Influence of pH on Inulin Conversion to 2,3-Butanediol by Bacillus licheniformis 24: A Gene Expression Assay
Abstract
1. Introduction
2. Results
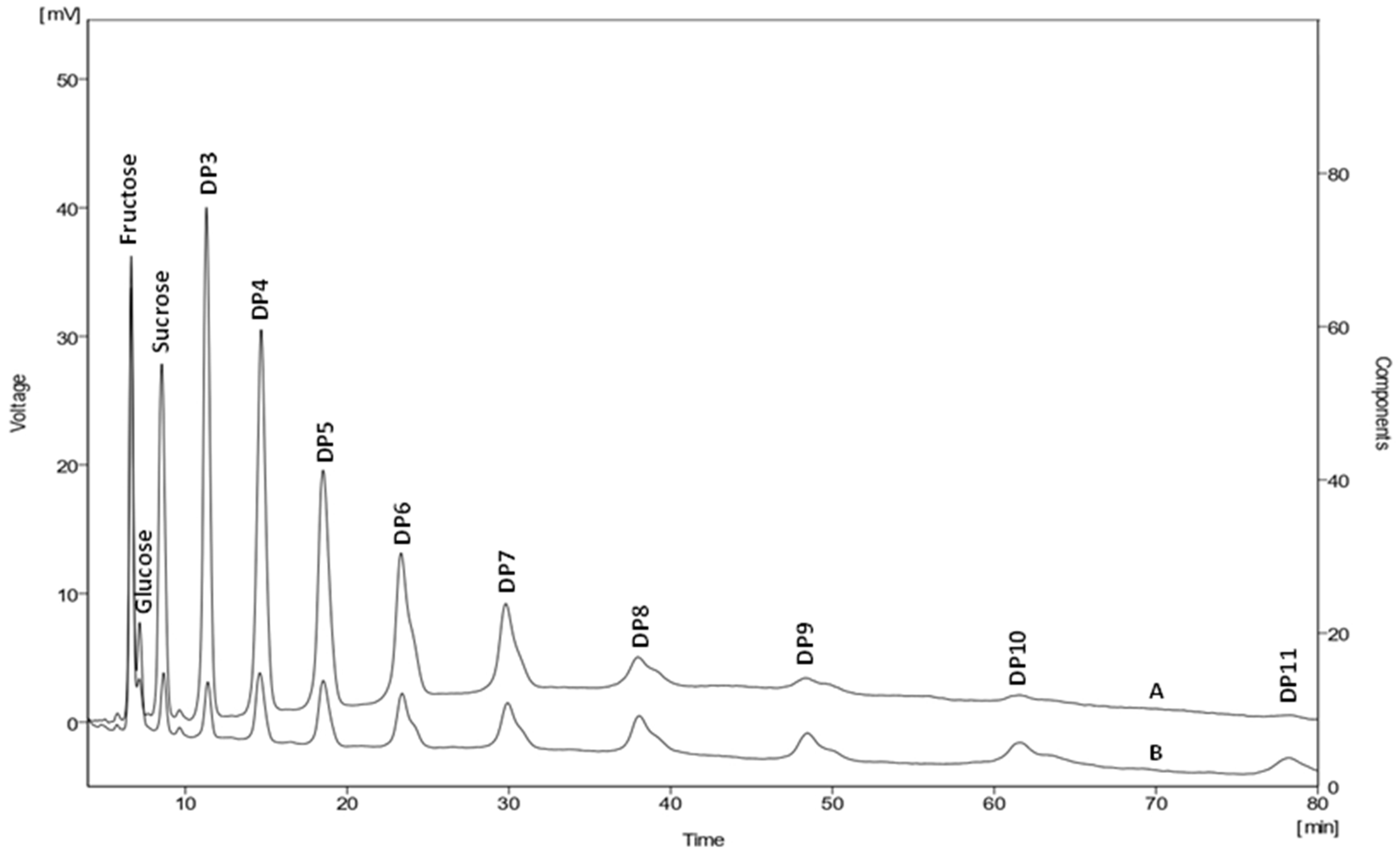
2.1. Soluble and Insoluble Chicory Flour as a Substrate for 2,3-BD Production
2.2. Flask-Batch Fermentation of Inulin by B. licheniformis 24
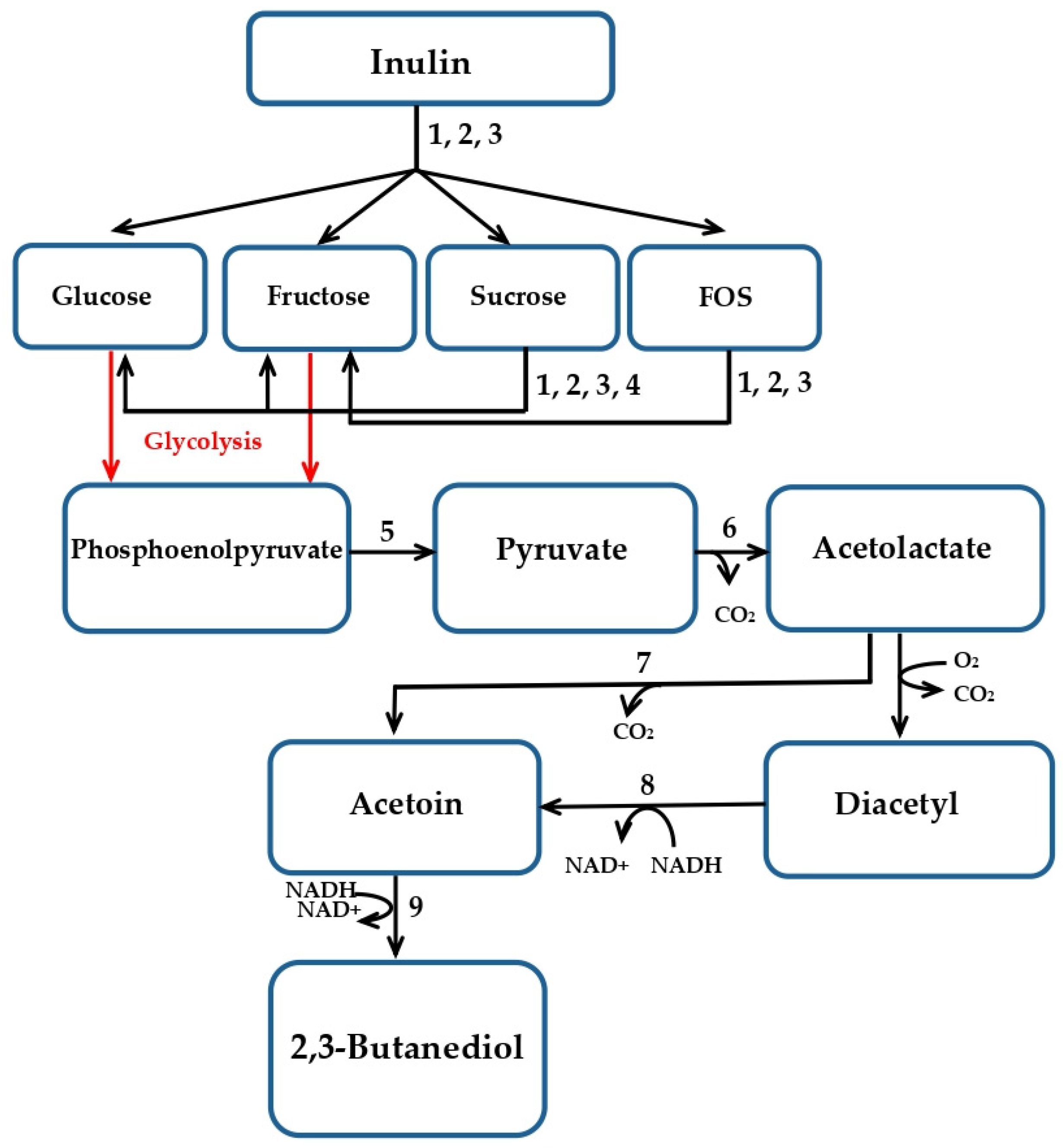
2.3. Effect of pH on Inulin Conversion to 2,3-BD
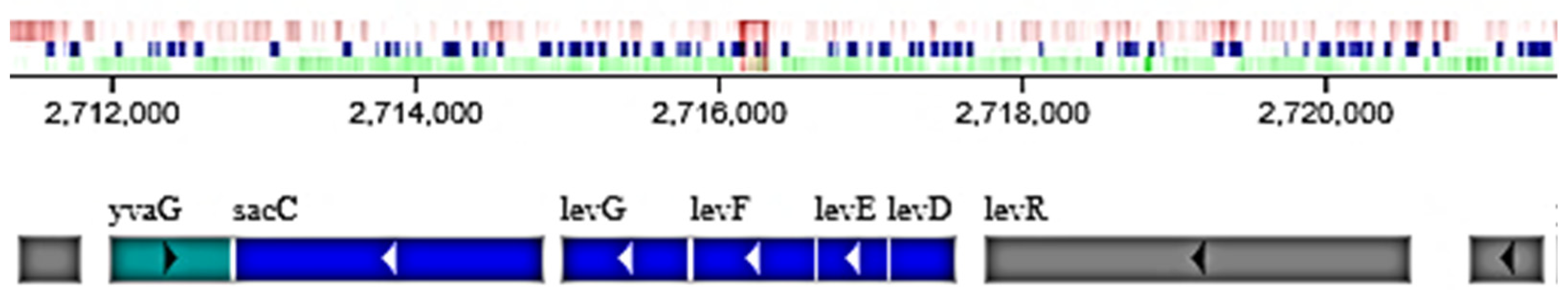
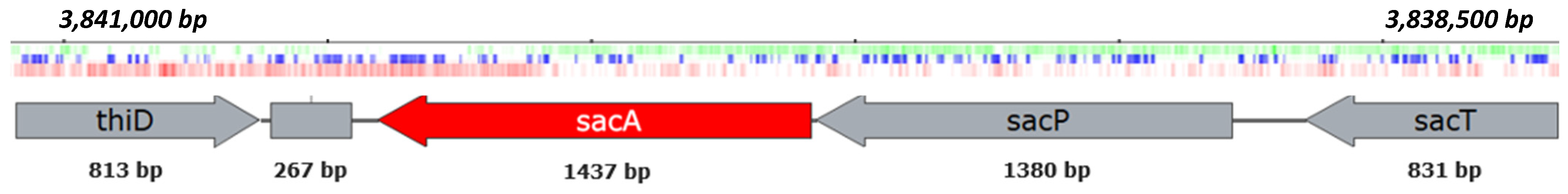
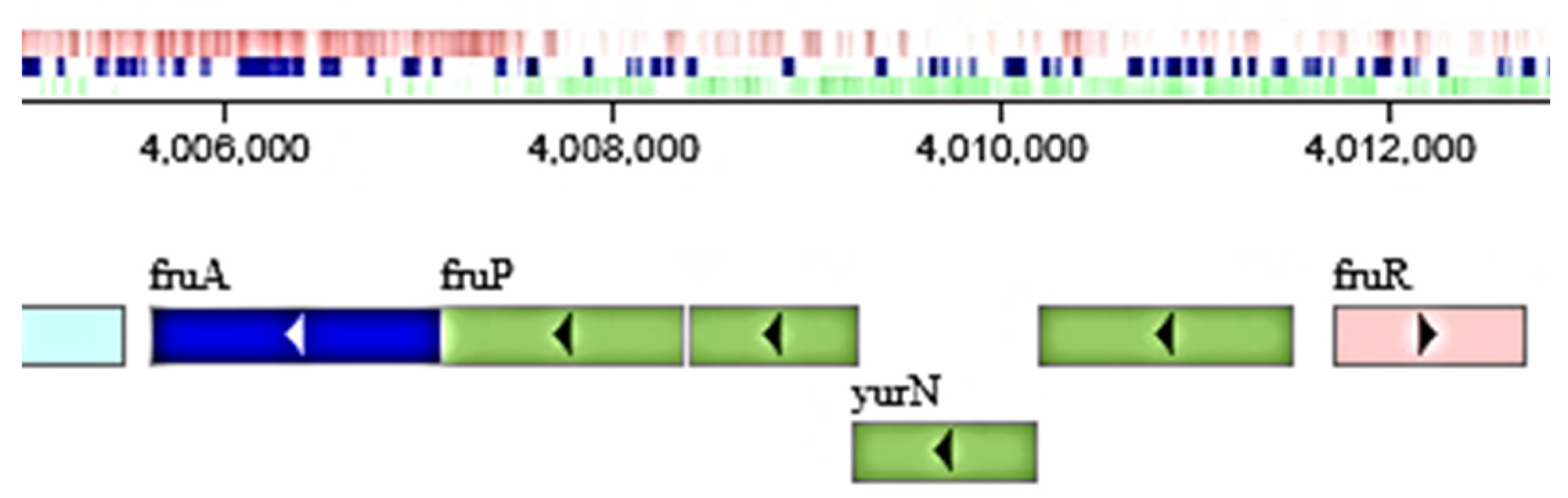
2.4. Sequencing of GH Genes Involved in Inulin Hydrolysis by B. licheniformis 24
2.5. Real-Time Reverse Transcription PCR (RT-PCR)
3. Discussion
4. Materials and Methods
4.1. Strain, Media, and Cultivation Conditions
4.2. DNA Isolation, PCR, and Sequencing
4.3. Real-Time-RT PCR
4.4. Analytical Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- The EU’s Green Deal Industrial Plan. Available online: https://www.edelmanglobaladvisory.com/insights/european-green-deal-industrial-plan (accessed on 6 June 2023).
- Tinôco, D.; Borschiver, S.; Coutinho, P.L.; Freire, D.M.G. Technological development of the bio-based 2,3-butanediol process. Biofuels Bioprod. Biorefining 2021, 15, 357–376. [Google Scholar] [CrossRef]
- 2,3-Butanediol Market. Available online: https://www.transparencymarketresearch.com/2-3-butanediol-market.html (accessed on 4 August 2023).
- Petrov, K.; Petrova, P. Current Advances in Microbial Production of Acetoin and 2,3-Butanediol by Bacillus spp. Fermentation 2021, 7, 307. [Google Scholar] [CrossRef]
- Song, D.; Cho, S.-Y.; Vu, T.-T.; Duong, H.-P.-Y.; Kim, E. Dehydration of 2,3-Butanediol to 1,3-Butadiene and Methyl Ethyl Ketone: Modeling, Numerical Analysis and Validation Using Pilot-Scale Reactor Data. Catalysts 2021, 11, 999. [Google Scholar] [CrossRef]
- Maina, S.; Prabhu, A.A.; Vivek, N.; Vlysidis, A.; Koutinas, A.; Kumar, V. Prospects on bio-based 2,3-butanediol and acetoin production: Recent progress and advances. Biotechnol. Adv. 2022, 54, 107783. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Lee, D.Y.; Lee, S.K.; Kim, H.R.; Chun, Y.; Yoo, H.Y.; Kwak, H.S.; Park, C.; Lee, J.H.; Kim, S.W. Development of 2,3-Butanediol Production Process from Klebsiella aerogenes ATCC 29007 Using Extracted Sugars of Chlorella pyrenoidosa and Biodiesel-Derived Crude Glycerol. Processes 2021, 9, 517. [Google Scholar] [CrossRef]
- Kallbach, M.; Horn, S.; Kuenz, A.; Prusse, U. Screening of novel bacteria for the 2,3-butanediol production. Appl. Microbiol. Biotechnol. 2017, 101, 1025–1033. [Google Scholar] [CrossRef]
- Song, C.W.; Rathnasingh, C.; Park, J.M.; Lee, J.; Song, H. Isolation and evaluation of Bacillus strains for industrial production of 2,3-butanediol. J. Microbiol. Biotechnol. 2018, 28, 409–417. [Google Scholar] [CrossRef]
- Ge, Y.S.; Li, K.; Li, L.X.; Gao, C.; Zhang, L.J.; Ma, C.Q.; Xu, P. Contracted but effective: Production of enantiopure 2,3-butanediol by thermophilic and GRAS Bacillus licheniformis. Green Chem. 2016, 18, 4693–4703. [Google Scholar] [CrossRef]
- Petrova, P.; Petlichka, S.; Petrov, K. New Bacillus spp. with potential for 2,3-butanediol production from biomass. J. Biosci. Bioeng. 2020, 130, 20–28. [Google Scholar] [CrossRef]
- Häßler, T.; Schieder, D.; Pfaller, R.; Faulstich, M.; Sieber, V. Enhanced fed-batch fermentation of 2,3-butanediol by Paenibacillus polymyxa DSM 365. Bioresour. Technol. 2012, 124, 237–244. [Google Scholar] [CrossRef]
- Li, J.; Wang, W.; Ma, Y.H.; Zeng, A.P. Medium optimization and proteome analysis of (R,R)-2,3-butanediol production by Paenibacillus polymyxa ATCC 12321. Appl. Microbiol. Biotechnol. 2013, 97, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Tsigoriyna, L.; Ganchev, D.; Petrova, P.; Petrov, K. Highly Efficient 2,3-Butanediol Production by Bacillus licheniformis via Complex Optimization of Nutritional and Technological Parameters. Fermentation 2021, 7, 118. [Google Scholar] [CrossRef]
- Jurchescu, I.M.; Hamann, J.; Zhou, X.; Ortmann, T.; Kuenz, A.; Prusse, U.; Lang, S. Enhanced 2,3-butanediol production in fed batch cultures of free and immobilized Bacillus licheniformis DSM 8785. Appl. Microbiol. Biotechnol. 2013, 97, 6715–6723. [Google Scholar] [CrossRef]
- Tsigoriyna, L.; Petrov, K. Production of 2,3-butanediol from Fructose by Bacillus licheniformis 24. Acta Microbiol. Bulg. 2021, 37, 183–187. Available online: https://actamicrobio.bg/archive/issue-4-2021/amb-4-2021-article-2.pdf (accessed on 18 March 2023).
- Perego, P.; Converti, A.; del Borghi, M. Effects of temperature, inoculum size and starch hydrolyzate concentration on butanediol production by Bacillus licheniformis. Bioresour. Technol. 2003, 89, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Li, L.X.; Li, K.; Wang, K.; Chen, C.; Gao, C.; Ma, C.Q.; Xu, P. Efficient production of 2,3-butanediol from corn stover hydrolysate by using a thermophilic Bacillus licheniformis strain. Bioresour. Technol. 2014, 170, 256–261. [Google Scholar] [CrossRef]
- Petrova, P.; Velikova, P.; Popova, L.; Petrov, K. Direct conversion of chicory flour into L(+)-lactic acid by the highly effective inulinase producer Lactobacillus paracasei DSM 23505. Bioresour. Technol. 2015, 186, 329–333. [Google Scholar] [CrossRef]
- Park, J.M.; Oh, B.-R.; Kang, I.Y.; Heo, S.-Y.; Seo, J.-W.; Park, S.-M.; Hong, W.-K.; Kim, C.H. Enhancement of 2,3-butanediol production from Jerusalem artichoke tuber extract by a recombinant Bacillus sp. strain BRC1 with increased inulinase activity. J. Ind. Microbiol. Biotechnol. 2017, 44, 1107–1113. [Google Scholar] [CrossRef]
- Sun, L.H.; Wang, X.D.; Dai, J.Y.; Xiu, Z.L. Microbial production of 2,3-butanediol from Jerusalem artichoke tubers by Klebsiella pneumoniae. Appl. Microbiol. Biotechnol. 2009, 82, 847–852. [Google Scholar] [CrossRef]
- Gao, J.-H.; Li, Q.-J.; Feng, X.-H.; Li, S. Optimization of medium for one-step fermentation of inulin extract from Jerusalem artichoke tubers using Paenibacillus polymyxa ZJ-9 to produce R, R-2,3-butanediol. Bioresour. Technol. 2010, 101, 7087–7093. [Google Scholar] [CrossRef]
- Guo, Z.-W.; Ni, Z.-F.; Zong, M.-H.; Lou, W.-Y. Modular Metabolic Engineering of Bacillus licheniformis for Efficient 2,3-Butanediol Production by Consolidated Bioprocessing of Jerusalem Artichoke Tubers. ACS Sustain. Chem. Eng. 2022, 10, 9624–9634. [Google Scholar] [CrossRef]
- Li, L.X.; Chen, C.; Li, K.; Wang, Y.; Gao, C.; Ma, C.Q.; Xu, P. Efficient simultaneous saccharification and fermentation of inulin to 2,3-butanediol by thermophilic Bacillus licheniformis ATCC 14580. Appl. Environ. Microbiol. 2014, 80, 6458–6464. [Google Scholar] [CrossRef] [PubMed]
- Mera, A.; de Lima, M.Z.T.; Bernardes, A.; Garcia, W.; Muniz, J.R.C. Low-resolution structure, oligomerization and its role on the enzymatic activity of a sucrose-6-phosphate hydrolase from Bacillus licheniformis. Amino Acids 2019, 51, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Holyavka, M.; Artyukhov, V.; Kovaleva, T. Structural and functional properties of inulinases: A review. Biocatal. Biotransform. 2016, 34, 1–17. [Google Scholar] [CrossRef]
- He, C.; Yang, Y.; Zhao, R.; Qu, J.; Jin, L.; Lu, L.; Xu, L.; Xiao, M. Rational designed mutagenesis of levansucrase from Bacillus licheniformis 8-37-0-1 for product specificity study. Appl. Microbiol. Biotechnol. 2018, 102, 3217–3228. [Google Scholar] [CrossRef]
- Doan, C.T.; Tran, T.N.; Nguyen, T.T.; Tran, T.P.H.; Nguyen, V.B.; Tran, T.D.; Nguyen, A.D.; Wang, S.-L. Production of Sucrolytic Enzyme by Bacillus licheniformis by the Bioconversion of Pomelo Albedo as a Carbon Source. Polymers 2021, 13, 1959. [Google Scholar] [CrossRef]
- Klaewkla, M.; Pichyangkura, R.; Chunsrivirot, S. Computational Design of Oligosaccharide-Producing Levansucrase from Bacillus licheniformis RN-01 to Increase Its Stability at High Temperature. J. Phys. Chem. B 2021, 125, 5766–5774. [Google Scholar] [CrossRef]
- Porras-Domínguez, J.R.; Ávila-Fernández, Á.; Rodríguez-Alegría, M.E.; Miranda-Molina, A.; Escalante, A.; González-Cervantes, R.; Olvera, C.; López-Munguía, A. Levan-type FOS production using a Bacillus licheniformis endolevanase. Process Biochem. 2014, 49, 783–790. [Google Scholar] [CrossRef]
- Tsigoriyna, L.; Arsov, A.; Petrova, P.; Gergov, E.; Petrov, K. Heterologous Expression of Inulinase Gene in Bacillus licheniformis 24 for 2,3-Butanediol Production from Inulin. Catalysts 2023, 13, 841. [Google Scholar] [CrossRef]
- Petrova, P.; Arsov, A.; Ivanov, I.; Tsigoriyna, L.; Petrov, K. New Exopolysaccharides Produced by Bacillus licheniformis 24 Display Substrate-Dependent Content and Antioxidant Activity. Microorganisms 2021, 9, 2127. [Google Scholar] [CrossRef]
- Rey, M.W.; Ramaiya, P.; Nelson, B.A.; Brody-Karpin, S.D.; Zaretsky, E.; Tang, M.; Lopez de Leon, A.; Xiang, H.; Gusti, V.; Clausen, I.G.; et al. Complete genome sequence of the industrial bacterium Bacillus licheniformis and comparisons with closely related Bacillus species. Genome Biol. 2004, 5, R77. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.-Y.; Guan, W.-T.; Xiu, Z.-L. Bioconversion of inulin to 2,3-butanediol by a newly isolated Klebsiella pneumoniae producing inulinase. Proc. Biochem. 2020, 98, 247–253. [Google Scholar] [CrossRef]
- Samolińska, W.; Grela, E.R. Comparative Effects of Inulin with Different Polymerization Degrees on Growth Performance, Blood Trace Minerals, and Erythrocyte Indices in Growing-Finishing Pigs. Biol. Trace Elem. Res. 2017, 176, 130–142. [Google Scholar] [CrossRef] [PubMed]
- Fages, J.; Mulard, D.; Rouquet, J.J.; Wilhelm, J.L. 2,3-Butanediol production from Jerusalem artichoke, Helianthus Tuberosus, by Bacillus polymyxa ATCC 12321. Optimization of kL a profile. Appl. Microb. Biotechnol. 1986, 25, 197–202. [Google Scholar] [CrossRef]
- Méndez-Lorenzo, L.; Porras-Domínguez, J.R.; Raga-Carbajal, E.; Olvera, C.; Rodríguez-Alegría, M.E.; Carrillo-Nava, E.; Costas, M.; Munguía, A.L. Intrinsic Levanase Activity of Bacillus subtilis 168 Levansucrase (SacB). PLoS ONE 2015, 10, e0143394. [Google Scholar] [CrossRef]
- Wanker, E.; Huber, A.; Schwab, H. Purification and characterization of the Bacillus subtilis levanase produced in Escherichia coli. Appl. Environ. Microbiol. 1995, 61, 1953–1958. [Google Scholar] [CrossRef] [PubMed]
- Marciniak, B.C.; Pabijaniak, M.; de Jong, A.; Dűhring, R.; Seidel, G.; Hillen, W.; Kuipers, O.P. High- and low-affinity cre boxes for CcpA binding in Bacillus subtilis revealed by genome-wide analysis. BMC Genom. 2012, 13, 401. [Google Scholar] [CrossRef]
- Marvasi, M.; Visscher, P.T.; Casillas Martinez, L. Exopolymeric Substances (EPS) from Bacillus subtilis: Polymers and Genes Encoding Their Synthesis. FEMS Microbiol. Lett. 2010, 313, 1–9. [Google Scholar] [CrossRef]
- Martin-Verstraete, I.; Débarbouillé, M.; Klier, A.; Rapoport, G. Levanase Operon of Bacillus Subtilis Includes a Fructose-Specific Phosphotransferase System Regulating the Expression of the Operon. J. Mol. Biol. 1990, 214, 657–671. [Google Scholar] [CrossRef] [PubMed]
- Domżał-Kędzia, M.; Ostrowska, M.; Lewińska, A.; Łukaszewicz, M. Recent Developments and Applications of Microbial Levan, A Versatile Polysaccharide-Based Biopolymer. Molecules 2023, 28, 5407. [Google Scholar] [CrossRef]
- Seibel, J.; Jördening, H.-J.; Buchholz, K. Glycosylation with activated sugars using glycosyltransferases and transglycosidases. Biocatal. Biotransform. 2006, 24, 311–342. [Google Scholar] [CrossRef]
- Weijers, C.A.; Franssen, M.C.; Visser, G.M. Glycosyltransferase-catalyzed synthesis of bioactive oligosaccharides. Biotechnol. Adv. 2008, 26, 436–456. [Google Scholar] [CrossRef] [PubMed]
- Perez Oseguera, M.A.; Guereca, L.; Lopez-Munguia, A. Properties of levansucrase from Bacillus circulans. Appl. Microbiol. Biotechnol. 1996, 45, 465–471. [Google Scholar] [CrossRef]
- Öner, E.T.; Hernández, L.; Combie, J. Review of Levan polysaccharide: From a century of past experiences to future prospects. Biotechnol. Adv. 2016, 34, 827–844. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Ni, D.; Zhang, W.; Guang, C.; Zhang, T.; Mu, W. Recent advances in Levansucrase and Inulosucrase: Evolution, characteristics, and application. Crit. Rev. Food Sci. Nutr. 2019, 59, 3630–3647. [Google Scholar] [CrossRef]
- Nakapong, S.; Pichyangkura, R.; Ito, K.; Iizuka, M.; Pongsawasdi, P. High expression level of levansucrase from Bacillus licheniformis RN-01 and synthesis of levan nanoparticles. Int. J. Biol. Macromol. 2013, 54, 30–36. [Google Scholar] [CrossRef]
- Lu, L.; Fu, F.; Zhao, R.; Jin, L.; He, C.; Xu, L.; Xiao, M. A recombinant levansucrase from Bacillus licheniformis 8-37-0-1 catalyzes versatile transfructosylation reactions. Process Biochem. 2014, 49, 1503–1510. [Google Scholar] [CrossRef]
- Xavier, J.R.; Ramana, K.V. Optimization of levan production by cold-active Bacillus licheniformis ANT 179 and fructooligosaccharide synthesis by its levansucrase. Appl. Biochem. Biotechnol. 2017, 181, 986–1006. [Google Scholar] [CrossRef]
- Daguer, J.-P. Autogenous modulation of the Bacillus subtilis sacB-levB-yveA levansucrase operon by the levB transcript. Microbiology 2004, 150, 3669–3679. [Google Scholar] [CrossRef][Green Version]
- Jensen, S.L.; Diemer, M.B.; Lundmark, M.; Larsen, F.H.; Blennow, A.; Mogensen, H.K.; Nielsen, T.H. Levanase from Bacillus subtilis hydrolyses β-2,6 fructosyl bonds in bacterial levans and in grass fructans. Int. J. Biol. Macromol. 2016, 85, 514–521. [Google Scholar] [CrossRef]
- Pereira, Y.; Petit-Glatron, M.F.; Chambert, R. YveB, Encoding Endolevanase LevB, Is Part of the SacB-YveB-YveA Levansucrase Tricistronic Operon in Bacillus subtilis. Microbiology 2001, 147, 3413–3419. [Google Scholar] [CrossRef] [PubMed]
- Deutscher, J.; Francke, C.; Postma, P.W. How phosphotransferase system-related protein phosphorylation regulates carbohydrate metabolism in bacteria. Microbiol. Mol. Biol. Rev. 2006, 70, 939–1031. [Google Scholar] [CrossRef] [PubMed]
- Debarbouille, M.; Arnaud, M.; Fouet, A.; Klier, A.; Rapoport, G. The sacT gene regulating the sacPA operon in Bacillus subtilis shares strong homology with transcriptional antiterminators. J. Bacteriol. 1990, 172, 3966–3973. [Google Scholar] [CrossRef] [PubMed]
- Morabbi Heravi, K.; Altenbuchner, J. Cross Talk among Transporters of the Phosphoenolpyruvate-Dependent Phosphotransferase System in Bacillus subtilis. J. Bacteriol. 2018, 200, e00213-18. [Google Scholar] [CrossRef]
- Ye, J.; Coulouris, G.; Zaretskaya, I.; Cutcutache, I.; Rozen, S.; Madden, T. Primer-BLAST: A tool to design target-specific primers for polymerase chain reaction. BMC Bioinform. 2012, 13, 134. [Google Scholar] [CrossRef]




| Gene | Size (bp) | Enzyme (EC Number) | Enzyme (Name) | Localization | Amino Acids (Number) | CAZy (Family) | GenBank Accession No. |
|---|---|---|---|---|---|---|---|
| sacA | 1437 | 3.2.1.26 | β-Fructofuranosidase | Cytoplasmic | 479 | 32 | OR400366 |
| sacB | 1449 | 2.4.1.10 | Levansucrase | Extracellular | 482 | 68 | OR400367 |
| sacC | 2034 | 3.2.1.80 | Fructan β-fructosidase | Extracellular | 677 | 32 | OR400368 |
| levB | 1548 | 3.2.1.65 | Levanase | Membrane | 516 | 32 | OR400369 |
| fruA | 1479 | 3.2.1.26 | β-Fructofuranosidase | Cytoplasmic | 492 | 32 | OR400370 |
| Gene | Time (h) | FC | ||||
|---|---|---|---|---|---|---|
| pH 5.25 | pH 5.50 | pH 5.75 | pH 6.00 | pH 6.25 | ||
| sacA | 0 | 1.00 | 0.20 | 0.06 | 0.61 | 0.29 |
| 24 | 0.25 | 0.88 | 10.34 | ND | 66.26 | |
| 48 | 0.41 | 0.97 | 0.58 | 3.32 | 1.32 | |
| 72 | 1.00 | 1.02 | 9.51 | 1.87 | 1.08 | |
| sacB | 0 | 1.00 | 0.91 | 1.34 | 1.41 | 1.17 |
| 24 | 0.74 | 1.39 | 12.82 | ND | 196.72 | |
| 48 | 0.67 | 1.99 | 0.37 | 1.85 | 1.58 | |
| 72 | 0.29 | 1.73 | 7.16 | 5.31 | 0.13 | |
| sacC | 0 | 1.00 | 0.20 | 0.80 | 0.43 | 0.49 |
| 24 | 0.85 | 1.19 | 23.59 | 22.63 | 163.14 | |
| 48 | 1.11 | 2.62 | 3.89 | 3.56 | 1.21 | |
| 72 | 2.11 | ND | 1.96 | 1.31 | 0.22 | |
| levB | 0 | 1.00 | 0.30 | 0.57 | 0.11 | 0.33 |
| 24 | 0.41 | 2.75 | 1.02 | 1.04 | 0.40 | |
| 48 | 0.36 | 1.53 | 0.56 | 1.06 | 0.74 | |
| 72 | 0.61 | 2.58 | ND | 0.86 | 0.84 | |
| fruA | 0 | 1.00 | 0.11 | 0.13 | 0.52 | 0.63 |
| 24 | 0.90 | 1.17 | 8.06 | 2.91 | 53.82 | |
| 48 | 0.43 | 1.16 | 0.56 | 7.67 | 3.46 | |
| 72 | 2.50 | 4.14 | 5.10 | 8.40 | 0.42 | |
| Primer | Sequence (5′-3′) | PCR Product (bp) | Position in Genome * |
|---|---|---|---|
| sacA_F | atgaatcaagatcaggagcttcgtcaaaaggcaat | 1437 | 3,838,486–3,839,922 |
| sacA_R | ttatgccattgtccaggatgtcacattcattatga | ||
| sacB_F | atgaacatcaaaaacattgctaaaaaagcgtcagc | 1449 | 3,535,232–3,536,680 |
| sacB_R | ttatttgtttaccgttagttgtccctgttcaagga | ||
| sacC_F | cgctgcctggatgcttcgcaaaggggtgaatcc | 2556 † | 2,712,789–2,714,822 |
| sacC_R | gaccgtcaatacggttatgccgggctcaacc | ||
| levB_F | ttgaagaaggcagtatataagcggatcagcatttt | 1548 | 3,536,757–3,538,304 |
| levB_R | taatcgcggattgaacgcaaatgtttgatcttaaga | ||
| fruA_F | atgaacagaattcagcaggcagaagaagcattaaa | 1479 | 4,005,611–4,007,089 |
| fruA_R | tcatttggcttcatcacctttccaaatatctttca |
| Primer | Sequence (5’-3’) | PCR Product (bp) | Position in Gene * |
|---|---|---|---|
| 16S_F | gagtacgaccgcaaggttga | 100 | 875–895 |
| 16S_R | cctggtaaggttcttcgcgt | 975–955 | |
| sacA_RTF | aagagatcgccctcacgccgagcgactggttt | 125 | 255–286 |
| sacA_RTR | atttccctcgccgtctctgacattccccgtgt | 379–348 | |
| sacB_RTF | caacagagcctactacgggggcagcaagaagt | 117 | 861–892 |
| sacB_RTR | tcgatgattccgagagcgccgttagccagcga | 977–946 | |
| sacC_RTF | gccgctcgttgccatttatacgcaggaccgga | 64 | 375–406 |
| sacC_RTR | gctgtaggcgatgctttgcacttgttccccgc | 438–407 | |
| levB_RTF | gcatactggacaggcagcttcaacggcaacga | 121 | 784–815 |
| levB_RTR | cgttcgtttcgccgtcctcaaatgtcacgccc | 904–873 | |
| fruA_RTF | gggagtcagagatgccgacgaaagcagacgga | 62 | 893–924 |
| fruA_RTR | ttcacgcggcaaagttaatgccccgcaccatc | 954–923 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsigoriyna, L.; Arsov, A.; Gergov, E.; Petrova, P.; Petrov, K. Influence of pH on Inulin Conversion to 2,3-Butanediol by Bacillus licheniformis 24: A Gene Expression Assay. Int. J. Mol. Sci. 2023, 24, 14065. https://doi.org/10.3390/ijms241814065
Tsigoriyna L, Arsov A, Gergov E, Petrova P, Petrov K. Influence of pH on Inulin Conversion to 2,3-Butanediol by Bacillus licheniformis 24: A Gene Expression Assay. International Journal of Molecular Sciences. 2023; 24(18):14065. https://doi.org/10.3390/ijms241814065
Chicago/Turabian StyleTsigoriyna, Lidia, Alexander Arsov, Emanoel Gergov, Penka Petrova, and Kaloyan Petrov. 2023. "Influence of pH on Inulin Conversion to 2,3-Butanediol by Bacillus licheniformis 24: A Gene Expression Assay" International Journal of Molecular Sciences 24, no. 18: 14065. https://doi.org/10.3390/ijms241814065
APA StyleTsigoriyna, L., Arsov, A., Gergov, E., Petrova, P., & Petrov, K. (2023). Influence of pH on Inulin Conversion to 2,3-Butanediol by Bacillus licheniformis 24: A Gene Expression Assay. International Journal of Molecular Sciences, 24(18), 14065. https://doi.org/10.3390/ijms241814065








