Vitamin D Status Modestly Regulates NOD-Like Receptor Family with a Pyrin Domain 3 Inflammasome and Interleukin Profiles among Arab Adults
Abstract
:1. Introduction
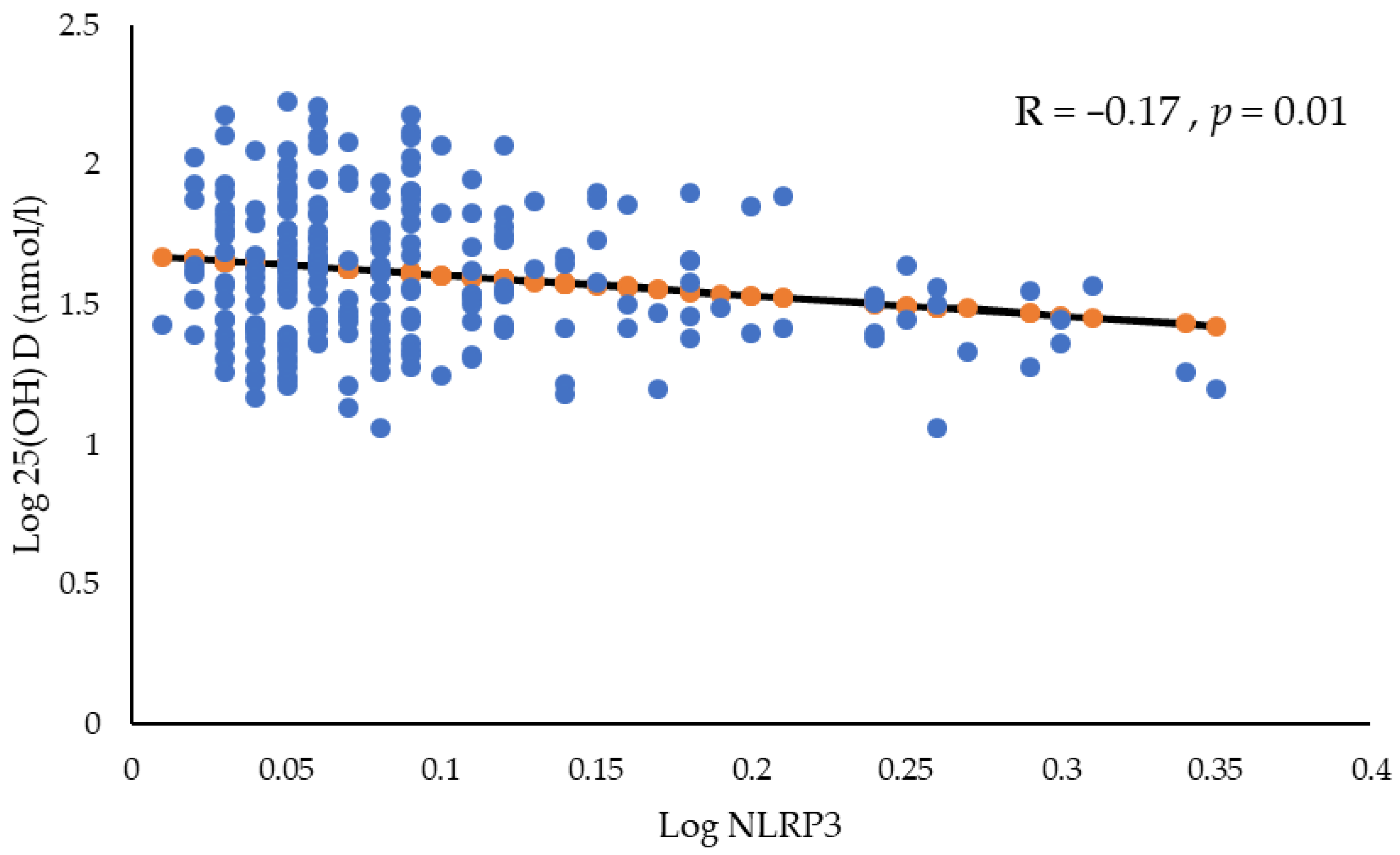
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Design and Participants
4.2. Sample Collection and Anthropometrics
4.3. Lipid Profile and Biochemical Estimations
4.4. Serum NLRP3 Inflammasome (NLRP3 and Caspase–1) and Interleukin (IL–1α, IL–1β, IL–18, IL–33 and IL–37) Estimations
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-Alyani, H.; Al-Turki, H.A.; Al-Essa, O.N.; Alani, F.M.; Sadat-Ali, M. Vitamin D deficiency in Saudi Arabians: A reality or simply hype: A meta-analysis (2008–2015). J. Fam. Community Med. 2018, 25, 1–4. [Google Scholar]
- Al-Mogbel, E.S. Vitamin D status among Adult Saudi Females visiting Primary Health Care Clinics. Int. J. Health Sci. Qassim. 2012, 6, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Elsammak, M.Y.; Al-Wosaibi, A.A.; Al-Howeish, A.; Alsaeed, J. Vitamin d deficiency in Saudi Arabs. Horm. Metab. Res. 2010, 42, 364–368. [Google Scholar] [CrossRef]
- Darraj, H.; Hakami, K.M.; Maghrabi, R.; Bakri, N.; Alhazmi, M.H.; Names, A.A.; Akkur, A.; Sayegh, M.; Alhazmi, A.; Khubrani, S.M.; et al. Nutritional Rickets Among Children: A Retrospective Study from Saudi Arabia. Pediatr. Health Med. Ther. 2023, 14, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Al-Daghri, N.M.; Ansari, M.G.A.; Sabico, S.; Al-Saleh, Y.; Aljohani, N.J.; Alfawaz, H.; Alharbi, M.; Al-Othman, A.M.; Alokail, M.S.; Wimalawansa, S.J. Efficacy of different modes of vitamin D supplementation strategies in Saudi adolescents. J. Steroid Biochem. Mol. Biol. 2018, 180, 23–28. [Google Scholar] [CrossRef]
- AlGhamdi, S.A.; Enaibsi, N.N.; Alsufiani, H.M.; Alshaibi, H.F.; Khoja, S.O.; Carlberg, C. A Single Oral Vitamin D3 Bolus Reduces Inflammatory Markers in Healthy Saudi Males. Int. J. Mol. Sci. 2022, 23, 11992. [Google Scholar] [CrossRef]
- Feldman, D.; Krishnan, A.V.; Swami, S.; Giovannucci, E.; Feldman, B.J. The role of vitamin D in reducing cancer risk and progression. Nat. Rev. Cancer 2014, 14, 342–357. [Google Scholar] [CrossRef]
- Alamro, A.A.; Al-Malky, M.M.; Ansari, M.G.A.; Amer, O.E.; Alnaami, A.M.; Hussain, S.D.; Barhoumi, T.A.; Alghamdi, A.A.; Haq, S.H.; Sabico, S.; et al. The effects of melatonin and vitamin D3 on the gene expression of BCl-2 and BAX in MCF-7 breast cancer cell line. J. King Saud. Univ. Sci. 2021, 33, 101287. [Google Scholar] [CrossRef]
- Autier, P.; Boniol, M.; Pizot, C.; Mullie, P. Vitamin D status and ill health: A systematic review. Lancet Diabetes Endocrinol. 2014, 2, 76–89. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D and immune function: Understanding common pathways. Curr. Osteoporos. Rep. 2009, 7, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D: A d-lightful solution for health. J. Investig. Med. 2011, 59, 872–880. [Google Scholar] [CrossRef] [PubMed]
- Hossein-nezhad, A.; Holick, M.F. Vitamin D for health: A global perspective. Mayo Clin. Proc. 2013, 88, 720–755. [Google Scholar] [CrossRef] [PubMed]
- Zabihiyeganeh, M.; Amini Kadijani, A.; Akbari, A.; Yahyazadeh, H.; Mirzaei, A. Association of serum vitamin D status with serum pro-inflammatory cytokine levels and clinical severity of fibromyalgia patients. Clin. Nutr. ESPEN 2023, 55, 71–75. [Google Scholar] [CrossRef]
- Cederberg, K.L.J.; Silvestri, R.; Walters, A.S. Vitamin D and Restless Legs Syndrome: A Review of Current Literature. Tremor Other Hyperkinet. Mov. N. Y. 2023, 13, 12. [Google Scholar] [CrossRef] [PubMed]
- AlNafea, H.M.; Korish, A.A. The interplay between hypovitaminosis D and the immune dysfunction in the arteriovenous thrombotic complications of the sever coronavirus disease 2019 (COVID-19) infection. Blood Coagul. Fibrinolysis 2023, 34, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.G.A.; Sabico, S.; Clerici, M.; Khattak, M.N.K.; Wani, K.; Al-Musharaf, S.; Amer, O.E.; Alokail, M.S.; Al-Daghri, N.M. Vitamin D Supplementation Is Associated with Increased Glutathione Peroxidase-1 Levels in Arab Adults with Prediabetes. Antioxidants 2020, 9, 118. [Google Scholar] [CrossRef]
- Bouillon, R.; Marcocci, C.; Carmeliet, G.; Bikle, D.; White, J.H.; Dawson-Hughes, B.; Lips, P.; Munns, C.F.; Lazaretti-Castro, M.; Giustina, A.; et al. Skeletal and Extraskeletal Actions of Vitamin D: Current Evidence and Outstanding Questions. Endocr. Rev. 2019, 40, 1109–1151. [Google Scholar] [CrossRef]
- Ansari, M.G.A.; Mohammed, A.K.; Wani, K.A.; Hussain, S.D.; Alnaami, A.M.; Abdi, S.; Aljohani, N.J.; Al-Daghri, N.M. Vitamin D Receptor Gene Variants Susceptible to Osteoporosis in Arab Post-Menopausal Women. Curr. Issues Mol. Biol. 2021, 43, 1325–1334. [Google Scholar] [CrossRef]
- Sassi, F.; Tamone, C.; D’Amelio, P. Vitamin D: Nutrient, Hormone, and Immunomodulator. Nutrients 2018, 10, 1656. [Google Scholar] [CrossRef]
- Liu, P.T.; Stenger, S.; Li, H.; Wenzel, L.; Tan, B.H.; Krutzik, S.R.; Ochoa, M.T.; Schauber, J.; Wu, K.; Meinken, C.; et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 2006, 311, 1770–1773. [Google Scholar] [CrossRef]
- Hewison, M. Vitamin D and immune function: Autocrine, paracrine or endocrine? Scand. J. Clin. Lab. Investig. Suppl. 2012, 243, 92–102. [Google Scholar]
- Aranow, C. Vitamin D and the immune system. J. Investig. Med. 2011, 59, 881–886. [Google Scholar] [CrossRef]
- Lu, L.; Lu, Q.; Chen, W.; Li, J.; Li, C.; Zheng, Z. Vitamin D(3) Protects against Diabetic Retinopathy by Inhibiting High-Glucose-Induced Activation of the ROS/TXNIP/NLRP3 Inflammasome Pathway. J. Diabetes Res. 2018, 2018, 8193523. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Ridker, P.M.; Hansson, G.K. Progress and challenges in translating the biology of atherosclerosis. Nature 2011, 473, 317–325. [Google Scholar] [CrossRef]
- Cao, R.; Ma, Y.; Li, S.; Shen, D.; Yang, S.; Wang, X.; Cao, Y.; Wang, Z.; Wei, Y.; Li, S.; et al. 1,25(OH)(2) D(3) alleviates DSS-induced ulcerative colitis via inhibiting NLRP3 inflammasome activation. J. Leukoc. Biol. 2020, 108, 283–295. [Google Scholar] [CrossRef]
- Huang, H.; Hong, J.Y.; Wu, Y.J.; Wang, E.Y.; Liu, Z.Q.; Cheng, B.H.; Mei, L.; Liu, Z.G.; Yang, P.C.; Zheng, P.Y. Vitamin D receptor interacts with NLRP3 to restrict the allergic response. Clin. Exp. Immunol. 2018, 194, 17–26. [Google Scholar] [CrossRef]
- Rao, Z.; Chen, X.; Wu, J.; Xiao, M.; Zhang, J.; Wang, B.; Fang, L.; Zhang, H.; Wang, X.; Yang, S.; et al. Vitamin D Receptor Inhibits NLRP3 Activation by Impeding Its BRCC3-Mediated Deubiquitination. Front. Immunol. 2019, 10, 2783. [Google Scholar] [CrossRef]
- Al-Daghri, N.M.; Guerini, F.R.; Al-Attas, O.S.; Alokail, M.S.; Alkharfy, K.M.; Draz, H.M.; Agliardi, C.; Costa, A.S.; Saulle, I.; Mohammed, A.K.; et al. Vitamin D receptor gene polymorphisms are associated with obesity and inflammosome activity. PLoS ONE 2014, 9, e102141. [Google Scholar] [CrossRef]
- Swanson, K.V.; Deng, M.; Ting, J.P. The NLRP3 inflammasome: Molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019, 19, 477–489. [Google Scholar] [CrossRef]
- Schroder, K.; Tschopp, J. The inflammasomes. Cell 2010, 140, 821–832. [Google Scholar] [CrossRef]
- Wani, K.; AlHarthi, H.; Alghamdi, A.; Sabico, S.; Al-Daghri, N.M. Role of NLRP3 Inflammasome Activation in Obesity-Mediated Metabolic Disorders. Int. J. Environ. Res. Public. Health 2021, 18, 511. [Google Scholar] [CrossRef] [PubMed]
- Alfadul, H.; Sabico, S.; Ansari, M.G.A.; Alnaami, A.M.; Amer, O.E.; Hussain, S.D.; Wani, K.; Khattak, M.N.K.; Clerici, M.; Al-Daghri, N.M. Differences and Associations of NLRP3 Inflammasome Levels with Interleukins 1α, 1β, 33 and 37 in Adults with Prediabetes and Type 2 Diabetes Mellitus. Biomedicines 2023, 11, 1315. [Google Scholar] [CrossRef] [PubMed]
- Saresella, M.; La Rosa, F.; Piancone, F.; Zoppis, M.; Marventano, I.; Calabrese, E.; Rainone, V.; Nemni, R.; Mancuso, R.; Clerici, M. The NLRP3 and NLRP1 inflammasomes are activated in Alzheimer’s disease. Mol. Neurodegener. 2016, 11, 23. [Google Scholar] [CrossRef]
- Alfadul, H.; Sabico, S.; Al-Daghri, N.M. The role of interleukin-1beta in type 2 diabetes mellitus: A systematic review and meta-analysis. Front. Endocrinol. 2022, 13, 901616. [Google Scholar] [CrossRef]
- Al-Daghri, N.M.; Wani, K.; AlHarthi, H.; Alghamdi, A.; Alnaami, A.M.; Yakout, S.M. Sex-Specific Signature in the Circulating NLRP3 Levels of Saudi Adults with Metabolic Syndrome. J. Clin. Med. 2021, 10, 3288. [Google Scholar] [CrossRef]
- Tunbridge, M.; França Gois, P.H. Vitamin D and the NLRP3 Inflammasome. Appl. Sci. 2020, 10, 8462. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, W.; Sun, T.; Huang, Y.; Wang, Y.; Deb, D.K.; Yoon, D.; Kong, J.; Thadhani, R.; Li, Y.C. 1,25-Dihydroxyvitamin D promotes negative feedback regulation of TLR signaling via targeting microRNA-155-SOCS1 in macrophages. J. Immunol. 2013, 190, 3687–3695. [Google Scholar] [CrossRef]
- He, Y.; Hara, H.; Nunez, G. Mechanism and Regulation of NLRP3 Inflammasome Activation. Trends Biochem. Sci. 2016, 41, 1012–1021. [Google Scholar] [CrossRef]
- Wang, T.T.; Nestel, F.P.; Bourdeau, V.; Nagai, Y.; Wang, Q.; Liao, J.; Tavera-Mendoza, L.; Lin, R.; Hanrahan, J.W.; Mader, S.; et al. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J. Immunol. 2004, 173, 2909–2912. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.T.; Stenger, S.; Tang, D.H.; Modlin, R.L. Cutting edge: Vitamin D-mediated human antimicrobial activity against Mycobacterium tuberculosis is dependent on the induction of cathelicidin. J. Immunol. 2007, 179, 2060–2063. [Google Scholar] [CrossRef] [PubMed]
- Latz, E.; Xiao, T.S.; Stutz, A. Activation and regulation of the inflammasomes. Nat. Rev. Immunol. 2013, 13, 397–411. [Google Scholar] [CrossRef]
- Roffe-Vazquez, D.N.; Huerta-Delgado, A.S.; Castillo, E.C.; Villarreal-Calderon, J.R.; Gonzalez-Gil, A.M.; Enriquez, C.; Garcia-Rivas, G.; Elizondo-Montemayor, L. Correlation of Vitamin D with Inflammatory Cytokines, Atherosclerotic Parameters, and Lifestyle Factors in the Setting of Heart Failure: A 12-Month Follow-Up Study. Int. J. Mol. Sci. 2019, 20, 5811. [Google Scholar] [CrossRef]
- Mallat, Z.; Corbaz, A.; Scoazec, A.; Graber, P.; Alouani, S.; Esposito, B.; Humbert, Y.; Chvatchko, Y.; Tedgui, A. Interleukin-18/interleukin-18 binding protein signaling modulates atherosclerotic lesion development and stability. Circ. Res. 2001, 89, E41–E45. [Google Scholar] [CrossRef] [PubMed]
- Zirlik, A.; Abdullah, S.M.; Gerdes, N.; MacFarlane, L.; Schonbeck, U.; Khera, A.; McGuire, D.K.; Vega, G.L.; Grundy, S.; Libby, P.; et al. Interleukin-18, the metabolic syndrome, and subclinical atherosclerosis: Results from the Dallas Heart Study. Arter. Thromb. Vasc. Biol. 2007, 27, 2043–2049. [Google Scholar] [CrossRef] [PubMed]
- Baeke, F.; Takiishi, T.; Korf, H.; Gysemans, C.; Mathieu, C. Vitamin D: Modulator of the immune system. Curr. Opin. Pharmacol. 2010, 10, 482–496. [Google Scholar] [CrossRef]
- Vilaysane, A.; Chun, J.; Seamone, M.E.; Wang, W.; Chin, R.; Hirota, S.; Li, Y.; Clark, S.A.; Tschopp, J.; Trpkov, K.; et al. The NLRP3 inflammasome promotes renal inflammation and contributes to CKD. J. Am. Soc. Nephrol. 2010, 21, 1732–1744. [Google Scholar] [CrossRef]
- Zhang, Y.; Kong, J.; Deb, D.K.; Chang, A.; Li, Y.C. Vitamin D receptor attenuates renal fibrosis by suppressing the renin-angiotensin system. J. Am. Soc. Nephrol. 2010, 21, 966–973. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Leung, D.Y.; Richers, B.N.; Liu, Y.; Remigio, L.K.; Riches, D.W.; Goleva, E. Vitamin D inhibits monocyte/macrophage proinflammatory cytokine production by targeting MAPK phosphatase-1. J. Immunol. 2012, 188, 2127–2135. [Google Scholar] [CrossRef] [PubMed]
- Al-Daghri, N.; Al-Attas, O.; Alokail, M.; Alkharfy, K.; Yousef, M.; Nadhrah, H.; Al-Othman, A.; Al-Saleh, Y.; Sabico, S.; Chrousos, G. Hypovitaminosis D and cardiometabolic risk factors among non-obese youth. Open Med. 2010, 5, 752–757. [Google Scholar] [CrossRef]
- Al-Daghri, N.M.; Hussain, S.D.; Ansari, M.G.A.; Khattak, M.N.K.; Aljohani, N.; Al-Saleh, Y.; Al-Harbi, M.Y.; Sabico, S.; Alokail, M.S. Decreasing prevalence of vitamin D deficiency in the central region of Saudi Arabia (2008–2017). J. Steroid Biochem. Mol. Biol. 2021, 212, 105920. [Google Scholar] [CrossRef] [PubMed]
- Al-Daghri, N.M.; Al-Attas, O.S.; Alokail, M.; Alkharfy, K.; Wani, K.; Amer, O.E.; Haq, S.U.; Rahman, S.; Alnaami, A.M.; Livadas, S.; et al. Does visceral adiposity index signify early metabolic risk in children and adolescents?: Association with insulin resistance, adipokines, and subclinical inflammation. Pediatr. Res. 2014, 75, 459–463. [Google Scholar] [CrossRef] [PubMed]
- Al-Saleh, Y.; Sulimani, R.; Sabico, S.; Alshahrani, F.M.; Fouda, M.A.; Almohaya, M.; Alaidarous, S.B.; Alkhawashki, H.M.; Alshaker, M.; Alrayes, H.; et al. Diagnosis and management of osteoporosis in Saudi Arabia: 2023 key updates from the Saudi Osteoporosis Society. Arch. Osteoporos. 2023, 18, 75. [Google Scholar] [CrossRef]
- Sabico, S.; Wani, K.; Grant, W.B.; Al-Daghri, N.M. Improved HDL Cholesterol through Vitamin D Status Correction Substantially Lowers 10-Year Atherosclerotic Cardiovascular Disease Risk Score in Vitamin D-Deficient Arab Adults. Nutrients 2023, 15, 551. [Google Scholar] [CrossRef] [PubMed]
| Parameters | Males | p-Value | Females | p-Value | ||
|---|---|---|---|---|---|---|
| Sufficient (>50 nmol/L) | Deficient (<50 nmol/L) | Sufficient (>50 nmol/L) | Deficient (<50 nmol/L) | |||
| N | 32 | 96 | 95 | 115 | ||
| Age (years) | 42.9 ± 10.4 | 39.2 ± 9.8 | 0.07 | 42.7 ± 7.7 | 41.3 ± 8.8 | 0.11 |
| BMI (kg/m2) | 30.4 ± 6.6 | 29.0 ± 5.5 | 0.31 | 33.1 ± 6.6 | 31.8 ± 6.8 | 0.18 |
| WHR | 0.91 ± 0.1 | 0.92 ± 0.1 | 0.99 | 0.85 ± 0.1 | 0.85 ± 0.1 | 0.91 |
| Systolic BP (mmHg) | 123.1 ± 9.3 | 124.6 ± 13.9 | 0.59 | 126.1 ± 13.3 | 123.0 ± 14.8 | 0.13 |
| Diastolic BP (mmHg) | 76.8 ± 9.1 | 76.3 ± 10.3 | 0.53 | 78.8 ± 11.6 | 76.8 ± 10.6 | 0.19 |
| Total Cholesterol (mmol/L) | 4.7 ± 1.0 | 5.31 ± 1.0 | 0.008 | 5.03 ± 1.1 | 5.22 ± 1.1 | 0.22 |
| HDL Cholesterol (mmol/L) | 1.0 ± 0.2 | 1.02 ± 0.3 | 0.99 | 1.15 ± 0.3 | 1.14 ± 0.4 | 0.86 |
| Triglycerides (mmol/L) | 1.7 (1.3–2.2) | 1.62 (1.1–2.3) | 0.93 | 1.35 (1.11–2.0) | 1.59 (1.1–2.1) | 0.23 |
| Insulin (uU/mL) | 18.8 (16–20) | 16.1 (11–17) | 0.07 | 16.5 (15–19) | 15.9 (11–19) | 0.54 |
| Glucose (mmol/L) | 5.7 (5.4–6.3) | 5.42 (4.9–6.2) | 0.30 | 6.10 (5.3–7.4) | 5.94 (5.2–7.5) | 0.62 |
| HbA1c (%) | 5.84 ± 0.8 | 5.50 ± 0.9 | 0.07 | 5.75 ± 1.3 | 6.10 ± 1.5 | 0.08 |
| Calcium (mmol/L) | 2.21 ± 0.1 | 2.20 ± 0.2 | 0.68 | 2.21 ± 0.2 | 2.21 ± 0.2 | 0.89 |
| 25(OH)D (nmol/L) | 71.3 (58–81) | 30.4 (26–38) | <0.001 | 75.7 (62–94) | 28.7 (22–41) | <0.001 |
| Parameters | VD Status | p-Value | p-Value * | |
|---|---|---|---|---|
| Sufficient (≥50 nmol/L) | Deficient (<50 nmol/L) | |||
| IL–1α (pg/mL) | 0.6 (0.5–0.8) | 0.6 (0.5–0.9) | 0.84 | 0.91 |
| IL–1β (pg/mL) | 0.8 (0.7–3.0) | 1.4 (0.7–2.9) | 0.43 | 0.51 |
| IL–18 (pg/mL) | 9.2 (1.0–23.4) | 10.5 (0.8–33.0) | 0.69 | 0.92 |
| IL–33 (pg/mL) | 3.5 (3.0–4.0) | 3.7 (3.2–4.0) | 0.01 | 0.06 |
| IL–37 (pg/mL) | 2.9 (2.1–7.0) | 4.2 (2.3–7.6) | 0.53 | 0.49 |
| Caspase–1 (ng/mL) | 0.9 (0.5–2.7) | 0.7 (0.5–2.1) | 0.27 | 0.44 |
| NLRP3 (ng/mL) | 0.16 (0.12–0.24) | 0.18 (0.11–0.32) | 0.01 | 0.05 |
| Parameters | Sufficient | Deficient | ||
|---|---|---|---|---|
| R | p-Value | R | p-Value | |
| Age (years) | −0.12 | 0.28 | −0.20 | 0.02 |
| BMI (kg/m2) | −0.22 | 0.06 | −0.13 | 0.12 |
| WHR | 0.09 | 0.48 | −0.04 | 0.64 |
| Systolic BP (mmHg) | 0.00 | 0.99 | −0.05 | 0.56 |
| Diastolic BP (mmHg) | −0.03 | 0.77 | −0.03 | 0.70 |
| Cholesterol (mmol/L) | 0.04 | 0.71 | −0.03 | 0.69 |
| Glucose (mmol/L) | 0.05 | 0.64 | −0.09 | 0.29 |
| HDL Cholesterol (mmol/L) | 0.03 | 0.77 | −0.12 | 0.14 |
| Triglycerides (mmol/L) | 0.01 | 0.91 | 0.01 | 0.92 |
| HbA1c | −0.02 | 0.86 | −0.11 | 0.18 |
| 25(OH)D (nmol/L) | −0.10 | 0.39 | −0.13 | 0.12 |
| IL–18 (pg/mL) | −0.12 | 0.34 | −0.26 | 0.02 |
| IL–1α (pg/mL) | 0.33 | 0.006 | 0.28 | 0.001 |
| IL–β (pg/mL) | 0.00 | 0.98 | 0.03 | 0.79 |
| IL–33 (pg/mL) | 0.37 | 0.001 | 0.20 | 0.02 |
| IL–37 (pg/mL) | −0.04 | 0.72 | 0.10 | 0.34 |
| Caspase–1 (ng/mL) | −0.11 | 0.40 | 0.02 | 0.83 |
| Insulin (uU/mL) | −0.23 | 0.18 | −0.25 | 0.08 |
| Albumin (g/L) | −0.03 | 0.80 | 0.06 | 0.64 |
| Calcium (mmol/L) | −0.33 | 0.01 | 0.06 | 0.65 |
| Parameters | β ± SE | St. β | p-Value |
|---|---|---|---|
| Insulin | 0.05 ± 0.2 | 0.38 | 0.005 |
| NLRP3 | −1.33 ± 0.59 | −0.29 | 0.03 |
| Adjusted R2 | 18.3 | 0.004 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yakout, S.M.; Alfadul, H.; Ansari, M.G.A.; Khattak, M.N.K.; Al-Daghri, N.M. Vitamin D Status Modestly Regulates NOD-Like Receptor Family with a Pyrin Domain 3 Inflammasome and Interleukin Profiles among Arab Adults. Int. J. Mol. Sci. 2023, 24, 16377. https://doi.org/10.3390/ijms242216377
Yakout SM, Alfadul H, Ansari MGA, Khattak MNK, Al-Daghri NM. Vitamin D Status Modestly Regulates NOD-Like Receptor Family with a Pyrin Domain 3 Inflammasome and Interleukin Profiles among Arab Adults. International Journal of Molecular Sciences. 2023; 24(22):16377. https://doi.org/10.3390/ijms242216377
Chicago/Turabian StyleYakout, Sobhy M., Hend Alfadul, Mohammed G. A. Ansari, Malak N. K. Khattak, and Nasser M. Al-Daghri. 2023. "Vitamin D Status Modestly Regulates NOD-Like Receptor Family with a Pyrin Domain 3 Inflammasome and Interleukin Profiles among Arab Adults" International Journal of Molecular Sciences 24, no. 22: 16377. https://doi.org/10.3390/ijms242216377
APA StyleYakout, S. M., Alfadul, H., Ansari, M. G. A., Khattak, M. N. K., & Al-Daghri, N. M. (2023). Vitamin D Status Modestly Regulates NOD-Like Receptor Family with a Pyrin Domain 3 Inflammasome and Interleukin Profiles among Arab Adults. International Journal of Molecular Sciences, 24(22), 16377. https://doi.org/10.3390/ijms242216377







