Inactivation of Group 1B Phospholipase A2 Enhances Disease Recovery and Reduces Experimental Colitis in Mice
Abstract
1. Introduction
2. Results
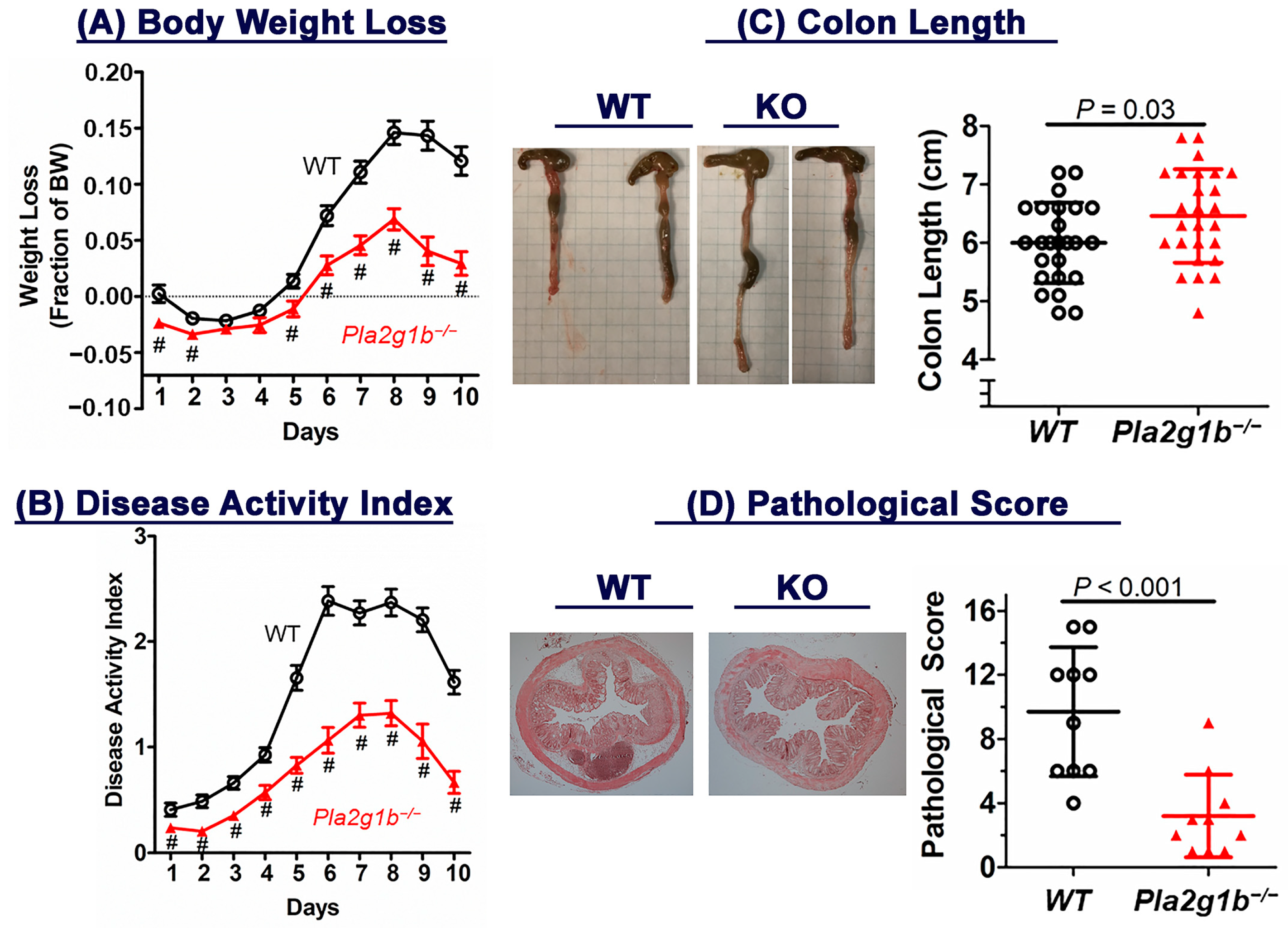
2.1. Genetic Inactivation of PLA2G1B Protects against DSS-Induced Colitis
2.2. Resistance of Pla2g1b−/− Mice to DSS-Induced Colitis Is Unrelated to Lysophospholipid Content in Colon
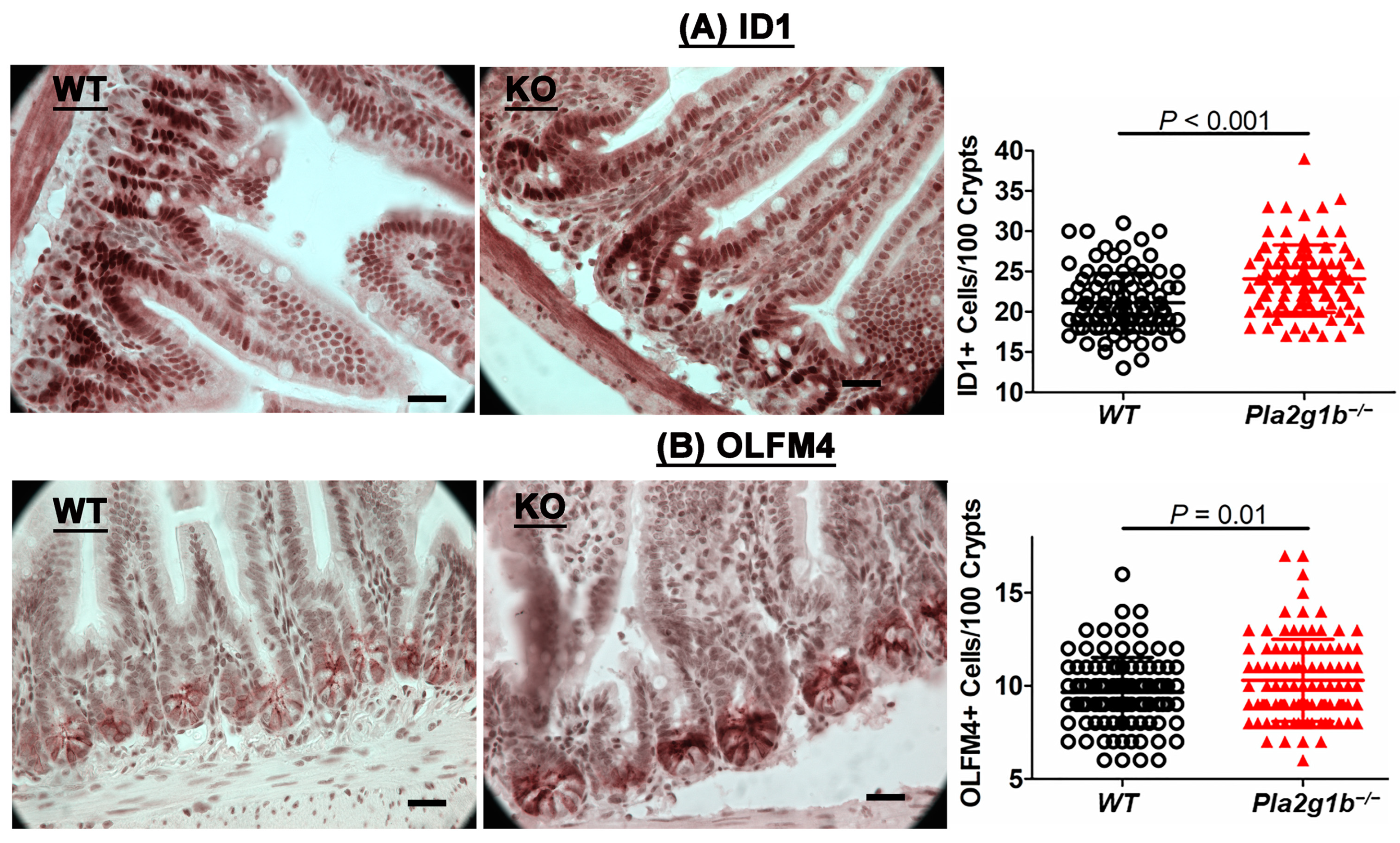
2.3. PLA2G1B Inactivation Increases Intestinal Stem Cells
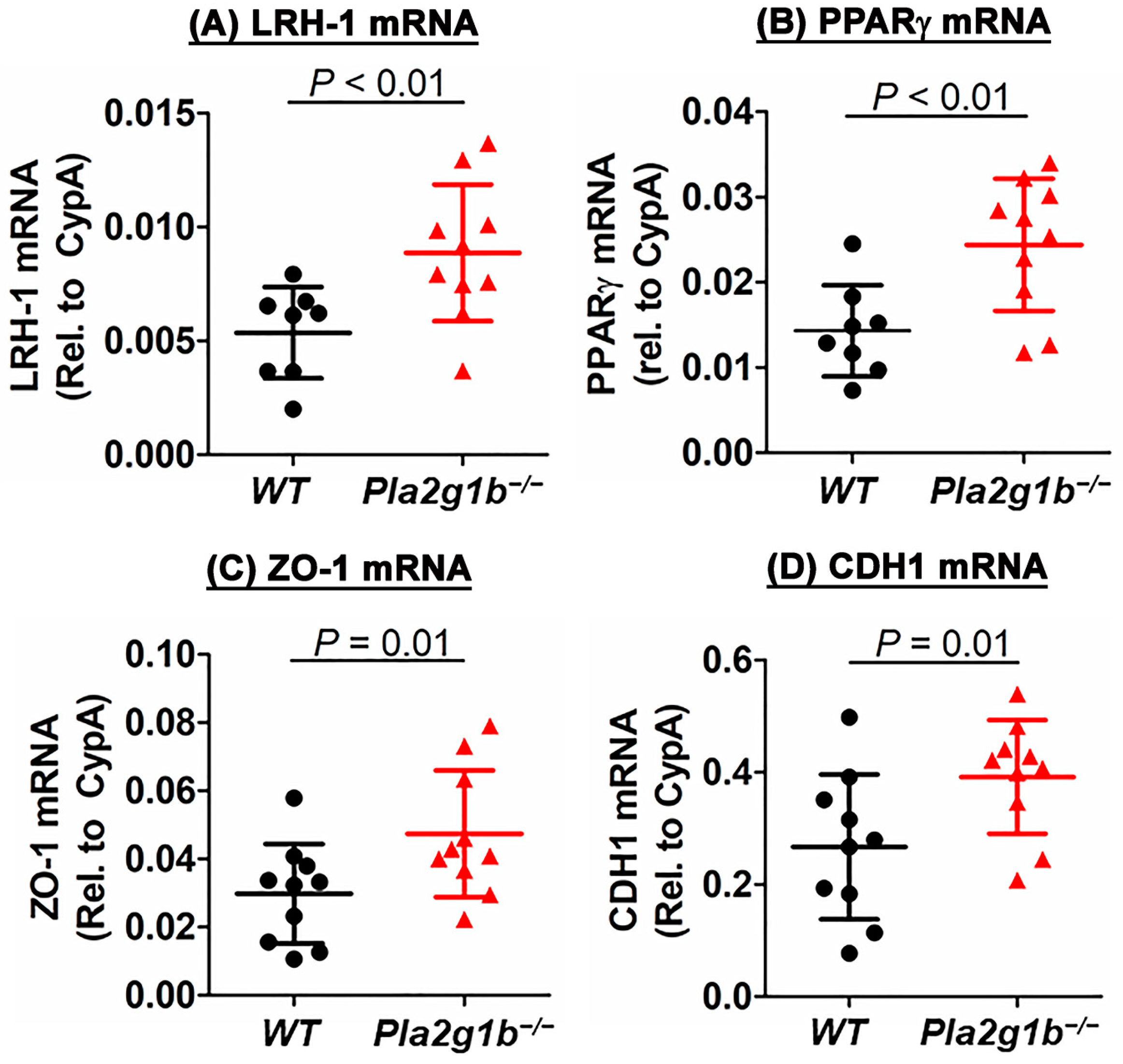
2.4. PLA2G1B Inactivation Increases Expression of Genes That Promote Epithelial Repair
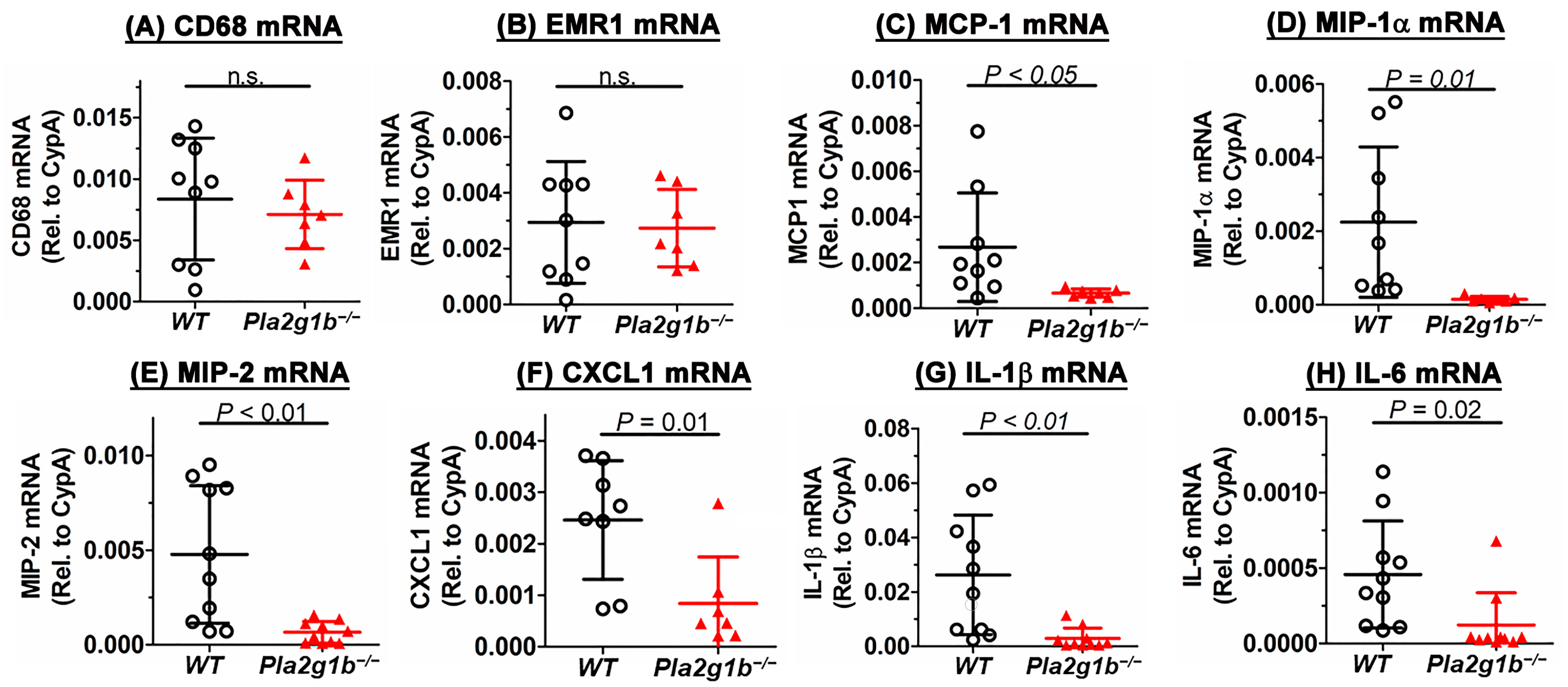
2.5. PLA2G1B Inactivation Reduces DSS-Induced Inflammation
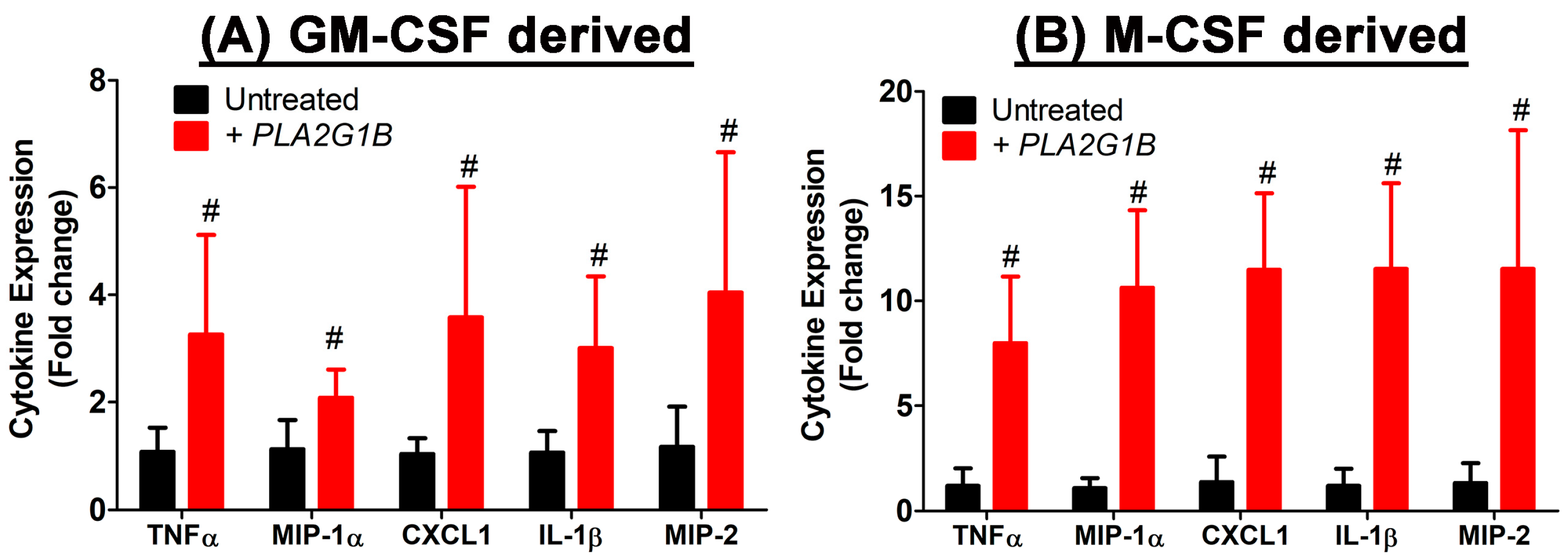
2.6. PLA2G1B Directly Activates Inflammatory Cytokine Production in Myeloid Cells
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. DSS-Induced Colitis
4.3. Colon Histology
4.4. Colonic Lysophosphatidylcholine (LPC) and Lysophosphatidic Acid (LPA) Measurements
4.5. Intestinal Stem and Progenitor Cell Identification
4.6. RNA Expression in Colon and Bone Marrow-Derived Myeloid Cells
4.7. Plasma Cytokine Measurement
4.8. PLA2G1B-Induced Cytokine Expression In Vitro
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Scaldaferri, F.; Fiocchi, C. Inflammatory bowel disease: Progress and current concepts of etiopathogenesis. J. Dig. Dis. 2007, 8, 171–178. [Google Scholar] [CrossRef]
- Mentella, M.C.; Scaldaferri, F.; Pizzoferrato, M.; Gasbarrini, A.; Miggiano, G.A.D. Nutrition, IBD, and gut microbiota: A review. Nutrients 2020, 12, 944. [Google Scholar] [CrossRef]
- Sakamoto, N.; Kono, S.; Wakai, K.; Fukuda, Y.; Satomi, M.; Shimoyama, T.; Inaba, Y.; Miyake, Y.; Sasaki, S.; Okamoto, K.; et al. Dietary risk factors for inflammatory bowel disease: A multicenter case-control study in Japan. Inflamm. Bowel Dis. 2005, 11, 154–163. [Google Scholar] [CrossRef]
- Gearry, R.B.; Richardson, A.K.; Frampton, C.M.; Dodgshun, A.J.; Barclay, M.L. Population based cases control study of inflammatory bowel disease risk factors. J. Gastroenterol. Hepatol. 2010, 25, 325–333. [Google Scholar] [CrossRef]
- Scoville, E.A.; Allaman, M.M.; Brown, C.T.; Motley, A.K.; Horst, S.N.; Williams, C.S.; Koyama, T.; Zhao, Z.; Adams, D.W.; Beaulieu, D.B.; et al. Alterations in lipid, amino acid, and energy metabolism distinguish Crohn’s disease from ulcerative colitis and control subjects by serum metabolomic profiling. Metabolomics 2018, 14, 17. [Google Scholar] [CrossRef]
- Diab, J.; Hansen, T.; Goll, R.; Stenlund, H.; Ahnlund, M.; Jensen, E.; Moritz, T.; Florholmen, J.; Forsdahl, G. Lipidomics in ulcerative colitis reveal alteration in mucosal lipid composition associated with the disease state. J. Inflamm. Bowel Dis. 2019, 25, 1780–1787. [Google Scholar] [CrossRef]
- Braun, A.; Treede, I.; Gotthardt, D.; Tietje, A.; Zahn, A.; Ruhwald, R.; Schoenfeld, U.; Welsch, T.; Kienle, P.; Erben, G.; et al. Alterations of phospholipid concentration and species composition of the intestinal mucus barrier in ulcerative colitis: A clue to pathogenesis. Inflamm. Bowel Dis. 2009, 15, 1705–1720. [Google Scholar] [CrossRef]
- Dennis, E.A.; Cao, J.; Hsu, Y.-H.; Magrioti, V.; Kokotos, G. Phospholipase A2 enzymes: Physical structure, biological function, disease implication, chemical inhibition, and therapeutic intervention. Chem. Rev. 2011, 111, 6130–6185. [Google Scholar] [CrossRef]
- Wu, W.-H.; Li, W.-X.; Huang, C.-H. Phospholipase A2 a nonnegligible enzyme superfamily in gastrointestinal diseases. Biochimie 2022, 194, 79–95. [Google Scholar] [CrossRef]
- Sonnweber, T.; Pizzini, A.; Nairz, M.; Weiss, G.; Tancevski, I. Arachidonic acid metabolites in cardiovascular and metabolic diseases. Int. J. Mol. Sci. 2018, 19, 3285. [Google Scholar] [CrossRef]
- Jiao, L.; Inhoffen, J.; Gan-Schreier, H.; Tuma-Kellner, S.; Stremmel, W.; Sun, Z.; Chamulitrat, W. Deficiency of Group VIA phospholipase A2 (iPLA2b) renders susceptibility for chemical-induced colitis. Dig. Dis. Sci. 2015, 60, 3590–3602. [Google Scholar] [CrossRef] [PubMed]
- Rosengarten, M.; Hadad, N.; Solomonov, Y.; Lamprecht, S.; Levy, R. Cytosolic phospholipase A2a has a crucial role in the pathogenesis of DSS-induced colitis in mice. Eur. J. Immunol. 2016, 46, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Zhai, L.; Huang, T.; Xiao, H.-T.; Wu, P.-G.; Lin, C.-Y.; Ning, Z.-W.; Zhao, L.; Kwan, H.Y.A.; Hu, X.-J.; Wong, H.L.X.; et al. Berberine suppresses colonic inflammation in dextran sulfate sodium-induced murine colitis through inhibition of cytosolic phospholipase A2 activity. Front. Pharmacol. 2020, 11, 576496. [Google Scholar] [CrossRef] [PubMed]
- Murase, R.; Taketomi, Y.; Miki, Y.; Nishito, Y.; Saito, M.; Fukami, K.; Yamamoto, K.; Murakami, M. Group III phospholipase A2 promotes colitis and colorectal cancer. Sci. Rep. 2017, 7, 12261. [Google Scholar] [CrossRef]
- Murase, R.; Sato, H.; Yamamoto, K.; Ushida, A.; Nishito, Y.; Ikeda, K.; Kobayashi, T.; Yamamoto, T.; Taketomi, Y.; Murakami, M. Group X secreted phospholipase A2 release w3 polyunsaturated fatty acids, suppresses colitis, and promotes sperm fertility. J. Biol. Chem. 2016, 291, 6895–6911. [Google Scholar] [CrossRef]
- Schewe, M.; Franken, P.F.; Sacchetti, A.; Schmitt, M.; Joosten, R.; Bottcher, R.; van Royen, M.E.; Jeammet, L.; Payre, C.; Scott, P.M.; et al. Secreted phospholipase A2 are intestinal stem cell niche factors with distinct roles in homeostasis, inflammation, and cancer. Cell Stem Cell 2016, 19, 38–51. [Google Scholar] [CrossRef]
- Woodruff, T.M.; Arumugam, T.V.; Shiels, I.A.; Newman, M.L.; Ross, P.A.; Reid, R.C.; Fairlie, D.P.; Taylor, S.M. A potent and selective inhibitor of group IIa secretory phospholipase A2 protects rats from TNBS-induced colitis. Int. Immunopharmacol. 2005, 5, 883–892. [Google Scholar] [CrossRef]
- Labonté, E.D.; Kirby, R.J.; Schildmeyer, N.M.; Cannon, A.M.; Huggins, K.W.; Hui, D.Y. Group 1B phospholipase A2-mediated lysophospholipid absorption directly contributes to postprandial hyperglycemia. Diabetes 2006, 55, 935–941. [Google Scholar] [CrossRef]
- Labonté, E.D.; Pfluger, P.T.; Cash, J.G.; Kuhel, D.G.; Roja, J.C.; Magness, D.P.; Jandacek, R.J.; Tschop, M.H.; Hui, D.Y. Postprandial lysophospholipid suppresses hepatic fatty acid oxidation: The molecular link between group 1B phospholipase A2 and diet-induced obesity. FASEB J. 2010, 24, 2516–2524. [Google Scholar] [CrossRef]
- Hollie, N.I.; Cash, J.G.; Matlib, M.A.; Wortman, M.; Basford, J.E.; Abplanalp, W.; Hui, D.Y. Micromolar changes in lysophosphatidylcholine concentration cause minor effects on mitochondrial permeability but major alterations in function. Biochim. Biophys. Acta 2014, 1841, 888–895. [Google Scholar] [CrossRef]
- Hollie, N.I.; Hui, D.Y. Group 1B phospholipase A2 deficiency protects against diet-induced hyperlipidemia in mice. J. Lipid Res. 2011, 52, 2005–2011. [Google Scholar] [CrossRef] [PubMed]
- Hollie, N.I.; Konaniah, E.S.; Goodin, C.; Hui, D.Y. Group 1B phospholipase A2 inactivation suppresses atherosclerosis and metabolic diseases in LDL receptor-deficient mice. Atherosclerosis 2014, 234, 377–380. [Google Scholar] [CrossRef] [PubMed]
- Hui, D.Y. Group 1B phospholipase A2 in metabolic and inflammatory disease modulation. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1846, 784–788. [Google Scholar] [CrossRef] [PubMed]
- Entwistle, L.J.; Pelly, V.S.; Coomes, S.M.; Kannan, Y.; Perez-Lloret, J.; Czieso, S.; Silva dos Santos, M.; MacRae, J.I.; Collinson, L.; Sesay, A.; et al. Epithelial-cell-derived phospholipase A2 group 1b is an endogenous anthelmintic. Cell Host Microbe 2017, 22, 484–493. [Google Scholar] [CrossRef]
- Okayasu, I.; Hatakeyama, S.; Yamada, M.; Ohkusa, T.; Inagaki, Y.; Nakaya, R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology 1990, 98, 694–701. [Google Scholar] [CrossRef]
- Bayrer, J.R.; Wang, H.; Nattiv, R.; Suzawa, M.; Escusa, H.S.; Fletterick, R.J.; Klein, O.D.; Moore, D.D.; Ingraham, H.A. LRH-1 mitigates intestinal inflammatory disease by maintaining epithelial homeostasis and cell survival. Nat. Commun. 2018, 9, 4055. [Google Scholar] [CrossRef]
- Coste, A.; Dubuquoy, L.; Barnouin, R.; Annicotte, J.-S.; Magnier, B.; Notti, M.; Corazza, N.; Antal, M.C.; Metzger, D.; Desreumaux, P.; et al. LRH-1-mediated glucocorticoid synthesis in enterocytes protects against inflammatory bowel disease. Proc. Natl. Acad. Sci. USA 2007, 104, 13098–13103. [Google Scholar] [CrossRef]
- Bouguen, G.; Langlois, A.; Djouina, M.; Branche, J.; Koriche, D.; Dewaeles, E.; Mongy, A.; Auwerx, J.; Columbel, J.-F.; Desreumaux, P.; et al. Intestinal steroidogenesis controls PPARg expression in the colon and is impaired during ulcerative colitis. Gut 2015, 64, 901–910. [Google Scholar] [CrossRef]
- Adachi, M.; Kurotani, R.; Morimura, K.; Shah, Y.M.; Sanford, M.; Madison, B.B.; Gumucio, D.L.; Marin, H.E.; Peters, J.M.; Young, H.A.; et al. Peroxisome proliferator activated receptor g in colonic epithelial cells protects against experimental inflammatory bowel disease. Gut 2006, 55, 1104–1113. [Google Scholar] [CrossRef]
- Su, C.G.; Wen, X.; Bailey, S.T.; Jiang, W.; Rangwala, S.M.; Keilbaugh, S.A.; Flanigan, A.; Murthy, S.; Lazar, M.A.; Wu, G.D. A novel therapy for colitis utilizing PPAR-g ligands to inhibit the epithelial inflammatory response. J. Clin. Investig. 1999, 104, 383–389. [Google Scholar] [CrossRef]
- Fang, J.; Wang, H.D.; Xue, Z.; Cheng, Y.; Zhang, X. PPARg: The central mucus barrier coordinator in ulcerative colitis. Inflamm. Bowel Dis. 2021, 27, 732–741. [Google Scholar] [CrossRef] [PubMed]
- Kuo, W.-T.; Odenwald, M.A.; Turner, J.R.; Zuo, L. Tight junction proteins occludin and ZO-1 as regulators of epithelial proliferation and survival. Ann. N. Y. Acad. Sci. 2022, 1514, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Schnoor, M. E-cadherin is important for the maintenance of intestinal epithelial homeostasis under basal and inflammatory conditions. Dig. Dis. Sci. 2015, 60, 816–818. [Google Scholar] [CrossRef] [PubMed]
- Frantz, A.L.; Bruno, M.E.C.; Rogier, E.W.; Tuna, H.; Cohen, D.A.; Bondada, S.; Chelvarajan, R.L.; Brandon, J.A.; Jennings, C.D.; Kaetzel, C.S. Multifactorial patterns of gene expression in colonic epithelial cells predict disease phenotypes in experimental colitis. Inflamm. Bowel Dis. 2012, 18, 2138–2148. [Google Scholar] [CrossRef][Green Version]
- Tomita, Y.; Jyoyama, H.; Kobayashi, M.; Kuwabara, K.; Furue, S.; Ueno, M.; Yamada, K.; Ono, T.; Teshirogi, T.; Nomura, K.; et al. Role of group IIA phospholipase A2 in rat colitis induced by dextran sulfate sodium. Eur. J. Pharmacol. 2003, 472, 147–158. [Google Scholar] [CrossRef]
- Mournier, C.M.; Wendum, D.; Greenspan, E.; Flejou, J.-F.; Rosenberg, D.W.; Lambeau, G. Distinct expression pattern of the full set of secreted phospholipase A2 in human colorectal adenocarcinomas: sPLA2-III as a biomarker candidate. Br. J. Cancer 2008, 98, 587–595. [Google Scholar] [CrossRef]
- Barker, N. Adult intestinal stem cells: Critical drivers of epithelial homeostasis and regeneration. Nat. Rev. Mol. Cell Biol. 2014, 15, 19–33. [Google Scholar] [CrossRef]
- Sorrentino, G.; Perino, A.; Yildiz, E.; El Alam, G.; Bou Sleiman, M.; Gioiello, A.; Pellicciari, R.; Schoonjans, K. Bile acids signal via TGR5 to activate intestinal stem cells and epithelial regeneration. Gastroenterology 2020, 159, 956–968. [Google Scholar] [CrossRef]
- Borgström, B. Importance of phospholipids, pancreatic phospholipase A2 and fatty acid for the digestion of dietary fat. In vitro experiments with the porcine enzymes. Gastroenterology 1980, 78, 954–962. [Google Scholar] [CrossRef]
- Petruzzelli, M.; Piccinin, E.; Pinto, C.; Peres, C.; Bellafante, E.; Moschetta, A. Biliary phospholipids sustain enterocyte proliferation and intestinal tumor progression via nuclear receptor LRH1 in mice. Sci. Rep. 2016, 6, 39278. [Google Scholar] [CrossRef]
- Zerlotin, R.; Arconzo, M.; Piccinin, E.; Moschetta, A. Another one bites the gut: Nuclear receptor LRH-1 in intestinal regeneration and cancer. Cancers 2021, 13, 896. [Google Scholar] [CrossRef] [PubMed]
- Mackay, K.; Starr, J.R.; Lawn, R.M.; Ellsworth, J.L. Phosphatidylcholine hydrolysis is required for pancreatic cholesterol esterase- and phospholipase A2-facilitated cholesterol uptake into intestinal Caco-2 cells. J. Biol. Chem. 1997, 272, 13380–13389. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Rong, X.; Palladino, E.N.D.; Wang, J.; Fogelman, A.M.; Martin, M.G.; Alrefai, W.A.; Ford, D.A.; Tontonoz, P. Phospholipid remodeling and cholesterol availability regulate intestinal stemness and tumorigenesis. Cell Stem Cell 2018, 22, 206–220. [Google Scholar] [CrossRef] [PubMed]
- Castro-Dopico, T.; Fleming, A.; Dennison, T.W.; Ferdinand, J.R.; Harcourt, K.; Stewart, B.J.; Cader, Z.; Tuong, Z.K.; Jing, C.; Lok, L.S.C.; et al. GM-CSF calibrates macrophage defense and wound healing programs during intestinal infection and inflammation. Cell Rep. 2020, 32, 107857. [Google Scholar] [CrossRef]
- Granata, F.; Petraroli, A.; Biolard, E.; Bezzine, S.; Bollinger, J.G.; Del Vecchio, L.; Gelb, M.H.; Lambeau, G.; Marone, G.; Triggiani, M. Activation of cytokine production by secreted phospholipase A2 in human lung macrophages expressing the M type receptor. J. Immunol. 2005, 174, 464–474. [Google Scholar] [CrossRef]
- Cooper, H.S.; Murthy, S.N.S.; Shah, R.S.; Sedergran, D.J. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab. Investig. 1993, 69, 238–249. [Google Scholar]
- Cash, J.G.; Konaniah, E.S.; Hegde, N.; Kuhel, D.G.; Watanabe, M.; Romick-Rosendale, L.; Hui, D.Y. Therapeutic reduction of lysophospholipids in the digestive tract recapitulates the metabolic benefits of bariatric surgery and promotes diabetes remission. Mol. Metab. 2018, 16, 55–64. [Google Scholar] [CrossRef]


| Score | Weight Loss | Stool Consistency | Blood in Stool |
|---|---|---|---|
| 0 | None | Normal pellets | None |
| 1 | 1–5% | Slightly loose but shaped | Hemoccult positive |
| 2 | 5–10% | Loose pellets | Visible slight bleeding |
| 3 | 10–15% | Loose feces and no shape | Obvious bleeding, no adhesion around anus |
| 4 | >15% | Diarrhea | Gross bleeding, blood encrustation around anus |
| Histology Grade | Area | Immune Cell Infiltration | |||
|---|---|---|---|---|---|
| 0 | Normal morphology | ×1 | 0–25% | 0 | None |
| 1 | <1/3 crypt damage | ×2 | 26–50% | 1 | Crypt base |
| 2 | Loss of crypt | ×3 | 51–75% | 2 | Mucosa |
| 3 | Crypt and epithelium loss | ×4 | 76–100% | 3 | Submucosa |
| Name | Forward Primer | Reverse Primer |
|---|---|---|
| Cyclophilin | TCATGTGCCAGGGTGGTGAC | CCATTCAGTCTTGGCAGTGC |
| CD68 | TTTCTCCAGCTGTTCACCTTGA | CCCGAAGTGTCCCTTGTCA |
| EMR1 | TGTCTGACAATTGGGATCTGCCCT | ATACGTTCCGAGAGTGTTGTGGCA |
| MCP-1 | CTTCCTCCACCACCATGCA | CCAGCCGGCAACTGTGA |
| MIP-1α | TTTGAAACCAGCAGCCTTTGCTCC | TCAGGCATTCAGTTCCAGGTCAGT |
| MIP-2 | CCCTCAACGGAAGAACCAAA | AGGCACATCAGGTACGATCCA |
| CXCL1 | TGGCTGGGATTCACCTCAAGAACA | TGTGGCTATGACTTCGGTTTGGGT |
| IL-1β | CTACAGGCTCCGAGATGAACAAC | TCCATTGAGGTGGAGAGCTTTC |
| IL-6 | CCGGGAACGAAAGAGAAGCT | GCGCTTGTGGAGAAGGAGTT |
| TNFα | ATCCGCGACGTGGAACTG | ACCGCCTGGAGTTCTGGAA |
| PPARγ | CTGCAGGCCCTGGAACTG | CGATCTGCCTGAGGTCTGTCA |
| LRH-1 | TCACATCTCCCATTAGCATGACA | GGAAAGTGACCATAGGGTTGGTAA |
| ZO-1 | CCTCCGTTGCCCTCACAGTA | GGGCGCCCTTGGAATG |
| CDH1 | TGTGGGTCAGGAAATCACATCTT | CCGATACGTGATCTTCTGATCCA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haller, A.M.; Wolfkiel, P.R.; Jaeschke, A.; Hui, D.Y. Inactivation of Group 1B Phospholipase A2 Enhances Disease Recovery and Reduces Experimental Colitis in Mice. Int. J. Mol. Sci. 2023, 24, 16155. https://doi.org/10.3390/ijms242216155
Haller AM, Wolfkiel PR, Jaeschke A, Hui DY. Inactivation of Group 1B Phospholipase A2 Enhances Disease Recovery and Reduces Experimental Colitis in Mice. International Journal of Molecular Sciences. 2023; 24(22):16155. https://doi.org/10.3390/ijms242216155
Chicago/Turabian StyleHaller, April M., Patrick R. Wolfkiel, Anja Jaeschke, and David Y. Hui. 2023. "Inactivation of Group 1B Phospholipase A2 Enhances Disease Recovery and Reduces Experimental Colitis in Mice" International Journal of Molecular Sciences 24, no. 22: 16155. https://doi.org/10.3390/ijms242216155
APA StyleHaller, A. M., Wolfkiel, P. R., Jaeschke, A., & Hui, D. Y. (2023). Inactivation of Group 1B Phospholipase A2 Enhances Disease Recovery and Reduces Experimental Colitis in Mice. International Journal of Molecular Sciences, 24(22), 16155. https://doi.org/10.3390/ijms242216155






