Comparative Analysis of Anticonvulsant Activity of Trans and Cis 5,5′-Diphenylhydantoin Schiff Bases
Abstract
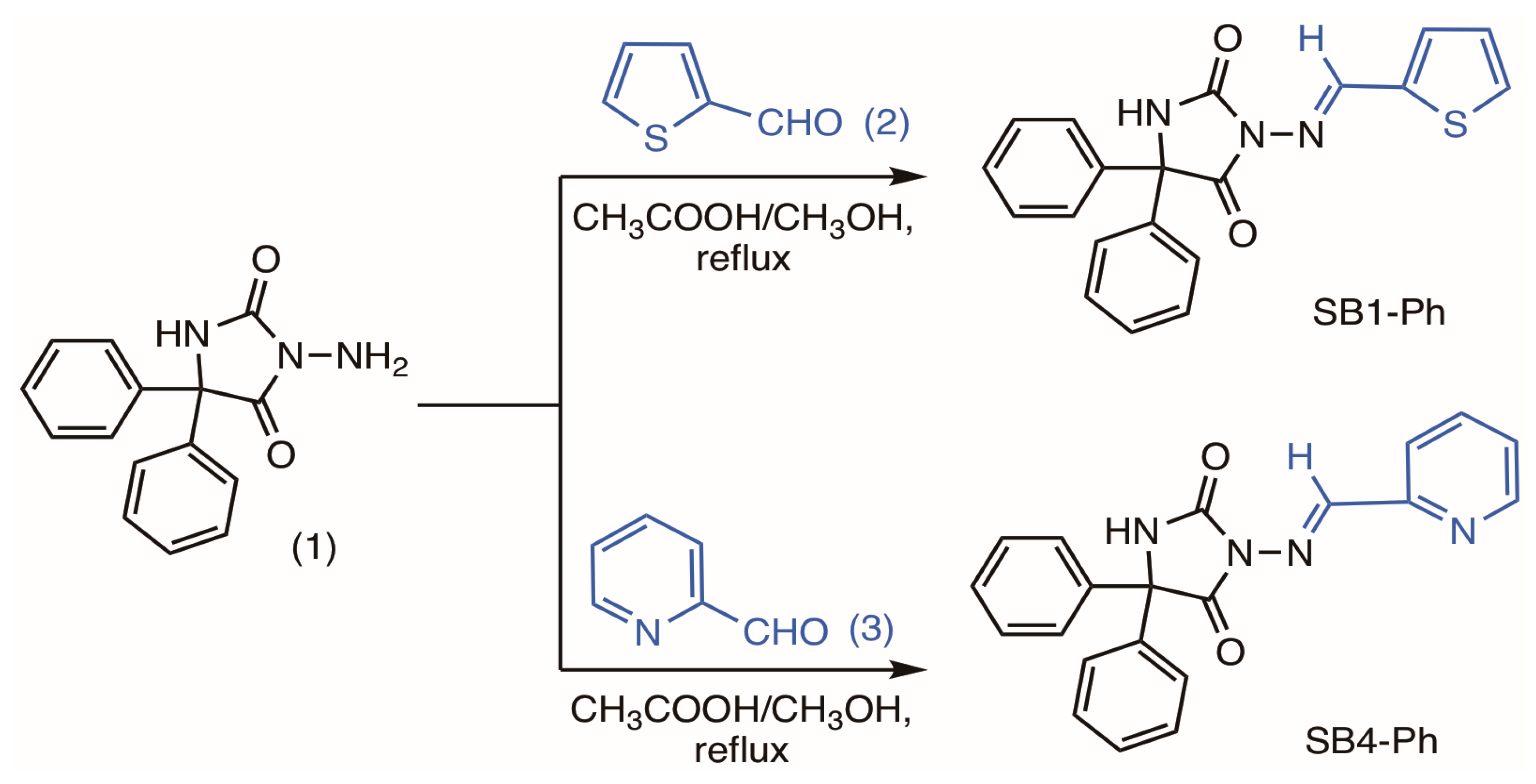
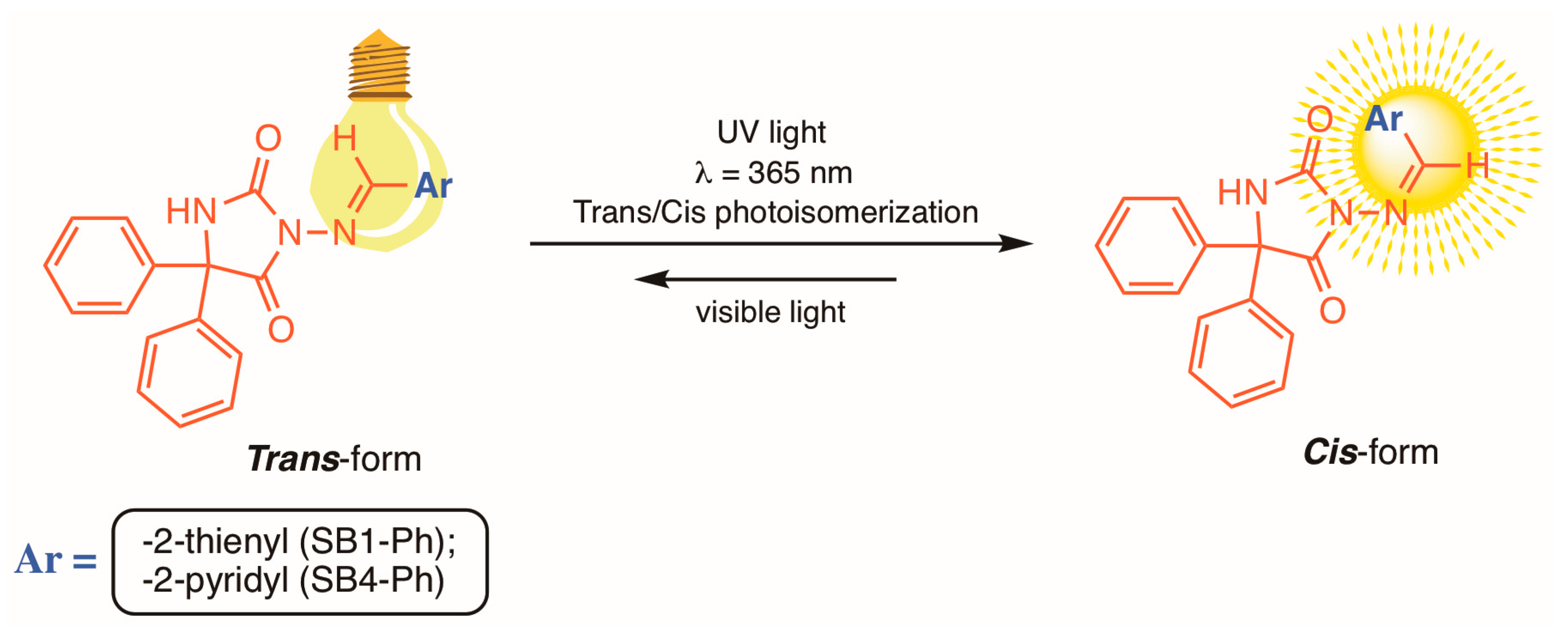
:1. Introduction
2. Results
2.1. Grip Strength and Rotarod
2.2. Maximal Electroshock Test
2.3. Kainate-Induced Status Epilepticus
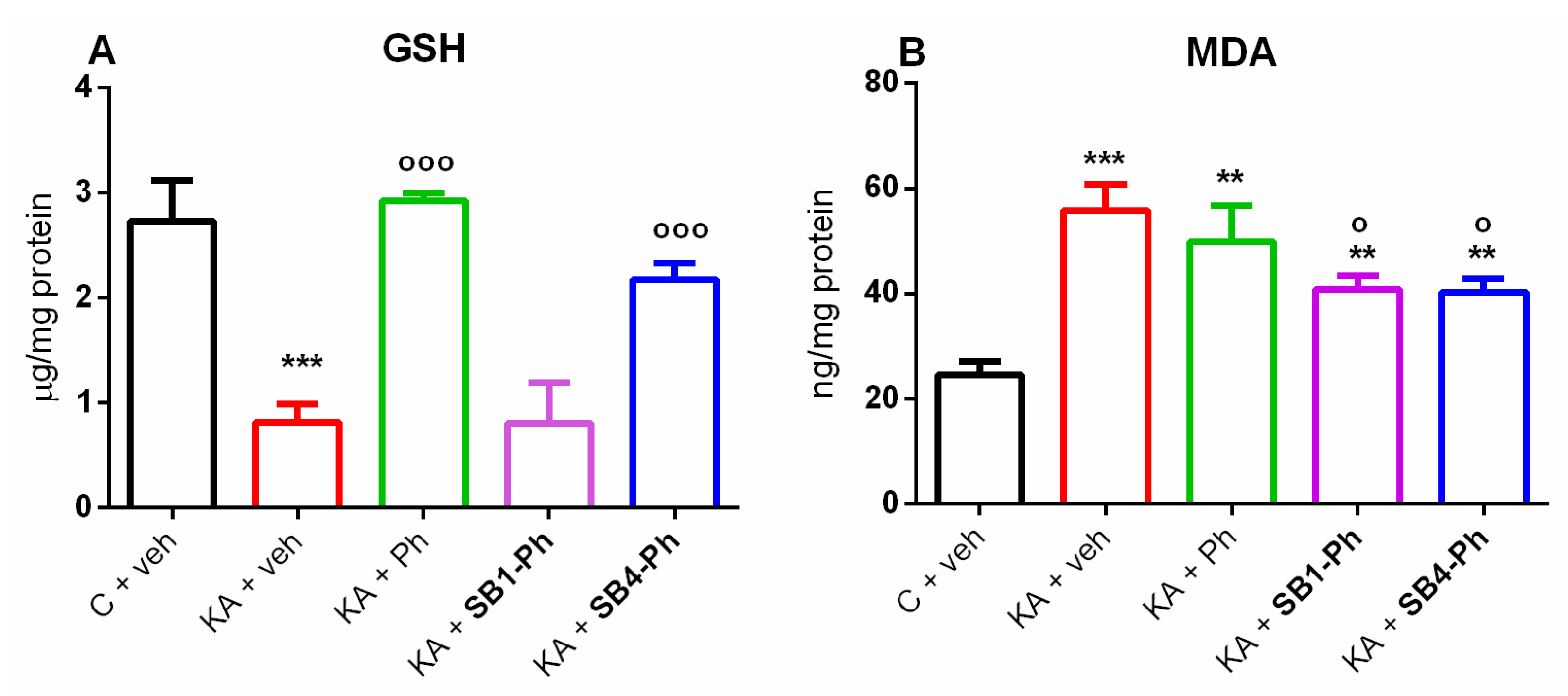
2.4. Effects of Cis Isomers of SB1-Ph and SB4-Ph Derivates on the KA-Induced Oxidative Stress
3. Discussion
4. Materials and Methods
4.1. Chemicals and Instrumentation
4.2. Experimental Rodents
4.3. Experimental Design
4.4. Drugs and Treatment
4.5. Rotarod Test
4.6. Muscle Strength
4.7. Anticonvulsant Activity
4.7.1. Maximal Electroshock (MES) Test
4.7.2. Kainate-Induced Status Epilepticus
4.8. Measurement of Glutathione (GSH) and Malondialdehyde (MDA) in the Hippocampus
4.9. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McNamara, J.O. Emerging insights into the genesis of epilepsy. Nature 1999, 399, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Fattorusso, A.; Matricardi, S.; Mencaroni, E.; Dell’Isola, G.B.; Di Cara, G.; Striano, P.; Verrotti, A. The Pharmacoresistant Epilepsy: An Overview on Existant and New Emerging Therapies. Front. Neurol. 2021, 12, 674483. [Google Scholar] [CrossRef] [PubMed]
- Perucca, P.; Gilliam, F.G. Adverse effects of antiepileptic drugs. Lancet Neurol. 2012, 11, 792–802. [Google Scholar] [CrossRef] [PubMed]
- Browne, T.R.; Holmes, G.L. Epilepsy. N. Engl. J. Med. 2001, 344, 1145–1151. [Google Scholar] [CrossRef] [PubMed]
- Wadher, S.J.; Puranik, M.P.; Karande, N.A.; Yeole, P.G. Synthesis and Biological Evaluation of Schiff Base of Dapsone and Their Derivative as Antimicrobial Agents. Int. J. PharmTech Res. 2009, 1, 22–23. [Google Scholar]
- Cates, L.A.; Rasheed, M.S. Phosphorus GABA Analogues as Potential Prodrugs. Pharm. Res. 1984, 1, 271–273. [Google Scholar] [CrossRef] [PubMed]
- Paneersalvam, P.; Raj, T.; Ishar, M.P.; Singh, B.; Sharma, V.; Rather, B.A. Anticonvulsant Activity of Schiff Bases of 3-Amino-6,8-dibromo-2-phenyl-quinazolin-4(3H)-ones. Indian J. Pharm. Sci. 2010, 72, 375–378. [Google Scholar] [CrossRef] [PubMed]
- Kajal, A.; Bala, S.; Kamboj, S.; Sharma, N.; Saini, V. Schiff Bases: A Versatile Pharmacophore. J. Catal. 2013, 14, 893512. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, G.; Zhao, R.; Zhang, Z. Tetraphenylethene-Based cis/trans Isomers for Targeted Fluorescence Sensing and Biomedical Applications. Chem.—A Eur. J. 2023, 29, e202300539. [Google Scholar] [CrossRef] [PubMed]
- Todorov, P.; Georgieva, S.; Peneva, P.; Rusew, R.; Shivachevc, B.; Georgiev, A. Experimental and theoretical study of bidirectional photoswitching behavior of 5,5′-diphenylhydantoin Schiff bases: Synthesis, crystal structure and kinetic approaches. New J. Chem. 2020, 44, 15081–15099. [Google Scholar] [CrossRef]
- Dugave, C.; Demange, L. Cis–Trans Isomerization of Organic Molecules and Biomolecules: Implications and Applications. Chem. Rev. 2003, 103, 2475–2532. [Google Scholar] [CrossRef] [PubMed]
- Hadidi, S.; Farshad, S.; Mohammadsaleh, N. Conversion mechanism and isomeric preferences of the cis and trans isomers of anti-cancer medicine carmustine; A double hybrid DFT calculation. Chem. Phys. 2019, 522, 39–43. [Google Scholar] [CrossRef]
- Ning, Y.; Liu, Y.-W.; Yang, Z.-S.; Yao, Y.; Kang, L.; Sessler, L.J.; Zhang, J.-L. Split and Use: Structural Isomers for Diagnosis and Therapy. J. Am. Chem. Soc. 2020, 142, 6761–6768. [Google Scholar] [CrossRef] [PubMed]
- Bhurta, D.; Bharate, S.B. Styryl Group, a Friend or Foe in Medicinal Chemistry. ChemMedChem 2022, 17, 7. [Google Scholar] [CrossRef] [PubMed]
- Racine, R.; Rose, P.A.; Burnham, W.M. Afterdischarge thresholds and kindling rates in dorsal and ventral hippocampus and dentate gyrus. Can. J. Neurol. Sci. 1977, 4, 273–278. [Google Scholar] [CrossRef] [PubMed]



| Group/Treatment | Dose (mg/kg). i.p. | Neuromuscular Strength (N) | Rotarod Test N/F |
|---|---|---|---|
| Control (saline) | 0 | 2.08 ± 0.48 | 0/8 |
| trans SB2-Ph | 10 | 1.56 ± 0.28 | 1/6 |
| 20 | 1.62 ± 0.39 | 2/6 | |
| 40 | 1.74 ± 0.29 | 2/6 | |
| cis SB2-Ph | 10 | 2.17 ± 0.36 | 1/6 |
| 20 | 1.41 ± 0.21 | 1/6 | |
| 40 | 1.84 ± 0.36 | 1/6 | |
| trans SB4-Ph | 10 | 2.0 ± 0.41 | 0/6 |
| 20 | 1.94 ± 0.4 | 1/6 | |
| 40 | 1.93 ± 0.32 | 3/6 | |
| cis SB4-Ph | 10 | 2.52 ± 0.14 | 1/6 |
| 20 | 1.75 ± 0.35 | 1/6 | |
| 40 | 2.01 ± 0.13 | 1/6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tchekalarova, J.; Todorov, P.; Stoyanova, T.; Atanasova, M. Comparative Analysis of Anticonvulsant Activity of Trans and Cis 5,5′-Diphenylhydantoin Schiff Bases. Int. J. Mol. Sci. 2023, 24, 16071. https://doi.org/10.3390/ijms242216071
Tchekalarova J, Todorov P, Stoyanova T, Atanasova M. Comparative Analysis of Anticonvulsant Activity of Trans and Cis 5,5′-Diphenylhydantoin Schiff Bases. International Journal of Molecular Sciences. 2023; 24(22):16071. https://doi.org/10.3390/ijms242216071
Chicago/Turabian StyleTchekalarova, Jana, Petar Todorov, Tsveta Stoyanova, and Milena Atanasova. 2023. "Comparative Analysis of Anticonvulsant Activity of Trans and Cis 5,5′-Diphenylhydantoin Schiff Bases" International Journal of Molecular Sciences 24, no. 22: 16071. https://doi.org/10.3390/ijms242216071
APA StyleTchekalarova, J., Todorov, P., Stoyanova, T., & Atanasova, M. (2023). Comparative Analysis of Anticonvulsant Activity of Trans and Cis 5,5′-Diphenylhydantoin Schiff Bases. International Journal of Molecular Sciences, 24(22), 16071. https://doi.org/10.3390/ijms242216071









