GSK2656157, a PERK Inhibitor, Alleviates Pyroptosis of Macrophages Induced by Mycobacterium Bacillus Calmette–Guerin Infection
Abstract
:1. Introduction
2. Results
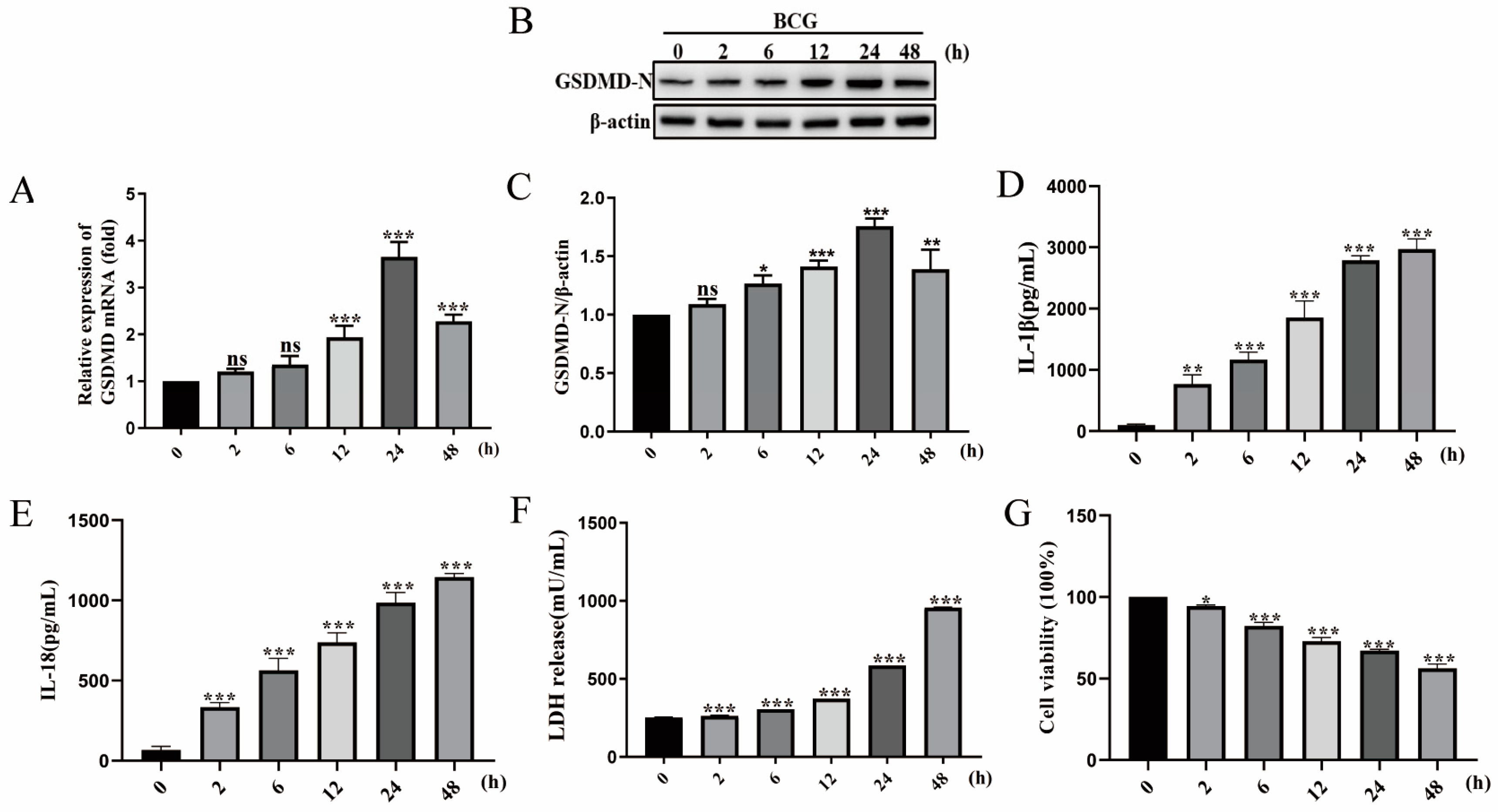
2.1. BCG Infection Induced THP-1 Macrophage Pyroptosis
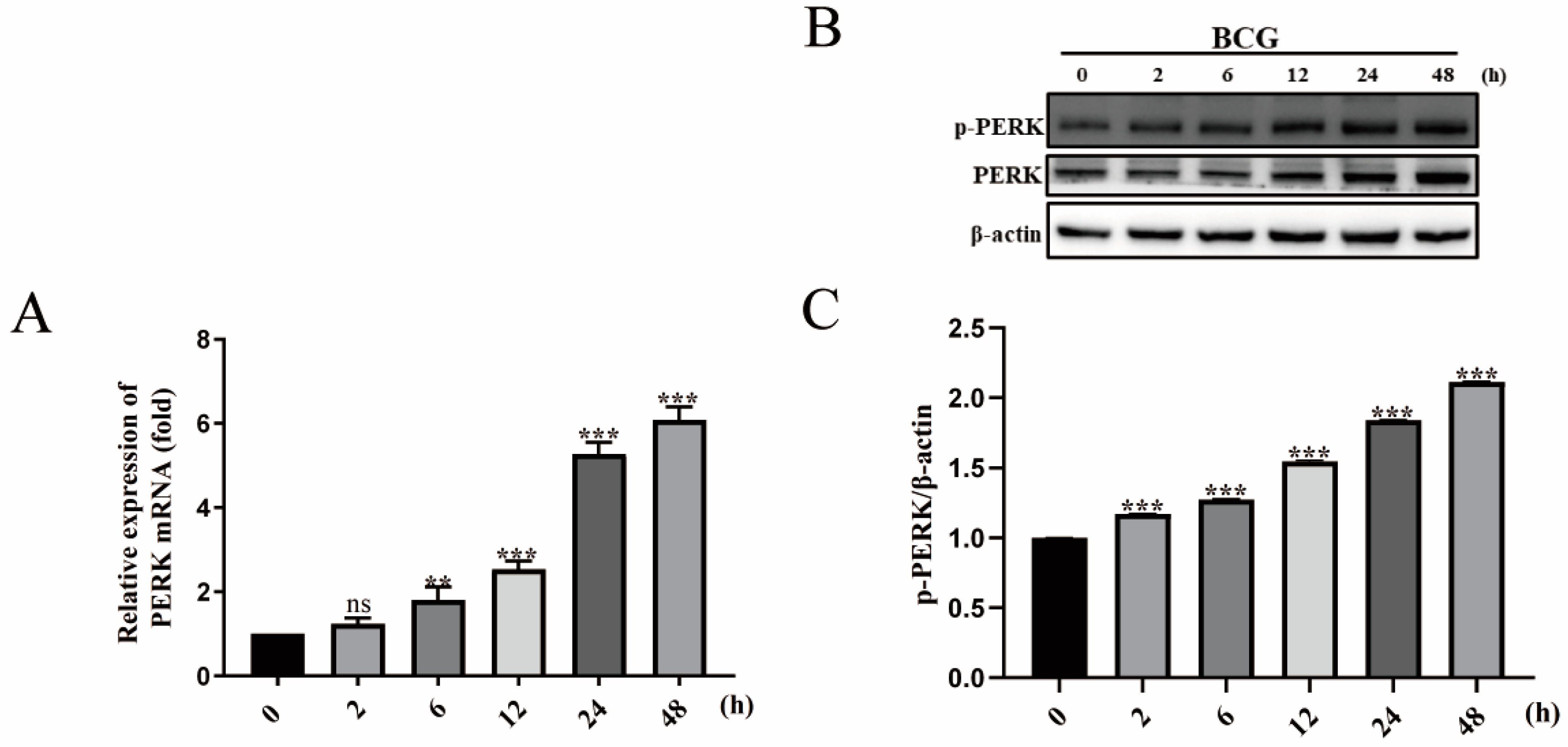
2.2. BCG Infection Activates PERK of THP-1 Macrophage
2.3. GSK2656157 Suppressed BCG Infection-Induced Pyroptosis of THP-1 Macrophages
2.4. GSK2656157 Suppressed BCG-Infected Pyroptosis in the Lung of Mice
2.5. GSK2656157 Inhibited the Expression of IL-1β and IL-18 in the Lung of BCG-Infected Mice
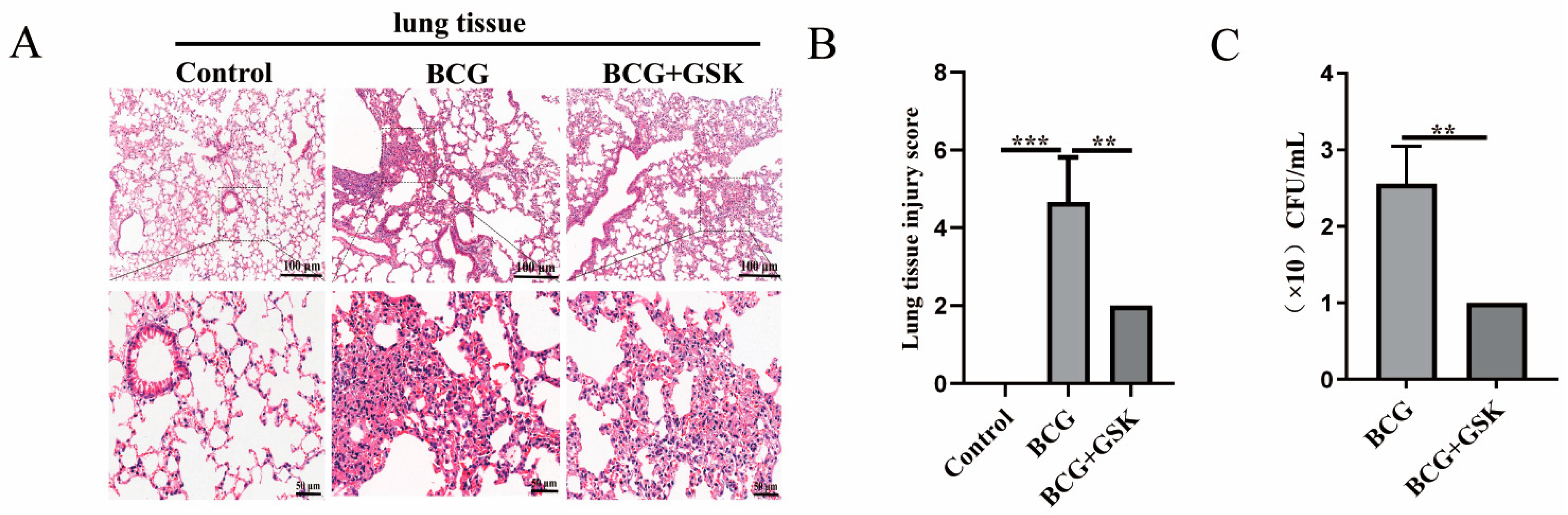
2.6. GSK2656157 Attenuated the Level of Lung Damage, Inflammatory Infiltration, and Bacterial Colonization in BCG-Infected Mice
3. Discussion
4. Materials and Methods
4.1. BCG, Cell Lines, Antibodies, Reagents, and Animals
4.2. THP-1 Cell Culture and BCG Infection
4.3. Western Blotting Analysis
4.4. Quantitative Real-Time PCR (qRT-PCR)
4.5. ELISA
4.6. LDH
4.7. CCK-8
4.8. Immunofluorescence Analysis
4.9. Transmission Electron Microscopy (TEM)
4.10. Animal Challenge Experiments
4.11. H&E Staining
4.12. IHC
4.13. Bacterial Colony-Forming Unit (CFU) Count
4.14. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Furin, J.; Cox, H.; Pai, M. Tuberculosis. Lancet 2019, 393, 1642–1656. [Google Scholar] [CrossRef] [PubMed]
- Bell, L.C.K.; Noursadeghi, M. Pathogenesis of HIV-1 and Mycobacterium tuberculosis co-infection. Nat. Rev. Microbiol. 2018, 16, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Borrell, S.; Gagneux, S. Strain diversity, epistasis and the evolution of drug resistance in Mycobacterium tuberculosis. Clin. Microbiol. Infect. 2011, 17, 815–820. [Google Scholar] [CrossRef] [PubMed]
- Sia, J.K.; Rengarajan, J. Immunology of Mycobacterium tuberculosis Infections. Microbiol. Spectr. 2019, 7, 1110–1128. [Google Scholar] [CrossRef]
- Yu, P.; Zhang, X.; Liu, N.; Tang, L.; Peng, C.; Chen, X. Pyroptosis: Mechanisms and diseases. Signal Transduct. Target. Ther. 2021, 6, 128–148. [Google Scholar] [CrossRef]
- Zheng, M.; Kanneganti, T.D. The regulation of the ZBP1-NLRP3 inflammasome and its implications in pyroptosis, apoptosis, and necroptosis (PANoptosis). Immunol. Rev. 2020, 297, 26–38. [Google Scholar] [CrossRef]
- Paik, S.; Kim, J.K.; Silwal, P.; Sasakawa, C.; Jo, E.K. An update on the regulatory mechanisms of NLRP3 inflammasome activation. Cell. Mol. Immunol. 2021, 18, 1141–1160. [Google Scholar] [CrossRef]
- Sharma, B.R.; Kanneganti, T.D. NLRP3 inflammasome in cancer and metabolic diseases. Nat. Immunol. 2021, 22, 550–559. [Google Scholar] [CrossRef]
- Huang, Y.; Xu, W.; Zhou, R. NLRP3 inflammasome activation and cell death. Cell. Mol. Immunol. 2021, 18, 2114–2127. [Google Scholar] [CrossRef]
- Shi, J.; Zhao, Y.; Wang, Y.; Gao, W.; Ding, J.; Li, P.; Hu, L.; Shao, F. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature 2014, 514, 187–192. [Google Scholar] [CrossRef]
- Shi, J.; Zhao, Y.; Wang, K.; Shi, X.; Wang, Y.; Huang, H.; Zhuang, Y.; Cai, T.; Wang, F.; Shao, F. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 2015, 526, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yang, T.; Xiao, J.; Xu, C.; Alippe, Y.; Sun, K.; Kanneganti, T.D.; Monahan, J.B.; Abu-Amer, Y.; Lieberman, J.; et al. NLRP3 inflammasome activation triggers gasdermin D-independent inflammation. Sci. Immunol. 2021, 6, eabj3859–eabj3885. [Google Scholar] [CrossRef] [PubMed]
- D’souza, C.A.; Heitman, J. Dismantling the Cryptococcus coat. Trends Microbiol. 2001, 9, 112–113. [Google Scholar] [CrossRef] [PubMed]
- Phillips, F.C.; Gurung, P.; Kanneganti, T.D. Microbiota and caspase-1/caspase-8 regulate IL-1β-mediated bone disease. Gut Microbes 2016, 7, 334–341. [Google Scholar] [CrossRef]
- Qu, Z.; Zhou, J.; Zhou, Y.; Xie, Y.; Jiang, Y.; Wu, J.; Luo, Z.; Liu, G.; Yin, L.; Zhang, X.L.; et al. Mycobacterial EST12 activates a RACK1-NLRP3-gasdermin D pyroptosis-IL-1β immune pathway. Sci. Adv. 2020, 6, eaba4733–eaba4749. [Google Scholar] [CrossRef]
- Wassermann, R.; Gulen, M.F.; Sala, C.; Perin, S.G.; Lou, Y.; Rybniker, J.; Schmid-Burgk, J.L.; Schmidt, T.; Hornung, V.; Cole, S.T.; et al. Mycobacterium tuberculosis Differentially Activates cGAS- and Inflammasome-Dependent Intracellular Immune Responses through ESX-1. Cell Host Microbe 2015, 17, 799–810. [Google Scholar] [CrossRef]
- Sano, R.; Reed, J.C. ER stress-induced cell death mechanisms. Biochim. Biophys. Acta 2013, 1833, 3460–3470. [Google Scholar] [CrossRef]
- Shacham, T.; Patel, C.; Lederkremer, G.Z. PERK Pathway and Neurodegenerative Disease: To Inhibit or to Activate? Biomolecules 2021, 11, 354. [Google Scholar] [CrossRef]
- Bettigole, S.E.; Glimcher, L.H. Endoplasmic reticulum stress in immunity. Annu. Rev. Immunol. 2015, 33, 107–138. [Google Scholar] [CrossRef]
- Keestra-Gounder, A.M.; Byndloss, M.X.; Seyffert, N.; Young, B.M.; Chávez-Arroyo, A.; Tsai, A.Y.; Cevallos, S.A.; Winter, M.G.; Pham, O.H.; Tiffany, C.R.; et al. NOD1 and NOD2 signalling links ER stress with inflammation. Nature 2016, 532, 394–397. [Google Scholar] [CrossRef]
- Raines, L.N.; Zhao, H.; Wang, Y.; Chen, H.Y.; Gallart-Ayala, H.; Hsueh, P.C.; Cao, W.; Koh, Y.; Alamonte-Loya, A.; Liu, P.S.; et al. PERK is a critical metabolic hub for immunosuppressive function in macrophages. Nat. Immunol. 2022, 23, 431–445. [Google Scholar] [CrossRef] [PubMed]
- Di Conza, G.; Ho, P.C. ER Stress Responses: An Emerging Modulator for Innate Immunity. Cells 2020, 9, 695. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Wang, F.; Bao, C.; Han, J.; Guo, Y.; Liu, F.; Zhang, Y. BAG2 ameliorates endoplasmic reticulum stress-induced cell apoptosis in Mycobacterium tuberculosis-infected macrophages through selective autophagy. Autophagy 2020, 16, 1453–1467. [Google Scholar] [CrossRef] [PubMed]
- Bussi, C.; Gutierrez, M.G. Mycobacterium tuberculosis infection of host cells in space and time. FEMS Microbiol. Rev. 2019, 43, 341–361. [Google Scholar] [CrossRef]
- Cohen, S.B.; Gern, B.H.; Delahaye, J.L.; Adams, K.N.; Plumlee, C.R.; Winkler, J.K.; Sherman, D.R.; Gerner, M.Y.; Urdahl, K.B. Alveolar Macrophages Provide an Early Mycobacterium tuberculosis Niche and Initiate Dissemination. Cell Host Microbe 2018, 24, 439–446.e4. [Google Scholar] [CrossRef]
- Guo, Q.; Bi, J.; Wang, H.; Zhang, X. Mycobacterium tuberculosis ESX-1-secreted substrate protein EspC promotes mycobacterial survival through endoplasmic reticulum stress-mediated apoptosis. Emerg. Microbes Infect. 2021, 10, 19–36. [Google Scholar] [CrossRef]
- Silwal, P.; Paik, S.; Kim, J.K.; Yoshimori, T.; Jo E., K. Regulatory Mechanisms of Autophagy-Targeted Antimicrobial Therapeutics against Mycobacterial Infection. Front. Cell. Infect. Microbiol. 2021, 11, 633360–633372. [Google Scholar] [CrossRef]
- Allen, I.C.; Mcelvania-Tekippe, E.; Wilson, J.E.; Lich, J.D.; Arthur, J.C.; Sullivan, J.T.; Braunstein, M.; Ting, J.P. Characterization of NLRP12 during the in vivo host immune response to Klebsiella pneumoniae and Mycobacterium tuberculosis. PLoS ONE 2013, 8, e60842. [Google Scholar] [CrossRef]
- Moossavi, M.; Parsamanesh, N.; Bahrami, A.; Atkin, S.L.; Sahebkar, A. Role of the NLRP3 inflammasome in cancer. Mol. Cancer 2018, 17, 158–170. [Google Scholar] [CrossRef]
- Tuladhar, S.; Kanneganti, T.D. NLRP12 in innate immunity and inflammation. Mol. Asp. Med. 2020, 76, 100887–100909. [Google Scholar] [CrossRef]
- Kesavardhana, S.; Malireddi RK, S.; Kanneganti, T.D. Caspases in Cell Death, Inflammation, and Pyroptosis. Annu. Rev. Immunol. 2020, 38, 567–595. [Google Scholar] [CrossRef] [PubMed]
- Beckwith, K.S.; Beckwith, M.S.; Ullmann, S.; Sætra, R.S.; Kim, H.; Marstad, A.; Åsberg, S.E.; Strand, T.A.; Haug, M.; Niederweis, M.; et al. Plasma membrane damage causes NLRP3 activation and pyroptosis during Mycobacterium tuberculosis infection. Nat. Commun. 2020, 11, 2270. [Google Scholar] [CrossRef] [PubMed]
- Dorhoi, A.; Nouailles, G.; Jörg, S.; Hagens, K.; Heinemann, E.; Pradl, L.; Oberbeck-Müller, D.; Duque-Correa, M.A.; Reece, S.T.; Ruland, J.; et al. Activation of the NLRP3 inflammasome by Mycobacterium tuberculosis is uncoupled from susceptibility to active tuberculosis. Eur. J. Immunol. 2012, 42, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Arora, G.; Misra, R.; Sajid, A. Model Systems for Pulmonary Infectious Diseases: Paradigms of Anthrax and Tuberculosis. Curr. Top. Med. Chem. 2017, 17, 2077–2099. [Google Scholar] [CrossRef]
- Sandstrom, A.; Mitchell, P.S.; Goers, L.; Mu, E.W.; Lesser, C.F.; Vance, R.E. Functional degradation: A mechanism of NLRP1 inflammasome activation by diverse pathogen enzymes. Science 2019, 364, 6435–6451. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zheng, X.; Li, Y.; Ma, B.; Nie, X.; Li, M.; Liu, Y.; Xu, J.; Yang, Y. Wnt5a regulates autophagy in Bacille Calmette-Guérin (BCG)-Infected pulmonary epithelial cells. Microb. Pathog. 2022, 173 Pt A, 105826. [Google Scholar] [CrossRef]
- Kaufmann, E.; Sanz, J.; Dunn, J.L.; Khan, N.; Mendonça, L.E.; Pacis, A.; Tzelepis, F.; Pernet, E.; Dumaine, A.; Grenier, J.C.; et al. BCG Educates Hematopoietic Stem Cells to Generate Protective Innate Immunity against Tuberculosis. Cell 2018, 172, 176–190.e19. [Google Scholar] [CrossRef]
- Tiwari, S.; Casey, R.; Goulding, C.W.; Hingley-Wilson, S.; Jacobs, W.R., Jr. Infect and Inject: How Mycobacterium tuberculosis Exploits Its Major Virulence-Associated Type VII Secretion System, ESX-1. Microbiol. Spectr. 2019, 7, 113–126. [Google Scholar] [CrossRef]
- Almeida, L.M.; Pinho, B.R.; Duchen, M.R.; Oliveira, J.M.A. The PERKs of mitochondria protection during stress: Insights for PERK modulation in neurodegenerative and metabolic diseases. Biol. Rev. Camb. Philos. Soc. 2022, 97, 1737–1748. [Google Scholar] [CrossRef]
- Bu, Y.; Diehl, J.A. PERK Integrates Oncogenic Signaling and Cell Survival during Cancer Development. J. Cell. Physiol. 2016, 231, 2088–2096. [Google Scholar] [CrossRef]
- Wang, H.; Rong, X.; Zhao, G.; Zhou, Y.; Xiao, Y.; Ma, D.; Jin, X.; Wu, Y.; Yan, Y.; Yang, H.; et al. The microbial metabolite trimethylamine N-oxide promotes antitumor immunity in triple-negative breast cancer. Cell Metab. 2022, 34, 581–594.e8. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Shi, Y.; Wang, Y.; Qi, H.; Chen, H.; Li, J.; Li, L. Cadmium-induced pyroptosis is mediated by PERK/TXNIP/NLRP3 signaling in SH-SY5Y cells. Environ. Toxicol. 2023, 38, 2219–2227. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Ma, Y.; Li, H.; Cui, J.; Guo, X.; Wang, G.; Ji, L. Endoplasmic reticulum stress promotes caspase-1-dependent acinar cell pyroptosis through the PERK pathway to aggravate acute pancreatitis. Int. Immunopharmacol. 2023, 120, 110293. [Google Scholar] [CrossRef]
- Lebeaupin, C.; Proics, E.; De Bieville, C.H.; Rousseau, D.; Bonnafous, S.; Patouraux, S.; Adam, G.; Lavallard, V.J.; Rovere, C.; Le Thuc, O.; et al. ER stress induces NLRP3 inflammasome activation and hepatocyte death. Cell Death Dis. 2015, 6, e1879. [Google Scholar] [CrossRef]
- Lin, Y.; Luo, T.; Weng, A.; Huang, X.; Yao, Y.; Fu, Z.; Li, Y.; Liu, A.; Li, X.; Chen, D.; et al. Gallic Acid Alleviates Gouty Arthritis by Inhibiting NLRP3 Inflammasome Activation and Pyroptosis through Enhancing Nrf2 Signaling. Front. Immunol. 2020, 11, 580593. [Google Scholar] [CrossRef] [PubMed]
- Chanput, W.; Mes, J.J.; Savelkoul, H.F.; Wichers, H.J. Characterization of polarized THP-1 macrophages and polarizing ability of LPS and food compounds. Food Funct. 2013, 4, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Li, M.; Chen, Q.; Ma, B.; Nie, X.; Liu, Y.; Yang, Y.; Xu, J.; Wang, Y. Ror2-mediated cholesterol accumulation regulates autophagic activity within BCG-infected macrophages. Microb. Pathog. 2022, 167, 105564. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, J.; Dai, F.; Zhang, D.; Li, W. DUSP1 mediates BCG induced apoptosis and inflammatory response in THP-1 cells via MAPKs/NF-κB signaling pathway. Sci. Rep. 2023, 13, 2606. [Google Scholar] [CrossRef]
- Sen, T.; Saha, P.; Gupta, R.; Foley, L.M.; Jiang, T.; Abakumova, O.S.; Hitchens, T.K.; Sen, N. Aberrant ER Stress Induced Neuronal-IFNβ Elicits White Matter Injury Due to Microglial Activation and T-Cell Infiltration after TBI. J. Neurosci. 2020, 40, 424–446. [Google Scholar] [CrossRef]



| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| Human-β-actin | CCTGGCACCCAGCACAAT | GGGCCGGACTCGTCATAC |
| Human-GSDMD | AGCCCTACTGCCTGGTGGTTAG | CACGCTGCACGTCTGGTTCC |
| Mouse-β-actin | GTGCTATGTTGCTCTAGACTTCG | ATGCCACAGGATTCCATACC |
| Mouse-pro-caspase-1 | ATACAACCACTCGTACACGTCTTGC | TCCTCCAGCAGCAACTTCATTTCTC |
| Mouse-GSDMD | CGATGGGAACATTCAGGGCAGAG | ACACATTCATGGAGGCACTGGAAC |
| Mouse-IL-1β | CTGTCGGACCCATATGAGCTGAAAG | TGTCGTTGCTTGGTTCTCCTTGTAC |
| Mouse-IL-18 | GGCTGCCATGTCAGAAGACTCTTG | AGTGAAGTCGGCCAAAGTTGTCTG |
| Mouse-caspase-11 | GACTTAGGCTACGATGTGGTGGTG | ATGTGCTGTCTGATGTCTGGTGTTC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, B.; Nie, X.; Liu, L.; Li, M.; Chen, Q.; Liu, Y.; Hou, Y.; Yang, Y.; Xu, J. GSK2656157, a PERK Inhibitor, Alleviates Pyroptosis of Macrophages Induced by Mycobacterium Bacillus Calmette–Guerin Infection. Int. J. Mol. Sci. 2023, 24, 16239. https://doi.org/10.3390/ijms242216239
Ma B, Nie X, Liu L, Li M, Chen Q, Liu Y, Hou Y, Yang Y, Xu J. GSK2656157, a PERK Inhibitor, Alleviates Pyroptosis of Macrophages Induced by Mycobacterium Bacillus Calmette–Guerin Infection. International Journal of Molecular Sciences. 2023; 24(22):16239. https://doi.org/10.3390/ijms242216239
Chicago/Turabian StyleMa, Boli, Xueyi Nie, Lei Liu, Mengyuan Li, Qi Chen, Yueyang Liu, Yuxin Hou, Yi Yang, and Jinrui Xu. 2023. "GSK2656157, a PERK Inhibitor, Alleviates Pyroptosis of Macrophages Induced by Mycobacterium Bacillus Calmette–Guerin Infection" International Journal of Molecular Sciences 24, no. 22: 16239. https://doi.org/10.3390/ijms242216239
APA StyleMa, B., Nie, X., Liu, L., Li, M., Chen, Q., Liu, Y., Hou, Y., Yang, Y., & Xu, J. (2023). GSK2656157, a PERK Inhibitor, Alleviates Pyroptosis of Macrophages Induced by Mycobacterium Bacillus Calmette–Guerin Infection. International Journal of Molecular Sciences, 24(22), 16239. https://doi.org/10.3390/ijms242216239





