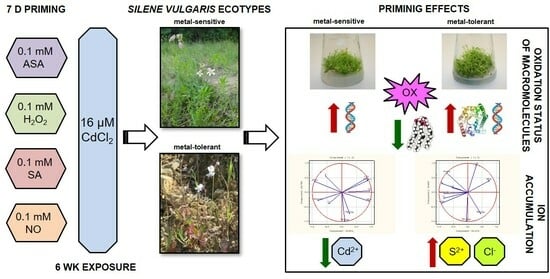
Response to Cadmium in Silene vulgaris Ecotypes Is Distinctly Affected by Priming-Induced Changes in Oxidation Status of Macromolecules
Abstract
:1. Introduction
2. Results
2.1. Growth Parameters
2.2. Enzymatic and Nonenzymatic Oxidation of Lipids
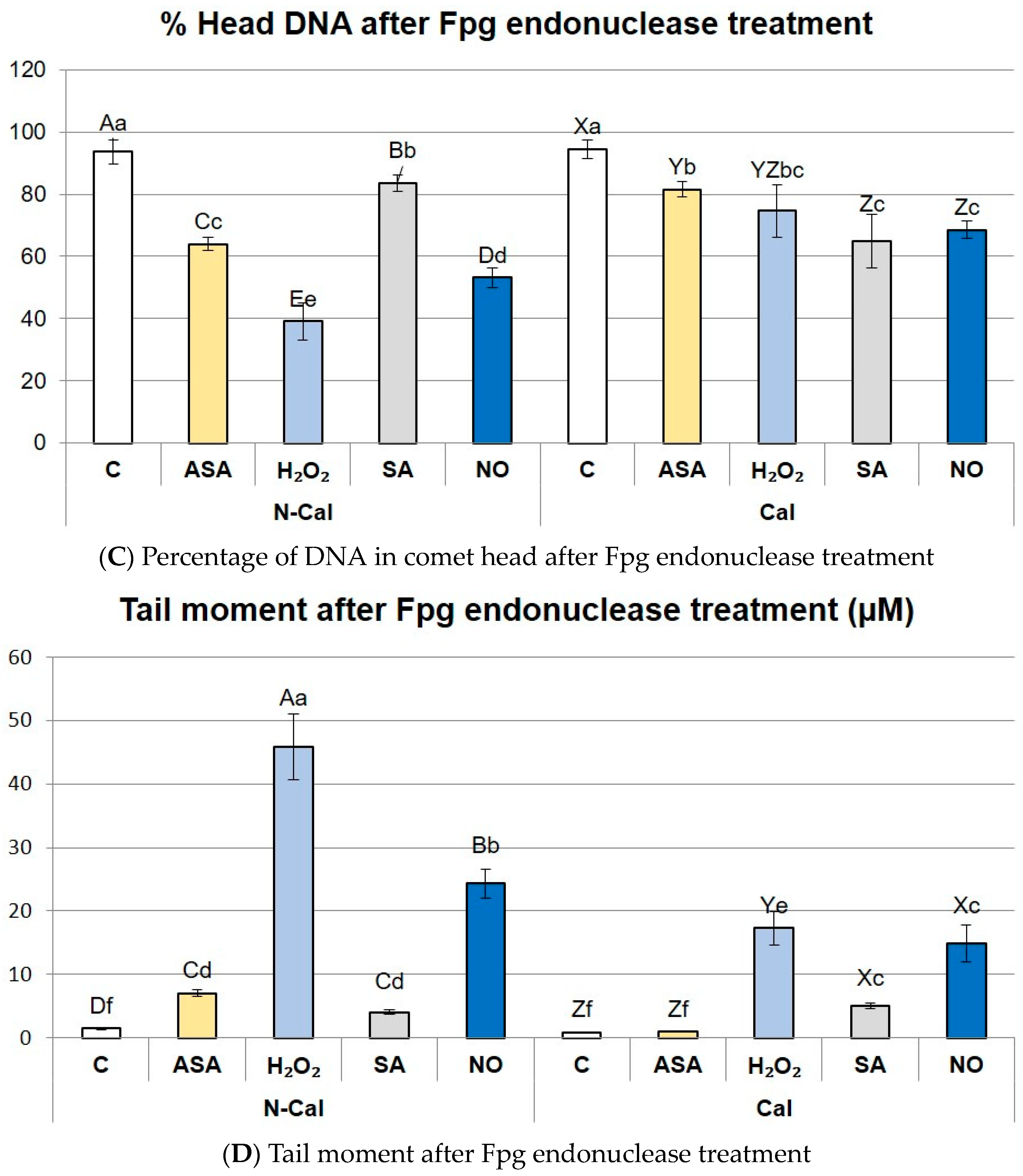
2.3. Assessment of DNA Damage and Excision of Oxidized Bases by Repair Enzyme
2.4. Protein Content and Its Oxidation Status
2.5. Cadmium Accumulation
2.6. Ionic Balance
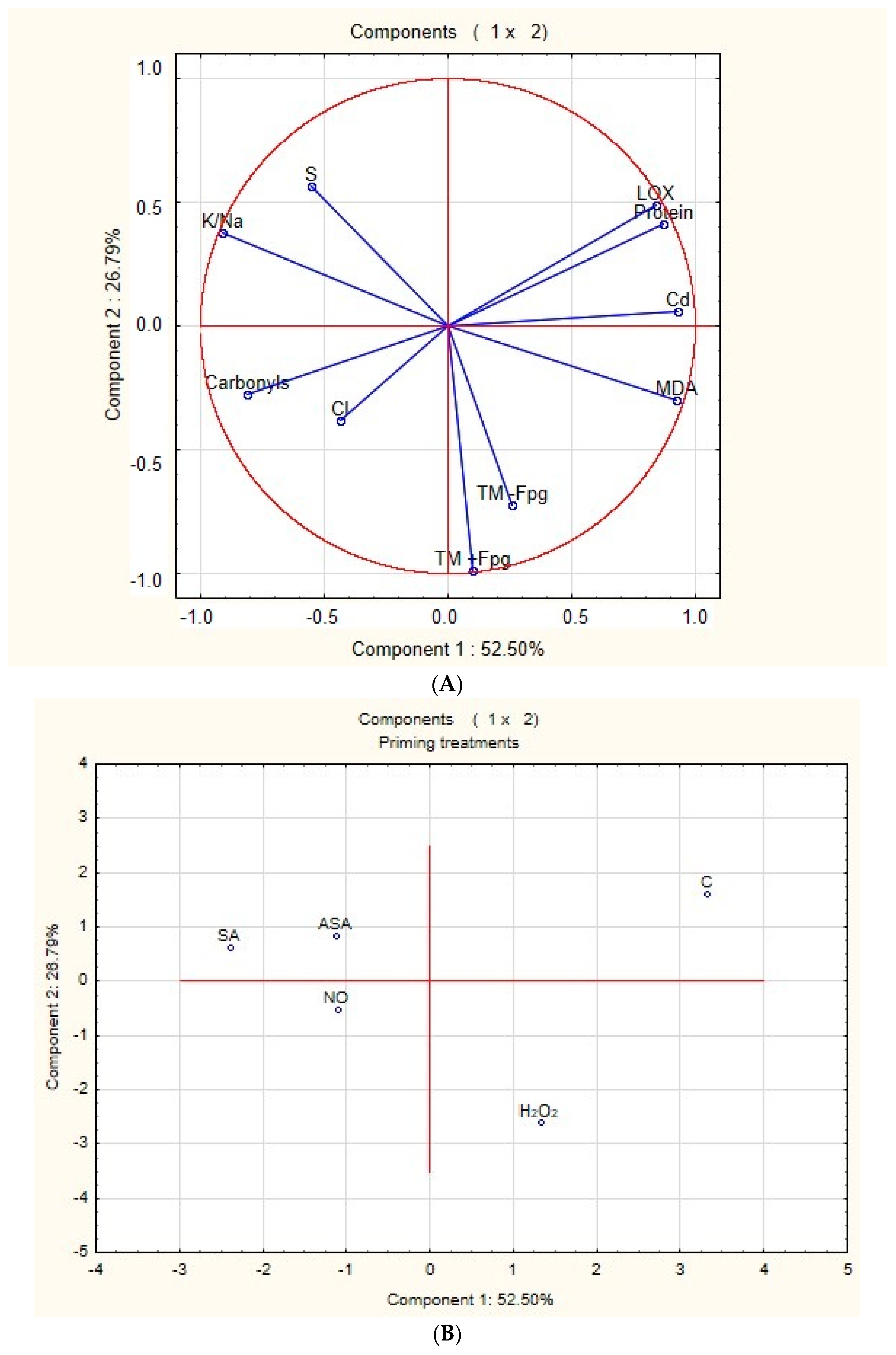
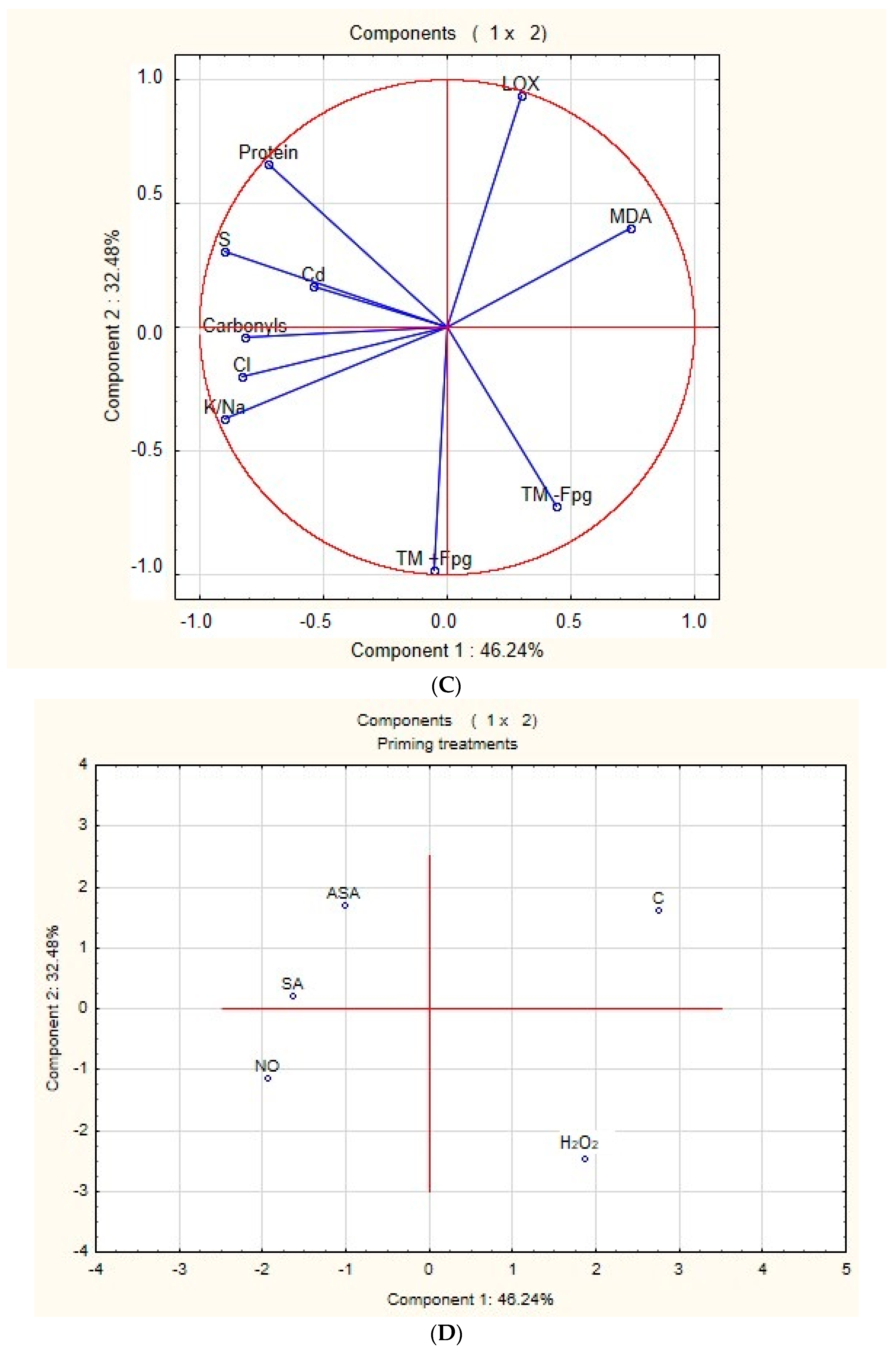
2.7. Principal Component Analysis (PCA)
3. Discussion
3.1. Ecotypes’ Growth Characteristics Were Similarly Modulated via Priming
3.2. Oxidation Status in Non-Primed Shoots Treated with Cd
3.3. Effect of Priming on Macromolecule Oxidation under Cd Treatment
4. Materials and Methods
4.1. Plant Material, Experimental Design, and Assessment of Growth Parameters
4.2. Biochemical Analyses
4.2.1. Malondialdehyde (MDA) Content
4.2.2. Lipoxygenase (LOX) Activity
4.2.3. Protein Carbonylation
4.3. Determination of DNA Oxidative Damage
4.4. Determination of Cd, Cl, K, Na, and S Contents
4.5. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Suhani, I.; Sahab, S.; Srivastava, V.; Singh, R.P. Impact of Cadmium Pollution on Food Safety and Human Health. Curr. Opin. Toxicol. 2021, 27, 1–7. [Google Scholar] [CrossRef]
- Kumar, A.; Subrahmanyam, G.; Mondal, R.; Cabral-Pinto, M.M.S.; Shabnam, A.A.; Jigyasu, D.K.; Malyan, S.K.; Fagodiya, R.K.; Khan, S.A.; Kumar, A.; et al. Bio-Remediation Approaches for Alleviation of Cadmium Contamination in Natural Resources. Chemosphere 2021, 268, 128855. [Google Scholar] [CrossRef]
- Cuypers, A.; Plusquin, M.; Remans, T.; Jozefczak, M.; Keunen, E.; Gielen, H.; Opdenakker, K.; Nair, A.R.; Munters, E.; Artois, T.J.; et al. Cadmium Stress: An Oxidative Challenge. Biometals 2010, 23, 927–940. [Google Scholar] [CrossRef] [PubMed]
- Graska, J.; Fidler, J.; Gietler, M.; Prabucka, B.; Nykiel, M.; Labudda, M. Nitric Oxide in Plant Functioning: Metabolism, Signaling, and Responses to Infestation with Ecdysozoa Parasites. Biology 2023, 12, 927. [Google Scholar] [CrossRef] [PubMed]
- Møller, I.M.; Jensen, P.E.; Hansson, A. Oxidative Modifications to Cellular Components in Plants. Annu. Rev. Plant Biol. 2007, 58, 459–481. [Google Scholar] [CrossRef] [PubMed]
- Anjum, N.A.; Sofo, A.; Scopa, A.; Roychoudhury, A.; Gill, S.S.; Iqbal, M.; Lukatkin, A.S.; Pereira, E.; Duarte, A.C.; Ahmad, I. Lipids and Proteins—Major Targets of Oxidative Modifications in Abiotic Stressed Plants. Environ. Sci. Pollut. Res. 2015, 22, 4099–4121. [Google Scholar] [CrossRef]
- Roldán-Arjona, T.; Ariza, R.R. Repair and Tolerance of Oxidative DNA Damage in Plants. Mutat. Res. 2009, 681, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Chmielowska-Bąk, J.; Izbiańska, K.; Deckert, J. Products of Lipid, Protein and RNA Oxidation as Signals and Regulators of Gene Expression in Plants. Front. Plant Sci. 2015, 6, 405. [Google Scholar] [CrossRef]
- Agger, J.W.; Isaksen, T.; Várnai, A.; Vidal-Melgosa, S.; Willats, W.G.T.; Ludwig, R.; Horn, S.J.; Eijsink, V.G.H.; Westereng, B. Discovery of LPMO Activity on Hemicelluloses Shows the Importance of Oxidative Processes in Plant Cell Wall Degradation. Proc. Natl. Acad. Sci. USA 2014, 111, 6287–6292. [Google Scholar] [CrossRef]
- Bazin, J.; Langlade, N.; Vincourt, P.; Arribat, S.; Balzergue, S.; El-Maarouf-Bouteau, H.; Bailly, C. Targeted mRNA Oxidation Regulates Sunflower Seed Dormancy Alleviation during Dry After-Ripening. Plant Cell 2011, 23, 2196–2208. [Google Scholar] [CrossRef]
- Wiszniewska, A. Priming Strategies for Benefiting Plant Performance under Toxic Trace Metal Exposure. Plants 2021, 10, 623. [Google Scholar] [CrossRef] [PubMed]
- Labudda, M.; Dziurka, K.; Fidler, J.; Gietler, M.; Rybarczyk-Płońska, A.; Nykiel, M.; Prabucka, B.; Morkunas, I.; Muszyńska, E. The Alleviation of Metal Stress Nuisance for Plants—A Review of Promising Solutions in the Face of Environmental Challenges. Plants 2022, 11, 2544. [Google Scholar] [CrossRef] [PubMed]
- Belkadhi, A.; De Haro, A.; Obregon, S.; Chaïbi, W.; Djebali, W. Positive Effects of Salicylic Acid Pretreatment on the Composition of Flax Plastidial Membrane Lipids under Cadmium Stress. Environ. Sci. Pollut. Res. 2015, 22, 1457–1467. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Nahar, K.; Gill, S.S.; Alharby, H.F.; Razafindrabe, B.H.N.; Fujita, M. Hydrogen Peroxide Pretreatment Mitigates Cadmium-Induced Oxidative Stress in Brassica napus L.: An Intrinsic Study on Antioxidant Defense and Glyoxalase Systems. Front. Plant Sci. 2017, 8, 115. [Google Scholar] [CrossRef]
- Kabała, K.; Zboińska, M.; Głowiak, D.; Reda, M.; Jakubowska, D.; Janicka, M. Interaction between the Signaling Molecules Hydrogen Sulfide and Hydrogen Peroxide and Their Role in Vacuolar H+-ATPase Regulation in Cadmium-Stressed Cucumber Roots. Physiol. Plant. 2019, 166, 688–704. [Google Scholar] [CrossRef]
- Kaya, C.; Ashraf, M.; Alyemeni, M.N.; Ahmad, P. Responses of Nitric Oxide and Hydrogen Sulfide in Regulating Oxidative Defence System in Wheat Plants Grown under Cadmium Stress. Physiol. Plant. 2020, 168, 345–360. [Google Scholar] [CrossRef]
- Agami, R.A.; Mohamed, G.F. Exogenous Treatment with Indole-3-Acetic Acid and Salicylic Acid Alleviates Cadmium Toxicity in Wheat Seedlings. Ecotoxicol. Environ. Saf. 2013, 94, 164–171. [Google Scholar] [CrossRef]
- Fu, M.-M.; Dawood, M.; Wang, N.-H.; Wu, F. Exogenous Hydrogen Sulfide Reduces Cadmium Uptake and Alleviates Cadmium Toxicity in Barley. Plant Growth Regul. 2019, 89, 227–237. [Google Scholar] [CrossRef]
- Karalija, E.; Selović, A.; Dahija, S.; Demir, A.; Samardžić, J.; Vrobel, O.; Ćavar Zeljković, S.; Parić, A. Use of Seed Priming to Improve Cd Accumulation and Tolerance in Silene sendtneri, Novel Cd Hyper-Accumulator. Ecotoxicol. Environ. Saf. 2021, 210, 111882. [Google Scholar] [CrossRef]
- Dolui, D.; Hasanuzzaman, M.; Saha, I.; Ghosh, A.; Adak, M.K. Amelioration of Sodium and Arsenic Toxicity in Salvinia natans L. with 2,4-D Priming through Physiological Responses. Environ. Sci. Pollut. Res. 2022, 29, 9232–9247. [Google Scholar] [CrossRef]
- Baker, A.J.M.; Ernst, W.H.O.; Van Der Ent, A.; Malaisse, F.; Ginocchio, R. Metallophytes: The Unique Biological Resource, Its Ecology and Conservational Status in Europe, Central Africa and Latin America. In Ecology of Industrial Pollution; Batty, L.C., Hallberg, K.B., Eds.; Cambridge University Press: Cambridge, UK, 2010; pp. 7–40. ISBN 978-0-521-51446-0. [Google Scholar]
- Muszyńska, E.; Labudda, M.; Kamińska, I.; Górecka, M.; Bederska-Błaszczyk, M. Evaluation of Heavy Metal-Induced Responses in Silene vulgaris Ecotypes. Protoplasma 2019, 256, 1279–1297. [Google Scholar] [CrossRef] [PubMed]
- Swęd, M.; Uzarowicz, Ł.; Duczmal-Czernikiewicz, A.; Kwasowski, W.; Pędziwiatr, A.; Siepak, M.; Niedzielski, P. Forms of Metal(Loid)s in Soils Derived from Historical Calamine Mining Waste and Tailings of the Olkusz Zn–Pb Ore District, Southern Poland: A Combined Pedological, Geochemical and Mineralogical Approach. Appl. Geochem. 2022, 139, 105218. [Google Scholar] [CrossRef]
- Muszyńska, E.; Labudda, M.; Kral, A. Ecotype-Specific Pathways of Reactive Oxygen Species Deactivation in Facultative Metallophyte Silene vulgaris (Moench) Garcke Treated with Heavy Metals. Antioxidants 2020, 9, 102. [Google Scholar] [CrossRef]
- Huang, Q.; Xu, R.; Zhang, Y.; Yan, Z.; Chen, H.; Shao, G. Salicylic Acid Ameliorates Cadmium Toxicity by Increasing Nutrients Uptake and Upregulating Antioxidant Enzyme Activity and Uptake/Transport-Related Genes in Oryza sativa L. Indica. J. Plant Growth Regul. 2023, 42, 1158–1170. [Google Scholar] [CrossRef]
- Senaratna, T.; Touchell, D.; Bunn, E.; Dixon, K. Acetyl Salicylic Acid (Aspirin) and Salicylic Acid Induce Multiple Stress Tolerance in Bean and Tomato Plants. Plant Growth Regul. 2000, 30, 157–161. [Google Scholar] [CrossRef]
- Yıldız, M.; Terzi, H.; Bingül, N. Protective Role of Hydrogen Peroxide Pretreatment on Defense Systems and BnMP1 Gene Expression in Cr(VI)-Stressed Canola Seedlings. Ecotoxicology 2013, 22, 1303–1312. [Google Scholar] [CrossRef] [PubMed]
- Guzel, S.; Terzi, R. Exogenous Hydrogen Peroxide Increases Dry Matter Production, Mineral Content and Level of Osmotic Solutes in Young Maize Leaves and Alleviates Deleterious Effects of Copper Stress. Bot. Stud. 2013, 54, 26. [Google Scholar] [CrossRef] [PubMed]
- Yaseen, M.; Ahmad, T.; Sablok, G.; Standardi, A.; Hafiz, I.A. Review: Role of Carbon Sources for in Vitro Plant Growth and Development. Mol. Biol. Rep. 2013, 40, 2837–2849. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Jiang, F.; Wu, Z. The Apoplastic Oxidative Burst as a Key Factor of Hyperhydricity in Garlic Plantlet in Vitro. Plant Cell Tiss. Organ. Cult. 2015, 120, 571–584. [Google Scholar] [CrossRef]
- Repetto, M.; Semprine, J.; Boveris, A. Lipid Peroxidation: Chemical Mechanism, Biological Implications and Analytical Determination. In Lipid Peroxidation; IntechOpen: London, UK, 2012; ISBN 978-953-51-0716-3. [Google Scholar]
- Farmer, E.E.; Mueller, M.J. ROS-Mediated Lipid Peroxidation and RES-Activated Signaling. Annu. Rev. Plant Biol. 2013, 64, 429–450. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Gupta, D.K.; Tian, S.; Yang, X.; Li, T. Lead Tolerance and Physiological Adaptation Mechanism in Roots of Accumulating and Non-Accumulating Ecotypes of Sedum alfredii. Environ. Sci. Pollut. Res. 2012, 19, 1640–1651. [Google Scholar] [CrossRef] [PubMed]
- Chakhchar, A.; Wahbi, S.; Lamaoui, M.; Ferradous, A.; El Mousadik, A.; Ibnsouda-Koraichi, S.; Filali-Maltouf, A.; El Modafar, C. Physiological and Biochemical Traits of Drought Tolerance in Argania spinosa. J. Plant Interact. 2015, 10, 252–261. [Google Scholar] [CrossRef]
- Zemanová, V.; Pavlík, M.; Kyjaková, P.; Pavlíková, D. Fatty Acid Profiles of Ecotypes of Hyperaccumulator Noccaea caerulescens Growing under Cadmium Stress. J. Plant Physiol. 2015, 180, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Andreou, A.; Feussner, I. Lipoxygenases—Structure and Reaction Mechanism. Phytochemistry 2009, 70, 1504–1510. [Google Scholar] [CrossRef] [PubMed]
- Grebner, W.; Stingl, N.E.; Oenel, A.; Mueller, M.J.; Berger, S. Lipoxygenase6-Dependent Oxylipin Synthesis in Roots Is Required for Abiotic and Biotic Stress Resistance of Arabidopsis. Plant Physiol. 2013, 161, 2159–2170. [Google Scholar] [CrossRef]
- Koźmińska, A.; Wiszniewska, A.; Hanus-Fajerska, E.; Boscaiu, M.; Al Hassan, M.; Halecki, W.; Vicente, O. Identification of Salt and Drought Biochemical Stress Markers in Several Silene vulgaris Populations. Sustainability 2019, 11, 800. [Google Scholar] [CrossRef]
- Wiszniewska, A.; Kamińska, I.; Hanus-Fajerska, E.; Sliwinska, E.; Koźmińska, A. Distinct Co-Tolerance Responses to Combined Salinity and Cadmium Exposure in Metallicolous and Non-Metallicolous Ecotypes of Silene vulgaris. Ecotoxicol. Environ. Saf. 2020, 201, 110823. [Google Scholar] [CrossRef]
- Filipič, M. Mechanisms of Cadmium Induced Genomic Instability. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2012, 733, 69–77. [Google Scholar] [CrossRef]
- Chakrabarti, M.; Mukherjee, A. Investigating the Underlying Mechanism of Cadmium-Induced Plant Adaptive Response to Genotoxic Stress. Ecotoxicol. Environ. Saf. 2021, 209, 111817. [Google Scholar] [CrossRef]
- López-Orenes, A.; Santos, C.; Dias, M.C.; Oliveira, H.; Ferrer, M.Á.; Calderón, A.A.; Silva, S. Genotoxicity and Cytotoxicity Induced in Zygophyllum fabago by Low Pb Doses Depends on the Population’s Redox Plasticity. Horticulturae 2021, 7, 455. [Google Scholar] [CrossRef]
- Al Khateeb, W.; Al-Qwasemeh, H. Cadmium, Copper and Zinc Toxicity Effects on Growth, Proline Content and Genetic Stability of Solanum nigrum L., a Crop Wild Relative for Tomato; Comparative Study. Physiol. Mol. Biol. Plants 2014, 20, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Gullì, M.; Marchi, L.; Fragni, R.; Buschini, A.; Visioli, G. Epigenetic Modifications Preserve the Hyperaccumulator Noccaea caerulescens from Ni Genotoxicity. Environ. Mol. Mutagen. 2018, 59, 464–475. [Google Scholar] [CrossRef]
- Wollgiehn, R.; Neumann, D. Metal Stress Response and Tolerance of Cultured Cells from Silene vulgaris and Lycopersicon peruvianum: Role of Heat Stress Proteins. J. Plant Physiol. 1999, 154, 547–553. [Google Scholar] [CrossRef]
- Kosová, K.; Vítámvás, P.; Prášil, I.T.; Renaut, J. Plant Proteome Changes under Abiotic Stress-Contribution of Proteomics Studies to Understanding Plant Stress Response. J. Proteom. 2011, 74, 1301–1322. [Google Scholar] [CrossRef] [PubMed]
- Ingle, R.A.; Smith, J.A.C.; Sweetlove, L.J. Responses to Nickel in the Proteome of the Hyperaccumulator Plant Alyssum lesbiacum. Biometals 2005, 18, 627–641. [Google Scholar] [CrossRef]
- Muszyńska, E.; Labudda, M.; Hanus-Fajerska, E. Changes in Proteolytic Activity and Protein Carbonylation in Shoots of Alyssum montanum Ecotypes under Multi-Metal Stress. J. Plant Physiol. 2019, 232, 61–64. [Google Scholar] [CrossRef]
- Muszyńska, E.; Labudda, M. Effects of Lead, Cadmium and Zinc on Protein Changes in Silene vulgaris Shoots Cultured in Vitro. Ecotoxicol. Environ. Saf. 2020, 204, 111086. [Google Scholar] [CrossRef]
- Romero-Puertas, M.C.; Palma, J.M.; Gómez, M.; Del Río, L.A.; Sandalio, L.M. Cadmium Causes the Oxidative Modification of Proteins in Pea Plants: Cadmium-Induced Protein Oxidation. Plant Cell Environ. 2002, 25, 677–686. [Google Scholar] [CrossRef]
- Ciąćka, K.; Tymiński, M.; Gniazdowska, A.; Krasuska, U. Carbonylation of Proteins—An Element of Plant Ageing. Planta 2020, 252, 12. [Google Scholar] [CrossRef]
- Savvides, A.; Ali, S.; Tester, M.; Fotopoulos, V. Chemical Priming of Plants Against Multiple Abiotic Stresses: Mission Possible? Trends Plant Sci. 2016, 21, 329–340. [Google Scholar] [CrossRef]
- Collins, A.R.; Azqueta, A. Single-Cell Gel Electrophoresis Combined with Lesion-Specific Enzymes to Measure Oxidative Damage to DNA. In Methods in Cell Biology; Elsevier: Amsterdam, The Netherlands, 2012; Volume 112, pp. 69–92. ISBN 978-0-12-405914-6. [Google Scholar]
- Hossain, M.A.; Bhattacharjee, S.; Armin, S.-M.; Qian, P.; Xin, W.; Li, H.-Y.; Burritt, D.J.; Fujita, M.; Tran, L.-S.P. Hydrogen Peroxide Priming Modulates Abiotic Oxidative Stress Tolerance: Insights from ROS Detoxification and Scavenging. Front. Plant Sci. 2015, 6, 420. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.-J.; Liu, L.-J.; Zhang, C.; Ge, Y.; Cheng, W. Effect of H2O2 Pretreatment on Cd Tolerance of Different Rice Cultivars. Rice Sci. 2011, 18, 29–35. [Google Scholar] [CrossRef]
- Talukdar, D. Arsenic-Induced Oxidative Stress in the Common Bean Legume, Phaseolus vulgaris L. Seedlings and Its Amelioration by Exogenous Nitric Oxide. Physiol. Mol. Biol. Plants 2013, 19, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Sharma, P.; Rathee, S.; Singh, H.P.; Batish, D.R.; Kohli, R.K. Salicylic Acid Pre-Treatment Modulates Pb2+-Induced DNA Damage Vis-à-Vis Oxidative Stress in Allium cepa Roots. Environ. Sci. Pollut. Res. Int. 2021, 28, 51989–52000. [Google Scholar] [CrossRef]
- Liu, S.; Zhu, S.; Jing, G. Nitric Oxide Reduces Mitochondrial DNA Damage by Regulating the Base Excision Repair Pathway in Peaches during Storage. Postharvest Biol. Technol. 2023, 197, 112200. [Google Scholar] [CrossRef]
- Pedroso, M.C.; Magalhaes, J.R.; Durzan, D. Nitric Oxide Induces Cell Death in Taxus Cells. Plant Sci. 2000, 157, 173–180. [Google Scholar] [CrossRef]
- Bai, S.; Li, M.; Yao, T.; Wang, H.; Zhang, Y.; Xiao, L.; Wang, J.; Zhang, Z.; Hu, Y.; Liu, W.; et al. Nitric Oxide Restrain Root Growth by DNA Damage Induced Cell Cycle Arrest in Arabidopsis thaliana. Nitric Oxide 2012, 26, 54–60. [Google Scholar] [CrossRef]
- Giorgio, M.; Dellino, G.I.; Gambino, V.; Roda, N.; Pelicci, P.G. On the Epigenetic Role of Guanosine Oxidation. Redox Biol. 2020, 29, 101398. [Google Scholar] [CrossRef]
- Yan, S.; Wang, W.; Marqués, J.; Mohan, R.; Saleh, A.; Durrant, W.E.; Song, J.; Dong, X. Salicylic Acid Activates DNA Damage Responses to Potentiate Plant Immunity. Mol. Cell 2013, 52, 602–610. [Google Scholar] [CrossRef]
- Plitta-Michalak, B.P.; Litkowiec, M.; Michalak, M. Epigenetic Marks, DNA Damage Markers, or Both? The Impact of Desiccation and Accelerated Aging on Nucleobase Modifications in Plant Genomic DNA. Cells 2022, 11, 1748. [Google Scholar] [CrossRef]
- Antoniou, C.; Savvides, A.; Christou, A.; Fotopoulos, V. Unravelling Chemical Priming Machinery in Plants: The Role of Reactive Oxygen–Nitrogen–Sulfur Species in Abiotic Stress Tolerance Enhancement. Curr. Opin. Plant Biol. 2016, 33, 101–107. [Google Scholar] [CrossRef] [PubMed]
- De Vos, C.H.R.; Vonk, M.J.; Vooijs, R.; Schat, H. Glutathione Depletion Due to Copper-Induced Phytochelatin Synthesis Causes Oxidative Stress in Silene cucubalus. Plant Physiol. 1992, 98, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Colmenero-Flores, J.M.; Franco-Navarro, J.D.; Cubero-Font, P.; Peinado-Torrubia, P.; Rosales, M.A. Chloride as a Beneficial Macronutrient in Higher Plants: New Roles and Regulation. Int. J. Mol. Sci. 2019, 20, 4686. [Google Scholar] [CrossRef]
- Geilfus, C.-M. Review on the Significance of Chlorine for Crop Yield and Quality. Plant Sci. 2018, 270, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Tousi, S.; Zoufan, P.; Ghahfarrokhie, A.R. Alleviation of Cadmium-Induced Phytotoxicity and Growth Improvement by Exogenous Melatonin Pretreatment in Mallow (Malva parviflora) Plants. Ecotoxicol. Environ. Saf. 2020, 206, 111403. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the Thiobarbituric Acid-Reactive-Substances Assay for Estimating Lipid Peroxidation in Plant Tissues Containing Anthocyanin and Other Interfering Compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Salcedo, C.L.; López De Mishima, B.A.; Nazareno, M.A. Walnuts and Almonds as Model Systems of Foods Constituted by Oxidisable, pro-Oxidant and Antioxidant Factors. Food Res. Int. 2010, 43, 1187–1197. [Google Scholar] [CrossRef]
- Gardner, H.W. Lipoxygenase and Associated Enzymes. In Handbook of Food Enzymology; CRC Press: Boca Raton, FL, USA, 2002; pp. 1–12. ISBN 978-0-429-22254-2. [Google Scholar]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Końca, K.; Lankoff, A.; Banasik, A.; Lisowska, H.; Kuszewski, T.; Góźdź, S.; Koza, Z.; Wojcik, A. A Cross-Platform Public Domain PC Image-Analysis Program for the Comet Assay. Mutat. Res. 2003, 534, 15–20. [Google Scholar] [CrossRef]





| Priming Agent | MC 1 | Shoot Dry Weight (g) | Specific Morphological Remarks after 6 Weeks of Cd Exposure |
|---|---|---|---|
| N-Cal ecotype | |||
| Control (H2O) | 8.1 ± 0.8 Bc | 0.369 ± 0.03 Bc | Typical shoot morphology |
| ASA | 7.5 ± 1.1 Bc | 0.395 ± 0.02 Bc | Typical shoot morphology |
| H2O2 | 8.8 ± 1.4 Bc | 0.217 ± 0.06 Cd | Narrow leaf blades, pale green |
| SA | 11.4 ± 0.6 Ab | 0.472 ± 0.03 Ab | Enlarged, wide leaf blades |
| NO | 9.2 ± 0.3 Bc | 0.441 ± 0.03 Abc | Intense branching, enlarged explants |
| Cal ecotype | |||
| Control (H2O) | 11.9 ± 1.3 Zb | 0.538 ± 0.01 Ya | Typical shoot morphology |
| ASA | 15.6 ± 0.3 Ya | 0.554 ± 0.01 Ya | Typical shoot morphology |
| H2O2 | 13.6 ± 1.1 Zb | 0.446 ± 0.03 Zbc | Red pigmentation |
| SA | 15.2 ± 0.8 Ya | 0.512 ± 0.04 Ya | Elongated internodes |
| NO | 13.1 ± 0.5 Zb | 0.577 ± 0.02 Ya | Flowering |
| Ecotype and Priming Treatment | Carbonyl Derivatives (nmol·mg−1 Protein) | |
|---|---|---|
| Aldehydes | Ketones | |
| N-Cal | ||
| Control (H2O) | 15.8 ± 3.0 Cd | 8.7 ± 1.8 Cd |
| ASA | 19.4 ± 2.3 BCcd | 10.5 ± 1.2 BCcd |
| H2O2 | 21.4 ± 4.1 ABCc | 12.2 ± 3.5 ABCc |
| SA | 28.0 ± 2.6 Aab | 16.0 ± 1.9 Aab |
| NO | 24.2 ± 2.1 ABbc | 13.6 ± 1.2 ABbc |
| Cal | ||
| Control (H2O) | 17.0 ± 1.7 Zd | 7.6 ± 0.9 Zd |
| ASA | 33.8 ± 1.6 Xa | 18.2 ± 0.7 Xa |
| H2O2 | 23.7 ± 1.6 Ybc | 13.3 ± 1.1 Ybc |
| SA | 27.1 ± 2.8 Xb | 14.0 ± 1.4 Xb |
| NO | 29.4 ± 1.2 Xa | 16.0 ± 0.7 Xa |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiszniewska, A.; Labudda, M.; Muszyńska, E. Response to Cadmium in Silene vulgaris Ecotypes Is Distinctly Affected by Priming-Induced Changes in Oxidation Status of Macromolecules. Int. J. Mol. Sci. 2023, 24, 16075. https://doi.org/10.3390/ijms242216075
Wiszniewska A, Labudda M, Muszyńska E. Response to Cadmium in Silene vulgaris Ecotypes Is Distinctly Affected by Priming-Induced Changes in Oxidation Status of Macromolecules. International Journal of Molecular Sciences. 2023; 24(22):16075. https://doi.org/10.3390/ijms242216075
Chicago/Turabian StyleWiszniewska, Alina, Mateusz Labudda, and Ewa Muszyńska. 2023. "Response to Cadmium in Silene vulgaris Ecotypes Is Distinctly Affected by Priming-Induced Changes in Oxidation Status of Macromolecules" International Journal of Molecular Sciences 24, no. 22: 16075. https://doi.org/10.3390/ijms242216075
APA StyleWiszniewska, A., Labudda, M., & Muszyńska, E. (2023). Response to Cadmium in Silene vulgaris Ecotypes Is Distinctly Affected by Priming-Induced Changes in Oxidation Status of Macromolecules. International Journal of Molecular Sciences, 24(22), 16075. https://doi.org/10.3390/ijms242216075