Gut Microbiota and Mitochondria: Health and Pathophysiological Aspects of Long COVID
Abstract
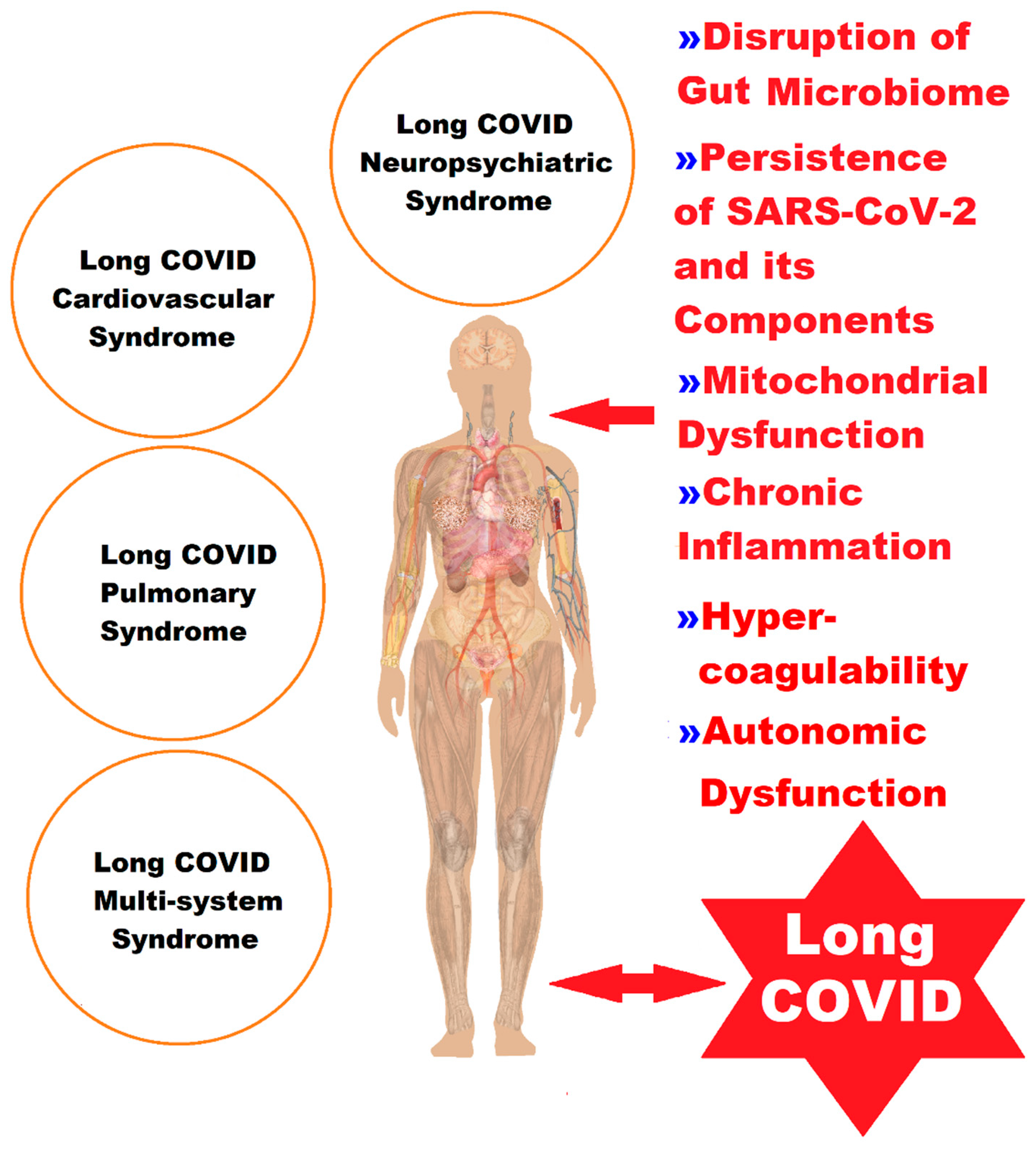
:1. Introduction
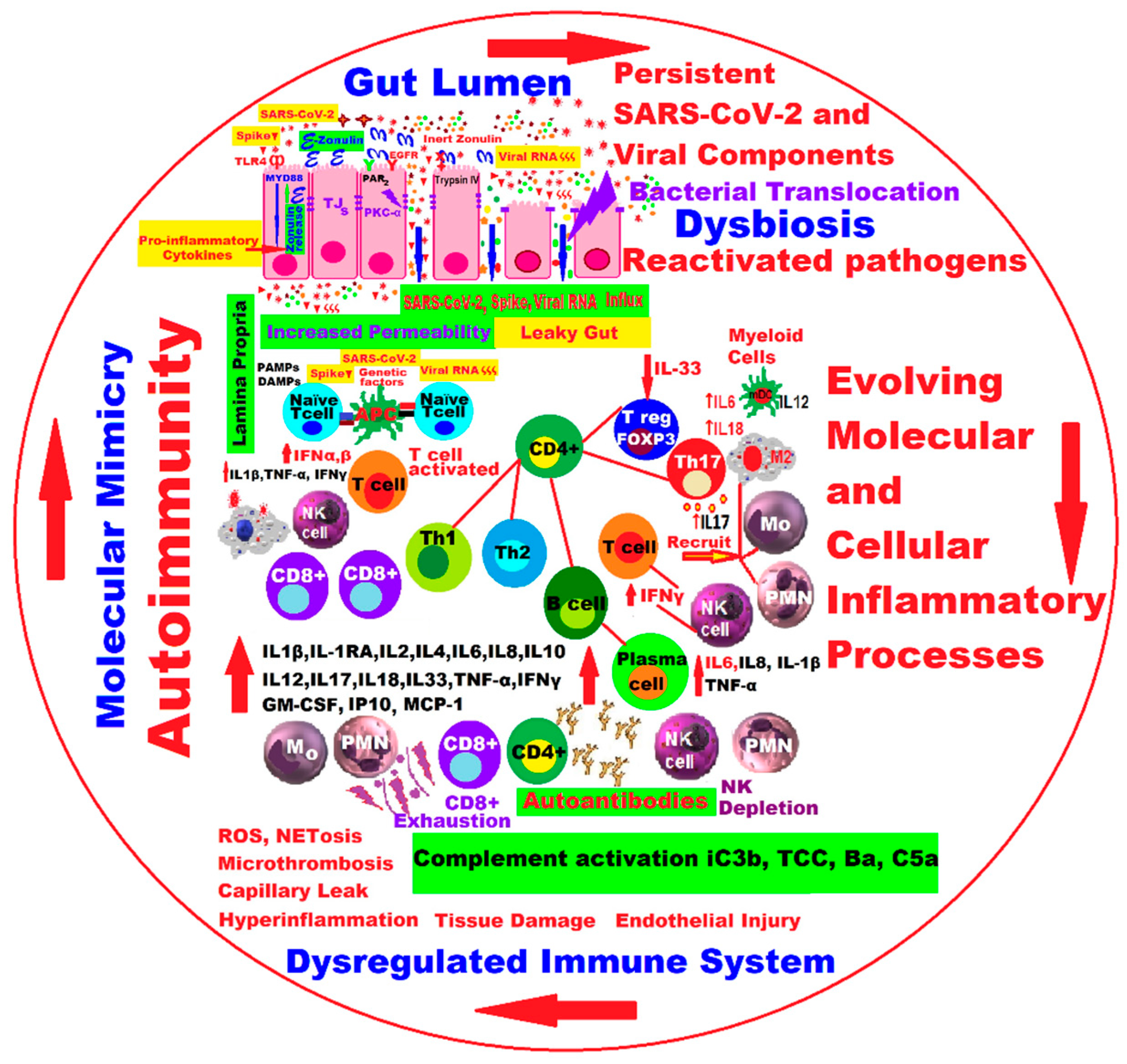
2. Molecular and Cellular Pathophysiological Mechanisms at Gut Level in Long COVID
3. Gut Microbiota Dynamics in Long COVID
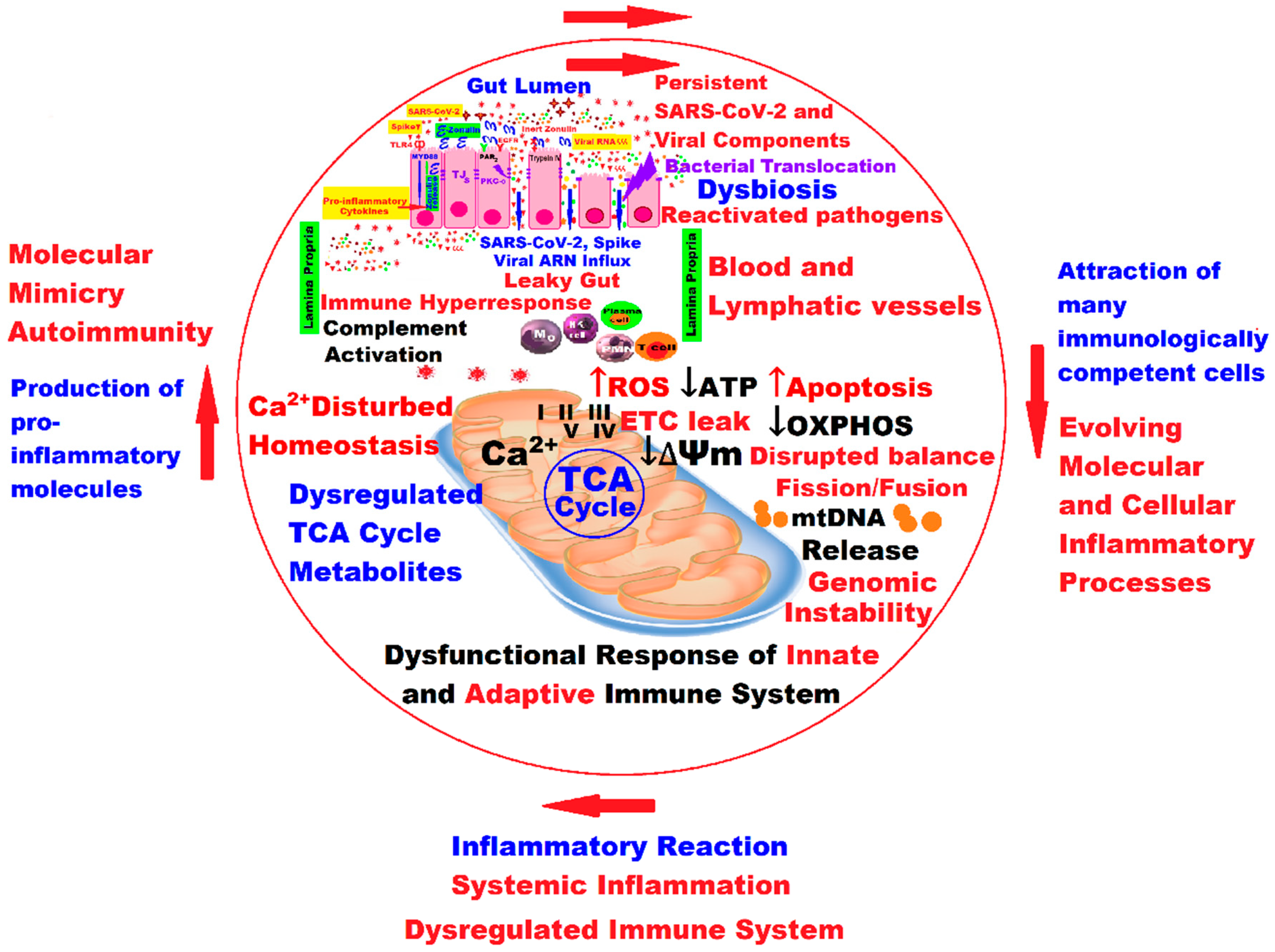
4. Mitochondria and Long COVID—The Hidden Molecular Connections and the Quantum Leap
5. Discussions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACE2 | angiotensin-converting enzyme 2 |
| ADEVs | astrocyte-derived EVs |
| AG-GIR | Autonomie Gérontologie Groupes Iso-Ressources |
| ATP | adenosine triphosphate |
| Ba | fragment of complement factor B that results from activation of the alternative pathway |
| B cell | B lymphocytes |
| BEVs | blood extracellular vesicles |
| C5a | complement component 5a; protein fragment released from the cleavage of complement component C5 by protease C5-convertase into C5a and C5b fragments |
| Ca2+ | calcium ions |
| CMV | cytomegalovirus |
| CCL18 | chemokine ligand 18 (PARC). |
| COVID-19 | coronavirus disease 2019 |
| CNS | central nervous system |
| CRM | rehabilitation center |
| CRP | C-reactive protein |
| CX3CR 3 | CX3C motif chemokine receptor 3 |
| DAMP | damage-associated molecular pattern |
| DNA | deoxyribonucleic acid |
| DNM1L | dynamin-1-like protein |
| ELISA | enzyme-linked immunosorbent assays |
| EV | extracellular vesicle |
| ESPA | European Spa Rehabilitation Association |
| ETC | electron transport chain |
| EWGSOP2 | European Working Group on Sarcopenia in Older People |
| FHWs | first-line healthcare workers |
| HBMECs | human brain microvascular endothelial cells |
| HCs | healthy controls |
| HIV | human immunodeficiency virus |
| HLA | human leukocyte antigen |
| HHV-6 | human herpesvirus 6 |
| HHV-7 | human herpesvirus 7 |
| HPBMC | human peripheral blood mononuclear cells |
| HUVEC | human umbilical vascular endothelial cells |
| GLIM | Global Leadership Initiative on Malnutrition |
| GSH | glutathione |
| iC3b | protein fragment part of the complement system, produced when complement factor I cleaves C3b |
| Ig | immunoglobulin |
| IgA | immunoglobulin A |
| IgG | immunoglobulin G |
| IgM | immunoglobulin M |
| ICAM | intercellular adhesion molecule |
| ICU | intensive care unit |
| IFN | interferon |
| IFN-α | interferon alpha |
| IP-10 | IFN-gamma-inducible protein 10 (IP-10, CXCL10) |
| IFN-γ | interferon-gamma |
| IL-1, IL-6, IL-8, IL-10, IL-12, IL-12p70, IL-15 | interleukin 1, 6, 8, 10, 12, 12p70, 15, 18, 27 |
| IL-1α | interleukin 1α |
| IL-1β | interleukin 1β |
| ITIM | immunoreceptor tyrosine-based inhibitory motif |
| LC | long COVID |
| LBP | lipopolysaccharide-binding protein |
| LTC | long-term care |
| MAVS | mitochondrial antiviral-signaling protein |
| ΔΨm | mitochondrial membrane potential |
| MPs | mitochondrial proteins |
| MFN2 | mitofusin-2 |
| Mo | monocyte |
| MR | mountain spa rehabilitation |
| ME/CFS | myalgic encephalomyelitis/chronic fatigue syndrome |
| mtDAMPs | damage-associated molecular patterns |
| mtDNA or mDNA | mitochondrial DNA |
| N | nucleoprotein |
| NAD+ | nicotinamide adenine dinucleotide |
| NK | natural killer |
| NDEVs | neuron-derived extracellular vesicles |
| NIHR | National Institute for Health Research |
| NIH | National Institutes of Health |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| NLRP3 | NLR family pyrin domain containing 3 |
| NMN | nicotinamide mononucleotide |
| NP | neuropsychiatric manifestations |
| NR | nicotinamide riboside |
| OXPHOS | oxidative phosphorylation or electron transport-linked phosphorylation |
| PAMP | pathogen-associated molecular pattern |
| PASC | post-acute sequelae of COVID-19 |
| PBMC | peripheral blood mononuclear cells |
| PCR | polymerase chain reaction |
| PC | post-COVID syndrome |
| PCC | post-COVID-19 condition |
| PD-1 | programmed cell death protein 1 |
| PD-L1 | programmed cell death protein ligand 1 |
| PD-L2 | programmed cell death protein ligand 2 |
| PELORA | PEnalized logistic regression analysis |
| PMN | polymorphonuclear |
| PINK1 | PTEN-induced kinase 1 |
| RPs | recovered patients |
| RBD | receptor binding domain |
| RT-qPCR | real-time quantitative reverse transcription PCR |
| ROS | reactive oxygen species |
| RBD | receptor binding domain |
| T cell | T lymphocytes |
| Tregs | regulatory T lymphocytes |
| RAS | renin–angiotensin system |
| RT-PCR | reverse transcriptase polymerase chain reaction |
| RNA | ribonucleic acid |
| S | spike protein |
| sRAGE | advanced glycation end product |
| SARS | severe acute respiratory syndrome |
| SAA | serum amyloid A |
| SARS-CoV-2 | severe acute respiratory syndrome coronavirus 2 |
| SCFAs | short-chain fatty acids |
| SHWs | second-line healthcare workers |
| SOD | superoxide dismutase |
| TBARS | thiobarbituric acid-reactive substances |
| TCA cycle | tricarboxylic acid cycle |
| TCC | terminal complement complex or membrane attack complex (MAC) |
| TIGIT | T-cell immunoreceptor with immunoglobulin and immunoreceptor tyrosine-based inhibitory motif domains |
| TEVs | total extracellular vesicles |
| Th1 | T-helper 1 or T-helper type 1 |
| Th2 | T-helper 2 or T-helper type 2 |
| Th17 | T-helper 17 or T-helper type 17 |
| TJs | tight junctions |
| TLR2 | toll-like receptor 2 |
| TLR4 | toll-like receptor 4 |
| TLR | toll-like receptors |
| TLR7 | toll-like receptor 7 |
| TMPRSS2 | transmembrane serine protease 2 |
| TMPRSS4 | transmembrane serine protease 4 |
| TNF-α | tumor necrosis factor alpha |
| TGF-β | transforming growth factor-β |
| TKI | tyrosine kinase inhibitors |
| US | United States |
| FDA | U.S. Food and Drug Administration |
| UK | United Kingdom |
| UKRI | UK Research and Innovation |
| VCAM-1 | vascular cell adhesion molecule 1 |
| WHO | World Health Organization |
| Increased | ↑ |
| Decreased | ↓ |
| Present | + |
| Absent/Missing | - |
References
- Gupta, A.; Madhavan, M.V.; Sehgal, K.; Nair, N.; Mahajan, S.; Sehrawat, T.S.; Bikdeli, B.; Ahluwalia, N.; Ausiello, J.C.; Wan, E.Y.; et al. Extrapulmonary manifestations of COVID-19. Nat. Med. 2020, 26, 1017–1032. [Google Scholar] [CrossRef]
- Callaway, E. Time to use the p-word? Coronavirus enters dangerous new phase. Nature 2020. [Google Scholar] [CrossRef]
- Machhi, J.; Herskovitz, J.; Senan, A.M.; Dutta, D.; Nath, B.; Oleynikov, M.D.; Blomberg, W.R.; Meigs, D.D.; Hasan, M.; Patel, M.; et al. The Natural History, Pathobiology, and Clinical Manifestations of SARS-CoV-2 Infections. J. Neuroimmune Pharmacol. 2020, 15, 359–386. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.F.; Yuan, S.; Kok, K.H.; To, K.K.; Chu, H.; Yang, J.; Xing, F.; Liu, J.; Yip, C.C.; Poon, R.W.; et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: A study of a family cluster. Lancet 2020, 395, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Cucinotta, D.; Vanelli, M. WHO Declares COVID-19 a Pandemic. Acta Biomed. 2020, 91, 157–160. [Google Scholar] [CrossRef] [PubMed]
- WHO Director-General’s Opening Remarks at the Media Briefing on COVID19-March 2020. Available online: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 (accessed on 5 August 2023).
- WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 5 August 2023).
- Cheng, E. China’s Population Drops for the First Time in Decades. Available online: https://www.cnbc.com/2023/01/17/chinas-population-drops-for-the-first-time-in-decades.html (accessed on 5 August 2023).
- Population Europe. Demography & COVID-19. Available online: https://population-europe.eu/network/news-network/demography-covid-19 (accessed on 5 August 2023).
- Post-COVID Food and Agricultural Situation. Prepared By: Alan Hallman and United States Department of Agriculture, China Staff. Approved By: Robert Hanson. Available online: https://apps.fas.usda.gov/newgainapi/api/Report/DownloadReportByFileName?fileName=Post-COVID%20Food%20and%20Agricultural%20Situation_Beijing_China%20-%20People%27s%20Republic%20of_CH2023-0022 (accessed on 5 August 2023).
- Africa Coronavirus Round-Up: Healthcare Systems in Crisis. Available online: https://country.eiu.com/article.aspx?articleid=719548655&Country=Equatorial%2520Guinea&topic=Economy_4 (accessed on 5 August 2023).
- Impact of COVID-19 on People’s Livelihoods, Their Health and OUR Food Systems. Available online: https://www.who.int/news/item/13-10-2020-impact-of-covid-19-on-people%27s-livelihoods-their-health-and-our-food-systems (accessed on 5 August 2023).
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Deng, L.; Zhang, L.; Cai, Y.; Cheung, C.W.; Xia, Z. Review of the Clinical Characteristics of Coronavirus Disease 2019 (COVID-19). J. Gen. Intern. Med. 2020, 35, 1545–1549. [Google Scholar] [CrossRef]
- Yue, H.; Bai, X.; Wang, J.; Yu, Q.; Liu, W.; Pu, J.; Wang, X.; Hu, J.; Xu, D.; Li, X.; et al. Clinical characteristics of coronavirus disease 2019 in Gansu province, China. Ann. Palliat. Med. 2020, 9, 1404–1412. [Google Scholar] [CrossRef]
- Wiersinga, W.J.; Rhodes, A.; Cheng, A.C.; Peacock, S.J.; Prescott, H.C. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. JAMA 2020, 324, 782–793. [Google Scholar] [CrossRef]
- Perego, E.; Callard, F.; Stras, L.; Melville-Jóhannesson, B.; Pope, R.; Alwan, A.N. Why the Patient-Made Term ‘Long COVID’ is needed [version 1; peer review: Awaiting peer review]. Wellcome Open Res. 2020, 5, 224. [Google Scholar] [CrossRef]
- Nabavi, N. Long COVID: How to define it and how to manage it. BMJ 2020, 370, m3489. [Google Scholar] [CrossRef]
- National Institute for Health Research’s (NIHR). Living with COVID: NIHR Publishes Dynamic Themed Review into ‘Ongoing COVID’ 15 October 2020. Available online: https://www.institutemh.org.uk/news/829-living-with-covid-nihr-publishes-dynamic-themed-review-into-ongoing-covid (accessed on 7 August 2023).
- Greenhalgh, T.; Knight, M.; A’Court, C.; Buxton, M.; Husain, L. Management of post-acute COVID-19 in primary care. BMJ 2020, 370, m3026. [Google Scholar] [CrossRef]
- Callard, F.; Perego, E. How and why patients made Long COVID. Soc. Sci. Med. 2021, 268, 113426. [Google Scholar] [CrossRef]
- Zimmermann, P.; Pittet, L.F.; Curtis, N. How Common is Long COVID in Children and Adolescents? Pediatr. Infect. Dis. J. 2021, 40, e482–e487. [Google Scholar] [CrossRef]
- Bonilla, H.; Quach, T.C.; Tiwari, A.; Bonilla, A.E.; Miglis, M.; Yang, P.C.; Eggert, L.E.; Sharifi, H.; Horomanski, A.; Subramanian, A.; et al. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome is common in post-acute sequelae of SARS-CoV-2 infection (PASC): Results from a post-COVID-19 multidisciplinary clinic. Front. Neurol. 2023, 14, 1090747. [Google Scholar] [CrossRef]
- Oliveira, C.R.; Jason, L.A.; Unutmaz, D.; Bateman, L.; Vernon, S.D. Improvement of Long COVID symptoms over one year. Front. Med. 2023, 9, 1065620. [Google Scholar] [CrossRef]
- A Clinical Case Definition of Post COVID-19 Condition by a Delphi Consensus, 6 October 2021. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-Post_COVID-19_condition-Clinical_case_definition-2021.1 (accessed on 7 August 2023).
- Fischer, A.; Zhang, L.; Elbéji, A.; Wilmes, P.; Oustric, P.; Staub, T.; Nazarov, P.V.; Ollert, M.; Fagherazzi, G. Long COVID Symptomatology After 12 Months and Its Impact on Quality of Life According to Initial Coronavirus Disease 2019 Disease Severity. Open Forum Infect. Dis. 2022, 9, ofac397. [Google Scholar] [CrossRef]
- Fischer, A.; Badier, N.; Zhang, L.; Elbéji, A.; Wilmes, P.; Oustric, P.; Benoy, C.; Ollert, M.; Fagherazzi, G. Long COVID Classification: Findings from a Clustering Analysis in the Predi-COVID Cohort Study. Int. J. Environ. Res. Public Health 2022, 19, 16018. [Google Scholar] [CrossRef]
- Deer, R.R.; Rock, M.A.; Vasilevsky, N.; Carmody, L.; Rando, H.; Anzalone, A.J.; Basson, M.D.; Bennett, T.D.; Bergquist, T.; Boudreau, E.A.; et al. Characterizing Long COVID: Deep Phenotype of a Complex Condition. EBioMedicine 2021, 74, 103722. [Google Scholar] [CrossRef]
- Kimmig, L.M.; Rako, Z.A.; Ziegler, S.; Richter, M.J.; GS, A.T.; Roller, F.; Grimminger, F.; Vadász, I.; Seeger, W.; Herold, S.; et al. Long-term comprehensive cardiopulmonary phenotyping of COVID-19. Respir. Res. 2022, 23, 263. [Google Scholar] [CrossRef]
- Reese, J.T.; Blau, H.; Casiraghi, E.; Bergquist, T.; Loomba, J.J.; Callahan, T.J.; Laraway, B.; Antonescu, C.; Coleman, B.; Gargano, M.; et al. Generalisable long COVID subtypes: Findings from the NIH N3C and RECOVER programmes. EBioMedicine 2023, 87, 104413. [Google Scholar] [CrossRef]
- Pfaff, E.R.; Madlock-Brown, C.; Baratta, J.M.; Bhatia, A.; Davis, H.; Girvin, A.; Hill, E.; Kelly, E.; Kostka, K.; Loomba, J.; et al. Coding long COVID: Characterizing a new disease through an ICD-10 lens. BMC Med. 2023, 21, 58. [Google Scholar] [CrossRef]
- Amenta, E.M.; Spallone, A.; Rodriguez-Barradas, M.C.; El Sahly, H.M.; Atmar, R.L.; Kulkarni, P.A. Postacute COVID-19: An Overview and Approach to Classification. Open Forum Infect. Dis. 2020, 7, ofaa509. [Google Scholar] [CrossRef]
- Poenaru, S.; Abdallah, S.J.; Corrales-Medina, V.; Cowan, J. COVID-19 and post-infectious myalgic encephalomyelitis/chronic fatigue syndrome: A narrative review. Ther. Adv. Infect. Dis. 2021, 8, 20499361211009385. [Google Scholar] [CrossRef]
- Renz-Polster, H.; Scheibenbogen, C. Post-COVID syndrome with fatigue and exercise intolerance: Myalgic encephalomyelitis/chronic fatigue syndrome. Inn. Med. (Heidelb. Ger.) 2022, 63, 830–839. [Google Scholar] [CrossRef]
- Caspersen, I.H.; Magnus, P.; Trogstad, L. Excess risk and clusters of symptoms after COVID-19 in a large Norwegian cohort. Eur. J. Epidemiol. 2022, 37, 539–548. [Google Scholar] [CrossRef]
- Sahanic, S.; Tymoszuk, P.; Ausserhofer, D.; Rass, V.; Pizzini, A.; Nordmeyer, G.; Hüfner, K.; Kurz, K.; Weber, P.M.; Sonnweber, T.; et al. Phenotyping of Acute and Persistent Coronavirus Disease 2019 Features in the Outpatient Setting: Exploratory Analysis of an International Cross-sectional Online Survey. Clin. Infect. Dis. 2022, 75, e418–e431. [Google Scholar] [CrossRef]
- Turner, S.; Khan, M.A.; Putrino, D.; Woodcock, A.; Kell, D.B.; Pretorius, E. Long COVID: Pathophysiological factors and abnormalities of coagulation. Trends Endocrinol. Metab. 2023, 34, 321–344. [Google Scholar] [CrossRef]
- Taylor, K.; Pearson, M.; Das, S.; Sardell, J.; Chocian, K.; Gardners, S. Genetic Risk Factors for Severe and Fatigue Dominant Long COVID and Commonalities with ME/CFS Identified by Combinatorial Analysis. J. Transl. Med. 2023, 21, 775. [Google Scholar] [CrossRef]
- Cutler, D.M.; Summers, L.H. The COVID-19 Pandemic and the $16 Trillion Virus. JAMA 2020, 324, 1495–1496. [Google Scholar] [CrossRef]
- Carfì, A.; Bernabei, R.; Landi, F. Gemelli Against COVID-19 Post-Acute Care Study Group. Persistent Symptoms in Patients After Acute COVID-19. JAMA 2020, 324, 603–605. [Google Scholar] [CrossRef]
- Tung-Chen, Y.; Blanco-Alonso, S.; Antón-Huguet, B.; Figueras-López, C.; Ugueto-Rodrigo, C. Persistent chest pain after resolution of coronavirus 2019 disease (COVID-19). Semergen 2020, 46 (Suppl. 1), 88–90. [Google Scholar] [CrossRef]
- Komaroff, A.L.; Lipkin, W.I. Insights from myalgic encephalomyelitis/chronic fatigue syndrome may help unravel the pathogenesis of postacute COVID-19 syndrome. Trends Mol. Med. 2021, 27, 895–906. [Google Scholar] [CrossRef]
- d’Ettorre, G.; Gentilini Cacciola, E.; Santinelli, L.; De Girolamo, G.; Spagnolello, O.; Russo, A.; Tarsitani, L.; Ciccozzi, M.; Mastroianni, C.M.; d’Ettorre, G.; et al. COVID-19 sequelae in working age patients: A systematic review. J. Med. Virol. 2022, 94, 858–868. [Google Scholar] [CrossRef]
- Twomey, R.; DeMars, J.; Franklin, K.; Culos-Reed, S.N.; Weatherald, J.; Wrightson, J.G. Chronic Fatigue and Postexertional Malaise in People Living with Long COVID: An Observational Study. Phys. Ther. 2022, 102, pzac005. [Google Scholar] [CrossRef]
- Mehandru, S.; Merad, M. Pathological sequelae of long-haul COVID. Nat. Immunol. 2022, 23, 194–202. [Google Scholar] [CrossRef]
- Wolff Sagy, Y.; Feldhamer, I.; Brammli-Greenberg, S.; Lavie, G. Estimating the economic burden of long-COVID: The additive cost of healthcare utilisation among COVID-19 recoverees in Israel. BMJ Glob. Health 2023, 8, e012588. [Google Scholar] [CrossRef]
- Biga, L.M.; Bronson, S.; Dawson, S.; Harwell, A.; Hopkins, R.; Kaufmann, J.; LeMaster, M.; Matern, P.; Morrison-Graham, K.; Oja, K.; et al. Chapter 22.7 Embryonic Development of the Respiratory System. In Anatomy & Physiology, 1st ed.; OpenStax/Oregon State University Corvallis: Corvallis, OR, USA, 2019; pp. 1529–1537. ISBN 978-1-955101-15-8. [Google Scholar]
- Barcik, W.; Boutin, R.C.T.; Sokolowska, M.; Finlay, B.B. The Role of Lung and Gut Microbiota in the Pathology of Asthma. Immunity 2020, 52, 241–255. [Google Scholar] [CrossRef]
- Wu, J.; Li, C.; Gao, P.; Zhang, C.; Zhang, P.; Zhang, L.; Dai, C.; Zhang, K.; Shi, B.; Liu, M.; et al. Intestinal microbiota links to allograft stability after lung transplantation: A prospective cohort study. Signal Transduct. Target. Ther. 2023, 8, 326. [Google Scholar] [CrossRef]
- Du, M.; Cai, G.; Chen, F.; Christiani, D.C.; Zhang, Z.; Wang, M. Multiomics Evaluation of Gastrointestinal and Other Clinical Characteristics of COVID-19. Gastroenterology 2020, 158, 2298–2301.e7. [Google Scholar] [CrossRef]
- Clerbaux, L.A.; Mayasich, S.A.; Muñoz, A.; Soares, H.; Petrillo, M.; Albertini, M.C.; Lanthier, N.; Grenga, L.; Amorim, M.J. Gut as an Alternative Entry Route for SARS-CoV-2: Current Evidence and Uncertainties of Productive Enteric Infection in COVID-19. J. Clin. Med. 2022, 11, 5691. [Google Scholar] [CrossRef] [PubMed]
- Sherif, Z.A.; Gomez, C.R.; Connors, T.J.; Henrich, T.J.; Reeves, W.B. Pathogenic mechanisms of post-acute sequelae of SARS-CoV-2 infection (PASC). Elife 2023, 12, e86002. [Google Scholar] [CrossRef] [PubMed]
- Vojdani, A.; Vojdani, E.; Saidara, E.; Maes, M. Persistent SARS-CoV-2 Infection, EBV, HHV-6 and Other Factors May Contribute to Inflammation and Autoimmunity in Long COVID. Viruses 2023, 15, 400. [Google Scholar] [CrossRef] [PubMed]
- Kenny, G.; Townsend, L.; Savinelli, S.; Mallon, P.W.G. Long COVID: Clinical characteristics, proposed pathogenesis and potential therapeutic targets. Front. Mol. Biosci. 2023, 10, 1157651. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhou, Y.; Ma, J.; Zhang, Q.; Shao, J.; Liang, S.; Yu, Y.; Li, W.; Wang, C. The long-term health outcomes, pathophysiological mechanisms and multidisciplinary management of long COVID. Signal Transduct Target Ther. 2023, 8, 416. [Google Scholar] [CrossRef] [PubMed]
- Souyris, M.; Mejía, J.E.; Chaumeil, J.; Guéry, J.C. Female predisposition to TLR7-driven autoimmunity: Gene dosage and the escape from X chromosome inactivation. Semin. Immunopathol. 2019, 41, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Conti, P.; Younes, A. Coronavirus COV-19/SARS-CoV-2 affects women less than men: Clinical response to viral infection. J. Biol. Regul. Homeost. Agents 2020, 34, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Conti, P.; Ronconi, G.; Caraffa, A.; Gallenga, C.E.; Ross, R.; Frydas, I.; Kritas, S.K. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): Anti-inflammatory strategies. J. Biol. Regul. Homeost. Agents 2020, 34, 327–331. [Google Scholar] [CrossRef]
- Miller, R.A.J.; Williams, A.P.; Kovats, S. Sex chromosome complement and sex steroid signaling underlie sex differences in immunity to respiratory virus infection. Front. Pharmacol. 2023, 14, 1150282. [Google Scholar] [CrossRef]
- Tosato, M.; Carfì, A.; Martis, I.; Pais, C.; Ciciarello, F.; Rota, E.; Tritto, M.; Salerno, A.; Zazzara, M.B.; Martone, A.M.; et al. Prevalence and Predictors of Persistence of COVID-19 Symptoms in Older Adults: A Single-Center Study. J. Am. Med. Dir. Assoc. 2021, 22, 1840–1844. [Google Scholar] [CrossRef]
- Michelen, M.; Manoharan, L.; Elkheir, N.; Cheng, V.; Dagens, A.; Hastie, C.; O’Hara, M.; Suett, J.; Dahmash, D.; Bugaeva, P.; et al. Characterising long COVID: A living systematic review. BMJ Glob. Health 2021, 6, e005427. [Google Scholar] [CrossRef] [PubMed]
- Daitch, V.; Yelin, D.; Awwad, M.; Guaraldi, G.; Milić, J.; Mussini, C.; Falcone, M.; Tiseo, G.; Carrozzi, L.; Pistelli, F.; et al. Characteristics of long-COVID among older adults: A cross-sectional study. Int. J. Infect. Dis. 2022, 125, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Nirantharakumar, K.; Hughes, S.; Myles, P.; Williams, T.; Gokhale, K.M.; Taverner, T.; Chandan, J.S.; Brown, K.; Simms-Williams, N.; et al. Symptoms and risk factors for long COVID in non-hospitalized adults. Nat. Med. 2022, 28, 1706–1714. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.A.; Pan, D.; Melbourne, C.; Teece, L.; Aujayeb, A.; Baggaley, R.F.; Bryant, L.; Carr, S.; Gregary, B.; Gupta, A.; et al. Risk factors associated with SARS-CoV-2 infection in a multiethnic cohort of United Kingdom healthcare workers (UK-REACH): A cross-sectional analysis. PLoS Med. 2022, 19, e1004015. [Google Scholar] [CrossRef] [PubMed]
- Norredam, M.; Hayward, S.; Deal, A.; Agyemang, C.; Hargreaves, S. Understanding and addressing long-COVID among migrants and ethnic minorities in Europe. Lancet Reg. Health 2022, 19, 100427. [Google Scholar] [CrossRef] [PubMed]
- Iturrieta-Zuazo, I.; Rita, C.G.; García-Soidán, A.; de Malet Pintos-Fonseca, A.; Alonso-Alarcón, N.; Pariente-Rodríguez, R.; Tejeda-Velarde, A.; Serrano-Villar, S.; Castañer-Alabau, J.L.; Nieto-Gañán, I. Possible role of HLA class-I genotype in SARS-CoV-2 infection and progression: A pilot study in a cohort of COVID-19 Spanish patients. Clin. Immunol. 2020, 219, 108572. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.; David, J.K.; Maden, S.K.; Wood, M.A.; Weeder, B.R.; Nellore, A.; Thompson, R.F. Human Leukocyte Antigen Susceptibility Map for Severe Acute Respiratory Syndrome Coronavirus 2. J. Virol. 2020, 94, e00510-20. [Google Scholar] [CrossRef]
- Tripathy, A.S.; Wagh, P.; Vishwakarma, S.; Akolkar, K.; Tripathy, S.; Jali, P.; Kakrani, A.L.; Barthwal, M.; Gurav, Y.; Kadgi, N.; et al. Association of human leukocyte antigen class I and class II alleles and haplotypes in COVID-19 infection in a western Indian population. Infect. Genet. Evol. 2023, 113, 105468. [Google Scholar] [CrossRef]
- Truong, T.T.; Ryutov, A.; Pandey, U.; Yee, R.; Goldberg, L.; Bhojwani, D.; Aguayo-Hiraldo, P.; Pinsky, B.A.; Pekosz, A.; Shen, L.; et al. Increased viral variants in children and young adults with impaired humoral immunity and persistent SARS-CoV-2 infection: A consecutive case series. EBioMedicine 2021, 67, 103355. [Google Scholar] [CrossRef]
- Cheung, C.C.L.; Goh, D.; Lim, X.; Tien, T.Z.; Lim, J.C.T.; Lee, J.N.; Tan, B.; Tay, Z.E.A.; Wan, W.Y.; Chen, E.X.; et al. Residual SARS-CoV-2 viral antigens detected in GI and hepatic tissues from five recovered patients with COVID-19. Gut 2022, 71, 226–229, Erratum in Gut 2022, 71, e9. [Google Scholar] [CrossRef]
- Swank, Z.; Senussi, Y.; Manickas-Hill, Z.; Yu, X.G.; Li, J.Z.; Alter, G.; Walt, D.R. Persistent Circulating Severe Acute Respiratory Syndrome Coronavirus 2 Spike Is Associated with Post-acute Coronavirus Disease 2019 Sequelae. Clin. Infect. Dis. 2023, 76, e487–e490. [Google Scholar] [CrossRef] [PubMed]
- Craddock, V.; Mahajan, A.; Spikes, L.; Krishnamachary, B.; Ram, A.K.; Kumar, A.; Chen, L.; Chalise, P.; Dhillon, N.K. Persistent circulation of soluble and extracellular vesicle-linked Spike protein in individuals with postacute sequelae of COVID-19. J. Med. Virol. 2023, 95, e28568. [Google Scholar] [CrossRef] [PubMed]
- Mohandas, S.; Jagannathan, P.; Henrich, T.J.; Sherif, Z.A.; Bime, C.; Quinlan, E.; Portman, M.A.; Gennaro, M.; Rehman, J. Immune mechanisms underlying COVID-19 pathology and post-acute sequelae of SARS-CoV-2 infection (PASC). eLife 2023, 12, e86014. [Google Scholar] [CrossRef] [PubMed]
- Files, J.K.; Boppana, S.; Perez, M.D.; Sarkar, S.; Lowman, K.E.; Qin, K.; Sterrett, S.; Carlin, E.; Bansal, A.; Sabbaj, S.; et al. Sustained cellular immune dysregulation in individuals recovering from SARS-CoV-2 infection. J. Clin. Investig. 2021, 131, e140491. [Google Scholar] [CrossRef] [PubMed]
- Phetsouphanh, C.; Darley, D.R.; Wilson, D.B.; Howe, A.; Munier, C.M.L.; Patel, S.K.; Juno, J.A.; Burrell, L.M.; Kent, S.J.; Dore, G.J.; et al. Immunological dysfunction persists for 8 months following initial mild-to-moderate SARS-CoV-2 infection. Nat. Immunol. 2022, 23, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Mudd, P.A.; Remy, K.E. Prolonged adaptive immune activation in COVID-19: Implications for maintenance of long-term immunity? J. Clin. Investig. 2021, 131, e143928. [Google Scholar] [CrossRef] [PubMed]
- Francisco, L.M.; Sage, P.T.; Sharpe, A.H. The PD-1 pathway in tolerance and autoimmunity. Immunol. Rev. 2010, 236, 219–242. [Google Scholar] [CrossRef]
- Fife, B.T.; Pauken, K.E. The role of the PD-1 pathway in autoimmunity and peripheral tolerance. Ann. N. Y. Acad. Sci. 2011, 1217, 45–59. [Google Scholar] [CrossRef]
- Gianchecchi, E.; Delfino, D.V.; Fierabracci, A. Recent insights into the role of the PD-1/PD-L1 pathway in immunological tolerance and autoimmunity. Autoimmun. Rev. 2013, 12, 1091–1100. [Google Scholar] [CrossRef]
- Wang, S.; Khan, F.I. Investigation of Molecular Interactions Mechanism of Pembrolizumab and PD-1. Int. J. Mol. Sci. 2023, 24, 10684. [Google Scholar] [CrossRef]
- Peluso, M.J.; Lu, S.; Tang, A.F.; Durstenfeld, M.S.; Ho, H.E.; Goldberg, S.A.; Forman, C.A.; Munter, S.E.; Hoh, R.; Tai, V.; et al. Markers of Immune Activation and Inflammation in Individuals with Postacute Sequelae of Severe Acute Respiratory Syndrome Coronavirus 2 Infection. J. Infect. Dis. 2021, 224, 1839–1848. [Google Scholar] [CrossRef] [PubMed]
- Peluso, M.J.; Sans, H.M.; Forman, C.A.; Nylander, A.N.; Ho, H.E.; Lu, S.; Goldberg, S.A.; Hoh, R.; Tai, V.; Munter, S.E.; et al. Plasma Markers of Neurologic Injury and Inflammation in People with Self-Reported Neurologic Postacute Sequelae of SARS-CoV-2 Infection. Neurol.-Neuroimmunol. Neuroinflamm. 2022, 9, e200003. [Google Scholar] [CrossRef] [PubMed]
- Comeau, D.; Martin, M.; Robichaud, G.A.; Chamard-Witkowski, L. Neurological manifestations of post-acute sequelae of COVID-19: Which liquid biomarker should we use? Front. Neurol. 2023, 14, 1233192. [Google Scholar] [CrossRef] [PubMed]
- Perumal, R.; Shunmugam, L.; Naidoo, K.; Abdool Karim, S.S.; Wilkins, D.; Garzino-Demo, A.; Brechot, C.; Parthasarathy, S.; Vahlne, A.; Nikolich, J.Ž. Long COVID: A review and proposed visualization of the complexity of long COVID. Front. Immunol. 2023, 14, 1117464. [Google Scholar] [CrossRef] [PubMed]
- Rojas, M.; Rodríguez, Y.; Acosta-Ampudia, Y.; Monsalve, D.M.; Zhu, C.; Li, Q.Z.; Ramírez-Santana, C.; Anaya, J.M. Autoimmunity is a hallmark of post-COVID syndrome. J. Transl. Med. 2022, 20, 129. [Google Scholar] [CrossRef]
- Vahabi, M.; Ghazanfari, T.; Sepehrnia, S. Molecular mimicry, hyperactive immune system, and SARS-CoV-2 are three prerequisites of the autoimmune disease triangle following COVID-19 infection. Int. Immunopharmacol. 2022, 112, 109183. [Google Scholar] [CrossRef]
- Dobrowolska, K.; Zarębska-Michaluk, D.; Poniedziałek, B.; Jaroszewicz, J.; Flisiak, R.; Rzymski, P. Overview of autoantibodies in COVID-19 convalescents. J. Med. Virol. 2023, 95, e28864. [Google Scholar] [CrossRef]
- Zebardast, A.; Hasanzadeh, A.; Ebrahimian Shiadeh, S.A.; Tourani, M.; Yahyapour, Y. COVID-19: A trigger of autoimmune diseases. Cell Biol. Int. 2023, 47, 848–858. [Google Scholar] [CrossRef]
- Zuo, T.; Zhang, F.; Lui, G.C.Y.; Yeoh, Y.K.; Li, A.Y.L.; Zhan, H.; Wan, Y.; Chung, A.C.K.; Cheung, C.P.; Chen, N.; et al. Alterations in Gut Microbiota of Patients with COVID-19 During Time of Hospitalization. Gastroenterology 2020, 159, 944–955.e8. [Google Scholar] [CrossRef]
- Yeoh, Y.K.; Zuo, T.; Lui, G.C.; Zhang, F.; Liu, Q.; Li, A.Y.; Chung, A.C.; Cheung, C.P.; Tso, E.Y.; Fung, K.S.; et al. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut 2021, 70, 698–706. [Google Scholar] [CrossRef]
- Abbasi, A.F.; Marinkovic, A.; Prakash, S.; Sanyaolu, A.; Smith, S. COVID-19 and the Human Gut Microbiome: An Under-Recognized Association. Chonnam Med. J. 2022, 58, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Mak, J.W.Y.; Su, Q.; Yeoh, Y.K.; Lui, G.C.; Ng, S.S.S.; Zhang, F.; Li, A.Y.L.; Lu, W.; Hui, D.S.; et al. Gut microbiota dynamics in a prospective cohort of patients with post-acute COVID-19 syndrome. Gut 2022, 71, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Mazzarelli, A.; Giancola, M.L.; Fontana, A.; Piselli, P.; Binda, E.; Trivieri, N.; Mencarelli, G.; Marchioni, L.; Vulcano, A.; De Giuli, C.; et al. Gut microbiota composition in COVID-19 hospitalized patients with mild or severe symptoms. Front. Microbiol. 2022, 13, 1049215. [Google Scholar] [CrossRef] [PubMed]
- Sajdel-Sulkowska, E.M. Neuropsychiatric Ramifications of COVID-19: Short-Chain Fatty Acid Deficiency and Disturbance of Microbiota-Gut-Brain Axis Signaling. BioMed Res. Int. 2021, 2021, 7880448. [Google Scholar] [CrossRef] [PubMed]
- Kamble, S.R.; Dandekar, M.P. Implication of microbiota gut-brain axis in the manifestation of obsessive-compulsive disorder: Preclinical and clinical evidence. Eur. J. Pharmacol. 2023, 957, 176014. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Guo, R.; Ma, Q.; Li, Y.; Wang, W.; Fan, Y.; Ju, Y.; Zhao, B.; Gao, Y.; Qian, L.; et al. Stressful events induce long-term gut microbiota dysbiosis and associated post-traumatic stress symptoms in healthcare workers fighting against COVID-19. J. Affect. Disord. 2022, 303, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Vestad, B.; Ueland, T.; Lerum, T.V.; Dahl, T.B.; Holm, K.; Barratt-Due, A.; Kåsine, T.; Dyrhol-Riise, A.M.; Stiksrud, B.; Tonby, K.; et al. Respiratory dysfunction three months after severe COVID-19 is associated with gut microbiota alterations. J. Intern. Med. 2022, 291, 801–812. [Google Scholar] [CrossRef]
- Liu, Q.; Su, Q.; Zhang, F.; Tun, H.M.; Mak, J.W.Y.; Lui, G.C.; Ng, S.S.S.; Ching, J.Y.L.; Li, A.; Lu, W.; et al. Multi-kingdom gut microbiota analyses define COVID-19 severity and post-acute COVID-19 syndrome. Nat. Commun. 2022, 13, 6806. [Google Scholar] [CrossRef]
- Tkacheva, O.N.; Klimenko, N.S.; Kashtanova, D.A.; Tyakht, A.V.; Maytesyan, L.V.; Akopyan, A.A.; Koshechkin, S.I.; Strazhesko, I.D. Gut Microbiome in Post-COVID-19 Patients Is Linked to Immune and Cardiovascular Health Status but Not COVID-19 Severity. Microorganisms 2023, 11, 1036. [Google Scholar] [CrossRef]
- Caio, R.; Cultrera, R.; Blanco-Míguez, A.; Armanini, F.; Asnicar, F.; Catozzi, C.; Nezi, L.; Lungaro, L.; Costanzini, A.; Guarino, M.; et al. Gut microbiome features in COVID-19: Analysis of a cohort of hospitalized patients. Microbiota Health Dis. 2023, 5, e818. [Google Scholar] [CrossRef]
- Zhang, D.; Zhou, Y.; Ma, Y.; Chen, P.; Tang, J.; Yang, B.; Li, H.; Liang, M.; Xue, Y.; Liu, Y.; et al. Gut Microbiota Dysbiosis Correlates with Long COVID-19 at One-Year After Discharge. J. Korean Med. Sci. 2023, 38, e120. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Weng, S.; Xia, C.; Ren, Y.; Liu, Z.; Xu, Y.; Yang, X.; Wu, R.; Peng, L.; Sun, L.; et al. Gastrointestinal symptoms of long COVID-19 related to the ectopic colonization of specific bacteria that move between the upper and lower alimentary tract and alterations in serum metabolites. BMC Med. 2023, 21, 264. [Google Scholar] [CrossRef] [PubMed]
- Gatti, P.; Ilamathi, H.S.; Todkar, K.; Germain, M. Mitochondria Targeted Viral Replication and Survival Strategies-Prospective on SARS-CoV-2. Front. Pharmacol. 2020, 11, 578599. [Google Scholar] [CrossRef] [PubMed]
- Shoraka, S.; Samarasinghe, A.E.; Ghaemi, A.; Mohebbi, S.R. Host mitochondria: More than an organelle in SARS-CoV-2 infection. Front. Cell. Infect. Microbiol. 2023, 13, 1228275. [Google Scholar] [CrossRef] [PubMed]
- San-Millán, I. The Key Role of Mitochondrial Function in Health and Disease. Antioxidants 2023, 12, 782. [Google Scholar] [CrossRef]
- Komaroff, A.L.; Bateman, L. Will COVID-19 Lead to Myalgic Encephalomyelitis/Chronic Fatigue Syndrome? Front. Med. 2021, 7, 606824. [Google Scholar] [CrossRef]
- Paul, B.D.; Lemle, M.D.; Komaroff, A.L.; Snyder, S.H. Redox imbalance links COVID-19 and myalgic encephalomyelitis/chronic fatigue syndrome. Proc. Natl. Acad. Sci. USA 2021, 118, e2024358118. [Google Scholar] [CrossRef]
- Trihandini, I.; Muhtar, M.; Karunia Sakti, D.A.; Erlianti, C.P. The effect of long-haul COVID-19 toward domains of the health-related quality of life among recovered hospitalized patients. Front. Public Health 2023, 11, 1068127. [Google Scholar] [CrossRef]
- Grossini, E.; Concina, D.; Rinaldi, C.; Russotto, S.; Garhwal, D.; Zeppegno, P.; Gramaglia, C.; Kul, S.; Panella, M. Association Between Plasma Redox State/Mitochondria Function and a Flu-Like Syndrome/COVID-19 in the Elderly Admitted to a Long-Term Care Unit. Front. Physiol. 2021, 12, 707587. [Google Scholar] [CrossRef]
- Levy, D.; Giannini, M.; Oulehri, W.; Riou, M.; Marcot, C.; Pizzimenti, M.; Debrut, L.; Charloux, A.; Geny, B.; Meyer, A. Long Term Follow-Up of Sarcopenia and Malnutrition after Hospitalization for COVID-19 in Conventional or Intensive Care Units. Nutrients 2022, 14, 912. [Google Scholar] [CrossRef]
- Ghanem, J.; Passadori, A.; Severac, F.; Dieterlen, A.; Geny, B.; Andrès, E. Effects of Rehabilitation on Long-COVID-19 Patient’s Autonomy, Symptoms and Nutritional Observance. Nutrients 2022, 14, 3027. [Google Scholar] [CrossRef] [PubMed]
- Guntur, V.P.; Nemkov, T.; de Boer, E.; Mohning, M.P.; Baraghoshi, D.; Cendali, F.I.; San-Millán, I.; Petrache, I.; D’Alessandro, A. Signatures of Mitochondrial Dysfunction and Impaired Fatty Acid Metabolism in Plasma of Patients with Post-Acute Sequelae of COVID-19 (PASC). Metabolites 2022, 12, 1026. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Resendiz, K.; Benitez-Trinidad, A.B.; Covantes-Rosales, C.E.; Toledo-Ibarra, G.A.; Ortiz-Lazareno, P.C.; Girón-Pérez, D.A.; Bueno-Durán, A.Y.; Pérez-Díaz, D.A.; Barcelos-García, R.G.; Girón-Pérez, M.I. Loss of mitochondrial membrane potential (ΔΨm) in leucocytes as post-COVID-19 sequelae. J. Leukoc. Biol. 2022, 112, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Resendiz, K.J.G.; Covantes-Rosales, C.E.; Benítez-Trinidad, A.B.; Navidad-Murrieta, M.S.; Razura-Carmona, F.F.; Carrillo-Cruz, C.D.; Frias-Delgadillo, E.J.; Pérez-Díaz, D.A.; Díaz-Benavides, M.V.; Zambrano-Soria, M.; et al. Effect of Fucoidan on the Mitochondrial Membrane Potential (ΔΨm) of Leukocytes from Patients with Active COVID-19 and Subjects That Recovered from SARS-CoV-2 Infection. Mar. Drugs 2022, 20, 99. [Google Scholar] [CrossRef]
- Pozzi, A. COVID-19 and Mitochondrial Non-Coding RNAs: New Insights from Published Data. Front. Physiol. 2022, 12, 805005. [Google Scholar] [CrossRef]
- Lage, S.L.; Amaral, E.P.; Hilligan, K.L.; Laidlaw, E.; Rupert, A.; Namasivayan, S.; Rocco, J.; Galindo, F.; Kellogg, A.; Kumar, P.; et al. Persistent Oxidative Stress and Inflammasome Activation in CD14highCD16− Monocytes From COVID-19 Patients. Front. Immunol. 2022, 12, 799558. [Google Scholar] [CrossRef] [PubMed]
- Peluso, M.J.; Deeks, S.G.; Mustapic, M.; Kapogiannis, D.; Henrich, T.J.; Lu, S.; Goldberg, S.A.; Hoh, R.; Chen, J.Y.; Martinez, E.O.; et al. SARS-CoV-2 and Mitochondrial Proteins in Neural-Derived Exosomes of COVID-19. Ann. Neurol. 2022, 91, 772–781. [Google Scholar] [CrossRef]
- Goetzl, E.J.; Yao, P.J.; Kapogiannis, D. Prediction of Post-Acute-Sequelae of COVID-19 by Cargo Protein Biomarkers of Blood Total Extracellular Vesicles in Acute COVID-19. Am. J. Med. 2023, 136, 824–829. [Google Scholar] [CrossRef]
- Siekacz, K.; Kumor-Kisielewska, A.; Miłkowska-Dymanowska, J.; Pietrusińska, M.; Bartczak, K.; Majewski, S.; Stańczyk, A.; Piotrowski, W.J.; Białas, A.J. Oxidative Biomarkers Associated with the Pulmonary Manifestation of Post-COVID-19 Complications. J. Clin. Med. 2023, 12, 4253. [Google Scholar] [CrossRef]
- Gvozdjáková, A.; Sumbalová, Z.; Kucharská, J.; Rausová, Z.; Kovalčíková, E.; Takácsová, T.; Navas, P.; López-Lluch, G.; Mojto, V.; Palacka, P. Mountain spa rehabilitation improved health of patients with post-COVID-19 syndrome: Pilot study. Environ. Sci. Pollut. Res. 2023, 30, 14200–14211. [Google Scholar] [CrossRef]
- Ailioaie, L.M.; Ailioaie, C.; Litscher, G. Infection, Dysbiosis and Inflammation Interplay in the COVID Era in Children. Int. J. Mol. Sci. 2023, 24, 10874. [Google Scholar] [CrossRef]
- Ailioaie, L.M.; Ailioaie, C.; Litscher, G.; Chiran, D.A. Celiac Disease and Targeting the Molecular Mechanisms of Autoimmunity in COVID Pandemic. Int. J. Mol. Sci. 2022, 23, 7719. [Google Scholar] [CrossRef] [PubMed]
- Motta, C.S.; Torices, S.; da Rosa, B.G.; Marcos, A.C.; Alvarez-Rosa, L.; Siqueira, M.; Moreno-Rodriguez, T.; Matos, A.d.R.; Caetano, B.C.; Martins, J.S.C.d.C.; et al. Human Brain Microvascular Endothelial Cells Exposure to SARS-CoV-2 Leads to Inflammatory Activation through NF-κB Non-Canonical Pathway and Mitochondrial Remodeling. Viruses 2023, 15, 745. [Google Scholar] [CrossRef] [PubMed]
- Martín Giménez, V.M.; Modrego, J.; Gómez-Garre, D.; Manucha, W.; de las Heras, N. Gut Microbiota Dysbiosis in COVID-19: Modulation and Approaches for Prevention and Therapy. Int. J. Mol. Sci. 2023, 24, 12249. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Yuan, D.; Chen, D.G.; Ng, R.H.; Wang, K.; Choi, J.; Li, S.; Hong, S.; Zhang, R.; Xie, J.; et al. Multiple early factors anticipate post-acute COVID-19 sequelae. Cell 2022, 185, 881–895.e20. [Google Scholar] [CrossRef] [PubMed]
- Picard, M.; Shirihai, O.S. Mitochondrial signal transduction. Cell Metab. 2022, 34, 1620–1653. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Ahmad, S.; Ahmad, T.; Ali, S.; Syed, M.A. Mitochondrial dynamics and mitophagy in lung disorders. Life Sci. 2021, 284, 119876. [Google Scholar] [CrossRef]
- Ailioaie, L.M.; Ailioaie, C.; Litscher, G. Implications of SARS-CoV-2 Infection in Systemic Juvenile Idiopathic Arthritis. Int. J. Mol. Sci. 2022, 23, 4268. [Google Scholar] [CrossRef]
- Baillie, K.; Davies, H.E.; Keat, S.B.K.; Ladell, K.; Miners, K.L.; Jones, S.A.; Mellou, E.; Toonen, E.J.M.; Price, D.A.; Morgan, B.P.; et al. Complement dysregulation is a predictive and therapeutically amenable feature of long COVID. medRxiv 2023. [Google Scholar] [CrossRef]
- Wong, A.C.; Devason, A.S.; Umana, I.C.; Cox, T.O.; Dohnalová, L.; Litichevskiy, L.; Perla, J.; Lundgren, P.; Etwebi, Z.; Izzo, L.T.; et al. Serotonin reduction in post-acute sequelae of viral infection. Cell 2023, 186, 4851–4867.e20. [Google Scholar] [CrossRef]
- Martínez-Reyes, I.; Chandel, N.S. Mitochondrial TCA cycle metabolites control physiology and disease. Nat. Commun. 2020, 11, 102. [Google Scholar] [CrossRef] [PubMed]
- Cantó, C.; Menzies, K.J.; Auwerx, J. NAD(+) Metabolism and the Control of Energy Homeostasis: A Balancing Act between Mitochondria and the Nucleus. Cell Metab. 2015, 22, 31–53. [Google Scholar] [CrossRef]
- Xie, N.; Zhang, L.; Gao, W.; Huang, C.; Huber, P.E.; Zhou, X.; Li, C.; Shen, G.; Zou, B. NAD+ metabolism: Pathophysiologic mechanisms and therapeutic potential. Signal Transduct. Target. Ther. 2020, 5, 227. [Google Scholar] [CrossRef] [PubMed]
- Block, T.; Kuo, J. Rationale for Nicotinamide Adenine Dinucleotide (NAD+) Metabolome Disruption as a Pathogenic Mechanism of Post-Acute COVID-19 Syndrome. Clin. Pathol. 2022, 15, 2632010X221106986. [Google Scholar] [CrossRef]
- Zheng, M.; Schultz, M.B.; Sinclair, D.A. NAD+ in COVID-19 and viral infections. Trends Immunol. 2022, 43, 283–295. [Google Scholar] [CrossRef] [PubMed]
- Owens, C.D.; Bonin Pinto, C.; Mukli, P.; Szarvas, Z.; Peterfi, A.; Detwiler, S.; Olay, L.; Olson, A.L.; Li, G.; Galvan, V.; et al. Vascular mechanisms leading to progression of mild cognitive impairment to dementia after COVID-19: Protocol and methodology of a prospective longitudinal observational study. PLoS ONE 2023, 18, e0289508. [Google Scholar] [CrossRef] [PubMed]
- Sano, H.; Kratz, A.; Nishino, T.; Imamura, H.; Yoshida, Y.; Shimizu, N.; Kitano, H.; Yachie, A. Nicotinamide mononucleotide (NMN) alleviates the poly(I:C)-induced inflammatory response in human primary cell cultures. Sci. Rep. 2023, 13, 11765. [Google Scholar] [CrossRef]
- Alegre, G.F.S.; Pastore, G.M. NAD+ Precursors Nicotinamide Mononucleotide (NMN) and Nicotinamide Riboside (NR): Potential Dietary Contribution to Health. Curr. Nutr. Rep. 2023, 12, 445–464. [Google Scholar] [CrossRef]
- Fang, J.; Chen, W.; Hou, P.; Liu, Z.; Zuo, M.; Liu, S.; Feng, C.; Han, Y.; Li, P.; Shi, Y.; et al. NAD+ metabolism-based immunoregulation and therapeutic potential. Cell Biosci. 2023, 13, 81. [Google Scholar] [CrossRef]
- Hool, L.C. Elucidating the role of the L-type calcium channel in excitability and energetics in the heart: The ISHR 2020 Research Achievement Award Lecture. J. Mol. Cell. Cardiol. 2022, 172, 100–108. [Google Scholar] [CrossRef]
- Guarnieri, J.W.; Dybas, J.M.; Fazelinia, H.; Kim, M.S.; Frere, J.; Zhang, Y.; Soto Albrecht, Y.; Murdock, D.G.; Angelin, A.; Singh, L.N.; et al. Core mitochondrial genes are down-regulated during SARS-CoV-2 infection of rodent and human hosts. Sci. Transl. Med. 2023, 15, eabq1533. [Google Scholar] [CrossRef] [PubMed]
| Host Conditions | Viral Agents | Downstream Effects |
|---|---|---|
| Age Sex Ethnicity Genetic factors Metabolic/endocrine diseases Chronic inflammation Immunological imbalances/autoimmune diseases | Occult persistence of the SARS-CoV-2 virus Persistence of SARS-CoV-2 viral components Reactivation of latent viruses [(Epstein–Barr virus (EBV), Cytomegalovirus (CMV), human immunodeficiency virus (HIV), herpes simplex virus 1, human herpesvirus 6 (HHV-6), and human herpesvirus 7 (HHV-7)] | Grade of lesions from primary acute SARS-CoV-2 infection Vascular endothelial abnormalities Microclots Thromboses Dysfunctional neurological signaling Reduction in tissue oxygen/hypoxia Disruption of the intestinal microbiome |
| Reference | Patients COVID-19/LC | Measured Parameters | Conclusions | |||
|---|---|---|---|---|---|---|
| Fecal Samples | Respiratory Tract Samples | Increased Opportunistic Pathogens | Reduced Microbial Biodiversity | |||
| [92] Liu, Q. et al., 2022; https://doi.org/10.1136/gutjnl-2021-325989 | G1: 106 patients with LC. G2: 68 non-COVID-19 patients | 258 stool samples | - | Yes | Yes | Evidence of gut microbiome composition changes in LC. Could its modulation be useful in LC recovery? |
| [93] Mazzarelli, A. et al., 2022; https://doi.org/10.3389/fmicb.2022.1049215 | 97 patients—SARS-CoV-2 infection | 97 rectal swabs | - | Yes | Yes | The gut microbiota profile varies with the severity of the SARS-CoV-2 infection and may be a prognostic biomarker. |
| [96] Gao, F. et al., 2022; https://doi.org/10.1016/j.jad.2022.02.024 | G1 = 71 FHW-treated patients with COVID-19. G2 = 104 SHWs who treated non-infected patients with COVID-19. | Bacterial genomic DNA was extracted and analyzed. | - | Yes | Yes | Stress-triggered intestinal dysbiosis in FHWs was persistent for at least 6 months. Neuropsychiatric symptoms in FHWs were correlated directly with the intestinal microbiome. |
| [97] Vestad, B. et al., 2022; https://doi.org/10.1111/joim.13458 | Randomized trial of 181 patients with COVID-19, divided into 3 subgroups. | S1 = Rectal swab material and 16S rRNA gene sequencing. S2 = lung function tests. S3 = rectal swabs and pulmonary function tests. | Pulmonary function tests. | Yes | Yes | Respiratory dysfunction in LC could be correlated with an altered gut microbiome and elevated LBP levels. Possible involvement of the gut–lung axis in LC. |
| [98] Liu, Q. et al., 2022; https://doi.org/10.1038/s41467-022-34535-8 | Cross-sectional and prospective study on a cohort of 133 COVID-19 patients followed for up to 6 months. | Integrated analysis: 296 fecal metagenomes. 79 fecal metabolomics. 1378 viral loads in respiratory tract samples. | Viral load in 1378 respiratory tract samples (sputum and nasopharyngeal sample). | Yes | Yes | Host phenotype and multikingdom microbiota profile could be prognostic factors for COVID-19. |
| [99] Tkacheva, O.N. et al., 2023; https://doi.org/10.3390/microorganisms11041036 | 178 patients with post-COVID-19 and contacts for SARS-CoV-2 but without infection. | Fecal samples | - | Yes | Yes | Three months after infection with SARS-CoV-2, the intestinal microbiota was restored, and no significant differences in its composition were found. Novel strategies for microbiome-tailored disease prevention and treatment are needed. |
| [100] Caio R. et al., 2023; https://doi.org/10.26355/mhd_20233_818 | 46 patients aged between 30 and 95, hospitalized with COVID-19, were grouped by clinical severity (i.e., non-critical or critical), type of hospitalization (non-intensive care or intensive therapy unit), and outcome. | Stool samples were analyzed by shotgun metagenomic sequencing. | - | Yes | Yes | Intestinal dysbiosis could underlie disease severity, persistent inflammation, and late complications in LC. |
| [101] Zhang, D. et al., 2023; https://doi.org/10.3346/jkms.2023.38.e120 | 187 RPs, among them, 84 (44.9%) reported LC one year after discharge. | In 130 RPs and 32 HCs: Stool samples collection and 16s rRNA sequencing. | - | Yes | Significantly reduced bacterial diversities and a lower relative abundance of SCFAs. | SCFAs and SCFA-producing commensal bacteria may delay recovery and sustain the persistence of LC. |
| [102] Zhang, D. et al., 2023; https://doi.org/10.1186/s12916-023-02972-x | Prospectively analyzed oral, fecal, and serum samples from 983 antibiotic-naïve subjects with mild COVID-19 were monitored for 3 months after discharge. | 45 fecal and saliva samples and 25 matched serum samples were collected from patients who had LC with digestive symptoms, compared to HCs. | 8 saliva and fecal samples were collected from patients with LC but without digestive symptoms. | Yes | Yes | Patients with digestive symptoms of LC after mild forms of COVID-19 may have an ectopic colonization of the oral microbiome with gut microbes and a disturbance of serum metabolites. |
| No | References | Study Design | Targets/Trial Protocol/Main Parameters Measured | Brief Results | Conclusions |
|---|---|---|---|---|---|
| 1. | [109] Grossini, E. et al., 2021. https://doi.org/10.3389/fphys.2021.707587 | 60 subjects mostly women (mean age 84 years), 12 years older than men, admitted to a LTC facility. All without cognitive impairment. | Plasma markers of lipidic peroxidation: thiobarbituric acid reactive substances (TBARS) release, 8-hydroxy 2 deoxyguanosine (8 OH-2dG), 8-isoprostanes, superoxide dismutase (SOD) activity, glutathione (GSH), and 25(OH) vitamin D. Thymosin β4 (human TMS β4). Cell viability, mitochondrial membrane potential, and ROS on HUVEC. | TBARS, 8 OH-2dG, and 8-isoprostanes exhibited an “oxidative” plasma status. The antioxidant system was well preserved. Vitamin D and GSH were within the physiological range. SOD activity was about 51%. HUVEC treatment with plasma has reduced cell viability by about 60% and increased ROS by about 80% compared to untreated HUVEC. | Assessment of mitochondrial function in the elderly hospitalized in LTC facilities is essential for estimating susceptibility to COVID-19 and identifying patients at high “risk” for the development of infections. |
| 2. | [110] Levy, D. et al., 2022; https://doi.org/10.3390/nu14040912 | 139 patients who survived after COVID-19 and admitted to the ICU. | Sarcopenia and weight evolution at 3 (M3) and 6(M6) months after ICU discharge. | At M3: Sarcopenia (n = 22), weight decrease > 5% (n = 13). At M6: Persistent sarcopenia: n = 6. Recovering from sarcopenia: n = 16. | The persistence of sarcopenia was associated with female sex, older age, and more severe baseline sarcopenia. In a holistic approach, sarcopenia is reversible through individualized nutritional programs and personalized physical rehabilitation. |
| 3. | [111] Ghanem, J. et al., 2022; https://doi.org/10.3390/nu14153027 | 37 patients hospitalized for a severe SARS-CoV-2 infection. | Long-term evaluation of autonomy, malnutrition, and LC symptoms. | An important decrease in autonomy is associated with malnutrition after ICU hospitalization. Beneficial effects of personalized rehabilitation. | 6 months after discharge: 20% are still without full autonomy; 70% are still with chronic fatigue. Need for personalized and persistent follow-up. |
| 4. | [112] Guntur, V.P. et al., 2022; https://doi.org/10.3390/metabo12111026 | Plasma samples from 75 patients divided into 3 groups: G1: LC. G2: fully recovered. G3: healthy controls. | Mass spectrometry-based untargeted metabolomics. | Higher levels of fatty acid metabolites; lower levels of mono-, di-, and tri-carboxylates; and depletion of tryptophan in plasma samples of patients with LC (G1). | The need for therapeutic intervention to restore mitochondrial fat-burning capacity in LC. |
| 5. | [113] Díaz-Resendiz, K. et al., 2022; https://doi.org/10.1002/JLB.3MA0322-279RRR | Human plasma study with 4 groups: HC, C-19, R1, and R2. | ΔΨm measured in human leucocytes for all 4 groups. | ΔΨm was decreased in all three groups compared to healthy controls, even 11 months post-infection; a sex-associated response. | The loss of ΔΨm could indicate a susceptibility to developing LC. |
| 6. | [114] Díaz-Resendiz, K.J.G. et al., 2022; https://doi.org/10.3390/md20020099 | 76 subjects, divided into different groups, were administered Fucoidan. Phase 1: HC (n = 24) C-19 (n = 31) R1 (n = 21). Phase 2: HC (n = 19) R2 (n = 19). | Ex-vivo fucoidan treatment in HPBMCs. ∆Ψm measurements. | COVID-19 induces an elevated inflammatory/ oxidative state, mitochondrial dysfunction, and ∆Ψm loss. | Fucoidan may constitute a potential treatment to prevent LC, with mitochondria as a therapeutic target to restore homeostasis and ∆Ψm. |
| 7. | [115] Pozzi, A., 2022. https://doi.org/10.3389/fphys.2021.805005 | RNA samples extracted from PBMC in patients recovering from COVID-19. | Expression of canonical and non-canonical genes encoded on the mitochondrial genome. | Only some non-canonical mitochondrial genes are disrupted by COVID-19, being limited to mt-sRNAs, without altering the overall mitochondrial transcription. | Further studies on the role of mt-sRNAs in LC are required. |
| 8. | [116] Lage, S.L. et al., 2022; https://doi.org/10.3389/fimmu.2021.799558 | 47 COVID-19 patients, enrolled from March 2020 to August 2020, divided into mild (n = 31) and moderate-severe (n = 16) groups. | Plasma biomarkers. Inflammasome and mitochondrial status. Lipid peroxidation. Intracellular GSH levels. Mitochondrial superoxide. Circulating monocyte subsets. | ↑↑CD14high CD16− classical monocytes compared to HCs. ↑Inflammasome activation. ↑Oxidative stress/NLRP3 signaling pathway. Target therapy to mitigate early hyperinflammation and LC outcome. | Sustained deregulated oxidative stress and inflammasome activation in monocytes after short-term recovery support one of the current hypotheses that LC is driven by persistent pathological inflammation and suggest the pathways involved as potential targets for the management of LC. |
| 9. | [117] Peluso, M.J. et al., 2022; https://doi.org/10.1002/ana.26350 | Human plasma study with 4 groups, relative to controls. G1: post-COVID, without LC, G2: LC without NP, G3: LC with NP, and G4: LC with severe NP. | Measurements of SARS-CoV-2 proteins and MPs in NDEVs and ADEVs. | S1 and N proteins were increased in all LC subgroups compared to controls; N concentrations were higher in LC with NP. | Development of new biomarkers and a faster effective technology to identify MPs or SARS-CoV-2′s protein abnormalities in NDEVs or ADEVs during acute infection to accurately predict the risk of developing LC. |
| 10. | [118] Goetzl, E.J. et al., 2023; https://doi.org/10.1016/j.amjmed.2023.03.026 | 4 study groups: G1= no infection, G2= acute infection, G3 = LC, and G4= post-acute COVID without LC. | Measurements of plasma TEVs proteins in all 4 groups. | For SARS-CoV-2 S1 (RBD) and N: - confirmation of the intracellular presence of the virus. - detection of a specific strain of SARS-CoV-2. For functional MP altered by SARS-CoV-2 in G3 (or LC): ↓MOTS-c, VDAC-1, and humanin. ↑SARM-1 in G2 that progressed to LC. | Management with anti-viral drugs. Abnormal levels of humanin, MOTS-c, and SARM-1 in LC predict neuropsychiatric symptoms. |
| 11. | [119] Siekacz, K. et al., 2023; https://doi.org/10.3390/jcm12134253 | 80 patients post-COVID-19 divided into two groups: 1. (P(+), n = 40) with persistent interstitial lung lesions on CT. 2. (P(−), n = 40) without lung lesions on CT. | Mitochondrial biomarkers by (ELISA). | P(+) compared to P(−): ↑PTEN-induced kinase 1 (PINK1). ↑Dynamin-1-like protein (DNM1L). ↑Mitofusin-2 (MFN2). ↑Chemokine ligand 18 (PARC, CCL18). ↑IL-6 and ↑ tumor necrosis factor-alpha (TNF-α). ↓Interferon alpha (IFN-α). In P(+) patients: correlations between: - advanced glycation end product (sRAGE) and TNF-α - between DNM1L and IFN-α. | SARS-CoV-2 could trigger mitochondrial dysfunction and chronic inflammation by deregulating PINK1, DNM1L, and MFN2. ↑↑ CLL18, TNF-α, and IL-6 could support long-term pulmonary complications in LC. TNF-α = a potential predictor. |
| 12. | [120] Gvozdjáková, A. et al., 2023; https://doi.org/10.1007/s11356-022-22949-2 | 2 groups: G1 = 14 LC patients compared to 15 healthy subjects (G2= CG), before and after MR. | Functional capacity of the lungs. Questionnaire for clinical symptoms before and after MR. Blood count and biochemical parameters. CoQ10 and TBARS. Mitochondrial bioenergetics in platelets. Citrate synthase as a mitochondrial marker. | Important adjustment of clinical symptoms, lung function, and regeneration of platelet mitochondrial metabolism after MR. | High-altitude SPA rehabilitation accelerates post-COVID recovery by improving mitochondrial bioenergetics. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ailioaie, L.M.; Ailioaie, C.; Litscher, G. Gut Microbiota and Mitochondria: Health and Pathophysiological Aspects of Long COVID. Int. J. Mol. Sci. 2023, 24, 17198. https://doi.org/10.3390/ijms242417198
Ailioaie LM, Ailioaie C, Litscher G. Gut Microbiota and Mitochondria: Health and Pathophysiological Aspects of Long COVID. International Journal of Molecular Sciences. 2023; 24(24):17198. https://doi.org/10.3390/ijms242417198
Chicago/Turabian StyleAilioaie, Laura Marinela, Constantin Ailioaie, and Gerhard Litscher. 2023. "Gut Microbiota and Mitochondria: Health and Pathophysiological Aspects of Long COVID" International Journal of Molecular Sciences 24, no. 24: 17198. https://doi.org/10.3390/ijms242417198
APA StyleAilioaie, L. M., Ailioaie, C., & Litscher, G. (2023). Gut Microbiota and Mitochondria: Health and Pathophysiological Aspects of Long COVID. International Journal of Molecular Sciences, 24(24), 17198. https://doi.org/10.3390/ijms242417198








