Evaluating Manganese, Zinc, and Copper Metal Toxicity on SH-SY5Y Cells in Establishing an Idiopathic Parkinson’s Disease Model
Abstract
:1. Introduction
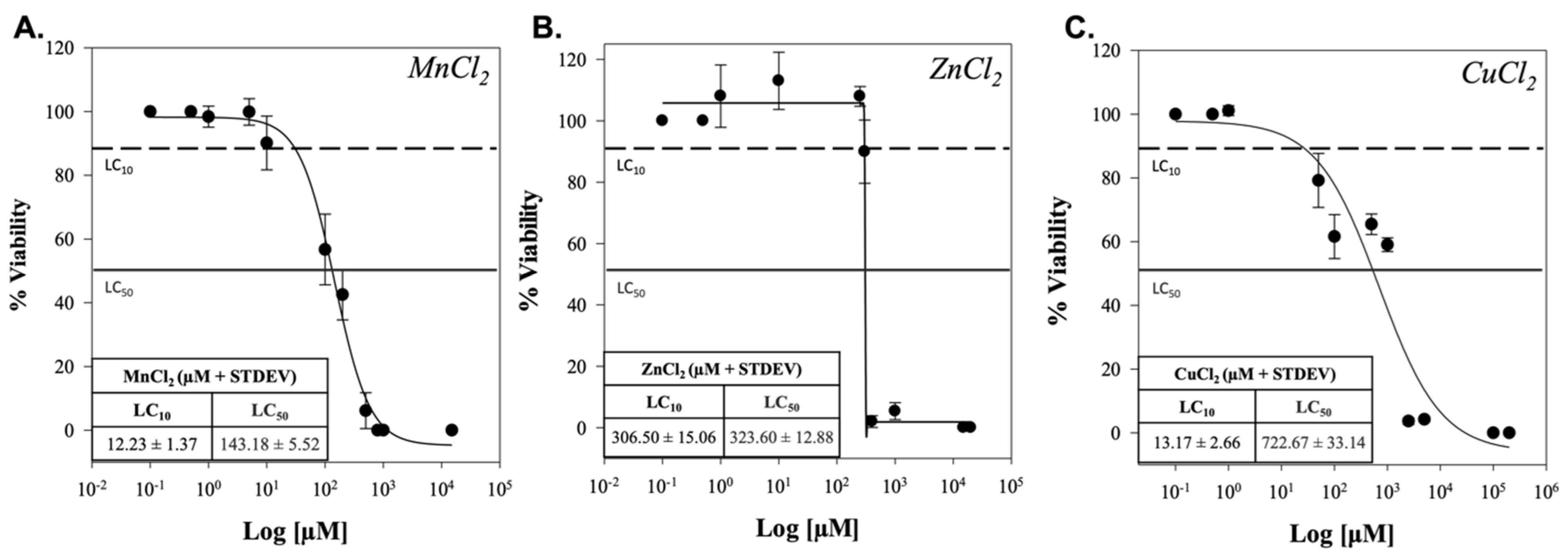
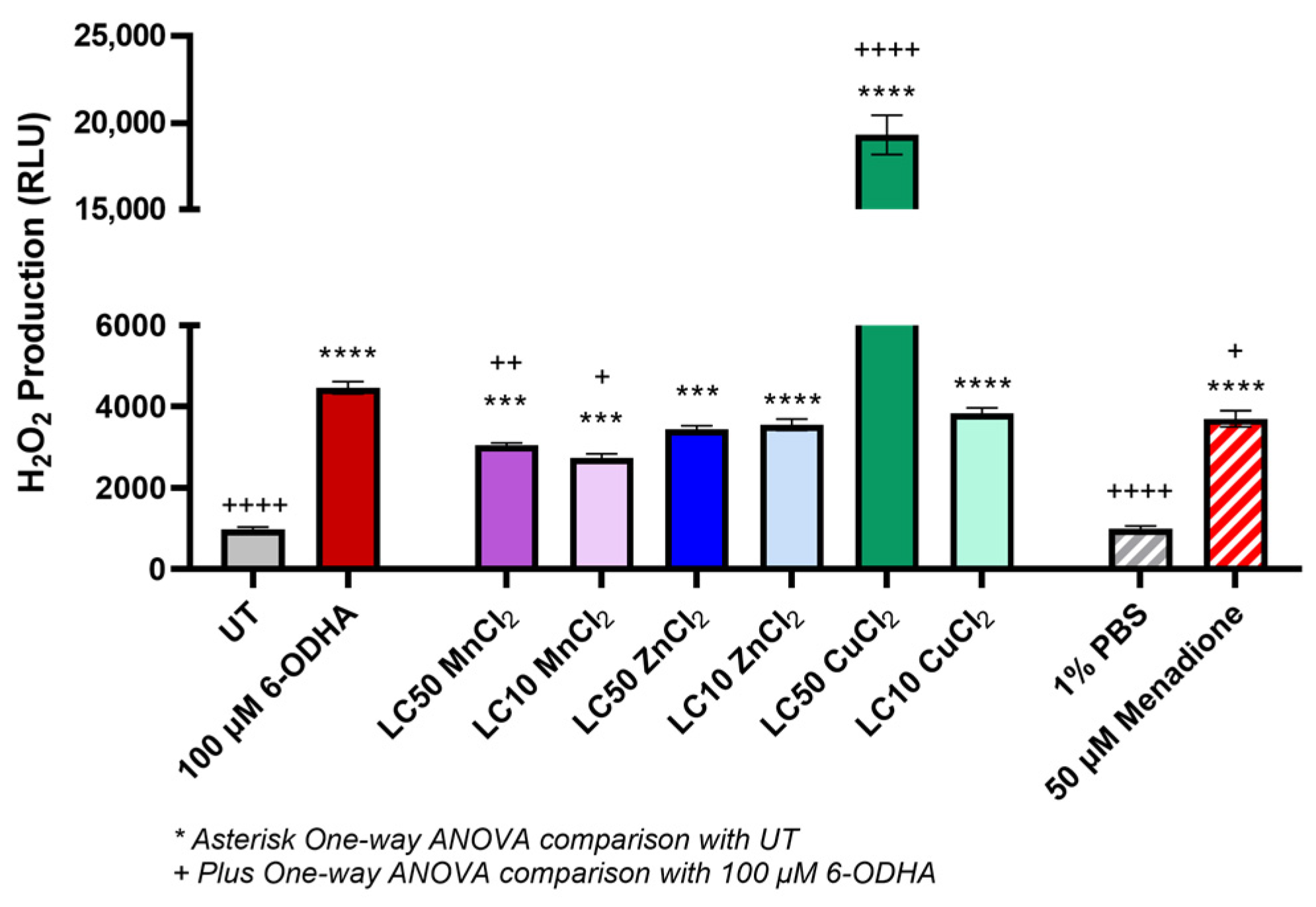
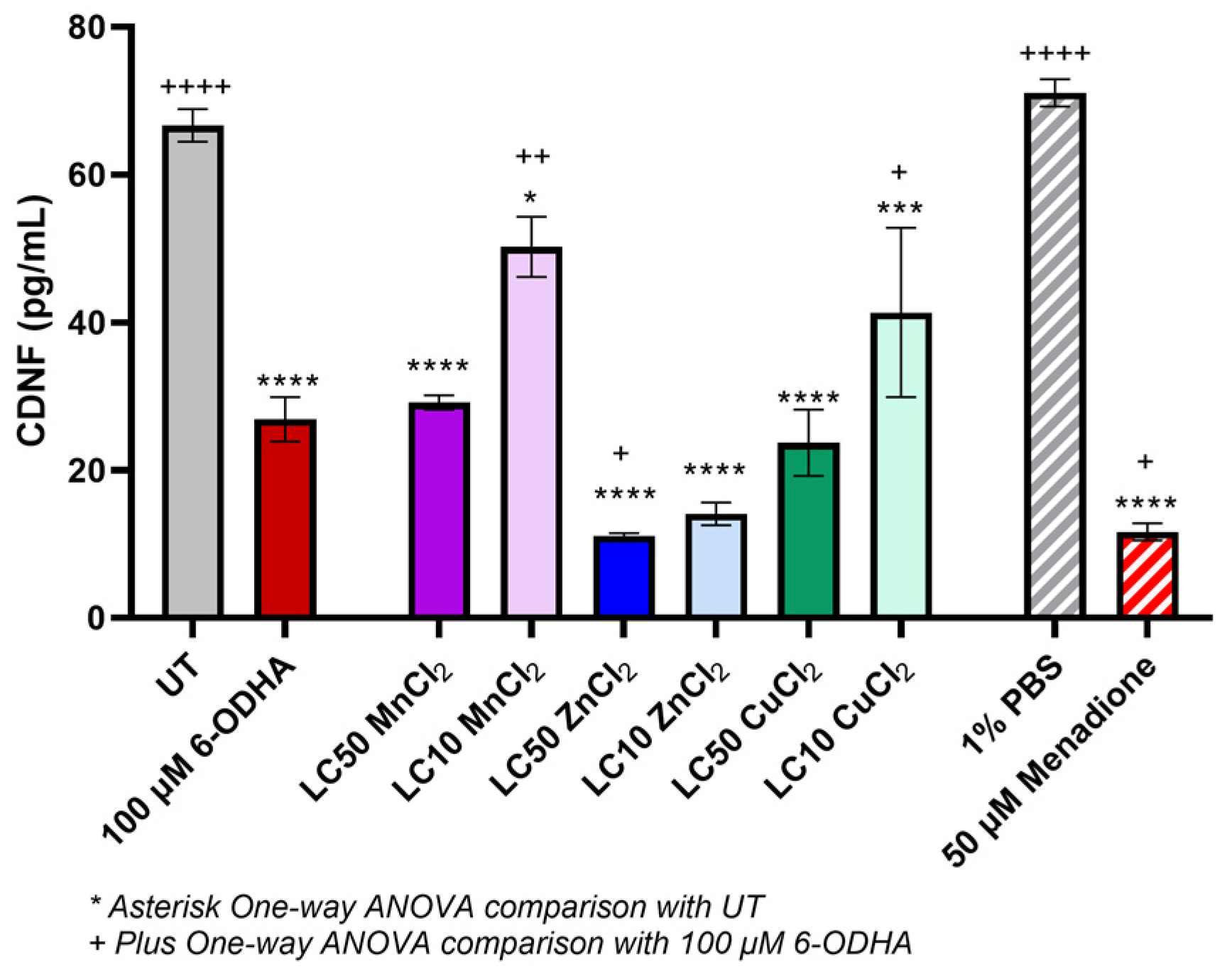
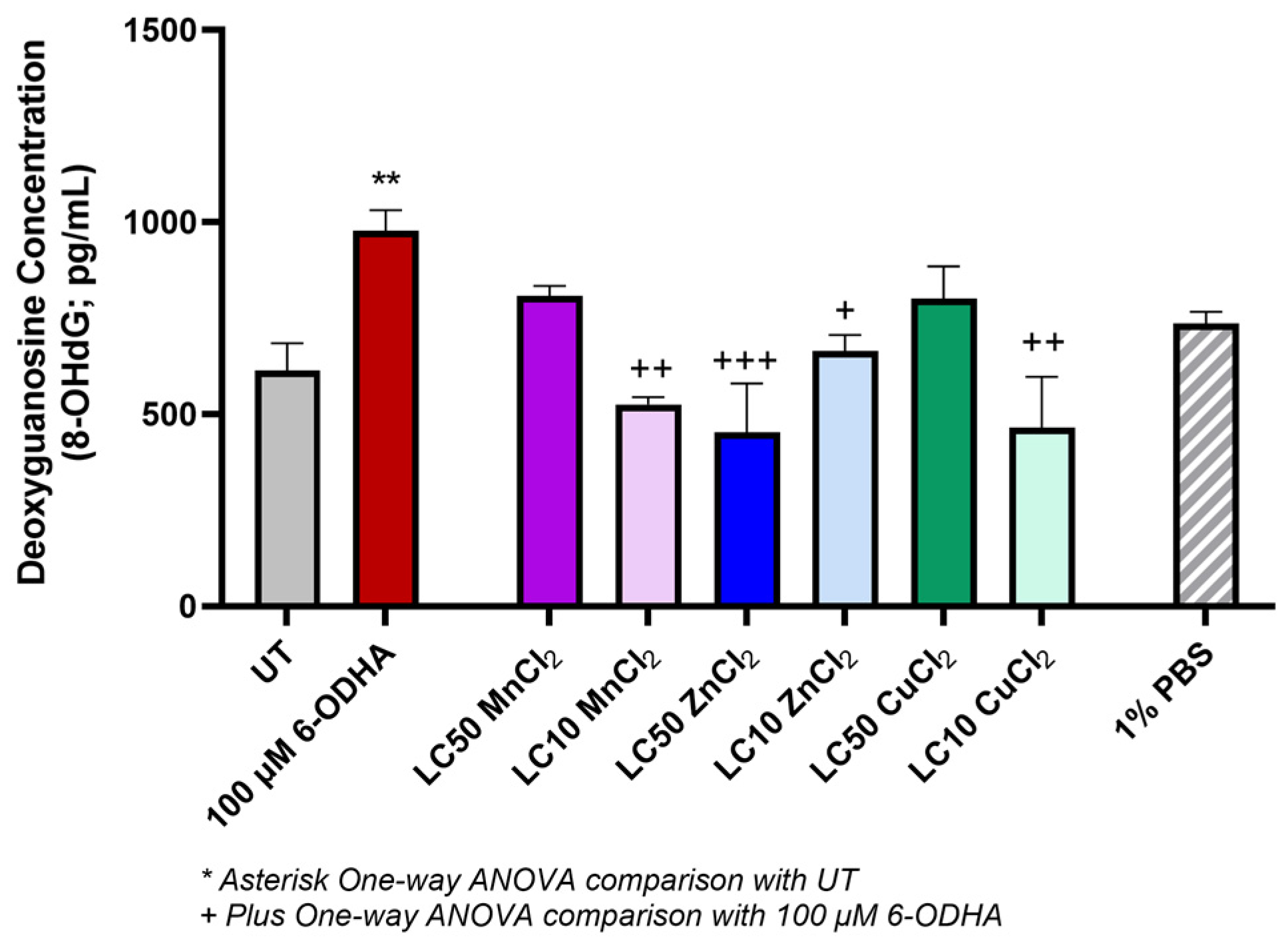
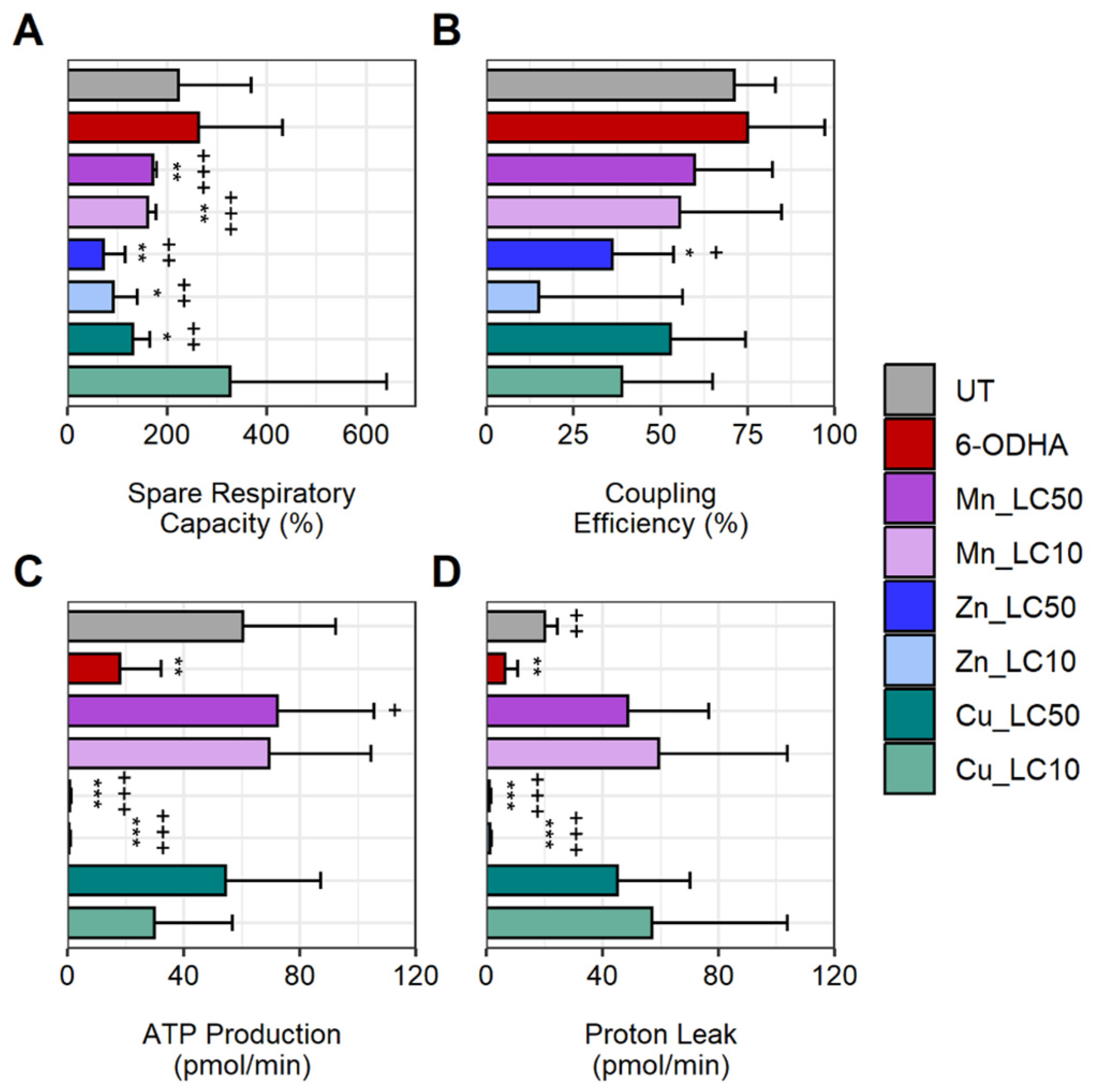
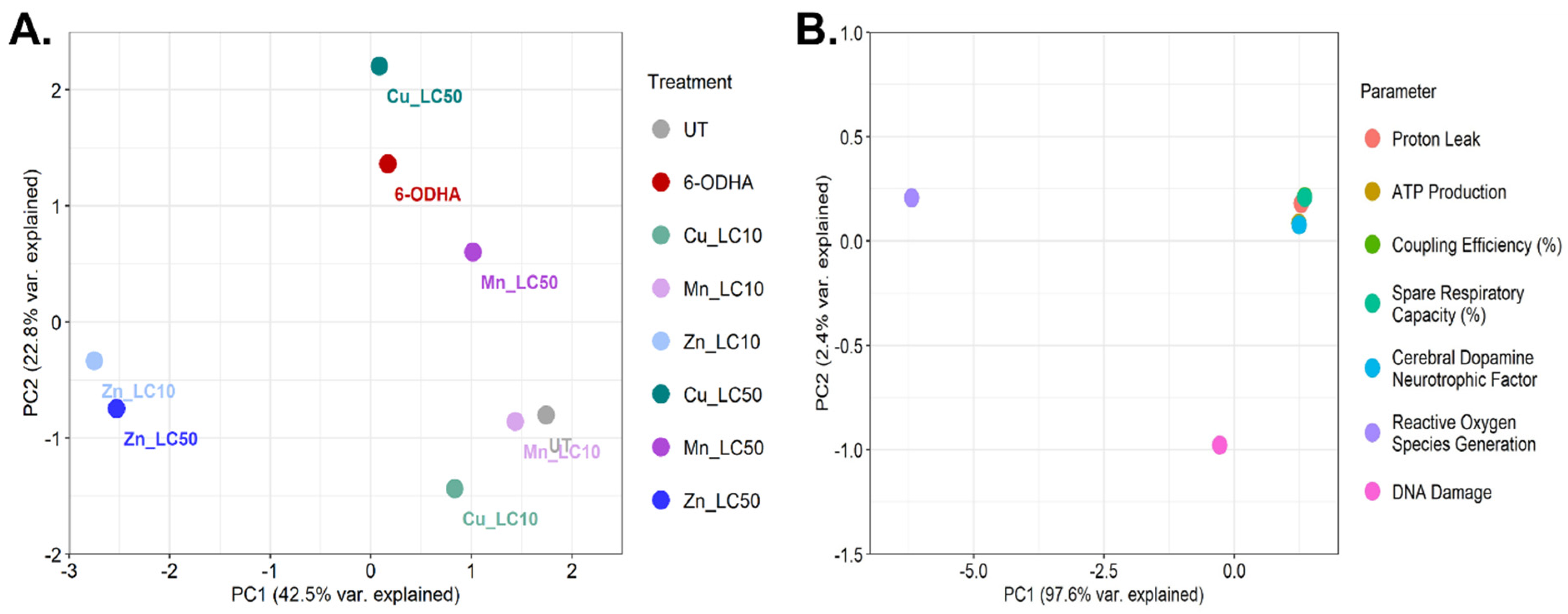
2. Results
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Maintaining Cell Culture
4.3. Dose–Response Curves
4.4. Oxidative Stress
4.5. Dopamine Concentration
4.6. DNA Damage
4.7. Mitochondrial Function
4.8. Principal Component Analysis
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nakmode, D.D.; Day, C.M.; Song, Y.; Garg, S. The Management of Parkinson’s Disease: An Overview of the Current Advancements in Drug Delivery Systems. Pharmaceutics 2023, 15, 1503. [Google Scholar] [CrossRef] [PubMed]
- Dorsey, E.; Sherer, T.; Okun, M.; Bloem, B. The emerging evidence of the Parkinson Pandemic. J. Park. Dis. 2018, 8 (Suppl. S1), S3–S8. [Google Scholar] [CrossRef] [PubMed]
- Bloem, B.R.; Okun, M.S.; Klein, C. Parkinson’s disease. Lancet 2021, 397, 2284–2303. [Google Scholar] [CrossRef] [PubMed]
- Ball, N.; Teo, W.-P.; Chandra, S.; Chapman, J. Parkinson’s disease and the environment. Front. Neurol. 2019, 10, 218. [Google Scholar] [CrossRef] [PubMed]
- Firestone, J.A.; Lundin, J.I.; Powers, K.M.; Smith-Weller, T.; Franklin, G.M.; Swanson, P.D.; Longstreth, W., Jr.; Checkoway, H. Occupational factors and risk of Parkinson’s disease: A population-based case–control study. Am. J. Ind. Med. 2010, 53, 217–223. [Google Scholar] [CrossRef]
- Nadig, A.P.; Huwaimel, B.; Alobaida, A.; Khafagy, E.-S.; Alotaibi, H.F.; Moin, A.; Lila, A.S.A.; Krishna, K. Manganese chloride (MnCl2) induced novel model of Parkinson’s disease in adult Zebrafish; Involvement of oxidative stress, neuroinflammation and apoptosis pathway. Biomed. Pharmacother. 2022, 155, 113697. [Google Scholar] [CrossRef]
- Pyatha, S.; Kim, H.; Lee, D.; Kim, K. Association between heavy metal exposure and Parkinson’s disease: A review of the mechanisms related to oxidative stress. Antioxidants 2022, 11, 2467. [Google Scholar] [CrossRef]
- Kim, H.; Lee, D.; Kim, K. Combined exposure to metals in drinking water alters the dopamine system in mouse striatum. Int. J. Environ. Res. Public Health 2021, 18, 6558. [Google Scholar] [CrossRef]
- Jellinger, K.A. Basic mechanisms of neurodegeneration: A critical update. J. Cell. Mol. Med. 2010, 14, 457–487. [Google Scholar] [CrossRef]
- Jurcau, A. Insights into the pathogenesis of neurodegenerative diseases: Focus on mitochondrial dysfunction and oxidative stress. Int. J. Mol. Sci. 2021, 22, 11847. [Google Scholar] [CrossRef]
- Engwa, G.A.; Ferdinand, P.U.; Nwalo, F.N.; Unachukwu, M.N. Mechanism and health effects of heavy metal toxicity in humans. Poisoning Mod. World-New Tricks Old Dog 2019, 10, 70–90. [Google Scholar]
- Wei, X.; Cai, M.; Jin, L. The function of the metals in regulating epigenetics during Parkinson’s disease. Front. Genet. 2021, 11, 616083. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Aschner, M.; Ghersi-Egea, J.-F. Brain barrier systems: A new frontier in metal neurotoxicological research. Toxicol. Appl. Pharmacol. 2003, 192, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Dong, X. Current strategies for brain drug delivery. Theranostics 2018, 8, 1481. [Google Scholar] [CrossRef] [PubMed]
- Genoud, S.; Roberts, B.R.; Gunn, A.P.; Halliday, G.M.; Lewis, S.J.; Ball, H.J.; Hare, D.J.; Double, K.L. Subcellular compartmentalisation of copper, iron, manganese, and zinc in the Parkinson’s disease brain. Metallomics 2017, 9, 1447–1455. [Google Scholar] [CrossRef] [PubMed]
- Elfawy, H.A.; Das, B. Crosstalk between mitochondrial dysfunction, oxidative stress, and age related neurodegenerative disease: Etiologies and therapeutic strategies. Life Sci. 2019, 218, 165–184. [Google Scholar] [CrossRef]
- Dusek, P.; Roos, P.M.; Litwin, T.; Schneider, S.A.; Flaten, T.P.; Aaseth, J. The neurotoxicity of iron, copper and manganese in Parkinson’s and Wilson’s diseases. J. Trace Elem. Med. Biol. 2015, 31, 193–203. [Google Scholar] [CrossRef]
- Li, G.J.; Zhang, L.-L.; Lu, L.; Wu, P.; Zheng, W. Occupational exposure to welding fume among welders: Alterations of manganese, iron, zinc, copper, and lead in body fluids and the oxidative stress status. J. Occup. Environ. Med. Am. Coll. Occup. Environ. Med. 2004, 46, 241. [Google Scholar] [CrossRef]
- Mezzaroba, L.; Alfieri, D.F.; Simão, A.N.C.; Reiche, E.M.V. The role of zinc, copper, manganese and iron in neurodegenerative diseases. Neurotoxicology 2019, 74, 230–241. [Google Scholar] [CrossRef]
- Ordonez-Librado, J.L.; Anaya-Martinez, V.; Gutierrez-Valdez, A.L.; Colín-Barenque, L.; Montiel-Flores, E.; Avila-Costa, M.R. Manganese inhalation as a Parkinson disease model. Park. Dis. 2011, 2011, 612989. [Google Scholar] [CrossRef]
- Kumar, V.; Singh, B.K.; Chauhan, A.K.; Singh, D.; Patel, D.K.; Singh, C. Minocycline rescues from zinc-induced nigrostriatal dopaminergic neurodegeneration: Biochemical and molecular interventions. Mol. Neurobiol. 2016, 53, 2761–2777. [Google Scholar] [CrossRef] [PubMed]
- Schober, A. Classic toxin-induced animal models of Parkinson’s disease: 6-OHDA and MPTP. Cell Tissue Res. 2004, 318, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Blum, D.; Torch, S.; Lambeng, N.; Nissou, M.-F.; Benabid, A.-L.; Sadoul, R.; Verna, J.-M. Molecular pathways involved in the neurotoxicity of 6-OHDA, dopamine and MPTP: Contribution to the apoptotic theory in Parkinson’s disease. Prog. Neurobiol. 2001, 65, 135–172. [Google Scholar] [CrossRef] [PubMed]
- Kavanagh, E.T.; Loughlin, J.P.; Herbert, K.R.; Dockery, P.; Samali, A.; Doyle, K.M.; Gorman, A.M. Functionality of NGF-protected PC12 cells following exposure to 6-hydroxydopamine. Biochem. Biophys. Res. Commun. 2006, 351, 890–895. [Google Scholar] [CrossRef]
- Solesio, M.E.; Saez-Atienzar, S.; Jordán, J.; Galindo, M.F. Characterization of mitophagy in the 6-hydoxydopamine Parkinson’s disease model. Toxicol. Sci. 2012, 129, 411–420. [Google Scholar] [CrossRef]
- Shipley, M.M.; Mangold, C.A.; Szpara, M.L. Differentiation of the SH-SY5Y human neuroblastoma cell line. J. Vis. Exp. JoVE 2016, 108, e53193. [Google Scholar]
- Tarale, P.; Sivanesan, S.; Daiwile, A.P.; Stöger, R.; Bafana, A.; Naoghare, P.K.; Parmar, D.; Chakrabarti, T.; Kannan, K. Global DNA methylation profiling of manganese-exposed human neuroblastoma SH-SY5Y cells reveals epigenetic alterations in Parkinson’s disease-associated genes. Arch. Toxicol. 2017, 91, 2629–2641. [Google Scholar] [CrossRef]
- Tarale, P.; Daiwile, A.P.; Sivanesan, S.; Stöger, R.; Bafana, A.; Naoghare, P.K.; Parmar, D.; Chakrabarti, T.; Krishnamurthi, K. Manganese exposure: Linking down-regulation of miRNA-7 and miRNA-433 with α-synuclein overexpression and risk of idiopathic Parkinson’s disease. Toxicol. Vitr. 2018, 46, 94–101. [Google Scholar] [CrossRef]
- Snoderly-Foster, L.J.; Olivas, W.M. Regulation of Parkinson’s disease-associated genes by Pumilio proteins and microRNAs in SH-SY5Y neuronal cells. PLoS ONE 2022, 17, e0275235. [Google Scholar] [CrossRef]
- Myers, J.E.; Fourie, M.; Zogoe, H.A.; Naik, I.; Theodorou, P.; Tassel, H.; Daya, A.; Thompson, M.L. Nervous system effects of occupational manganese exposure on South African manganese mineworkers. Neurotoxicology 2003, 24, 649–656. [Google Scholar] [CrossRef]
- Bowler, R.M.; Gysens, S.; Diamond, E.; Nakagawa, S.; Drezgic, M.; Roels, H.A. Manganese exposure: Neuropsychological and neurological symptoms and effects in welders. Neurotoxicology 2006, 27, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Dwane, S.; Durack, E.; Kiely, P.A. Optimising parameters for the differentiation of SH-SY5Y cells to study cell adhesion and cell migration. BMC Res. Notes 2013, 6, 366. [Google Scholar] [CrossRef] [PubMed]
- De Joode, B.V.W.; Barbeau, B.; Bouchard, M.F.; Mora, A.M.; Skytt, Å.; Córdoba, L.; Quesada, R.; Lundh, T.; Lindh, C.H.; Mergler, D. Manganese concentrations in drinking water from villages near banana plantations with aerial mancozeb spraying in Costa Rica: Results from the Infants’ Environmental Health Study (ISA). Environ. Pollut. 2016, 215, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Aiken, M.L.; Pace, C.E.; Ramachandran, M.; Schwabe, K.A.; Ajami, H.; Link, B.G.; Ying, S.C. Disparities in Drinking Water Manganese Concentrations in Domestic Wells and Community Water Systems in the Central Valley, CA, USA. Environ. Sci. Technol. 2023, 57, 1987–1996. [Google Scholar] [CrossRef]
- Song, W.; Zhang, J.; Guo, J.; Zhang, J.; Ding, F.; Li, L.; Sun, Z. Role of the dissolved zinc ion and reactive oxygen species in cytotoxicity of ZnO nanoparticles. Toxicol. Lett. 2010, 199, 389–397. [Google Scholar] [CrossRef]
- Lin, W.; Xu, Y.; Huang, C.-C.; Ma, Y.; Shannon, K.B.; Chen, D.-R.; Huang, Y.-W. Toxicity of nano-and micro-sized ZnO particles in human lung epithelial cells. J. Nanopart. Res. 2009, 11, 25–39. [Google Scholar] [CrossRef]
- Raj, K.; Kaur, P.; Gupta, G.; Singh, S. Metals associated neurodegeneration in Parkinson’s disease: Insight to physiological, pathological mechanisms and management. Neurosci. Lett. 2021, 753, 135873. [Google Scholar] [CrossRef]
- Chen, P.; Miah, M.R.; Aschner, M. Metals and neurodegeneration. F1000Research 2016, 5, 366. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Baronio, D.; Semenova, S.; Abdurakhmanova, S.; Panula, P. Cerebral dopamine neurotrophic factor regulates multiple neuronal subtypes and behavior. J. Neurosci. 2020, 40, 6146–6164. [Google Scholar] [CrossRef]
- Zhang, C.; Dischler, A.; Glover, K.; Qin, Y. Neuronal signalling of zinc: From detection and modulation to function. Open Biol. 2022, 12, 220188. [Google Scholar] [CrossRef]
- Tamano, H.; Morioka, H.; Nishio, R.; Takeuchi, A.; Takeda, A. AMPA-induced extracellular Zn2+ influx into nigral dopaminergic neurons causes movement disorder in rats. Neurotoxicology 2018, 69, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Lo, H.-S.; Chiang, H.-C.; Lin, A.M.; Chiang, H.-Y.; Chu, Y.-C.; Kao, L.-S. Synergistic effects of dopamine and Zn2+ on the induction of PC12 cell death and dopamine depletion in the striatum: Possible implication in the pathogenesis of Parkinson’s disease. Neurobiol. Dis. 2004, 17, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.-H.; Lee, S.R.; Park, Y.J.; Ra, M.; Lee, Y.; Pang, C.; Kim, K.H. Suppression of 6-hydroxydopamine-induced oxidative stress by hyperoside via activation of Nrf2/HO-1 signaling in dopaminergic neurons. Int. J. Mol. Sci. 2019, 20, 5832. [Google Scholar] [CrossRef] [PubMed]
- Di Marzo, N.; Chisci, E.; Giovannoni, R. The role of hydrogen peroxide in redox-dependent signaling: Homeostatic and pathological responses in mammalian cells. Cells 2018, 7, 156. [Google Scholar] [CrossRef]
- Dias, V.; Junn, E.; Mouradian, M.M. The role of oxidative stress in Parkinson’s disease. J. Park. Dis. 2013, 3, 461–491. [Google Scholar] [CrossRef]
- Guo, J.D.; Zhao, X.; Li, Y.; Li, G.R.; Liu, X.L. Damage to dopaminergic neurons by oxidative stress in Parkinson’s disease. Int. J. Mol. Med. 2018, 41, 1817–1825. [Google Scholar] [CrossRef]
- Umeno, A.; Biju, V.; Yoshida, Y. In vivo ROS production and use of oxidative stress-derived biomarkers to detect the onset of diseases such as Alzheimer’s disease, Parkinson’s disease, and diabetes. Free Radic. Res. 2017, 51, 413–427. [Google Scholar] [CrossRef]
- Lan, A.; Chen, J.; Chai, Z.; Hu, Y. The neurotoxicity of iron, copper and cobalt in Parkinson’s disease through ROS-mediated mechanisms. Biometals 2016, 29, 665–678. [Google Scholar] [CrossRef]
- Huang, X.; Cuajungco, M.P.; Atwood, C.S.; Hartshorn, M.A.; Tyndall, J.D.; Hanson, G.R.; Stokes, K.C.; Leopold, M.; Multhaup, G.; Goldstein, L.E. Cu (II) potentiation of Alzheimer Aβ neurotoxicity: Correlation with cell-free hydrogen peroxide production and metal reduction. J. Biol. Chem. 1999, 274, 37111–37116. [Google Scholar] [CrossRef]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, C. 8-hydroxy-2′-deoxyguanosine (8-OHdG): A critical biomarker of oxidative stress and carcinogenesis. J. Environ. Sci. Health Part C 2009, 27, 120–139. [Google Scholar] [CrossRef]
- Wang, Z.-X.; Li, Y.-L.; Pu, J.-L.; Zhang, B.-R. DNA damage-mediated neurotoxicity in Parkinson’s disease. Int. J. Mol. Sci. 2023, 24, 6313. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Mizuno, Y.; Hattori, N. Urinary 8-hydroxydeoxyguanosine levels as a biomarker for progression of Parkinson disease. Neurology 2005, 64, 1081–1083. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, A.P.; Schneider, J.A.; Nelson, B.C.; Atha, D.H.; Jain, A.; Soliman, K.F.; Aschner, M.; Mazzio, E.; Reams, R.R. Manganese-induced oxidative DNA damage in neuronal SH-SY5Y cells: Attenuation of thymine base lesions by glutathione and N-acetylcysteine. Toxicol. Lett. 2013, 218, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Kulikova, O.I.; Fedorova, T.N.; Lopachev, A.V.; Orlova, V.S.; Grachev, V.A. Effects of antioxidants on the viability of the human neuroblastoma SH-SY5Y cell culture under the conditions of heavy-metal toxicity. Biol. Med. 2016, 8, 1. [Google Scholar] [CrossRef]
- Zambrano, K.; Barba, D.; Castillo, K.; Noboa, L.; Argueta-Zamora, D.; Robayo, P.; Arizaga, E.; Caicedo, A.; Gavilanes, A.W. Fighting Parkinson’s disease: The return of the mitochondria. Mitochondrion 2022, 64, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Vergara, R.C.; Jaramillo-Riveri, S.; Luarte, A.; Moënne-Loccoz, C.; Fuentes, R.; Couve, A.; Maldonado, P.E. The energy homeostasis principle: Neuronal energy regulation drives local network dynamics generating behavior. Front. Comput. Neurosci. 2019, 13, 49. [Google Scholar] [CrossRef]
- Liu, H.Y.; Gale, J.R.; Reynolds, I.J.; Weiss, J.H.; Aizenman, E. The multifaceted roles of zinc in neuronal mitochondrial dysfunction. Biomedicines 2021, 9, 489. [Google Scholar] [CrossRef]
- Marchetti, P.; Fovez, Q.; Germain, N.; Khamari, R.; Kluza, J. Mitochondrial spare respiratory capacity: Mechanisms, regulation, and significance in non-transformed and cancer cells. FASEB J. 2020, 34, 13106–13124. [Google Scholar] [CrossRef]
- Yamamoto, H.; Morino, K.; Mengistu, L.; Ishibashi, T.; Kiriyama, K.; Ikami, T.; Maegawa, H. Amla enhances mitochondrial spare respiratory capacity by increasing mitochondrial biogenesis and antioxidant systems in a murine skeletal muscle cell line. Oxidative Med. Cell. Longev. 2016, 2016, 1735841. [Google Scholar] [CrossRef]
- Li, X.; Li, X.; Xiang, C.; Ye, F. Lead exposure represses mitochondrial metabolism by activation of heme-binding protein BACH1 in differentiated SH-SY5Y cell. Sci. Total Environ. 2022, 853, 158665. [Google Scholar] [CrossRef]
- Aring, L.; Choi, E.K.; Kopera, H.; Lanigan, T.; Iwase, S.; Klionsky, D.J.; Seo, Y.A. A neurodegeneration gene, WDR45, links impaired ferritinophagy to iron accumulation. J. Neurochem. 2022, 160, 356–375. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.; Hao, L.; Bijli, K.M.; Chandler, J.D.; Orr, M.; Hu, X.; Jones, D.P.; Go, Y.-M. From the cover: Manganese stimulates mitochondrial H2O2 production in SH-SY5Y human neuroblastoma cells over physiologic as well as toxicologic range. Toxicol. Sci. 2017, 155, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, M.; Li, Z.; Yue, J.; Xu, M.; Zhang, Y.; Yung, K.K.L.; Li, R. Fine particulate matter induces mitochondrial dysfunction and oxidative stress in human SH-SY5Y cells. Chemosphere 2019, 218, 577–588. [Google Scholar] [CrossRef] [PubMed]
- Maddirala, Y.; Tobwala, S.; Ercal, N. N-acetylcysteineamide protects against manganese-induced toxicity in SHSY5Y cell line. Brain Res. 2015, 1608, 157–166. [Google Scholar] [CrossRef]
- Baldovinos, Y.; Archer, A.; Salamanca, J.; Strongin, R.M.; Sayes, C.M. Chemical Interactions and Cytotoxicity of Terpene and Diluent Vaping Ingredients. Chem. Res. Toxicol. 2022, 36, 589–597. [Google Scholar] [CrossRef]
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J. Welcome to the tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Kassambara, A. Ggpubr:“Ggplot2” Based Publication Ready Plots (Version 0.1.7). Obtido Desde. 2018. Available online: https://CRAN.R-project.org/package=ggpubr (accessed on 28 September 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pradhan, S.H.; Liu, J.Y.; Sayes, C.M. Evaluating Manganese, Zinc, and Copper Metal Toxicity on SH-SY5Y Cells in Establishing an Idiopathic Parkinson’s Disease Model. Int. J. Mol. Sci. 2023, 24, 16129. https://doi.org/10.3390/ijms242216129
Pradhan SH, Liu JY, Sayes CM. Evaluating Manganese, Zinc, and Copper Metal Toxicity on SH-SY5Y Cells in Establishing an Idiopathic Parkinson’s Disease Model. International Journal of Molecular Sciences. 2023; 24(22):16129. https://doi.org/10.3390/ijms242216129
Chicago/Turabian StylePradhan, Sahar H., James Y. Liu, and Christie M. Sayes. 2023. "Evaluating Manganese, Zinc, and Copper Metal Toxicity on SH-SY5Y Cells in Establishing an Idiopathic Parkinson’s Disease Model" International Journal of Molecular Sciences 24, no. 22: 16129. https://doi.org/10.3390/ijms242216129
APA StylePradhan, S. H., Liu, J. Y., & Sayes, C. M. (2023). Evaluating Manganese, Zinc, and Copper Metal Toxicity on SH-SY5Y Cells in Establishing an Idiopathic Parkinson’s Disease Model. International Journal of Molecular Sciences, 24(22), 16129. https://doi.org/10.3390/ijms242216129






