytes [47]. | 1011 (e.g., Tyk2, Lrp10, Eng, Smg7, Prc1, Psd3, Irs2) |
| miR-26b | 1.14 | 0.02741 | Downregulated in periodontal inflammation [48]. | 1751 (e.g., Sema3d, Inpp5d, Urb2, Coro7, Actr6, Arf6) |
| Dysregulated miRNAs in an 8-Week T. forsythia Infection | ||||
|---|---|---|---|---|
| miRs | Fold Change | p-Value | Reported Function | Number of Target Genes |
| miR-375 | −2.38 | 0.0154379 | Down-regulated in oral squamous cell carcinoma [49] and salivary adenoid cystic carcinoma [50]. | 24 (e.g., Med13, C1qbp, Mtpn, Wdr26, Sept2) |
| miR-200c | −2.36 | 0.01351086 | Reduced levels observed in mice infected with LPS of P. gingivalis [51]. | 15 (e.g., Atp5b, Pls3, Mycn, Zeb1, Thap4) |
| miR-200b | −1.86 | 0.00822482 | Variations in the levels of miR-200b observed in gingival tissue of obese periodontitis subjects [52]. | 100 (e.g., Ythdf3, N4bp2, Apobec3, G6pc, Sc5d) |
| miR-34b-5p | −1.8 | 0.02779496 | Enhances the resistance to bleomycin by regulating its target gene TIMP3 during the pathogenesis of lung fibrosis [53]. | 14 (e.g., Mapk1, Mycn, Pou5f1, Sox2, Tfcp2l1) |
| miR-141 | −1.72 | 0.01359321 | Decreased levels reported in inflamed gingival tissues of periodontitis patients [54]. | 143 (e.g., Dlc1, Apob, Acox2, Helz2, Klf5) |
| miR-140 | −1.48 | 0.03988881 | Downregulated in cancer stem cells of DCIS tumors [55]. | |
| miR-129-5p | −1.45 | 0.01918338 | Downregulation fosters epithelial to mesenchymal transition in breast cancer [56]. | |
| miR-205 | −1.41 | 0.03628375 | Decreased levels reported in inflamed gingival tissues of periodontitis patients [54]. | |
| miR-423-3p | −1.4 | 0.00303573 | Upregulated expression in severe periodontal disease [26]. Reduced expression reported in obese periodontitis subjects [27]. | |
| miRNA | Accession # | Target Function |
|---|---|---|
| 8-Week Analysis | ||
| miR-592 | MIMAT0003730 | Increased expression of miR-592 during expansion of rat dental pulp stem cells and their implication in osteogenic differentiation [57]. |
| miR-2133 | MIMAT0011209 | Upregulated in mouse optic nerves exposed to oxygen glucose deprivation-treated MS-275 anticancer drug [58]. Downregulated in VCG vaccine immunized and Chlamydia-infected mice [59]. Reported in cardiac myocytes (Day 10) compared to normal P19 cells (Day 0) [60]. |
| miR-18a | MIMAT0000528 | Upregulated in obesity and associated periodontal disease condition [29]. Upregulated in periodontal ligament stem cells [61]. Associated with aortopathy patients [62]. Over-expression reduced fibrosis, hypertrophy, and apoptosis of cardiomyocytes in heart failure [63]. Elevated in the heart tissue of old age mice [64,65]. Aberrantly overexpressed in female CHD patient’s peripheral blood [66]. |
| miR-2136 | MIMAT0011212 | Upregulated in mouse brains with Alzheimer’s disease (PMID: 29057267). |
| miR-375 | MIMAT0000739 | Down-regulated in oral squamous cell carcinoma [49] and salivary adenoid cystic carcinoma [50]. |
| 16-Week Analysis | ||
| miR-339-5p | MIMAT0000584 | Downregulated in osteogenic differentiation conditions of human bone marrow mesenchymal stem cells and has a role in the NEAT-1-miR-339-5p-SPI1 feedback loop [67]. Potential biomarker for multiple system atrophy patients and Parkinson’s disease [68]. Upregulated during neonatal rat cardiomyocytes hypertrophy triggered by isoproterenol [69]. Upregulated in exercise non-responders and involved in angiogenesis, skeletal muscle function and inflammation [70]. Upregulated in animal models of left ventricular ischemia [71]. |
| miR-1892 | MIMAT0007871 | Reported in cardiac myocytes (Day 10) compared to normal P19 cells (Day 0) [60]. |
| miR-423-5p | MIMAT0004825 | Overexpression of miR-423-5p induced breast cancer cell invasion through the NF-κB signaling pathway [72]. |
| miR-146b | MIMAT0003475 | Elevated with the progression of periodontal disease [73]. Potential biomarker in periodontal disease and diabetes [74]. |
| miR-883-5p | MIMAT0004848 | Associated with neurotrophic pain and aging [75]. Bind to Cyp3a mRNAs and regulate Cyp gene expressions [76]. Increased in high-iodine exposed Wistar rats [77]. |
| Combined 8 and 16 Weeks | ||
| miR-592 | MIMAT0003730 | Shown in the 8-week analysis |
| miR-375 | MIMAT0000739 | Shown in the 8-week analysis |
| miR-18a | MIMAT0000528 | Shown in the 8-week analysis |
| miR-339-5p | MIMAT0000584 | Shown in the 16-week analysis |
| miR-466d-5p | MIMAT0004930 | Upregulated in rat alveolar epithelial cells [78] |
| miRNA | Accession # | Target Function |
|---|---|---|
| 8-week analysis | ||
| miR-592 | MIMAT0003730 | Increased expression of miR-592 during expansion of rat dental pulp stem cells and their implication in osteogenic differentiation [57]. |
| miR-1958 | MIMAT0009431 | Promoting Mycobacterium tuberculosis survival in RAW 264.7 cells [79]. |
| miR-381 | MIMAT0000746 | Upregulated in saliva samples of periodontitis patients [80]. Associated with chronic periodontitis [81]. Protective role in coronary heart disease patients [82]. miR-381 as novel vehicles for promoting the osteogenic differentiation of BMSCs via Mg2+ ions [83]. |
| miR-130b | MIMAT0000387 | Remarkably improved cardiac function and ameliorated morphological damage to heart tissue in LPS-induced mice [84]. Upregulation of cfa-miR-130b was observed in dogs with myxomatous mitral valve degeneration [85]. Contributing in the prediction of T2DM patients with CVD [86]. |
| miR-135b | MIMAT0000612 | Significantly associated with myocardium adipose and fibrosis in the primary cardiomyopathy patients [87]. Downregulated in the bone microvascular endothelial cells isolated from non-traumatic ONFH [88]. |
| 16-week analysis | ||
| miR-423-5p | MIMAT0004825 | Overexpression of miR-423-5p induced breast cancer cell invasion through NF-κB signaling pathway [72]. |
| miR-1892 | MIMAT0007871 | Reported in cardiac myocytes (Day 10) compared to normal P19 cells (Day 0) [60]. |
| miR-339-5p | MIMAT0000584 | Suppresses the invasion and migration of pancreatic cancer cells [89]. Promoting osteogenic differentiation [90]. Downregulated in metastatic gastric cancer patients [91], prostate cancer [92]. |
| miR-688 | MIMAT0003467 | Protective factor in acute kidney injury [93]. Expressed in the central nervous system of mice models [94]. |
| let-7d | MIMAT0000383 | Attenuates epithelial–mesenchymal transition in silica-induced pulmonary fibrosis [95]. |
| 8- and 16-week combined analysis | ||
| miR-592 | MIMAT0003730 | Increased expression of miR-592 during expansion of rat dental pulp stem cells and their implication in osteogenic differentiation [57]. |
| miR-375 | MIMAT0000739 | Down-regulated in oral squamous cell carcinoma [49] and salivary adenoid cystic carcinoma [50]. |
| miR-339-5p | MIMAT0000584 | Suppresses the invasion and migration of pancreatic cancer cells [89]. Promoting osteogenic differentiation [90]. Downregulated in metastatic gastric cancer patients [91], prostate cancer [92]. |
| miR-704 | MIMAT0003494 | Discovered in the late period of bleomycin-induced pulmonary fibrosis [96]. |
| miR-196a | MIMAT0000518 | Downregulated in the gingival tissue of obese periodontitis subjects [52]. Plays a role in immunity development [97]. The expression level of miR-196a in gingival sulcus was significantly higher in the periodontitis. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aravindraja, C.; Jeepipalli, S.; Duncan, W.; Vekariya, K.M.; Bahadekar, S.; Chan, E.K.L.; Kesavalu, L. Unique miRomics Expression Profiles in Tannerella forsythia-Infected Mandibles during Periodontitis Using Machine Learning. Int. J. Mol. Sci. 2023, 24, 16393. https://doi.org/10.3390/ijms242216393
Aravindraja C, Jeepipalli S, Duncan W, Vekariya KM, Bahadekar S, Chan EKL, Kesavalu L. Unique miRomics Expression Profiles in Tannerella forsythia-Infected Mandibles during Periodontitis Using Machine Learning. International Journal of Molecular Sciences. 2023; 24(22):16393. https://doi.org/10.3390/ijms242216393
Chicago/Turabian StyleAravindraja, Chairmandurai, Syam Jeepipalli, William Duncan, Krishna Mukesh Vekariya, Sakshee Bahadekar, Edward K. L. Chan, and Lakshmyya Kesavalu. 2023. "Unique miRomics Expression Profiles in Tannerella forsythia-Infected Mandibles during Periodontitis Using Machine Learning" International Journal of Molecular Sciences 24, no. 22: 16393. https://doi.org/10.3390/ijms242216393
APA StyleAravindraja, C., Jeepipalli, S., Duncan, W., Vekariya, K. M., Bahadekar, S., Chan, E. K. L., & Kesavalu, L. (2023). Unique miRomics Expression Profiles in Tannerella forsythia-Infected Mandibles during Periodontitis Using Machine Learning. International Journal of Molecular Sciences, 24(22), 16393. https://doi.org/10.3390/ijms242216393








