Exploring Reliable and Efficient Plasmonic Nanopatterning for Surface- and Tip-Enhanced Raman Spectroscopies
Abstract
:1. Introduction
2. Basic Principles of Raman Spectroscopy
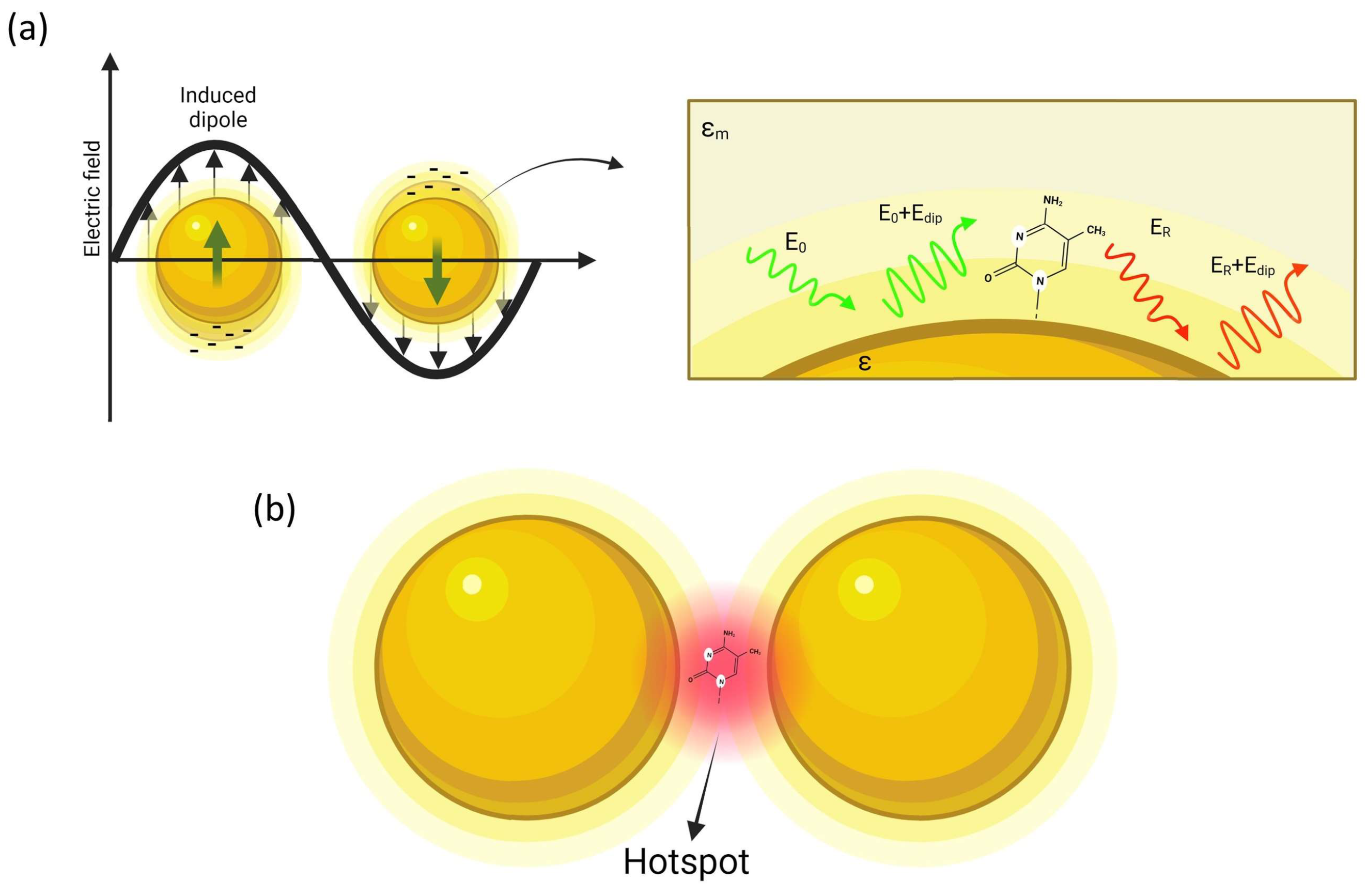
3. Surface- and Tip-Enhanced Raman Scattering
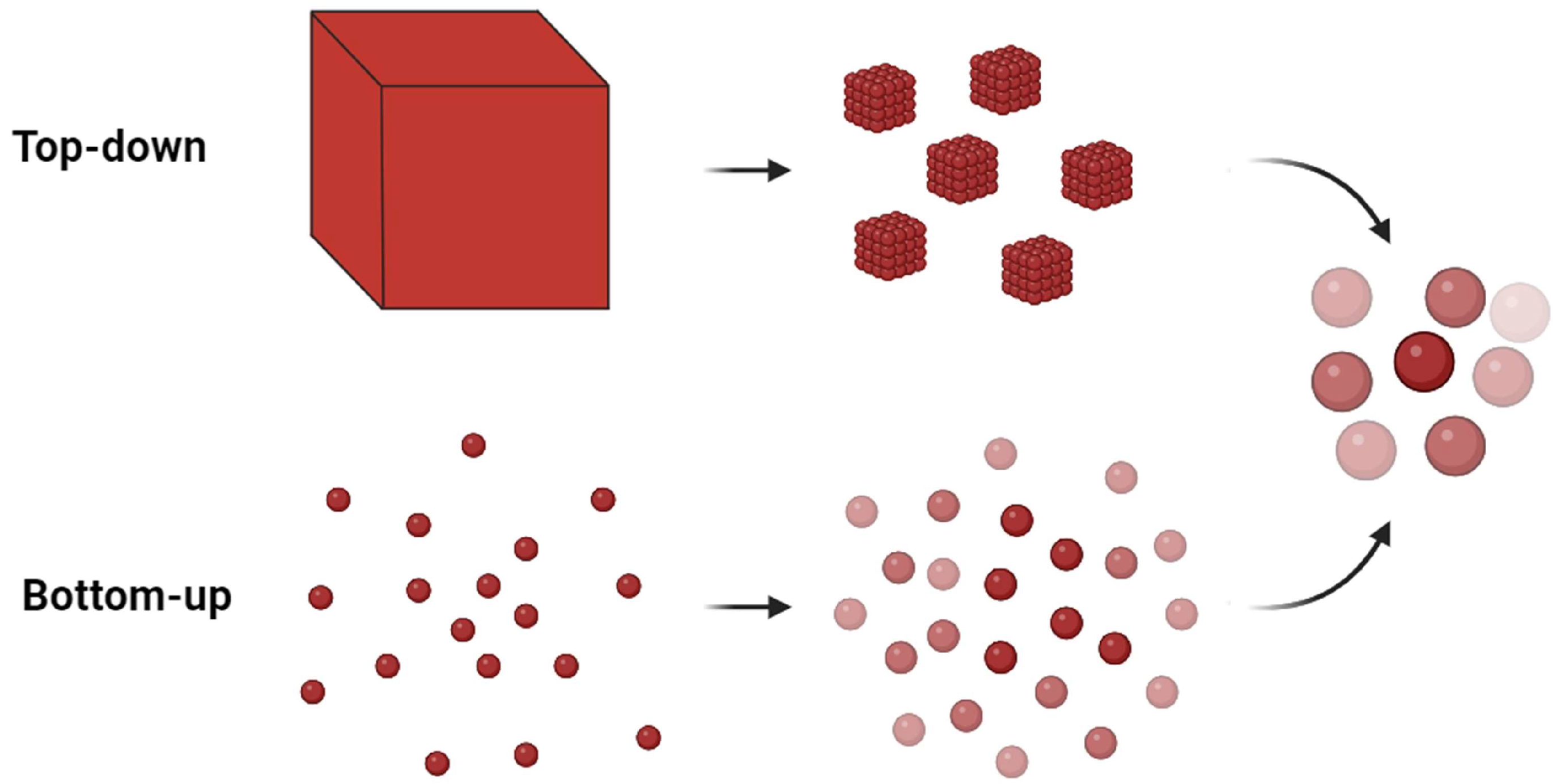
4. Development of Plasmonic Platforms
4.1. Bottom-Up Approaches
4.2. Top-Down Approaches
4.3. TERS Probe Production
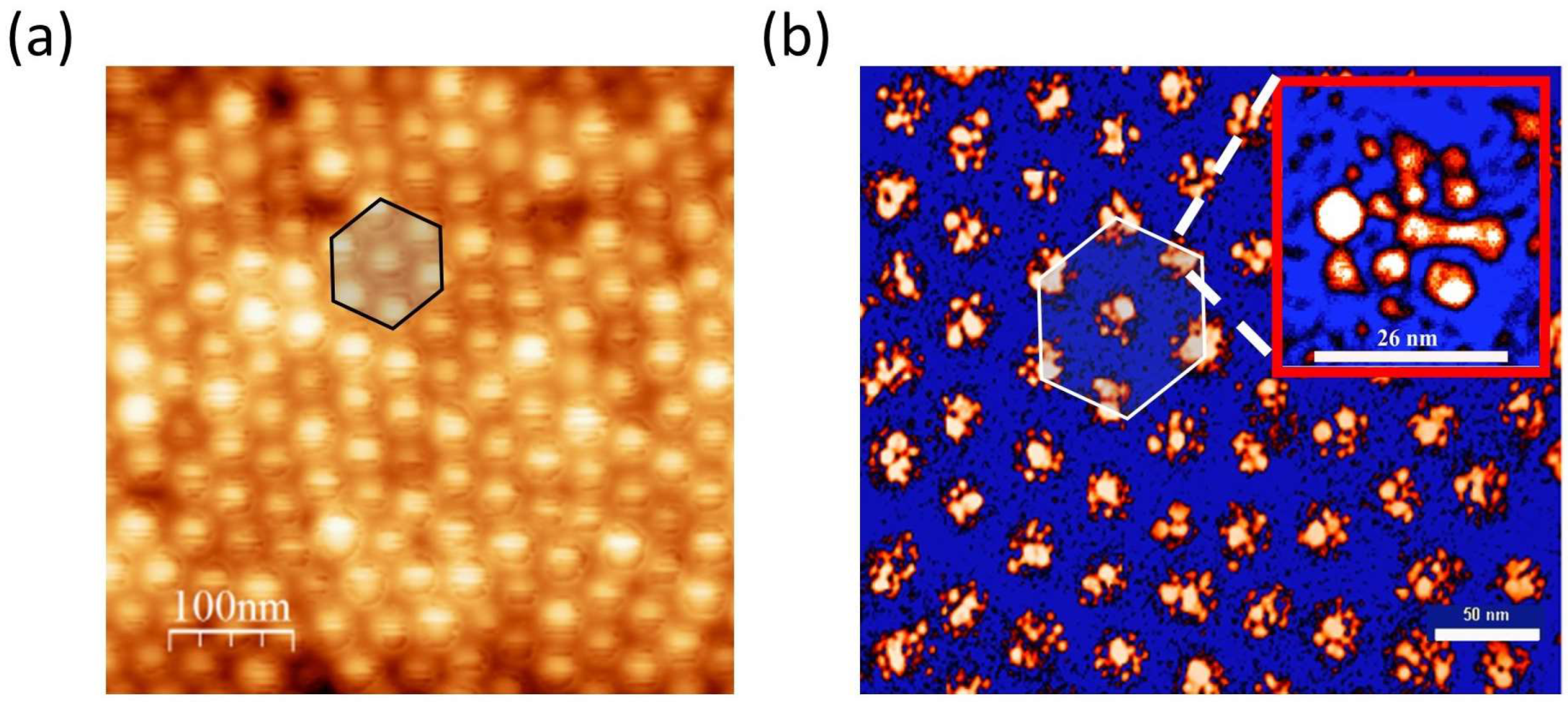
5. Silver-Based Nanopatterns Obtained by Self-Assembling of Block Copolymers
5.1. Fabrication of SERS Substrates and TERS Tips by Self-Assembling Block Copolymers
- (a)
- BCP selection;
- (b)
- Formation of the PS-b-P4VP crew-cut micelles in toluene solution;
- (c)
- Incorporation of the silver precursor (silver ions) into the P4VP micelle core;
- (d)
- Reduction by BaBH4 of the coordinated silver ions to silver NPs;
- (e)
- Centrifugation and phase separation of the solution;
- (f)
- Spin-coating of the solution on the glass substrate;
- (g)
- Polymer removal by UV-light exposure.
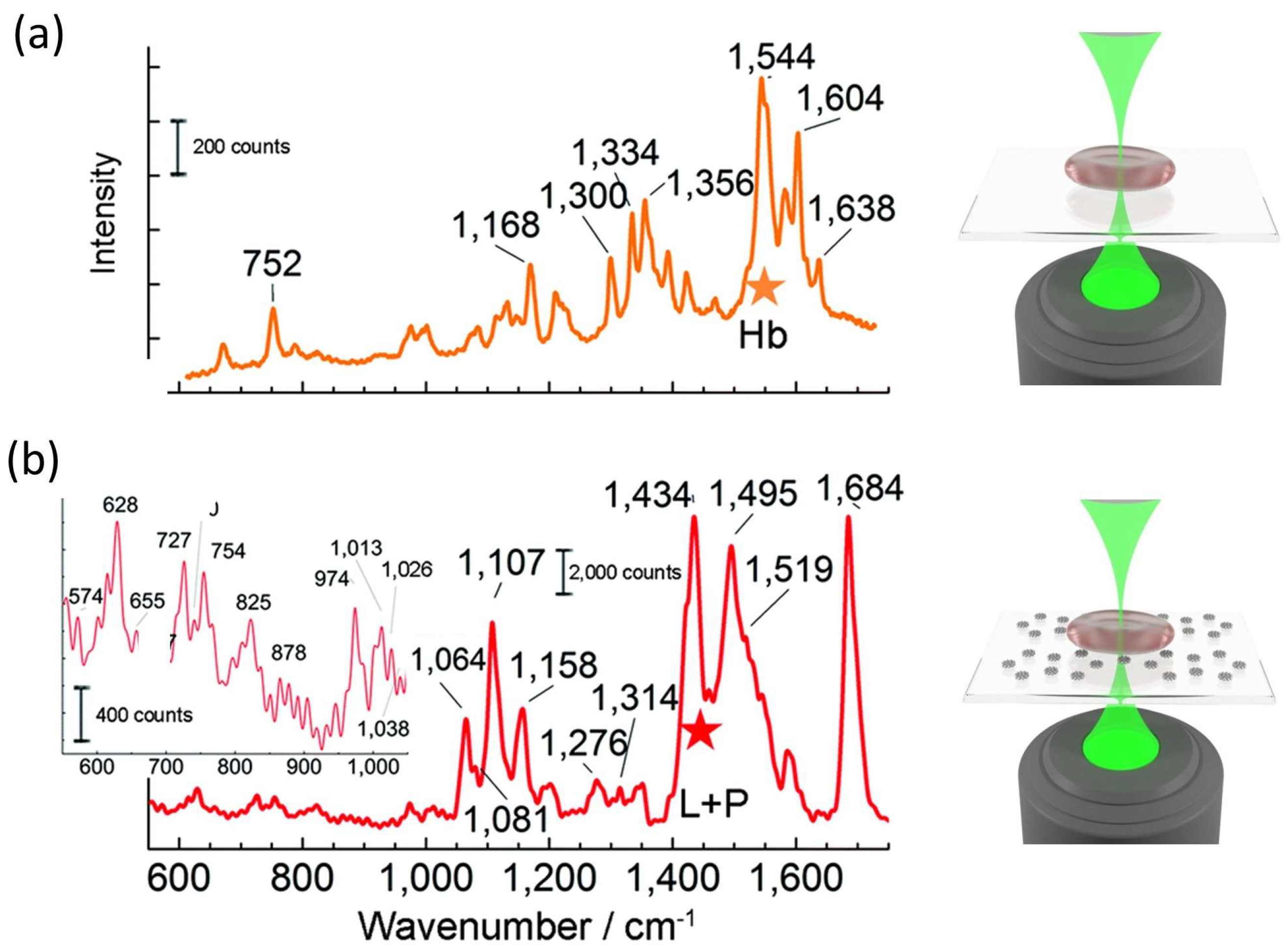
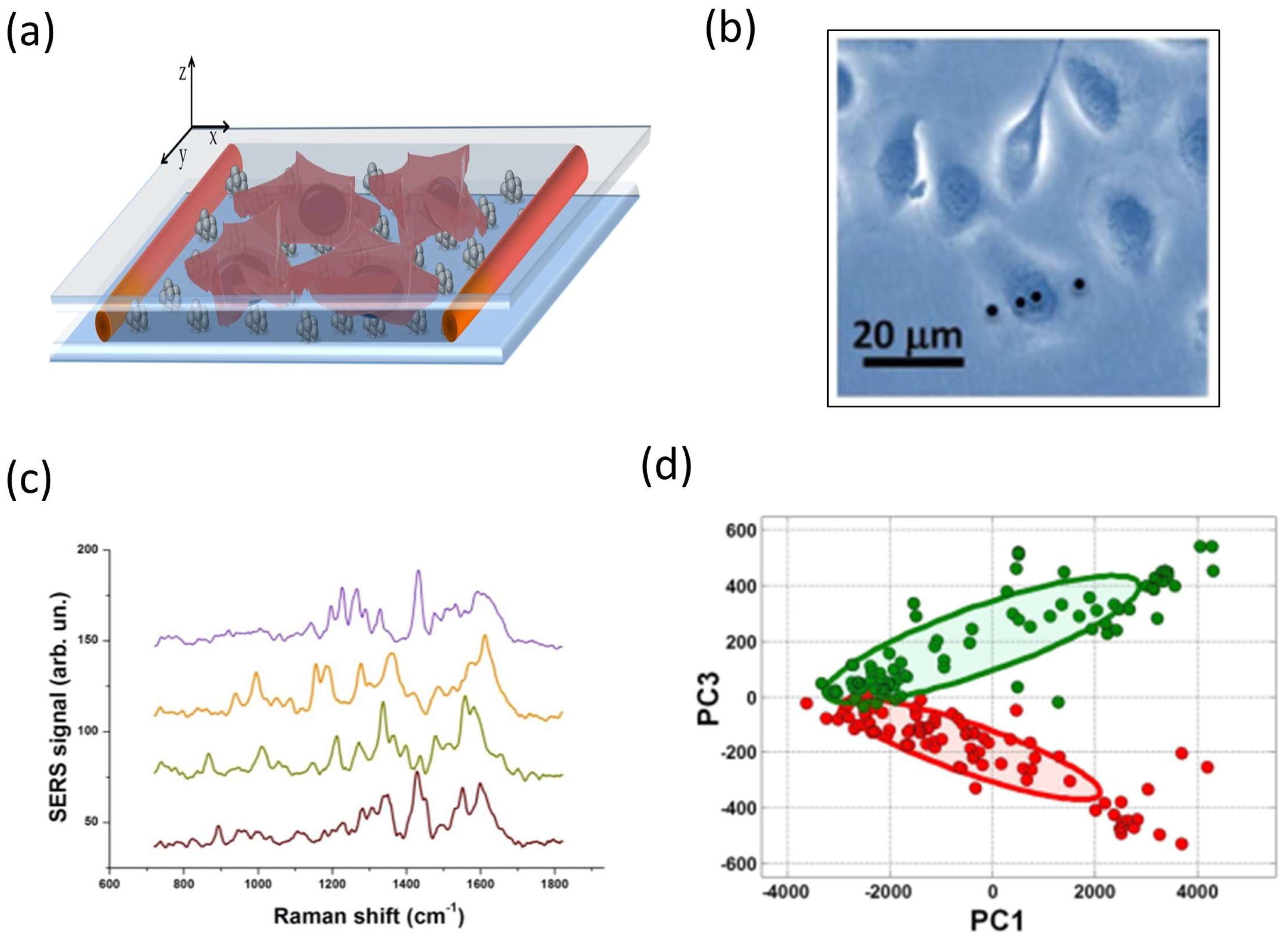
5.2. Study of Cell Membranes Using SERS
5.3. Study of Protein Overexpression in Cancer Cells
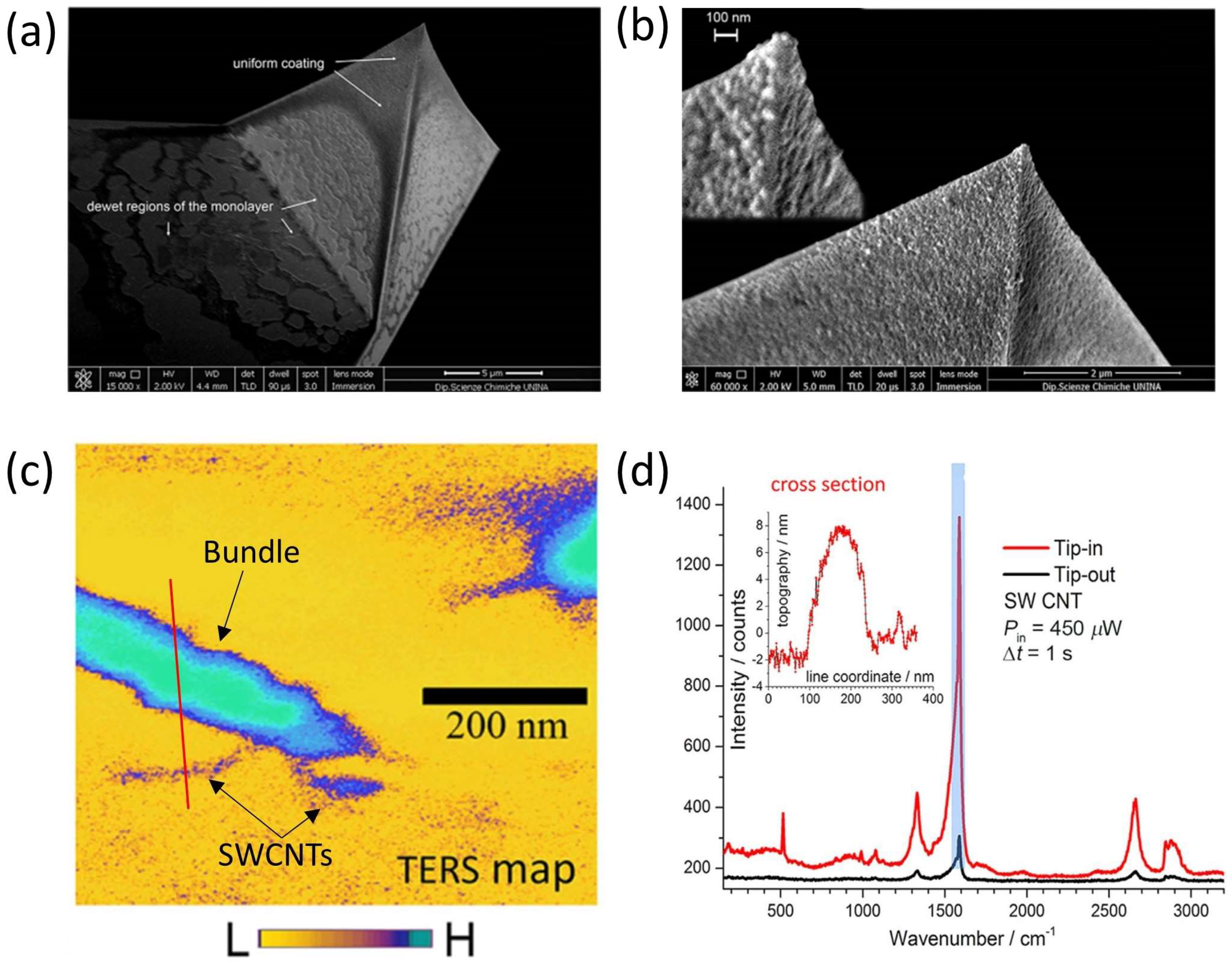
5.4. TERS Tip Fabrication and Characterization
6. Nanostructured Ag Films using Solid-State Dewetting
- (i)
- The reactive species (atomic oxygen) produced in the discharge can oxidize the silver layer [132]. As the oxide layer grows, internal stresses due to mechanical or thermal excursions can induce the cracking of the formed layer [133]. However, as experimentally observed by the same authors and by our group, this cracking-induced process is not sufficient to explain the growth of nanostructures on the silver surface.
- (ii)
- Another mechanism is oxide diffusion at the interface (Kirkendall effect [134]). The different diffusion rates of metals and oxides produces a disequilibrium of the material flow that is compensated with the inter-diffusion of vacancies; the condensation of excess vacancies can give rise to void formation (Kirkendall voids), which leads to the formation of nanopores [135]. Nevertheless, since the Kirkendall effect cannot be activated unless the material is exposed to an elevated temperature, the authors of ref. [136] suggest that the temperature could rise locally due to the exothermic nature of the chemical oxidation reaction of silver. Another explanation could be that the energy carried by the oxygen atoms is converted into heat in the oxide layer [133]. Nevertheless, the Kirkendall effect is considered responsible for the formation of nanovoids inside nanocrystals and below the formed oxide layer. In fact, this effect is used to produce hollow structures, as reported in several works [137,138,139].
- (iii)
- Compared to the works of El Mel’s group, we suggest that the formation of porous nanostructures could be triggered by the solid-state dewetting of the oxidized silver layer. To minimize the energy of the system as the exposure time to the RF plasma increases, the structure of the oxide film rearranges to form grain boundaries along which atoms can easily migrate to form islands. In fact, a similar process is also reported in the work of Zhao et al. [140], where the Kirkendall voids have been proposed to explain the initial stage of thermally induced SSD of Cu films deposited on native Si oxide substrates. Our assumption is that the cracking process and the Kirkendall effect might weaken and destabilize the thin oxide layer, promoting the SSD of the silver oxide. Moreover, the morphology of the silver layer is more like the classical picture of spinodal dewetting because the nanostructures exhibit similar spatial correlation.
6.1. Fabrication of SERS Substrates and TERS Probes
- Oxidation of the Si AFM probe. This is necessary to modify the refractive index from the value of silicon (n = 4.4) to that of SiO2 (n = 1.5) in order to match better the refractive index of the air and tune the plasmon resonance in the visible region. Oxidation is obtained by heating the tips in a muffle furnace at 1000 °C for 10 h to produce a 200 nm SiO2 thick layer.
- Coating of the AFM tip using magnetron sputtering. This is done to improve the adhesion of the Ag layer on the tip. First, a bi-layer of Cr-Au (with respective thicknesses of 3 nm and 10 nm) is deposited, and then the layer of Ag is deposited with an optimized thickness of 30 nm.
- Plasma treatment in air. The AFM tip is exposed to the plasma of a radio-frequency discharge (inductively coupling plasma, ICP) sustained by synthetic air. The optimal exposure around 90 s was chosen following a procedure described in [141]. After this treatment, a structured Ag film covered by a Ag oxide (AgO and Ag2O [142]) layer is formed.
- Plasma treatment in argon. Further exposure to the Ar-plasma is necessary to reduce the formed Ag oxide. In this case, the exposure time is optimized by monitoring the presence of the oxide using X-ray diffraction (XRD) until it disappears, but preserving the metallic silver nanostructure.
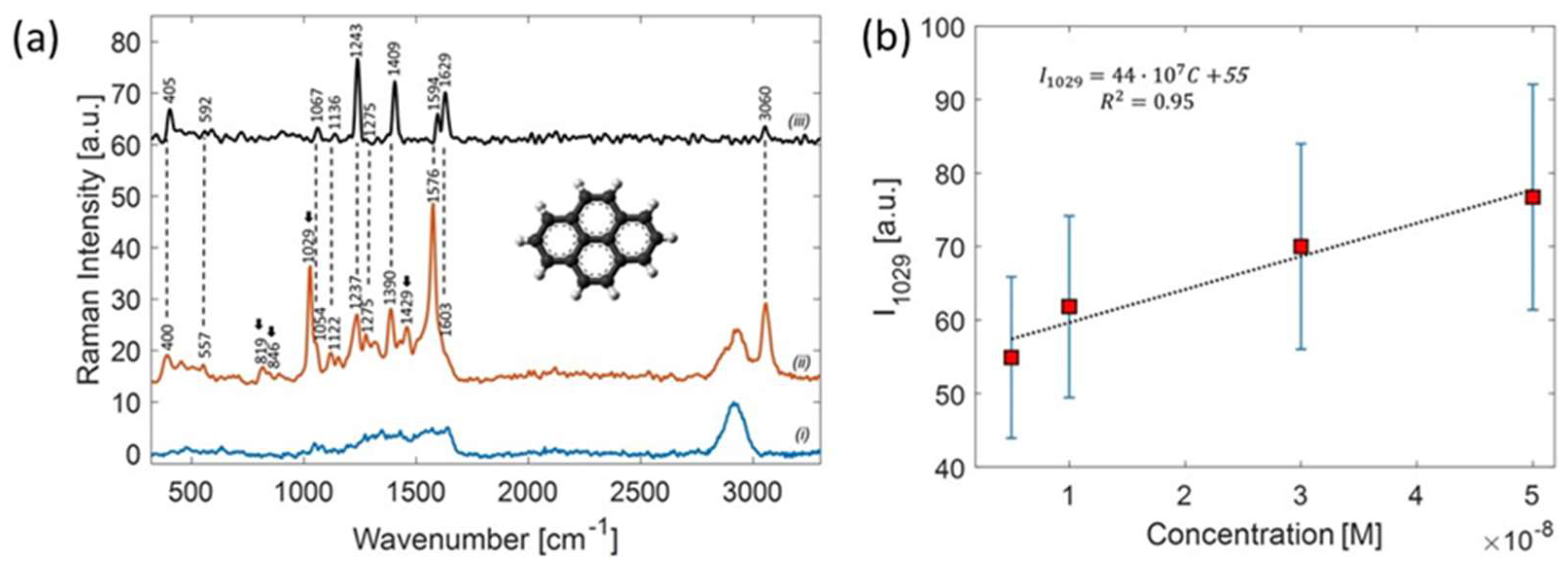
6.2. SERS-Active Sensors for the Detection of Pollutant Molecules
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Keiser, G. Biophotonics Concept and Applications, 1st ed.; Springer: Singapore, 2016. [Google Scholar]
- Prasad, P.N. Introduction to Biophotonics; Wiley: Hoboken, NJ, USA, 2003. [Google Scholar]
- Tsia, K. Understanding Biophotonics: Fundamentals, Advances, and Applications, 1st ed.; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Pavesi, L.; Fauchet, P. Biophotonics; Springer-Verlag: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Meglinski, I. Biophotonics for Medical Applications, 1st ed.; Woodhead Publishing: Sawston, UK, 2015. [Google Scholar]
- Jensen, E.C. Types of imaging, part 2: An overview of fluorescence microscopy. Anat. Rec. 2012, 295, 1621–1627. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.L. Basic Confocal Microscopy. Curr. Protoc. Neurosci. 2011, 56, 2. [Google Scholar] [CrossRef] [PubMed]
- Wäldchen, S.; Lehmann, J.; Klein, T.; van de Linde, S.; Sauer, M. Light-induced cell damage in live-cell super-resolution microscopy. Sci. Rep. 2015, 5, 15348. [Google Scholar] [CrossRef] [PubMed]
- Raman, C.V. A change of wavelength in light scattering. Nature 1928, 121, 619. [Google Scholar] [CrossRef]
- Krafft, C.; Popp, J. The many facets of Raman spectroscopy for biomedical analysis. Anal. Bioanal. Chem. 2015, 407, 699–717. [Google Scholar] [CrossRef] [PubMed]
- Kumar, C.S.S.R. Raman Spectroscopy for Nanomaterials Characterization, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar] [CrossRef]
- Gregoriou, V.G.; Braiman, M.S. Vibrational Spectroscopy of Biological and Polymeric Materials, 1st ed.; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Siebert, F.; Hildebrandt, P. Vibrational Spectroscopy in Life Science; Wiley: Hoboken, NJ, USA, 2008. [Google Scholar]
- Lasch, P.; Kneipp, J. Biomedical Vibrational Spectroscopy; Wiley-Interscience: Hoboken, NJ, USA, 2008. [Google Scholar]
- Rusciano, G.; Capriglione, P.; Pesce, G.; Carnovale, V.; Sasso, A. Raman spectroscopy as a new tool for early detection of bacteria in patients with cystic fibrosis. Laser Phys. Lett. 2013, 10, 075603. [Google Scholar] [CrossRef]
- Qi, Z.; Akhmetzhanov, T.; Pavlova, A.; Smirnov, E. Reusable SERS Substrates Based on Gold Nanoparticles for Peptide Detection. Sensors 2023, 23, 6352. [Google Scholar] [CrossRef]
- Fleischmann, M.; Hendra, P.J.; McQuillan, A.J. Raman spectra of pyridine adsorbed at a silver electrode. Chem. Phys. Lett. 1974, 26, 163–166. [Google Scholar] [CrossRef]
- Langer, J.; Jimenez de Aberasturi, D.; Aizpurua, J.; Alvarez-Puebla, R.A.; Auguié, B.; Baumberg, J.J.; Bazan, G.C.; Bell, S.E.; Boisen, A.; Brolo, A.G.; et al. Present and future of surface-enhanced Raman scattering. ACS Nano 2020, 14, 28–117. [Google Scholar] [CrossRef]
- Sharma, B.; Frontiera, R.R.; Henry, A.-I.; Ringe, E.; Van Duyne, R.P. SERS: Materials, applications, and the future. Mater. Today 2012, 15, 16–25. [Google Scholar] [CrossRef]
- Bailo, E.; Deckert, V. Tip-enhanced Raman scattering. Chem. Soc. Rev. 2008, 37, 921–930. [Google Scholar] [CrossRef] [PubMed]
- Domke, K.F.; Pettinger, B. Studying surface chemistry beyond the diffraction limit: 10 years of TERS. Chem. Phys. Chem. 2010, 11, 1365–1373. [Google Scholar] [CrossRef] [PubMed]
- Verma, P. Tip-enhanced Raman spectroscopy: Technique and recent advances. Chem. Rev. 2017, 117, 6447–6466. [Google Scholar] [CrossRef]
- Shao, F.; Zenobi, R. Tip-enhanced Raman spectroscopy: Principles, practice, and applications to nanospectroscopic imaging of 2D materials. Anal. Bioanal. Chem. 2019, 411, 37–61. [Google Scholar] [CrossRef] [PubMed]
- Gremlichl, H.-U.; Bing, Y. Infrared and Raman Spectroscopy of Biological Materials, 1st ed.; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Butler, H.; Ashton, L.; Bird, B.; Cinque, G.; Curtis, K.; Dorney, J.; Esmonde-White, K.; Fullwood, N.J.; Gardner, B.; Martin-Hirsch, P.L.; et al. Using Raman spectroscopy to characterize biological materials. Nat. Protoc. 2016, 11, 664–687. [Google Scholar] [CrossRef]
- Rusciano, G.; De Luca, A.C.; Pesce, G.; Sasso, A. Phase-sensitive detection in Raman Tweezers. Appl. Phys. Lett. 2006, 89, 261116. [Google Scholar] [CrossRef]
- Rusciano, G.; De Luca, A.C.; Pesce, G.; Sasso, A. Enhancing Raman tweezers by phase-sensitive detection. Anal. Chem. 2007, 79, 3708–3715. [Google Scholar] [CrossRef]
- De Luca, A.C.; Rusciano, G.; Ciancia, R.; Martinelli, V.; Pesce, G.; Rotoli, B.; Sasso, A. Resonance Raman spectroscopy and mechanics of single red blood cell manipulated by optical tweezers. Haematologica 2007, 92, 174. [Google Scholar]
- De Luca, A.C.; Rusciano, G.; Ciancia, R.; Martinelli, V.; Pesce, G.; Rotoli, B.; Sasso, A. Spectroscopical and mechanical characterization of normal and thalassemic red blood cells by Raman tweezers. Opt. Exp. 2008, 16, 7943–7957. [Google Scholar] [CrossRef]
- Kneipp, K.; Kneipp, H.; Kartha, V.B.; Manoharan, R.; Deinum, G.; Itzkan, I.; Dasari, R.R.; Feld, M.S. Detection and identification of a single DNA base molecule using surface-enhanced Raman scattering (SERS). Phys. Rev. E 1998, 57, R6281–R6284. [Google Scholar] [CrossRef]
- Jeanmaire, D.L.; Van Duyne, R.P. Surface Raman spectroelectrochemistry; Part I. Heterocyclic, aromatic, and aliphatic amines adsorbed on the anodized silver electrode. J. Electroanal. Chem. 1977, 84, 1–20. [Google Scholar] [CrossRef]
- Albrecht, M.G.; Creighton, J.A. Anomalously intense Raman spectra of pyridine at a silver electrode. J. Am. Chem. Soc. 1977, 99, 5215–5217. [Google Scholar] [CrossRef]
- Lin, D.-Y.; Yu, C.-Y.; Ku, C.-A.; Chung, C.-K. Design, fabrication, and applications of SERS substrates for food safety detection: Review. Micromachines 2023, 14, 1343. [Google Scholar] [CrossRef] [PubMed]
- Beeram, R.; Vepa, K.R.; Soma, V.R. Recent trends in SERS-based plasmonic sensors for disease diagnostics, biomolecules detection, and machine learning techniques. Biosensors 2023, 13, 328. [Google Scholar] [CrossRef] [PubMed]
- Jebakumari, K.A.E.; Murugasenapathi, N.K.; Palanisamy, T. Engineered two-dimensional nanostructures as SERS substrates for biomolecule sensing: A review. Biosensors 2023, 13, 102. [Google Scholar] [CrossRef] [PubMed]
- Muniz-Miranda, M.; Gellini, C.; Innocenti, M. SERS Spectroscopy and Microscopy. In Raman Spectroscopy for Nanomaterials Characterization, 1st ed.; Kumar, C.S.S.R., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 553–586. [Google Scholar] [CrossRef]
- Capasso, R.; Pannico, M.; Manna, P.L.; Musto, P.; Rippa, M.; Mormile, P.; Petti, L. Gold Photonic Crystals and Photonics Quasi-crystals for Reproducible Surface-Enhanced Raman Substrates. In Nano-Structures for Optics and Photonics; Di Bartolo, B., Collins, J., Silvestri, L., Eds.; Springer: Dordrecht, The Netherlands, 2015; pp. 437–438. [Google Scholar] [CrossRef]
- Giugni, A.; Torre, B.; Allione, M.; Gentile, F.; Candeloro, P.; Coluccio, M.L.; Perozziello, G.; Limongi, T.; Marini, M.; Raimondo, R.; et al. Novel Plasmonic Probes and Smart Superhydrophobic Devices, New Tools for Forthcoming Spectroscopies at the Nanoscale. In Nano-Structures for Optics and Photonics; Di Bartolo, B., Collins, J., Silvestri, L., Eds.; Springer: Dordrecht, The Netherland, 2015; pp. 209–235. [Google Scholar] [CrossRef]
- Fazio, B.; Foti, A.; D’Andrea, C.; Messina, E.; Irrera, A.; Maragò, O.M.; Gucciardi, P.M. Polarized Surface-Enhanced Raman Scattering. In Handbook of Enhanced Spectroscopy, 1st ed.; de la Chapelle, M.L., Gucciardi, P.G., Lidgi-Guigui, N., Eds.; Jenny Stanford Publishing: New York, NY, USA, 2015; pp. 203–241. [Google Scholar] [CrossRef]
- Pilot, R.; Signorini, R.; Durante, C.; Orian, L.; Bhamidipati, M.; Fabris, L. A review on surface-enhanced Raman scattering. Biosensors 2019, 9, 57. [Google Scholar] [CrossRef]
- Bonifacio, A. Nanostructured Substrates for Surface-Enhanced Raman Scattering Spectroscopy. In Nanomaterials for Theranostics and Tissue Engineering: Techniques, Trends and Applications; Rossi, F., Rainer, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 137–174. [Google Scholar] [CrossRef]
- Das, G.M.; Managò, S.; Mangini, M.; De Luca, A.C. Biosensing using SERS active gold nanostructures. Nanomaterials 2021, 11, 2679. [Google Scholar] [CrossRef]
- Ong, T.T.X.; Blanch, E.W.; Jones, O.A.H. Surface enhanced Raman spectroscopy in environmental analysis, monitoring and assessment. Sci Total Environ. 2020, 720, 137601. [Google Scholar] [CrossRef]
- Qian, X.; Peng, X.H.; Ansari, D.O.; Yin-Goen, Q.; Chen, G.Z.; Shin, D.M.; Yang, L.; Young, A.N.; Wang, M.D.; Nie, S. In vivo tumor targeting and spectroscopic detection with surface-enhanced Raman nanoparticle tags. Nat. Biotechnol. 2008, 26, 83–90. [Google Scholar] [CrossRef]
- Rusciano, G.; Capaccio, A.; Sasso, A.; Capo, A.; Murillo Almuzara, C.; Staiano, M.; D’Auria, S.; Varriale, A. A SERS-based biosensor for the detection of biological macro-molecules: The case of the lipopolysaccharide endotoxin molecules. Int. J. Mol. Sci. 2023, 24, 12099. [Google Scholar] [CrossRef]
- Xu, M.L.; Gao, Y.; Han, X.X.; Zhao, B. Innovative application of SERS in food quality and safety: A brief review of recent trends. Foods 2022, 11, 2097. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Lin, C.; Li, Y.; Gao, Y.; Wang, J.; He, J.; Huang, Z.; Liu, J.; Luo, X.; Yang, Y. Identifying infectiousness of SARS-CoV-2 by ultra-sensitive SnS2 SERS biosensors with capillary effect. Matter 2022, 5, 694–709. [Google Scholar] [CrossRef] [PubMed]
- Le Ru, E.C.; Etchegoin, P.G. Principles of Surface-Enhanced Raman Spectroscopy and related Plasmonic Effects, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Kneipp, K.; Moskovits, M.; Kneipp, H. Surface-Enhanced Raman Scattering: Physics and Applications, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Anderson, D.J.; Moskovits, M. A SERS-active system based on silver nanoparticles tethered to a deposited silver film. J. Phys. Chem. B 2006, 110, 13722–13727. [Google Scholar] [CrossRef]
- Lin, C.; Liang, S.; Peng, Y.; Long, L.; Li, Y.; Huang, Z.; Long, N.V.; Luo, X.; Liu, J.; Li, Z.; et al. Visualized SERS imaging of single molecule by Ag/Black phosphorus nanosheets. Nano-Micro Lett. 2022, 14, 75. [Google Scholar] [CrossRef]
- Cong, S.; Liu, X.; Jiang, Y.; Zhang, W.; Zhao, Z. Surface enhanced Raman scattering revealed by interfacial charge-transfer transitions. Innovation 2020, 1, 100051. [Google Scholar] [CrossRef] [PubMed]
- Le Ru, E.C.; Etchegoin, P.G.; Meyer, M. Enhancement factor distribution around a single surface-enhanced Raman scattering hot spot and its relation to single molecule detection. J. Chem. Phys. 2006, 125, 204701. [Google Scholar] [CrossRef]
- Stöckle, R.M.; Suh, Y.D.; Deckert, V.; Zenobi, R. Nanoscale chemical analysis by tip-enhanced Raman spectroscopy. Chem. Phys. Lett. 2000, 318, 131–136. [Google Scholar] [CrossRef]
- Anderson, M.S. Locally Enhanced Raman Spectroscopy with an Atomic Force Microscope. Appl. Phys. Lett. 2000, 76, 3130–3132. [Google Scholar] [CrossRef]
- Hayazawa, N.; Inouye, Y.; Sekkat, Z.; Kawata, S. Metallized tip amplification of near-field Raman scattering. Opt. Commun. 2000, 183, 333–336. [Google Scholar] [CrossRef]
- Pettinger, B.; Ren, B.; Picardi, G.; Schuster, R.; Ertl, G. Nanoscale probing of adsorbed species by tip-enhanced Raman spectroscopy. Phys. Rev. Lett. 2004, 92, 096101. [Google Scholar] [CrossRef]
- Itoh, T.; Procházka, M.; Dong, Z.C.; Ji, W.; Yamamoto, Y.S.; Zhang, Y.; Ozaki, Y. Toward a new era of SERS and TERS at the nanometer scale: From fundamentals to innovative applications. Chem. Rev. 2023, 123, 1552–1634. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, P.; Deutsch, B.; Novotny, L. Optical Antennas. Adv. Opt. Photonics 2009, 1, 438–483. [Google Scholar] [CrossRef]
- Fan, Y.; Jin, D.; Wu, X.; Fang, H.; Yuan, X. Facilitating hotspot alignment in tip-enhanced Raman spectroscopy via the silver photoluminescence of the probe. Sensors 2020, 20, 6687. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, A.; Yu, J.; Verma, P.; Kawata, S. Optical antennas with multiple plasmonic nanoparticles for tip-enhanced Raman microscopy. Nanoscale 2015, 7, 17424–17433. [Google Scholar] [CrossRef]
- Novotny, L.; Van Hulst, N. Antennas for light. Nat. Photonics 2011, 5, 83–90. [Google Scholar] [CrossRef]
- Rusciano, G.; Zito, G.; Isticato, R.; Sirec, T.; Ricca, E.; Bailo, E.; Sasso, A. Nanoscale chemical imaging of bacillus subtilis spores by combining Tip-Enhanced Raman Scattering and advanced statistical tools. ACS Nano 2014, 8, 12300–12309. [Google Scholar] [CrossRef]
- Huang, T.X.; Cong, X.; Wu, S.S.; Lin, K.Q.; Yao, X.; He, Y.H.; Wu, J.B.; Bao, Y.F.; Huang, S.C.; Wang, X.; et al. Probing the edge-related properties of atomically thin MoS2 at nanoscale. Nat. Commun. 2019, 10, 5544. [Google Scholar] [CrossRef]
- Schultz, J.F.; Mahapatra, S.; Li, L.; Jiang, N. The expanding frontiers of tip-enhanced Raman spectroscopy. Appl. Spectrosc. 2020, 74, 313–1340. [Google Scholar] [CrossRef]
- Novotny, L.; Stranick, S.J. Near-field optical microscopy and spectroscopy with pointed probes. Annu. Rev. Phys. Chem. 2006, 57, 303–331. [Google Scholar] [CrossRef]
- Schmid, T.; Opilik, L.; Blum, C.; Zenobi, R. Nanoscale chemical imaging using tip-enhanced Raman spectroscopy: A critical review. Angew. Chem. Int. Ed. 2013, 52, 5940–5954. [Google Scholar] [CrossRef]
- Stadler, J.; Schmid, T.; Zenobi, R. Developments in and practical guidelines for tip-enhanced Raman spectroscopy. Nanoscale 2012, 4, 1856–1870. [Google Scholar] [CrossRef] [PubMed]
- Deckert, V.; Deckert-Gaudig, T.; Diegel, M.; Götz, I.; Langelüddecke, L.; Schneidewind, H.; Sharma, G.; Singh, P.; Zeisberger, M.; Zhang, Z.; et al. Spatial resolution in Raman spectroscopy. Faraday Discuss. 2015, 177, 9–20. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, Y.; Dong, Z.C.; Jiang, S.; Zhang, C.; Chen, L.G.; Zhang, L.; Liao, Y.; Aizpurua, J.; Luo, Y.; et al. Chemical mapping of a single molecule by plasmon-enhanced Raman scattering. Nature 2013, 498, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Sun, M. Tip-enhanced Raman spectroscopy. Rev. Phys. 2022, 8, 100067. [Google Scholar] [CrossRef]
- Shi, X.; Coca-López, N.; Janik, J.; Hartschuh, A. Advances in Tip-enhanced near-field Raman microscopy using nanoantennas. Chem. Rev. 2017, 117, 4945–4960. [Google Scholar] [CrossRef]
- Langeluddecke, L.; Singh, P.; Deckert, V. Exploring the nanoscale: Fifteen years of tip enhanced Raman spectroscopy applied spectroscopy. Appl. Spectrosc. 2015, 69, 1357–1371. [Google Scholar] [CrossRef]
- Kumar, N.; Mignuzzi, S.; Su, W.; Roy, D. Tip-enhanced Raman spectroscopy: Principles and applications. EPJ Tech. Instrum. 2015, 2, 9. [Google Scholar] [CrossRef]
- Pienpinijtham, P.; Kitahama, Y.; Ozaki, Y. Progress of tip-enhanced Raman scattering for the last two decades and its challenges in very recent years. Nanoscale 2022, 14, 5265–5288. [Google Scholar] [CrossRef]
- Kausar, A.S.M.Z.; Reza, A.W.; Latef, T.A.; Ullah, M.H.; Karim, M.E. Optical nano antennas: State of the art, scope and challenges as a biosensor along with human exposure to nano-toxicology. Sensors 2015, 15, 8787–8831. [Google Scholar] [CrossRef]
- Bartolomeo, G.L.; Goubert, G.; Zenobi, R. Tip recycling for atomic force microscopy-based tip-enhanced Raman spectroscopy. Appl Spectrosc. 2020, 74, 1358–1364. [Google Scholar] [CrossRef]
- Gan, Y.; Yuan, C.; Zhang, D. Tip modification methods for tip-enhanced Raman spectroscopy (TERS) and colloidal probe technique: A 10 years update (2006–2016) review. Rev. Sci. Instr. 2017, 88, 031101. [Google Scholar] [CrossRef]
- Yan, B.; Boriskina, S.V.; Reinhard, B.M. Design and implementation of noble metal nanoparticle cluster arrays for plasmon enhanced biosensing. J. Phys. Chem. C 2011, 115, 24437–24453. [Google Scholar] [CrossRef] [PubMed]
- Boss, P. Review of SERS substrates for chemical sensing. Nanomaterials 2017, 7, 142. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.X.; Kong, X. Review of recent progress of plasmonic materials and nano-structures for surface-enhanced Raman scattering. Materials 2015, 8, 3024–3052. [Google Scholar] [CrossRef]
- Matamoros-Ambrocio, M.; Sánchez-Mora, E.; Gómez-Barojas, E. Surface-enhanced Raman scattering (SERS) substrates based on Ag-nanoparticles and Ag-nanoparticles/poly (methyl methacrylate) composites. Polymers 2023, 15, 2624. [Google Scholar] [CrossRef] [PubMed]
- Evanoff, D.D.; Chumanov, G. Synthesis and optical properties of silver nanoparticles and arrays. Chem. Phys. Chem. 2015, 6, 1221–1231. [Google Scholar] [CrossRef] [PubMed]
- Toropov, N.; Vartanyan, T. 1.03—Noble Metal Nanoparticles: Synthesis and Optical Properties. In Comprehensive Nanoscience and Nanotechnology, 2nd ed.; Andrews, D.L., Lipson, R.H., Nann, T., Eds.; Academic Press: Cambridge, MA, USA, 2019; Volume 1, pp. 61–88. [Google Scholar]
- Lee, P.C.; Meisel, D. Adsorption and surface-enhanced Raman of dyes on silver and gold sols. J. Phys. Chem. 1982, 86, 3391–3395. [Google Scholar] [CrossRef]
- Creighton, J.A.; Blatchford, C.G.; Albrecht, M.G. Plasma resonance enhancement of Raman scattering by pyridine adsorbed on silver or gold sol particles of size comparable to the excitation wavelength. J. Chem. Soc. Faraday Trans. 2 1979, 75, 790–798. [Google Scholar] [CrossRef]
- Vlčková, B.; Solecká-Čermáková, K.; Matějka, P.; Baumruk, V. SERS spectroscopy with Ag colloids. J. Mol. Struct. 1997, 408–409, 149–154. [Google Scholar] [CrossRef]
- Si, M.Z.; Kang, Y.P.; Zhang, Z.G. Surface-enhanced Raman scattering (SERS) spectra of Methyl Orange in Ag colloids prepared by electrolysis method. Appl. Surf. Sci. 2009, 255, 6007–6010. [Google Scholar] [CrossRef]
- Yaffe, N.R.; Blanch, E.W. Effects and anomalies that can occur in SERS spectra of biological molecules when using a wide range of aggregating agents for hydroxylamine-reduced and citrate-reduced silver colloids. Vib. Spectr. 2008, 48, 196–201. [Google Scholar] [CrossRef]
- Plyuto, Y.; Berquier, J.-M.; Jacquiod, C.; Ricolleau, C. Ag nanoparticles synthesised in template-structured mesoporous silica films on a glass substrate. Chem. Commun. 1999, 17, 1653–1654. [Google Scholar] [CrossRef]
- Wang, T.C.; Rubner, M.F.; Cohen, R.E. Polyelectrolyte multilayer nanoreactors for preparing silver nanoparticle composites: Controlling metal concentration and nanoparticle size. Langmuir 2002, 18, 3370–3375. [Google Scholar] [CrossRef]
- Traci, R.; Malinsky, M.D.; Haynes, C.L.; Van Duyne, R.P. Nanosphere lithography: Tunable localized surface plasmon resonance spectra of silver nanoparticles. J. Phys. Chem. B 2000, 104, 10549–10556. [Google Scholar] [CrossRef]
- Zhao, Y.-P.; Ye, D.-X.; Wang, G.-C.; Lu, T.-M. Novel nano-column and nano-flower arrays by glancing angle deposition. Nano Lett. 2002, 2, 351–354. [Google Scholar] [CrossRef]
- Malynych, S.Z.; Chumanov, G. Vacuum deposition of silver island films on chemically modified surfaces. J. Vac. Sci. Technol. A 2003, 21, 723–727. [Google Scholar] [CrossRef]
- Mafuné, F.; Kohno, J.; Takeda, Y.; Kondow, T.; Sawabe, H. Structure and stability of silver nanoparticles in aqueous solution produced by laser ablation. J. Phys. Chem. B 2000, 104, 8333–8337. [Google Scholar] [CrossRef]
- Huang, H.H.; Ni, X.P.; Loy, G.L.; Chew, C.H.; Tan, K.L.; Loh, F.C.; Deng, J.F.; Xu, G.Q. Photochemical formation of silver nanoparticles in poly(N-vinylpyrrolidone). Langmuir 1996, 12, 909–912. [Google Scholar] [CrossRef]
- Abid, J.P.; Wark, A.W.; Brevet, P.F.; Girault, H.H. Preparation of silver nanoparticles in solution from a silver salt by laser irradiation. Chem. Commun. 2002, 9, 792–793. [Google Scholar] [CrossRef]
- Kotov, N.A.; Darbello Zaniquelli, M.E.; Meldrum, F.C.; Fendler, J.H. Two-dimensional silver electrocrystallization under monolayers spread on aqueous silver nitrate. Langmuir 1993, 9, 3710–3716. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, S.; Palchik, O.; Koltypin, Y.; Gedanken, A. Shape controlled synthesis of silver nanoparticles by pulse sonoelectrochemical methods. Langmuir 2000, 16, 6396–6399. [Google Scholar] [CrossRef]
- Tao, A.R.; Habas, S.; Yang, P. Shape control of colloidal metal nanocrystals. Small 2008, 4, 310–325. [Google Scholar] [CrossRef]
- Tantra, R.; Brown, R.J.C.; Milton, M.J.T. Strategy to improve the reproducibility of colloidal SERS. J. Raman Spectrosc. 2007, 38, 1469–1479. [Google Scholar] [CrossRef]
- Natan, M.J. Concluding remarks surface enhanced Raman scattering. Faraday Discuss. 2006, 132, 321–328. [Google Scholar] [CrossRef]
- Fischer, U.C.; Zingsheim, H.P. Submicroscopic pattern replication with visible light. J. Vac. Sci. Technol. 1981, 19, 881–885. [Google Scholar] [CrossRef]
- Berger, S.D.; Gibson, J.M.; Camarda, R.M.; Farrow, R.C.; Huggins, H.A.; Kraus, J.S.; Liddle, J.A. Projection electron-beam lithography: A new approach. J. Vac. Sci. Technol. B 1991, 9, 2996–2999. [Google Scholar] [CrossRef]
- Sánchez-Iglesias, A.; Aldeanueva-Potel, P.; Ni, W.; Pérez-Juste, J.; Pastoriza-Santos, I.; Alvarez-Puebla, R.A.; Mbenkum, B.N.; Liz-Marzán, L.M. Chemical seeded growth of Ag nanoparticle arrays and their application as reproducible SERS substrates. Nano Today 2010, 5, 21–27. [Google Scholar] [CrossRef]
- Van Schrojenstein Lantman, E.M.; Deckert-Gaudig, T.; Mank, A.J.G.; Deckert, V.; Weckhuysen, B.M. Catalytic processes monitored at the nanoscale with tip-enhanced Raman spectroscopy. Nat. Nanotech. 2012, 7, 583–586. [Google Scholar] [CrossRef]
- Huang, T.X.; Huang, S.C.; Li, M.H.; Zeng, Z.C.; Wang, X.; Ren, B. Tip-enhanced Raman spectroscopy: Tip-related issues. Anal. Bioanal. Chem. 2015, 407, 8177–8195. [Google Scholar] [CrossRef]
- Yeo, B.-S.; Schmid, T.; Zhang, W.; Zenobi, R. Towards rapid nanoscale chemical analysis using tip-enhanced Raman spectroscopy with Ag-coated dielectric tips. Anal. Bioanal. Chem. 2007, 387, 2655–2662. [Google Scholar] [CrossRef]
- Maouli, I.; Taguchi, A.; Saito, Y.; Kawata, S.; Verma, P. Optical antennas for tunable enhancement in tip-enhanced Raman spectroscopy imaging. Appl. Phys. Exp. 2015, 8, 032401. [Google Scholar] [CrossRef]
- Rodriguez, R.D.; Sheremet, E.; Deckert-Gaudig, T.; Chaneac, C.; Hietschold, M.; Deckert, V.; Zahnab, D.R.T. Surface- and tip-enhanced Raman spectroscopy reveals spin-waves in iron oxide nanoparticles. Nanoscale 2015, 7, 9545–9551. [Google Scholar] [CrossRef] [PubMed]
- Sanders, A.; Zhang, L.; Bowman, R.W.; Herrmann, L.O.; Baumberg, J.J. Facile fabrication of spherical nanoparticle-tipped AFM probes for plasmonic applications. Part. Part. Syst. Char. 2015, 32, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.-K.; Huang, T.-X.; Zeng, Z.-C.; Li, M.-H.; Wang, X.; Yang, F.-Z.; Ren, B. Rational fabrication of a gold-coated AFM TERS tip by pulsed electrodeposition. Nanoscale 2015, 7, 18225–18231. [Google Scholar] [CrossRef] [PubMed]
- Segalman, R.A. Patterning with block copolymer thin films. Mater. Sci. Eng. R Rep. 2005, 48, 191–226. [Google Scholar] [CrossRef]
- Park, C.; Yoon, J.; Thomas, E.L. Enabling nanotechnology with self assembled block copolymer patterns. Polymer 2003, 44, 6275–6760. [Google Scholar] [CrossRef]
- Taheri, S.M.; Fischer, S.; Förster, S. Routes to nanoparticle-polymer superlattices. Polymer 2011, 3, 662–673. [Google Scholar] [CrossRef]
- Hamley, I.W. Nanostructure fabrication using block copolymers. Nanotechnology 2003, 14, R39. [Google Scholar] [CrossRef]
- Park, S.; Wang, J.Y.; Kim, B.; Russell, T.P. From nanorings to nanodots by patterning with block copolymers. Nano Lett. 2008, 8, 1667–1672. [Google Scholar] [CrossRef]
- Hamley, I.W. The Physics of Block Copolymers, 1st ed.; Oxford University Press: New York, NY, USA, 1998. [Google Scholar]
- Park, S.; Kim, B.; Wang, J.-Y.; Russell, T.P. Fabrication of highly ordered silicon oxide dots and stripes from block copolymer thin films. Adv. Mat. 2008, 20, 681–685. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, Y.; Alvarez-Puebla, R.A.; Buriak, J.M.; Fenniri, H. Synthesis and SERS properties of nanocrystalline gold octahedra generated from thermal decomposition of HAuCl4 in block copolymers. Adv. Mater. 2006, 18, 3233–3237. [Google Scholar] [CrossRef]
- Lu, J.; Chamberlin, D.; Rider, D.A.; Liu, M.; Manners, I.; Russell, T.P. Using a ferrocenylsilane-based block copolymer as a template to produce nanotextured Ag surfaces: Uniformly enhanced surface enhanced Raman scattering active substrates. Nanotechnology 2006, 17, 5792. [Google Scholar] [CrossRef]
- Wang, Y.; Becker, M.; Wang, L.; Liu, J.; Scholz, R.; Peng, J.; Gösele, U.; Christiansen, S.; Kim, D.H.; Steinhart, M. Nanostructured gold films for SERS by block copolymer-templated galvanic displacement reactions. Nano Lett. 2009, 9, 2384–2389. [Google Scholar] [CrossRef]
- Zito, G.; Rusciano, G.; Pesce, G.; Dochshanov, A.; Sasso, A. Surface-enhanced Raman imaging of cell membrane by a highly homogeneous and isotropic silver nanostructure. Nanoscale 2015, 7, 8593–8606. [Google Scholar] [CrossRef]
- De Rosa, C.; Auriemma, F.; Diletto, C.; Di Girolamo, R.; Malafronte, A.; Morvillo, P.; Zito, G.; Rusciano, G.; Pesce, G.; Sasso, A. Toward hyperuniform disordered plasmonic nanostructures for reproducible surface-enhanced Raman spectroscopy. Phys. Chem. Chem. Phys. 2015, 17, 8061–8069. [Google Scholar] [CrossRef]
- Rusciano, G.; Sasso, E.; Capaccio, A.; Zambrano, N.; Sasso, A. Revealing membrane alteration in cells over expressing CA IX and eGFR by surface-enhanced Raman scattering. Sci. Rep. 2019, 9, 1832. [Google Scholar] [CrossRef] [PubMed]
- Zito, G.; Rusciano, G.; Vecchione, A.; Pesce, G.; Di Girolamo, R.; Malafronte, A.; Sasso, A. Nanometal skin of plasmonic heterostructures for highly efficient near-field scattering probes. Sci. Rep. 2016, 9, 31113. [Google Scholar] [CrossRef]
- Thompson, C.V. Solid-state dewetting of thin films. Annu. Rev. Mater. Res. 2012, 42, 399–434. [Google Scholar] [CrossRef]
- Geddes, C.D. Reviews in Plasmonics 2017, 1st ed.; Springer: Cham, Switzerland, 2019. [Google Scholar]
- Milewska, A.; Zivanovic, V.; Merk, V.; Arnalds, U.B.; Sigurjónsson, Ó.E.; Kneipp, J.; Leosson, K. Gold nanoisland substrates for SERS characterization of cultured cells. Biomed. Opt. Exp. 2019, 10, 6172–6188. [Google Scholar] [CrossRef]
- Bhalla, N.; Jain, A.; Lee, Y.; Shen, A.Q.; Lee, D. Dewetting metal nanofilms-effect of substrate on refractive index sensitivity of nanoplasmonic gold. Nanomaterials 2019, 9, 1530. [Google Scholar] [CrossRef]
- Pandey, P.; Kunwar, S.; Lee, J. Solid state dewetting of Ag/Pt bilayers for the stronger localized surface plasmon resonance (LSPR) properties: The dynamic control of surface morphology and elemental composition of AgPt and Pt nanostructures by the auxiliary Ag layer. J. Alloys Compd. 2020, 813, 152193. [Google Scholar] [CrossRef]
- El Mel, A.-A.; Stephant, N.; Hamon, J.; Thiry, D.; Chauvin, A.; Chettab, M.; Gautron, E.; Konstantinidis, A.; Graniera, A.; Tessiera, P.-Y. Creating nanoporosity in silver nanocolumns by direct exposure to radio-frequency air plasma. Nanoscale 2016, 8, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Rooij, A. The Oxidation of Silver by Atomic Oxygen. ESA J. 1989, 13, 363–382. [Google Scholar]
- Smigelskas, A.; Kirkendall, E. Zinc Diffusion in Alpha Brass. Trans. AIME 1947, 171, 130142. [Google Scholar]
- Nakamura, R.; Nakajima, H. Preparation of Hollow Nanostructures via Various Methods and Their Applications. In Nanowires-Implementations and Applications; Hashim, A., Ed.; Intech Open: London, UK, 2011. [Google Scholar]
- El Mel, A.-A.; Stephant, B.; Molina-Luna, L.; Gautron, E.; Haik, Y.; Tabet, N.; Tessier†, P.-Y.; Gautier, R. Kirkendall effect vs corrosion of silver nanocrystals by atomic oxygen: From solid metal silver to nanoporous silver oxide. J. Phys. Chem. C 2017, 121, 19497–19504. [Google Scholar] [CrossRef]
- El Mel, A.-A.; Nakamura, R.; Bittencourt, C. The Kirkendall effect and nanoscience: Hollow nanospheres and nanotubes. Beilstein J. Nanotechnol. 2015, 6, 1348–1361. [Google Scholar] [CrossRef] [PubMed]
- Ji, D.; Fan, L.; Tao, L.; Sun, Y.; Li, M.; Yang, G.; Tran, T.Q.; Ramakrishna, S.; Guo, S. The Kirkendall effect for engineering oxygen vacancy of hollow Co3O4 nanoparticles toward high-performance portable zinc-air batteries. Angew. Chem. 2019, 131, 13978–13982. [Google Scholar] [CrossRef]
- Tu, K.N.; Gösele, U. Hollow nanostructures based on the Kirkendall effect: Design and stability considerations. Appl. Phys. Lett. 2005, 86, 093111. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, K.; Yuan, S.; Li, G.; Ma, Y.; Wang, Q. Solid-state dewetting in polycrystalline co films on native oxide Si(100) by Kirkendall effects. J. Phys. Chem. C 2019, 123, 19572–19578. [Google Scholar] [CrossRef]
- Capaccio, A.; Sasso, A.; Tarallo, O.; Rusciano, G. Coral-like plasmonic probes for tip-enhanced Raman spectroscopy. Nanoscale 2020, 12, 24376–24384. [Google Scholar] [CrossRef]
- Kumar, N.; Spencer, S.J.; Imbraguglio, D.; Rossi, A.M.; Wain, A.J.; Weckhuysenb, B.M.; Roy, D. Extending the plasmonic lifetime of tip-enhanced Raman spectroscopy probes. Phys. Chem. Chem. Phys. 2016, 18, 13710–13716. [Google Scholar] [CrossRef] [PubMed]
- Capaccio, A.; Sasso, A.; Rusciano, G. A simple and reliable approach for the fabrication of nanoporous silver patterns for surface-enhanced Raman spectroscopy applications. Sci. Rep. 2021, 1, 22295. [Google Scholar] [CrossRef] [PubMed]
- La Verde, G.; Sasso, A.; Rusciano, G.; Capaccio, A.; Fusco, S.; Mayol, L.; Biondi, M.; Silvestri, T.; Netti, P.A.; La Commara, M.; et al. Characterization of hyaluronic acid-coated PLGA nanoparticles by surface-enhanced Raman spectroscopy. Int. J. Mol. Sci. 2022, 24, 601. [Google Scholar] [CrossRef] [PubMed]
- Rusciano, G.; De Luca, A.C.; D’Alessio, A.; Minutolo, P.; Pesce, G.; Sasso, A. Surface-enhanced Raman scattering study of nano-sized organic carbon particles produced in combustion processes. Carbon 2008, 46, 335–341. [Google Scholar] [CrossRef]
- Capaccio, A.; Sasso, A.; Rusciano, G. Feasibility of SERS-active porous Ag substrates for the effective detection of pyrene in water sensors. Sensors 2022, 22, 2764. [Google Scholar] [CrossRef]
- Le Ru, E.C.; Meyer, S.A.; Artur, C.; Etchegoin, P.G.; Grand, J.; Lang, P.; Maurel, F. Experimental demonstration of surface selection rules for SERS on flat metallic surfaces. Chem. Commun. 2011, 47, 3903–3905. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sasso, A.; Capaccio, A.; Rusciano, G. Exploring Reliable and Efficient Plasmonic Nanopatterning for Surface- and Tip-Enhanced Raman Spectroscopies. Int. J. Mol. Sci. 2023, 24, 16164. https://doi.org/10.3390/ijms242216164
Sasso A, Capaccio A, Rusciano G. Exploring Reliable and Efficient Plasmonic Nanopatterning for Surface- and Tip-Enhanced Raman Spectroscopies. International Journal of Molecular Sciences. 2023; 24(22):16164. https://doi.org/10.3390/ijms242216164
Chicago/Turabian StyleSasso, Antonio, Angela Capaccio, and Giulia Rusciano. 2023. "Exploring Reliable and Efficient Plasmonic Nanopatterning for Surface- and Tip-Enhanced Raman Spectroscopies" International Journal of Molecular Sciences 24, no. 22: 16164. https://doi.org/10.3390/ijms242216164
APA StyleSasso, A., Capaccio, A., & Rusciano, G. (2023). Exploring Reliable and Efficient Plasmonic Nanopatterning for Surface- and Tip-Enhanced Raman Spectroscopies. International Journal of Molecular Sciences, 24(22), 16164. https://doi.org/10.3390/ijms242216164






