Sulfated Polysaccharides as a Fighter with Protein Non-Physiological Aggregation: The Role of Polysaccharide Flexibility and Charge Density
Abstract
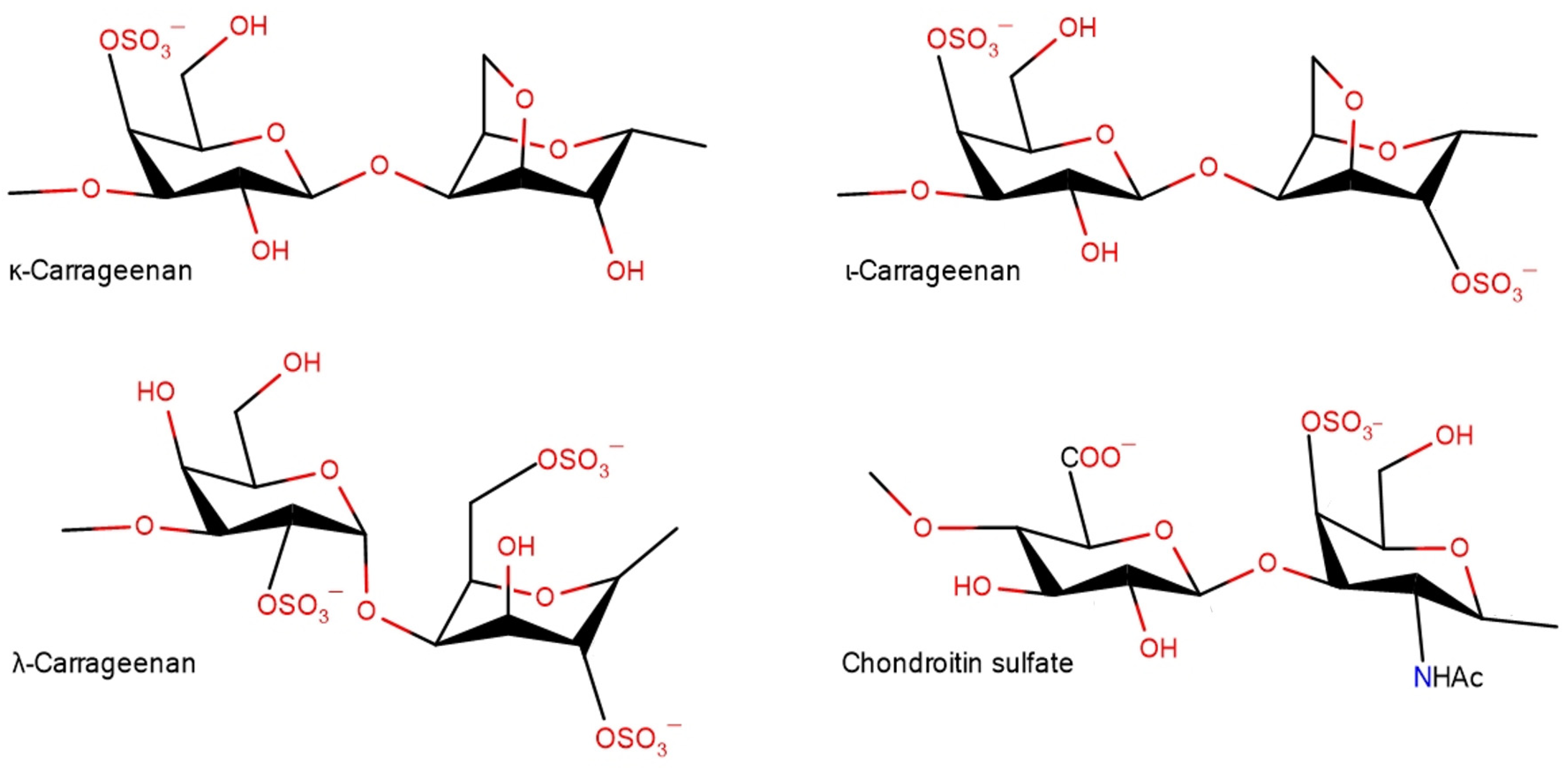
:1. Introduction
2. Results and Discussion
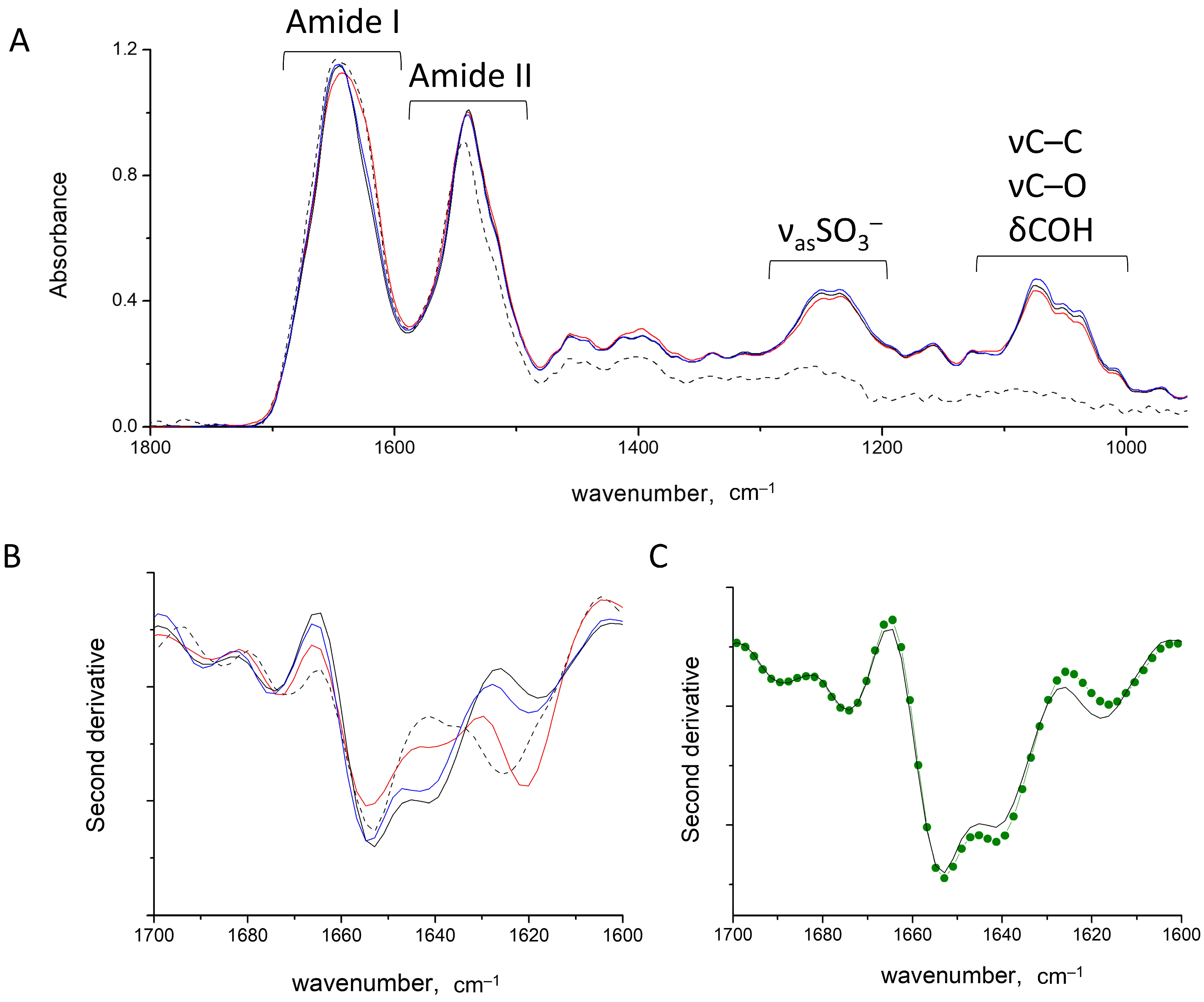
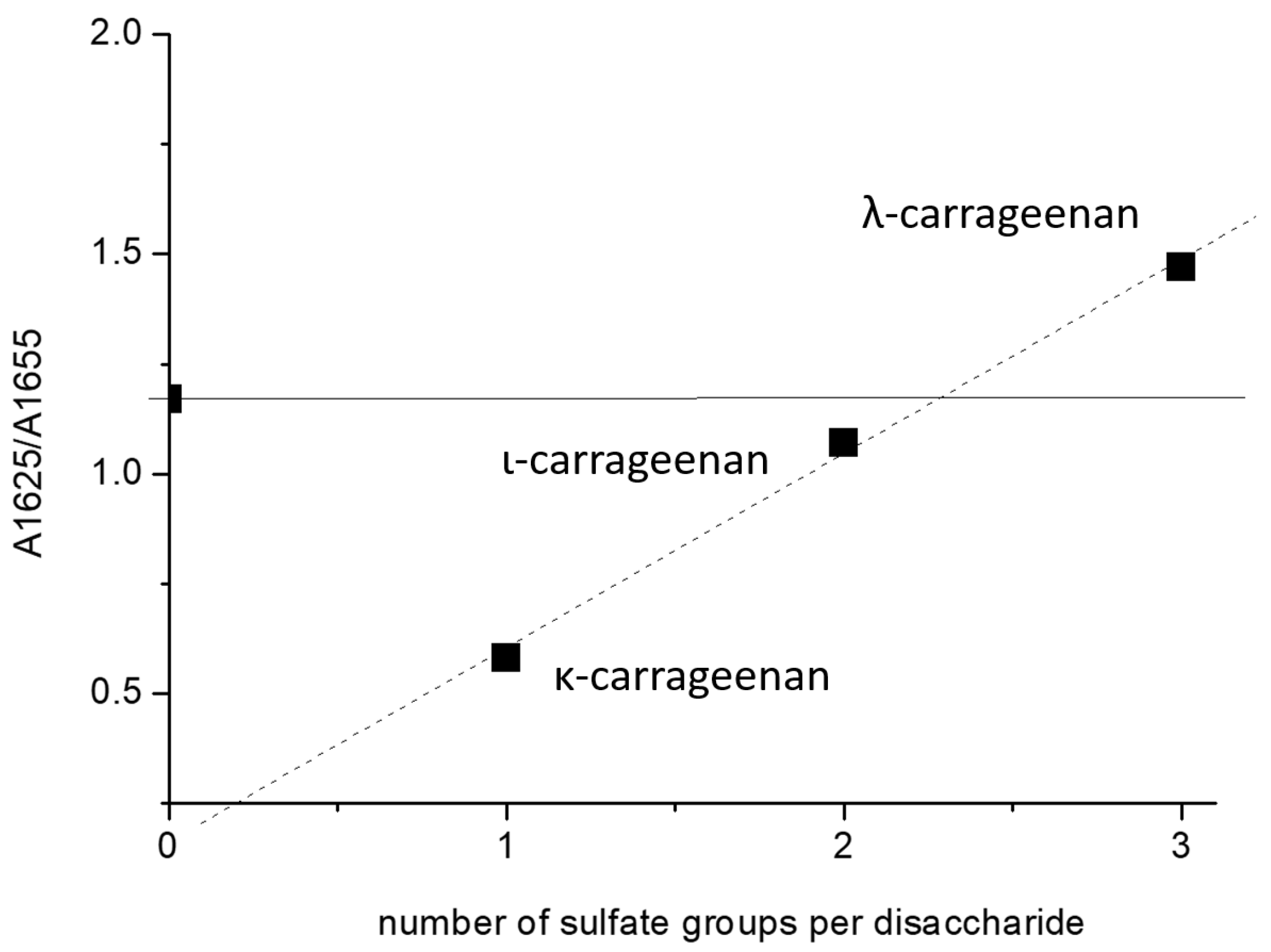
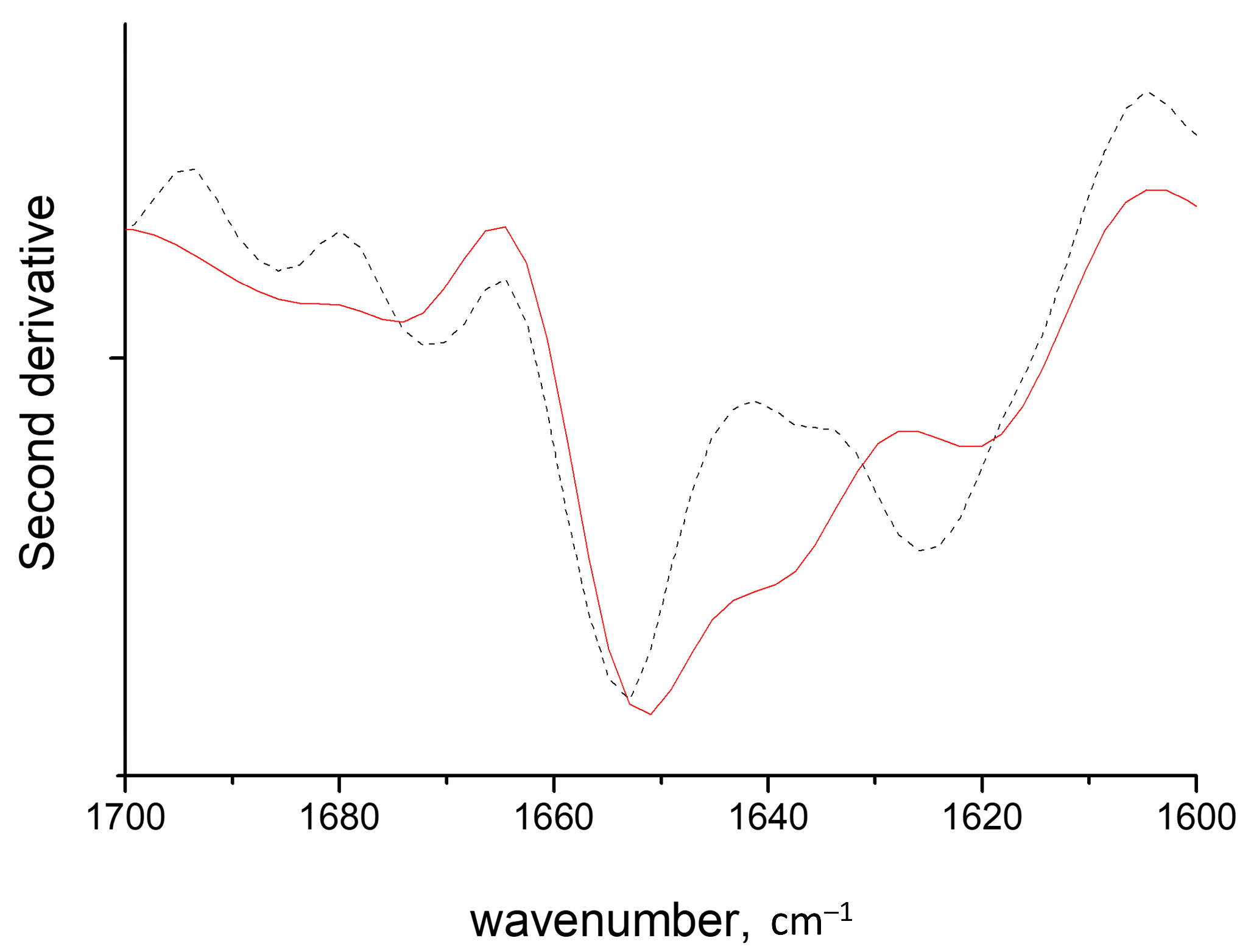
2.1. Band Assignments
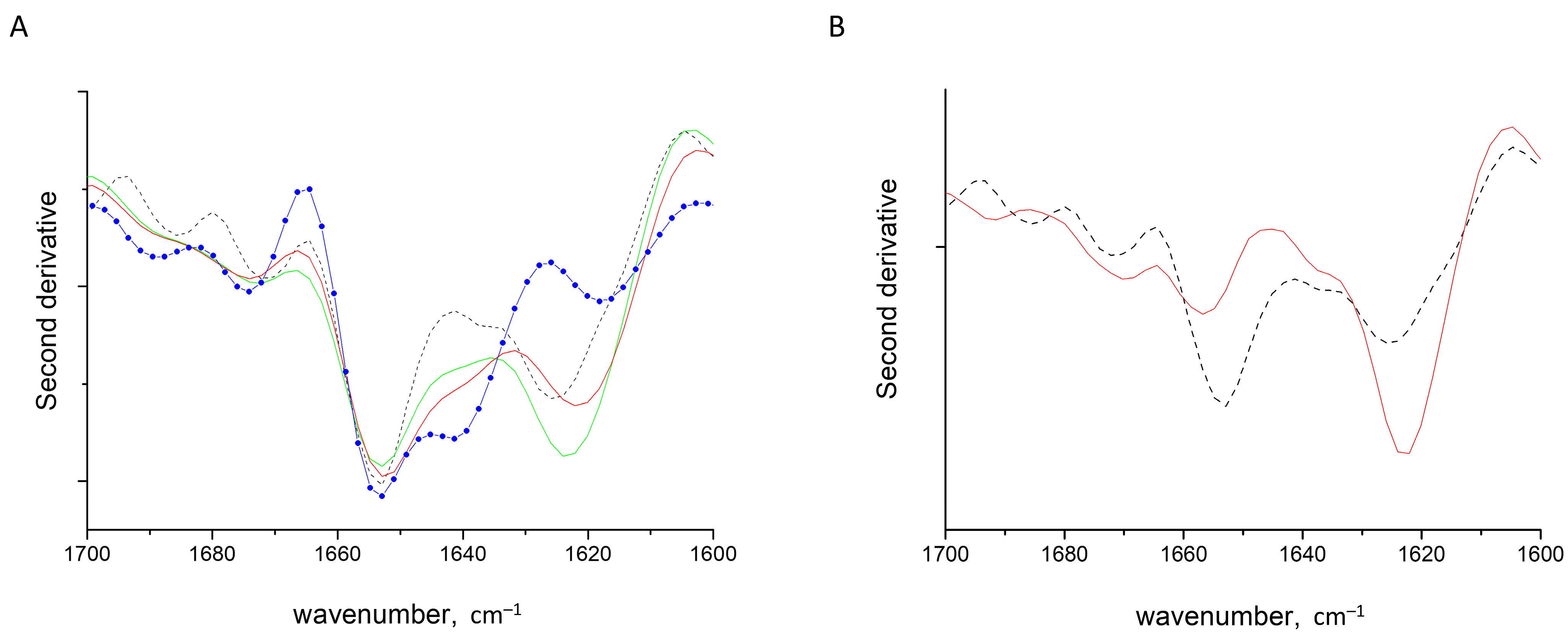
2.2. HEWL Fibrils–Flexible κ-Carrageenan Mixtures
2.3. HEWL Fibril Mixtures with Flexible ι- or λ-Carrageenans
2.4. HEWL Fibril–Rigid κ-Carrageenan Mixtures
2.5. HEWL Fibril–Chondroitin-4-Sulfate Mixtures
3. Materials and Methods
3.1. Materials and Fibril Preparation
3.2. HEWL Fibril–Polysaccharide Mixing
3.3. FTIR Spectroscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fink, A.L. Protein Aggregation: Folding Aggregates, Inclusion Bodies and Amyloid. Fold. Des. 1998, 3, R9–R23. [Google Scholar] [CrossRef]
- Sipe, J.D.; Benson, M.D.; Buxbaum, J.N.; Ikeda, S.I.; Merlini, G.; Saraiva, M.J.; Westermark, P. Amyloid Fibril Proteins and Amyloidosis: Chemical Identification and Clinical Classification International Society of Amyloidosis 2016 Nomenclature Guidelines. Amyloid 2016, 23, 209–213. [Google Scholar] [CrossRef]
- Uversky, V.N.; Fink, A.L. Conformational Constraints for Amyloid Fibrillation: The Importance of Being Unfolded. Biochim. Biophys. Acta 2004, 1698, 131–153. [Google Scholar] [CrossRef]
- Brudar, S.; Hribar-Lee, B. Effect of Buffer on Protein Stability in Aqueous Solutions: A Simple Protein Aggregation Model. J. Phys. Chem. B 2021, 125, 2504–2512. [Google Scholar] [CrossRef]
- Pedersen, J.T.; Heegaard, N.H. Analysis of Protein Aggregation in Neurodegenerative Disease. Anal. Chem. 2013, 85, 4215–4227. [Google Scholar] [CrossRef] [PubMed]
- Ghahghaei, A.; Faridi, N. Review: Structure of Amyloid Fibril in Diseases. J. Biomed. Sci. Eng. 2009, 2, 345–358. [Google Scholar] [CrossRef]
- Ke, P.C.; Zhou, R.; Serpell, L.C.; Riek, R.; Knowles, T.P.J.; Lashuel, H.A.; Gazit, E.; Hamley, I.W.; Davis, T.P.; Fandrich, M.; et al. Half a Century of Amyloids: Past, Present and Future. Chem. Soc. Rev. 2020, 49, 5473–5509. [Google Scholar] [CrossRef] [PubMed]
- Giorgetti, S.; Greco, C.; Tortora, P.; Aprile, F.A. Targeting Amyloid Aggregation: An Overview of Strategies and Mechanisms. Int. J. Mol. Sci. 2018, 19, 2677. [Google Scholar] [CrossRef] [PubMed]
- Holubová, M.; Štěpánek, P.; Hrubý, M. Polymer Materials as Promoters/Inhibitors of Amyloid Fibril Formation. Colloid Polym. Sci. 2021, 299, 343–362. [Google Scholar] [CrossRef]
- Alam, P.; Siddiqi, K.; Chturvedi, S.K.; Khan, R.H. Protein Aggregation: From Background to Inhibition Strategies. Int. J. Biol. Macromol. 2017, 103, 208–219. [Google Scholar] [CrossRef]
- Chiti, F.; Dobson, C.M. Protein Misfolding, Amyloid Formation, and Human Disease: A Summary of Progress over the Last Decade. Annu. Rev. Biochem. 2017, 86, 27–68. [Google Scholar] [CrossRef] [PubMed]
- Debnath, K.; Sarkar, A.K.; Jana, N.R.; Jana, N.R. Inhibiting Protein Aggregation by Small Molecule-Based Colloidal Nanoparticles. Acc. Mater. Res. 2022, 3, 54–66. [Google Scholar] [CrossRef]
- Albuquerque, H.M.T.; Nunes da Silva, R.; Pereira, M.; Maia, A.; Guieu, S.; Soares, A.R.; Santos, C.M.M.; Vieira, S.I.; Silva, A.M.S. Steroid-Quinoline Hybrids for Disruption and Reversion of Protein Aggregation Processes. ACS Med. Chem. Lett. 2022, 13, 443–448. [Google Scholar] [CrossRef]
- Johnson, L.; Faidra Angelerou, M.G.; Surikutchi, B.T.; Allen, S.; Zelzer, M.; Marlow, M. Low Molecular Weight Nucleoside Gelators: A Platform for Protein Aggregation Inhibition. Mol. Pharm. 2019, 16, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Peydayesh, M.; Kistler, S.; Zhou, J.; Lutz-Bueno, V.; Victorelli, F.D.; Meneguin, A.B.; Spósito, L.; Bauab, T.M.; Chorilli, M.; Mezzenga, R. Amyloid-Polysaccharide Interfacial Coacervates as Therapeutic Materials. Nat. Commun. 2023, 14, 1848. [Google Scholar] [CrossRef]
- Makshakova, O.N.; Zuev, Y.F. Interaction-Induced Structural Transformations in Polysaccharide and Protein-Polysaccharide Gels as Functional Basis for Novel Soft-Matter: A Case of Carrageenans. Gels 2022, 8, 287. [Google Scholar] [CrossRef] [PubMed]
- Derkach, S.R.; Voron’ko, N.G.; Kuchina, Y.A.; Kolotova, D.S.; Gordeeva, A.M.; Faizullin, D.A.; Gusev, Y.A.; Zuev, Y.F.; Makshakova, O.N. Molecular Structure and Properties of Kappa-Carrageenan-Gelatin Gels. Carbohydr. Polym. 2018, 197, 66–74. [Google Scholar] [CrossRef]
- Makshakova, O.; Antonova, M.; Bogdanova, L.; Faizullin, D.; Zuev, Y. Regulation of Intersubunit Interactions in Homotetramer of Glyceraldehyde-3-Phosphate Dehydrogenases Upon Its Immobilization in Protein-Kappa-Carrageenan Gels. Polymers 2023, 15, 676. [Google Scholar] [CrossRef]
- Makshakova, O.N.; Bogdanova, L.R.; Faizullin, D.A.; Ermakova, E.A.; Zuev, Y.F.; Sedov, I.A. Interaction-Induced Structural Transformation of Lysozyme and Kappa-Carrageenan in Binary Complexes. Carbohydr. Polym. 2021, 252, 117181. [Google Scholar] [CrossRef]
- Wang, X.; Nian, Y.; Zhang, Z.; Chen, Q.; Zeng, X.; Hu, B. High Internal Phase Emulsions Stabilized with Amyloid Fibrils and Their Polysaccharide Complexes for Encapsulation and Protection of Β-Carotene. Colloids Surf. B Biointerfaces 2019, 183, 110459. [Google Scholar] [CrossRef]
- Knowles, T.P.J.; Mezzenga, R. Amyloid Fibrils as Building Blocks for Natural and Artificial Functional Materials. Adv. Mater. 2016, 28, 6546–6561. [Google Scholar] [CrossRef] [PubMed]
- Makshakova, O.; Bogdanova, L.; Faizullin, D.; Khaibrakhmanova, D.; Ziganshina, S.; Ermakova, E.; Zuev, Y.; Sedov, I. The Ability of Some Polysaccharides to Disaggregate Lysozyme Amyloid Fibrils and Renature the Protein. Pharmaceutics 2023, 15, 624. [Google Scholar] [CrossRef] [PubMed]
- Iannuzzi, C.; Irace, G.; Sirangelo, I. The Effect of Glycosaminoglycans (Gags) on Amyloid Aggregation and Toxicity. Molecules 2015, 20, 2510–2528. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Ueno, M.; Zha, S.; Okimura, T.; Jiang, Z.; Yamaguchi, K.; Hatakeyama, T.; Oda, T. Sulfated Polysaccharide Ascophyllan Prevents Amyloid Fibril Formation of Human Insulin and Inhibits Amyloid-Induced Hemolysis and Cytotoxicity in Pc12 Cells. Biosci. Biotechnol. Biochem. 2021, 85, 2281–2291. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Sheng, A.; Chi, L. Glycosaminoglycan-Protein Interactions and Their Roles in Human Disease. Front. Mol. Biosci. 2021, 8, 639666. [Google Scholar] [CrossRef]
- Magnus, J.H.; Husby, G.; Kolset, S.O. Presence of Glycosaminoglycans in Purified Aa Type Amyloid Fibrils Associated with Juvenile Rheumatoid Arthritis. Ann. Rheum. Dis. 1989, 48, 215–219. [Google Scholar] [CrossRef]
- Torres-Bugeau, C.M.; Ávila, C.L.; Raisman-Vozari, R.; Papy-Garcia, D.; Itri, R.; Barbosa, L.R.; Cortez, L.M.; Sim, V.L.; Chehín, R.N. Characterization of Heparin-Induced Glyceraldehyde-3-Phosphate Dehydrogenase Early Amyloid-Like Oligomers and Their Implication in A-Synuclein Aggregation. J. Biol. Chem. 2012, 287, 2398–2409. [Google Scholar] [CrossRef]
- Perez, S.; Makshakova, O.; Angulo, J.; Bedini, E.; Bisio, A.; de Paz, J.L.; Fadda, E.; Guerrini, M.; Hricovini, M.; Hricovini, M.; et al. Glycosaminoglycans: What Remains to Be Deciphered? JACS Au 2023, 3, 628–656. [Google Scholar] [CrossRef]
- Bravo, R.; Arimon, M.; Valle-Delgado, J.J.; García, R.; Durany, N.; Castel, S.; Cruz, M.; Ventura, S.; Fernàndez-Busquets, X. Sulfated Polysaccharides Promote the Assembly of Amyloid Beta(1-42) Peptide into Stable Fibrils of Reduced Cytotoxicity. J. Biol. Chem. 2008, 283, 32471–32483. [Google Scholar] [CrossRef]
- Takase, H.; Tanaka, M.; Yamamoto, A.; Watanabe, S.; Takahashi, S.; Nadanaka, S.; Kitagawa, H.; Yamada, T.; Mukai, T. Structural Requirements of Glycosaminoglycans for Facilitating Amyloid Fibril Formation of Human Serum Amyloid A. Amyloid 2016, 23, 67–75. [Google Scholar] [CrossRef]
- Cohlberg, J.A.; Li, J.; Uversky, V.N.; Fink, A.L. Heparin and Other Glycosaminoglycans Stimulate the Formation of Amyloid Fibrils from Alpha-Synuclein in Vitro. Biochemistry 2002, 41, 1502–1511. [Google Scholar] [CrossRef] [PubMed]
- Mehra, S.; Ghosh, D.; Kumar, R.; Mondal, M.; Gadhe, L.G.; Das, S.; Anoop, A.; Jha, N.N.; Jacob, R.S.; Chatterjee, D.; et al. Glycosaminoglycans Have Variable Effects on A-Synuclein Aggregation and Differentially Affect the Activities of the Resulting Amyloid Fibrils. J. Biol. Chem. 2018, 293, 12975–12991. [Google Scholar] [CrossRef] [PubMed]
- Snow, A.D.; Cummings, J.A.; Lake, T. The Unifying Hypothesis of Alzheimer’s Disease: Heparan Sulfate Proteoglycans/Glycosaminoglycans Are Key as First Hypothesized over 30 Years Ago. Front. Aging Neurosci. 2021, 13, 710683. [Google Scholar] [CrossRef]
- Choudhary, S.; Save, S.N.; Vavilala, S.L. Unravelling the Inhibitory Activity of Chlamydomonas Reinhardtii Sulfated Polysaccharides against Alpha-Synuclein Fibrillation. Sci. Rep. 2018, 8, 5692. [Google Scholar] [CrossRef] [PubMed]
- Running, C.A.; Falshaw, R.; Janaswamy, S. Trivalent Iron Induced Gelation in Lambda-Carrageenan. Carbohydr. Polym. 2012, 87, 2735–2739. [Google Scholar] [CrossRef]
- Schefer, L.; Adamcik, J.; Mezzenga, R. Unravelling Secondary Structure Changes on Individual Anionic Polysaccharide Chains by Atomic Force Microscopy. Angew. Chem. Int. Ed. 2014, 53, 5376–5379. [Google Scholar] [CrossRef]
- Anderson, N.S.; Campbell, J.W.; Harding, M.M.; Rees, D.A.; Samuel, J.W.B. X-Ray Diffraction Studies of Polysaccharide Sulphates: Double Helix Models for Κ- and Ι-Carrageenans. J. Mol. Biol. 1969, 45, 85–97. [Google Scholar] [CrossRef]
- Ikeda, S.; Morris, V.J.; Nishinari, K. Microstructure of Aggregated and Nonaggregated Kappa-Carrageenan Helices Visualized by Atomic Force Microscopy. Biomacromolecules 2001, 2, 1331–1337. [Google Scholar] [CrossRef]
- Swaminathan, R.; Ravi, V.K.; Kumar, S.; Kumar, M.V.; Chandra, N. Lysozyme: A Model Protein for Amyloid Research. Adv. Protein Chem. Struct. Biol. 2011, 84, 63–111. [Google Scholar] [CrossRef]
- Eanes, E.D.; Glenner, G.G. X-ray Diffraction Studies on Amyloid Filaments. J. Histochem. Cytochem. 1968, 16, 673–677. [Google Scholar] [CrossRef]
- Willbold, D.; Strodel, B.; Schroder, G.F.; Hoyer, W.; Heise, H. Amyloid-Type Protein Aggregation and Prion-Like Properties of Amyloids. Chem. Rev. 2021, 121, 8285–8307. [Google Scholar] [CrossRef] [PubMed]
- Islam, Z.; Ali, M.H.; Popelka, A.; Mall, R.; Ullah, E.; Ponraj, J.; Kolatkar, P.R. Probing the Fibrillation of Lysozyme by Nanoscale-Infrared Spectroscopy. J. Biomol. Struct. Dyn. 2021, 39, 1481–1490. [Google Scholar] [CrossRef] [PubMed]
- Zurdo, J.; Guijarro, J.I.; Dobson, C.M. Preparation and Characterization of Purified Amyloid Fibrils. J. Am. Chem. Soc. 2001, 123, 8141–8142. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, F.S.; Longo, G.; Faggiano, S.; Lipiec, E.; Pastore, A.; Dietler, G. Infrared Nanospectroscopy Characterization of Oligomeric and Fibrillar Aggregates During Amyloid Formation. Nat. Commun. 2015, 6, 7831. [Google Scholar] [CrossRef]
- Sarroukh, R.; Goormaghtigh, E.; Ruysschaert, J.M.; Raussens, V. Atr-Ftir: A “Rejuvenated” Tool to Investigate Amyloid Proteins. Biochim. Biophys. Acta 2013, 1828, 2328–2338. [Google Scholar] [CrossRef]
- Ermakova, E.A.; Makshakova, O.N.; Zuev, Y.F.; Sedov, I.A. Fibril Fragments from the Amyloid Core of Lysozyme: An Accelerated Molecular Dynamics Study. J. Mol. Graph. Model. 2021, 106, 107917. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Li, Y.; Li, K.; Zhong, C. Biofilm-Inspired Amyloid-Polysaccharide Composite Materials. Appl. Mater. Today 2022, 27, 101497. [Google Scholar] [CrossRef]
- Usuelli, M.; Germerdonk, T.; Cao, Y.; Peydayesh, M.; Bagnani, M.; Handschin, S.; Nystrom, G.; Mezzenga, R. Polysaccharide-Reinforced Amyloid Fibril Hydrogels and Aerogels. Nanoscale 2021, 13, 12534–12545. [Google Scholar] [CrossRef]
- Dong, A.; Huang, P.; Caughey, W.S. Protein Secondary Structures in Water from Second-Derivative Amide I Infrared Spectra. Biochemistry 1990, 29, 3303–3308. [Google Scholar] [CrossRef]
- Susi, H.; Byler, D.M. Protein Structure by Fourier Transform Infrared Spectroscopy: Second Derivative Spectra. Biochem. Biophys. Res. Commun. 1983, 115, 391–397. [Google Scholar] [CrossRef]
- Kačuráková, M.; Capek, P.; Sasinková, V.; Wellner, N.; Ebringerová, A. Ft-Ir Study of Plant Cell Wall Model Compounds: Pectic Polysaccharides and Hemicelluloses. Carbohydr. Polym. 2000, 43, 195–203. [Google Scholar] [CrossRef]
- McCann, M.C.; Hammouri, M.; Wilson, R.; Belton, P.; Roberts, K. Fourier Transform Infrared Microspectroscopy Is a New Way to Look at Plant Cell Walls. Plant. Physiol. 1992, 100, 1940–1947. [Google Scholar] [CrossRef]
- Sekkal, M.; Legrand, P.; Huvenne, J.P.; Verdus, M.C. The Use of Ftir Microspectrometry as a New Tool for the Identification in Situ of Polygalactanes in Red Seaweeds. J. Mol. Struct. 1993, 294, 227–230. [Google Scholar] [CrossRef]
- Makshakova, O.N.; Faizullin, D.A.; Zuev, Y.F. Interplay between Secondary Structure and Ion Binding Upon Thermoreversible Gelation of Kappa-Carrageenan. Carbohydr. Polym. 2020, 227, 115342. [Google Scholar] [CrossRef]
- Belton, P.S.; Goodfellow, B.J.; Wilson, R.H. A Variable-Temperature Fourier-Transform Infrared Study of Gelation in Ι- and Κ-Carrageenans. Macromolecules 1989, 22, 1636–1642. [Google Scholar] [CrossRef]
- Roeters, S.J.; Iyer, A.; Pletikapić, G.; Kogan, V.; Subramaniam, V.; Woutersen, S. Evidence for Intramolecular Antiparallel Beta-Sheet Structure in Alpha-Synuclein Fibrils from a Combination of Two-Dimensional Infrared Spectroscopy and Atomic Force Microscopy. Sci. Rep. 2017, 7, 41051. [Google Scholar] [CrossRef] [PubMed]
- Antonov, Y.A.; Zhuravleva, I.L. Complexation of Lysozyme with Lambda Carrageenan: Complex Characterization and Protein Stability. Food Hydrocoll. 2019, 87, 519–529. [Google Scholar] [CrossRef]
- Antonov, Y.A.; Zhuravleva, I.L.; Cardinaels, R.; Moldenaers, P. Macromolecular Complexes of Lysozyme with Kappa Carrageenan. Food Hydrocoll. 2018, 74, 227–238. [Google Scholar] [CrossRef]
- Pace, C.N.; Vajdos, F.; Fee, L.; Grimsley, G.; Gray, T. How to Measure and Predict the Molar Absorption Coefficient of a Protein. Protein Sci. 1995, 4, 2411–2423. [Google Scholar] [CrossRef] [PubMed]
- Barth, A. Infrared Spectroscopy of Proteins. Biochim. Biophys. Acta (BBA) -Bioenerg. 2007, 1767, 1073–1101. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makshakova, O.N.; Bogdanova, L.R.; Faizullin, D.A.; Ermakova, E.A.; Zuev, Y.F. Sulfated Polysaccharides as a Fighter with Protein Non-Physiological Aggregation: The Role of Polysaccharide Flexibility and Charge Density. Int. J. Mol. Sci. 2023, 24, 16223. https://doi.org/10.3390/ijms242216223
Makshakova ON, Bogdanova LR, Faizullin DA, Ermakova EA, Zuev YF. Sulfated Polysaccharides as a Fighter with Protein Non-Physiological Aggregation: The Role of Polysaccharide Flexibility and Charge Density. International Journal of Molecular Sciences. 2023; 24(22):16223. https://doi.org/10.3390/ijms242216223
Chicago/Turabian StyleMakshakova, Olga N., Liliya R. Bogdanova, Dzhigangir A. Faizullin, Elena A. Ermakova, and Yuriy F. Zuev. 2023. "Sulfated Polysaccharides as a Fighter with Protein Non-Physiological Aggregation: The Role of Polysaccharide Flexibility and Charge Density" International Journal of Molecular Sciences 24, no. 22: 16223. https://doi.org/10.3390/ijms242216223
APA StyleMakshakova, O. N., Bogdanova, L. R., Faizullin, D. A., Ermakova, E. A., & Zuev, Y. F. (2023). Sulfated Polysaccharides as a Fighter with Protein Non-Physiological Aggregation: The Role of Polysaccharide Flexibility and Charge Density. International Journal of Molecular Sciences, 24(22), 16223. https://doi.org/10.3390/ijms242216223






