Browning of Mammary Fat Suppresses Pubertal Mammary Gland Development of Mice via Elevation of Serum Phosphatidylcholine and Inhibition of PI3K/Akt Pathway
Abstract
:1. Introduction
2. Results
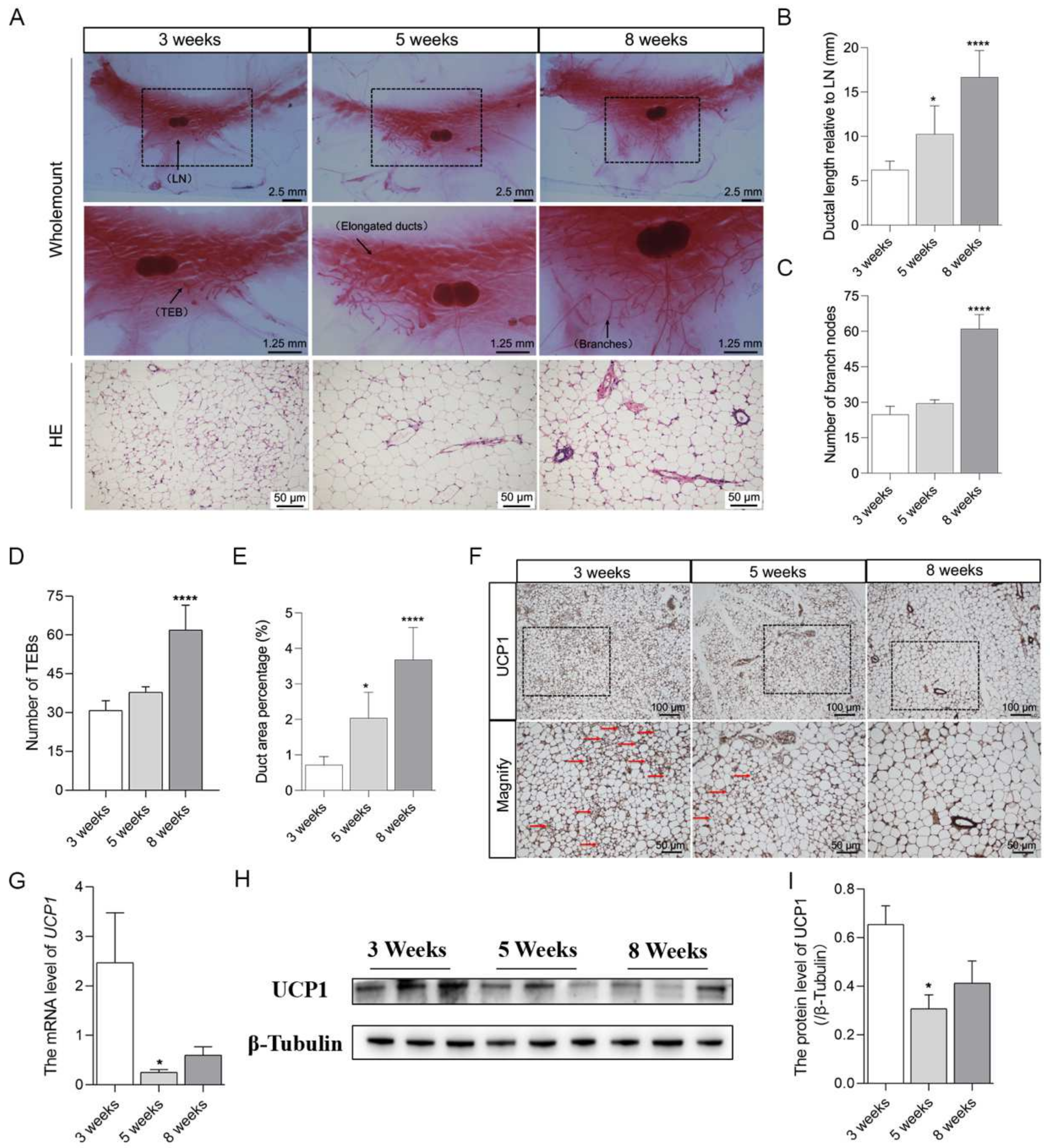
2.1. Brown Adipocytes in Mammary Fat Decreased with the Pubertal Development of Mice Mammary Glands
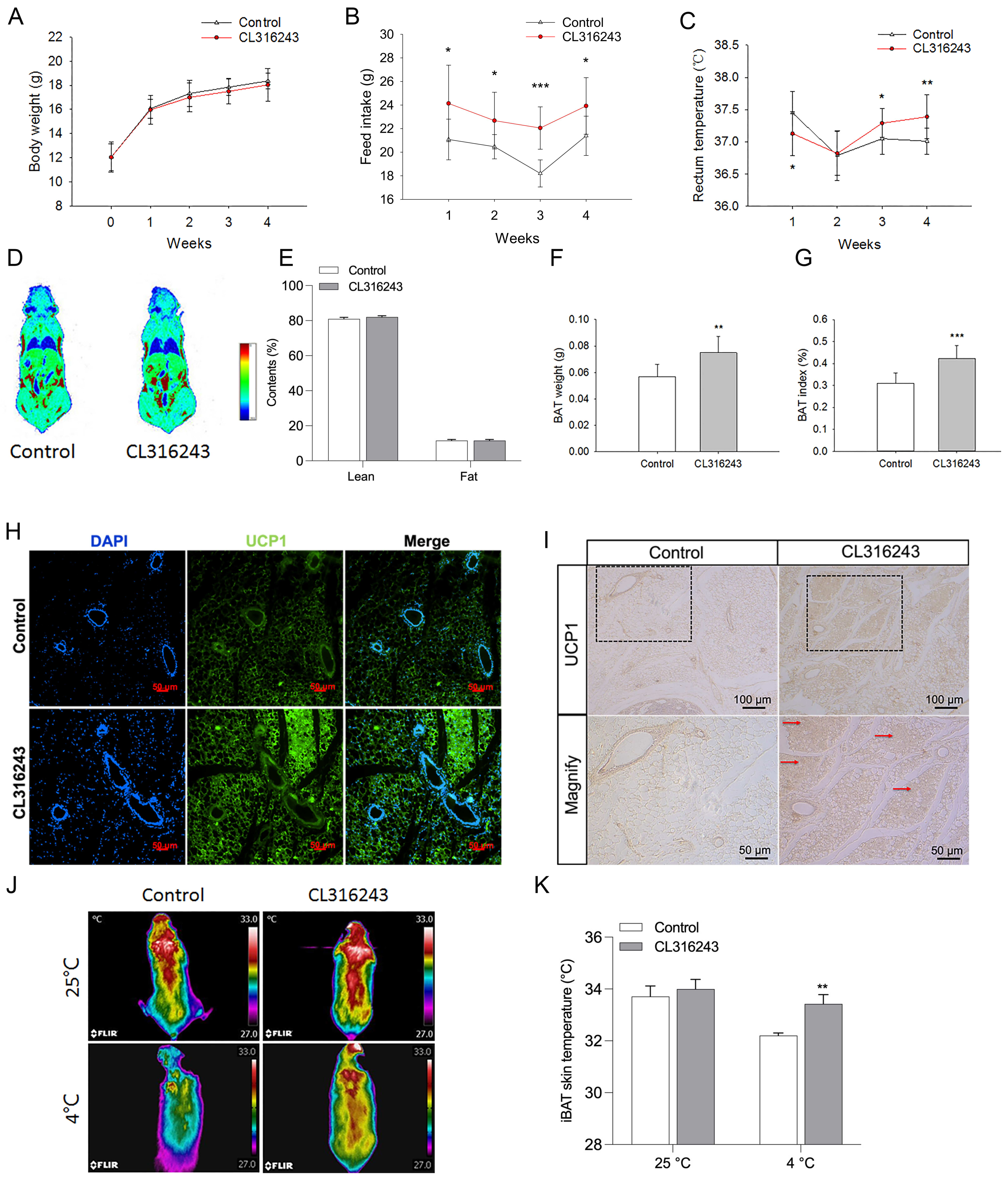
2.2. Pharmacological Induction of the Browning of Mammary Fat by Injection of CL316243
2.3. Browning of Mammary Fat Suppressed the Pubertal Development of Mice Mammary Glands
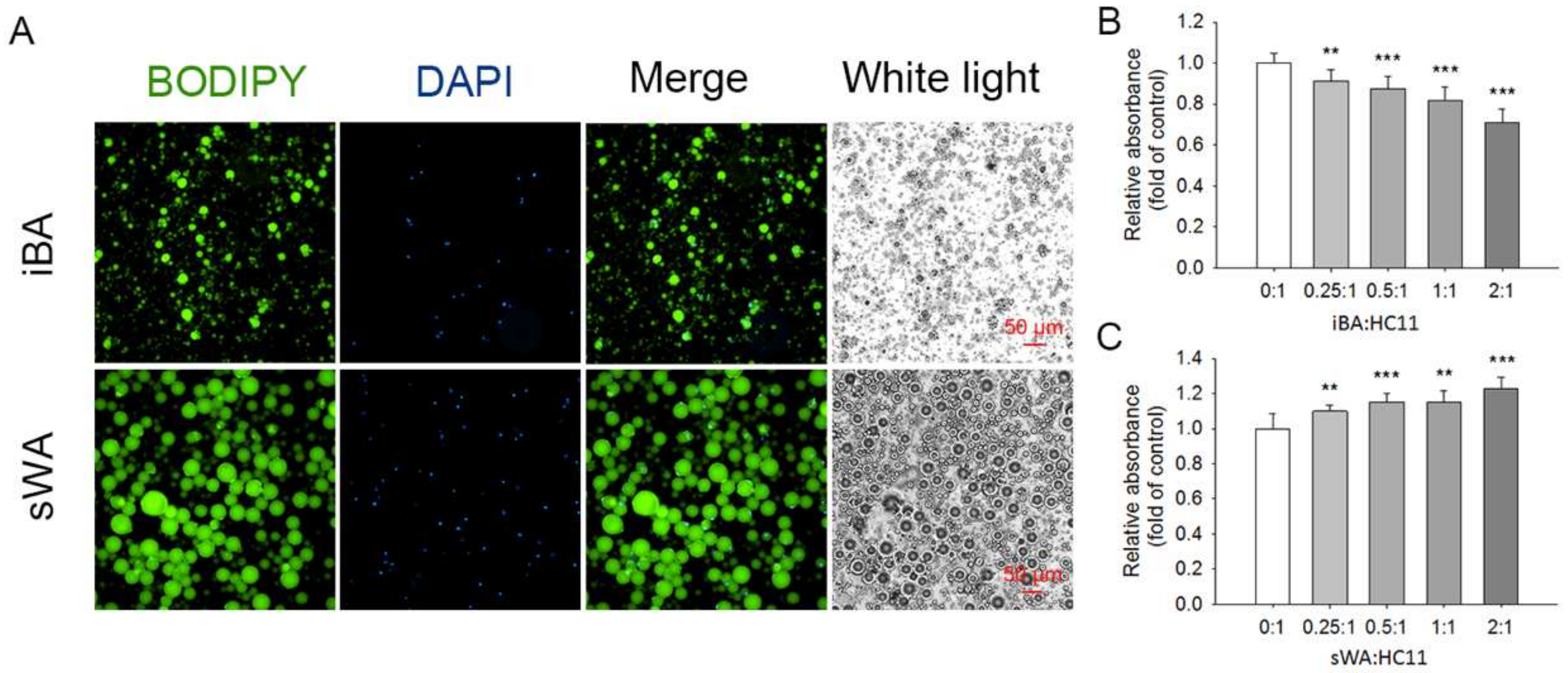
2.4. The Proliferation of HC11 Was Repressed When Co-Cultured with Brown Adipocytes
2.5. Serum Phosphatidylcholine Level Was Elevated in Response to the Browning of Mammary Fat
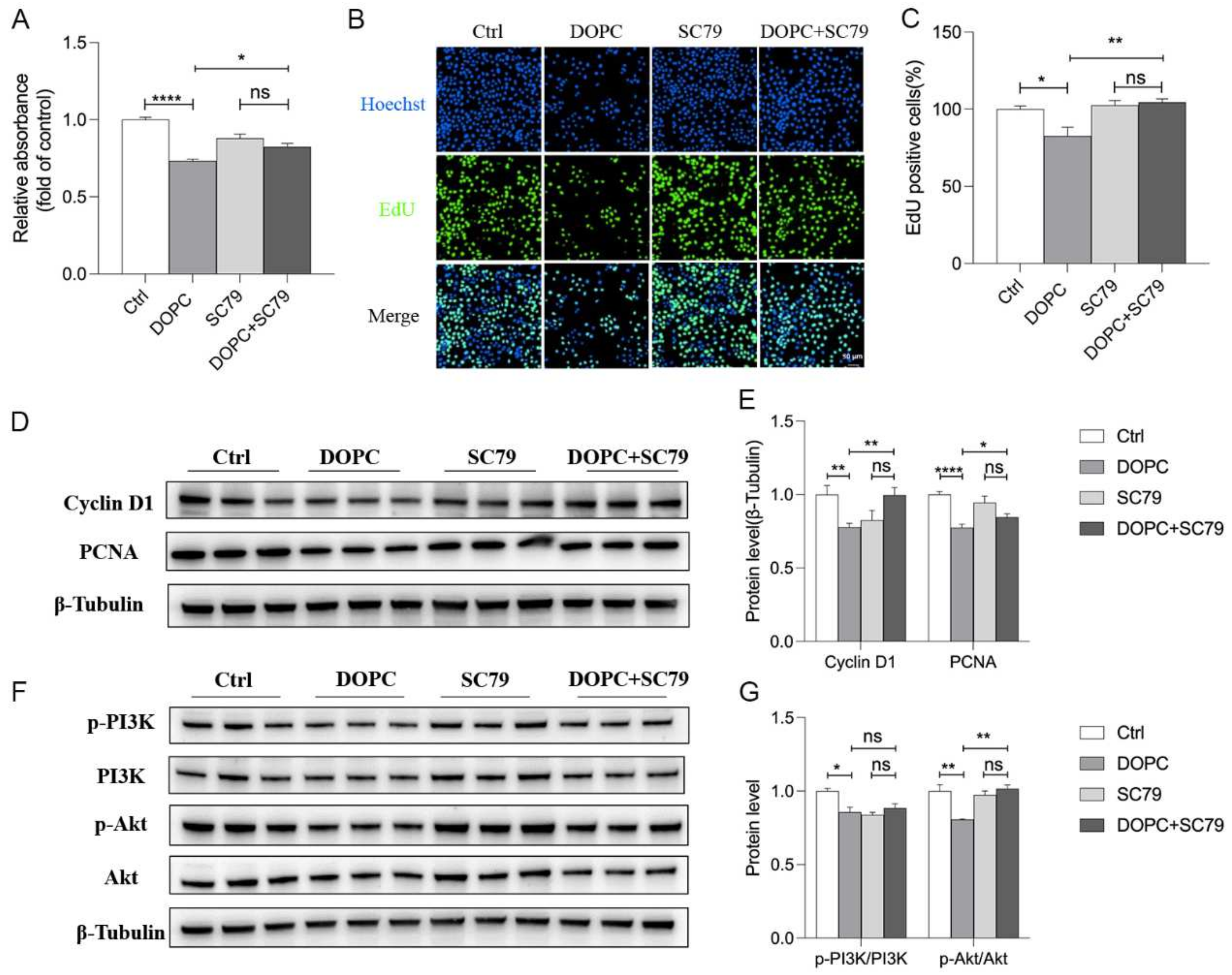
2.6. DOPC Exerted an Inhibitory Effect on the Proliferation of HC11
2.7. PI3K/Akt Pathway Was Involved in the DOPC-Inhibited Proliferation of HC11
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Wholemount and Hematoxylin and Eosin (H&E) Staining
4.3. Immunohistochemistry and Immunofluorescence Staining
4.4. Real-Time Quantitative PCR
4.5. Cell Culture and Treatments
4.6. CCK8 Assay and EdU Incorporation Assay
4.7. Western Blot
4.8. Body Composition and Infrared Thermography
4.9. Lipidomics Analysis
4.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Twigger, A.J.; Khaled, W.T. Mammary gland development from a single cell ‘omics view. Semin. Cell Dev. Biol. 2021, 114, 171–185. [Google Scholar] [CrossRef] [PubMed]
- Watson, C.J.; Khaled, W.T. Mammary development in the embryo and adult: A journey of morphogenesis and commitment. Development 2008, 135, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Kamikawa, A.; Ichii, O.; Yamaji, D.; Imao, T.; Suzuki, C.; Okamatsu-Ogura, Y.; Terao, A.; Kon, Y.; Kimura, K. Diet-induced obesity disrupts ductal development in the mammary glands of nonpregnant mice. Dev. Dyn. 2009, 238, 1092–1099. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Yuan, C.; Zhang, J.; Zhang, F.; Fu, Q.; Zhu, X.; Shu, G.; Wang, L.; Gao, P.; Xi, Q.; et al. Stearic acid suppresses mammary gland development by inhibiting PI3K/Akt signaling pathway through GPR120 in pubertal mice. Biochem. Biophys. Res. Commun. 2017, 491, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Farmer, C.; Palin, M.F.; Martel-Kennes, Y. Impact of diet deprivation and subsequent over-allowance during prepuberty. Part 1. Effects on growth performance, metabolite status, and mammary gland development in gilts. J. Anim. Sci. 2012, 90, 863–871. [Google Scholar] [CrossRef]
- Wu, W.-J.; Wang, S.-H.; Wu, C.-C.; Su, Y.-A.; Chiang, C.-Y.; Lai, C.-H.; Wang, T.-H.; Cheng, T.-L.; Kuo, J.-Y.; Hsu, T.-C.; et al. IL-4 and IL-13 Promote Proliferation of Mammary Epithelial Cells through STAT6 and IRS-1. Int. J. Mol. Sci. 2021, 22, 12008. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Zhang, J.; Zhang, F.; Ai, W.; Zhu, X.; Shu, G.; Wang, L.; Gao, P.; Xi, Q.; Zhang, Y.; et al. Lauric Acid Stimulates Mammary Gland Development of Pubertal Mice through Activation of GPR84 and PI3K/Akt Signaling Pathway. J. Agric. Food Chem. 2017, 65, 95–103. [Google Scholar] [CrossRef]
- Farmer, C. Nutritional impact on mammary development in pigs: A review. J. Anim. Sci. 2018, 96, 3748–3756. [Google Scholar] [CrossRef] [PubMed]
- Farmer, C.; Hurley, W.L. Mammary development. In The Gestating and Lactating Sow; Wageningen Academic Publishers: Wageningen, The Netherlands, 2015; pp. 73–94. [Google Scholar]
- Karayazi Atıcı, Ö.; Govindrajan, N.; Lopetegui-González, I.; Shemanko, C.S. Prolactin: A hormone with diverse functions from mammary gland development to cancer metastasis. Semin. Cell Dev. Biol. 2021, 114, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Inman, J.L.; Robertson, C.; Mott, J.D.; Bissell, M.J. Mammary gland development: Cell fate specification, stem cells and the microenvironment. Development 2015, 142, 1028–1042. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.; Alshaikh, B.; Schall, J.I.; Kelly, A.; Ford, E.; Zemel, B.S.; Umbach, D.M.; Adgent, M.; Stallings, V.A. Endocrine-sensitive physical endpoints in newborns: Ranges and predictors. Pediatr. Res. 2021, 89, 660–666. [Google Scholar] [CrossRef] [PubMed]
- Dzięgelewska, Ż.; Gajewska, M. Stromal-Epithelial Interactions during Mammary Gland Development. In Stromal Cells-Structure, Function, and Therapeutic Implications; IntechOpen: London, UK, 2019. [Google Scholar]
- Gouon-Evans, V.; Pollard, J.W. Unexpected deposition of brown fat in mammary gland during postnatal development. Mol. Endocrinol. 2002, 16, 2618–2627. [Google Scholar] [CrossRef] [PubMed]
- Ladoux, A.; Peraldi, P.; Chignon-Sicard, B.; Dani, C. Distinct Shades of Adipocytes Control the Metabolic Roles of Adipose Tissues: From Their Origins to Their Relevance for Medical Applications. Biomedicines 2021, 9, 40. [Google Scholar] [CrossRef]
- Sleeman, K.E.; Kendrick, H.; Robertson, D.; Isacke, C.M.; Ashworth, A.; Smalley, M.J. Dissociation of estrogen receptor expression and in vivo stem cell activity in the mammary gland. J. Cell Biol. 2007, 176, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Dunphy, K.A.; Tao, L.; Jerry, D.J. Mammary epithelial transplant procedure. J. Vis. Exp. 2010, 40, e1849. [Google Scholar]
- Olson, L.K.; Tan, Y.; Zhao, Y.; Aupperlee, M.D.; Haslam, S.Z. Pubertal exposure to high fat diet causes mouse strain-dependent alterations in mammary gland development and estrogen responsiveness. Int. J. Obes. 2010, 34, 1415–1426. [Google Scholar] [CrossRef]
- Dufau, J.; Shen, J.X.; Couchet, M.; De Castro Barbosa, T.; Mejhert, N.; Massier, L.; Griseti, E.; Mouisel, E.; Amri, E.Z.; Lauschke, V.M.; et al. In vitro and ex vivo models of adipocytes. Am. J. Physiol. Cell Physiol. 2021, 320, C822–C841. [Google Scholar] [CrossRef]
- Colleluori, G.; Perugini, J.; Barbatelli, G.; Cinti, S. Mammary gland adipocytes in lactation cycle, obesity and breast cancer. Rev. Endocr. Metab. Disord. 2021, 22, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, B.; Li, M.; Niu, C.; Wang, G.; Li, T.; Krol, E.; Jin, W.; Speakman, J.R. Brown adipocytes can display a mammary basal myoepithelial cell phenotype in vivo. Mol. Metab. 2017, 6, 1198–1211. [Google Scholar] [CrossRef] [PubMed]
- Pant, R.; Firmal, P.; Shah, V.K.; Alam, A.; Chattopadhyay, S. Epigenetic Regulation of Adipogenesis in Development of Metabolic Syndrome. Front. Cell Dev. Biol. 2020, 8, 619888. [Google Scholar] [CrossRef]
- Fan, L.; Xu, H.; Yang, R.; Zang, Y.; Chen, J.; Qin, H. Combination of Capsaicin and Capsiate Induces Browning in 3T3-L1 White Adipocytes via Activation of the Peroxisome Proliferator-Activated Receptor gamma/beta(3)-Adrenergic Receptor Signaling Pathways. J. Agric. Food Chem. 2019, 67, 6232–6240. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.; Liu, Z.; Chao, T.; Hou, L.; Fan, R.; He, R.; Wang, G.; Wang, J. Screening of miRNA profiles and construction of regulation networks in early and late lactation of dairy goat mammary glands. Sci. Rep. 2017, 7, 11933. [Google Scholar] [CrossRef] [PubMed]
- Pamarthy, S.; Mao, L.; Katara, G.K.; Fleetwood, S.; Kulshreshta, A.; Gilman-Sachs, A.; Beaman, K.D. The V-ATPase a2 isoform controls mammary gland development through Notch and TGF-beta signaling. Cell Death Dis. 2016, 7, e2443. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Wu, Y.; He, P.; Fan, Y.; Zhong, X.; Zheng, H.; Luo, T. PI3K/AKT/mTOR-Targeted Therapy for Breast Cancer. Cells 2022, 11, 2508. [Google Scholar] [CrossRef]
- Master, S.R.; Hartman, J.L.; D’Cruz, C.M.; Moody, S.E.; Keiper, E.A.; Ha, S.I.; Cox, J.D.; Belka, G.K.; Chodosh, L.A. Functional microarray analysis of mammary organogenesis reveals a developmental role in adaptive thermogenesis. Mol. Endocrinol. (Baltim. Md.) 2002, 16, 1185–1203. [Google Scholar] [CrossRef]
- Wu, Y.; Kinnebrew, M.A.; Kutyavin, V.I.; Chawla, A. Distinct signaling and transcriptional pathways regulate peri-weaning development and cold-induced recruitment of beige adipocytes. Proc. Natl. Acad. Sci. USA 2020, 117, 6883–6889. [Google Scholar] [CrossRef]
- Landskroner-Eiger, S.; Park, J.; Israel, D.; Pollard, J.W.; Scherer, P.E. Morphogenesis of the developing mammary gland: Stage-dependent impact of adipocytes. Dev. Biol. 2010, 344, 968–978. [Google Scholar] [CrossRef]
- Ridgway, N.D. The role of phosphatidylcholine and choline metabolites to cell proliferation and survival. Crit. Rev. Biochem. Mol. Biol. 2013, 48, 20–38. [Google Scholar] [CrossRef]
- Koeberle, A.; Shindou, H.; Koeberle, S.C.; Laufer, S.A.; Shimizu, T.; Werz, O. Arachidonoyl-phosphatidylcholine oscillates during the cell cycle and counteracts proliferation by suppressing Akt membrane binding. Proc. Natl. Acad. Sci. USA 2013, 110, 2546–2551. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Liu, F.; Yin, Q.; Jiang, T.; Fang, M.; Duan, L.; Quan, S.; Tian, X.; Shen, A.; Mi, K.; et al. Exploration of Lipid Metabolism Alterations in Children with Active Tuberculosis Using UHPLC-MS/MS. J. Immunol. Res. 2023, 2023, 8111355. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Du, M.; Wang, H.; Mao, X. Milk fat globule membrane and its component phosphatidylcholine induce adipose browning both in vivo and in vitro. J. Nutr. Biochem. 2020, 81, 108372. [Google Scholar] [CrossRef] [PubMed]
- Kuusik, K.; Kasepalu, T.; Zilmer, M.; Eha, J.; Paapstel, K.; Kilk, K.; Rehema, A.; Kals, J. Effects of RIPC on the Metabolomical Profile during Lower Limb Digital Subtraction Angiography: A Randomized Controlled Trial. Metabolites 2023, 13, 856. [Google Scholar] [CrossRef] [PubMed]
- Scow, R.O.; Chernick, S.S.; Fleck, T.R. Lipoprotein lipase and uptake of triacylglycerol, cholesterol and phosphatidylcholine from chylomicrons by mammary and adipose tissue of lactating rats in vivo. Biochim. Et Biophys. Acta (BBA)-Lipids Lipid Metab. 1977, 487, 297–306. [Google Scholar] [CrossRef]
- Zhe, L.; Krogh, U.; Lauridsen, C.; Nielsen, M.O.; Fang, Z.; Theil, P.K. Impact of dietary fat levels and fatty acid composition on milk fat synthesis in sows at peak lactation. J. Anim. Sci. Biotechnol. 2023, 14, 42. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Li, L. Degradation strategy of cyclin D1 in cancer cells and the potential clinical application. Front. Oncol. 2022, 12, 949688. [Google Scholar] [CrossRef] [PubMed]
- Choe, K.N.; Moldovan, G.L. Forging Ahead through Darkness: PCNA, Still the Principal Conductor at the Replication Fork. Mol. Cell 2017, 65, 380–392. [Google Scholar] [CrossRef] [PubMed]
- Matveyenka, M.; Rizevsky, S.; Kurouski, D. Unsaturation in the Fatty Acids of Phospholipids Drastically Alters the Structure and Toxicity of Insulin Aggregates Grown in Their Presence. J. Phys. Chem. Lett. 2022, 13, 4563–4569. [Google Scholar] [CrossRef]
- Gu, C.; Zhang, Q.; Li, Y.; Li, R.; Feng, J.; Chen, W.; Ahmed, W.; Soufiany, I.; Huang, S.; Long, J.; et al. The PI3K/AKT Pathway-The Potential Key Mechanisms of Traditional Chinese Medicine for Stroke. Front. Med. 2022, 9, 900809. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Sun, B.; Zhang, F.; Zhong, Z.; Zhang, Y.; Li, F.; Zhang, T.; Khatib, H.; Wang, X. lncRNA MPFAST Promotes Proliferation and Fatty Acid Synthesis of Bovine Mammary Epithelial Cell by Sponging miR-103 Regulating PI3K-AKT Pathway. J. Agric. Food Chem. 2022, 70, 12004–12013. [Google Scholar] [CrossRef]
- Phyu, S.M.; Tseng, C.C.; Smith, T.A.D. CDP-choline accumulation in breast and colorectal cancer cells treated with a GSK-3-targeting inhibitor. Magn. Reson. Mater. Phys. Biol. Med. 2019, 32, 227–235. [Google Scholar] [CrossRef] [PubMed]




| Gene | Primer | Primer Sequence (5′–3′) |
|---|---|---|
| UCP1 | Forward | ATTCAGAGGCAAATCAGCTTTG |
| Reverse | GTGTTTCTCTCCCTGAAGAGAA | |
| β-actin | Forward | GGTCATCACTATTGGCAACGAG |
| Reverse | GAGGTCTTTACGGATGTCAACG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lang, L.; Zheng, J.; Liang, S.; Zhang, F.; Fu, Y.; Deng, K.; Li, F.; Yang, X.; Wang, J.; Luo, Y.; et al. Browning of Mammary Fat Suppresses Pubertal Mammary Gland Development of Mice via Elevation of Serum Phosphatidylcholine and Inhibition of PI3K/Akt Pathway. Int. J. Mol. Sci. 2023, 24, 16171. https://doi.org/10.3390/ijms242216171
Lang L, Zheng J, Liang S, Zhang F, Fu Y, Deng K, Li F, Yang X, Wang J, Luo Y, et al. Browning of Mammary Fat Suppresses Pubertal Mammary Gland Development of Mice via Elevation of Serum Phosphatidylcholine and Inhibition of PI3K/Akt Pathway. International Journal of Molecular Sciences. 2023; 24(22):16171. https://doi.org/10.3390/ijms242216171
Chicago/Turabian StyleLang, Limin, Jisong Zheng, Shuyi Liang, Fenglin Zhang, Yiming Fu, Kaixin Deng, Fan Li, Xiaohua Yang, Junfeng Wang, Yuexiang Luo, and et al. 2023. "Browning of Mammary Fat Suppresses Pubertal Mammary Gland Development of Mice via Elevation of Serum Phosphatidylcholine and Inhibition of PI3K/Akt Pathway" International Journal of Molecular Sciences 24, no. 22: 16171. https://doi.org/10.3390/ijms242216171
APA StyleLang, L., Zheng, J., Liang, S., Zhang, F., Fu, Y., Deng, K., Li, F., Yang, X., Wang, J., Luo, Y., Zhang, S., Zhu, X., Wang, L., Gao, P., Zhu, C., Shu, G., Xi, Q., Zhang, Y., Jiang, Q., & Wang, S. (2023). Browning of Mammary Fat Suppresses Pubertal Mammary Gland Development of Mice via Elevation of Serum Phosphatidylcholine and Inhibition of PI3K/Akt Pathway. International Journal of Molecular Sciences, 24(22), 16171. https://doi.org/10.3390/ijms242216171








