Integrated Transcriptomic and Metabolomic Analysis Reveals the Mechanism of Gibberellic Acid Regulates the Growth and Flavonoid Synthesis in Phellodendron chinense Schneid Seedlings
Abstract
:1. Introduction
2. Results
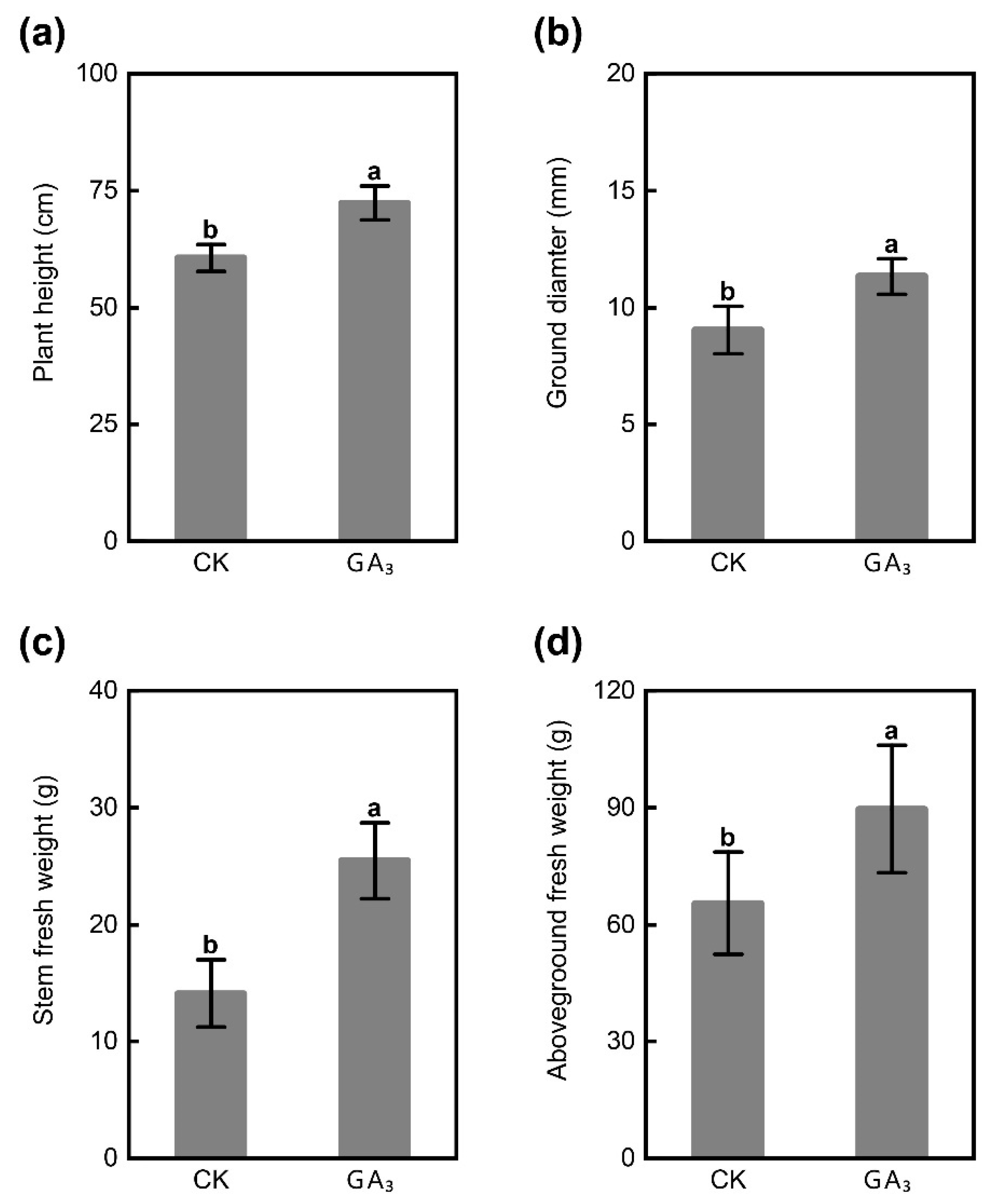
2.1. GA3 Promotes Plant Growth
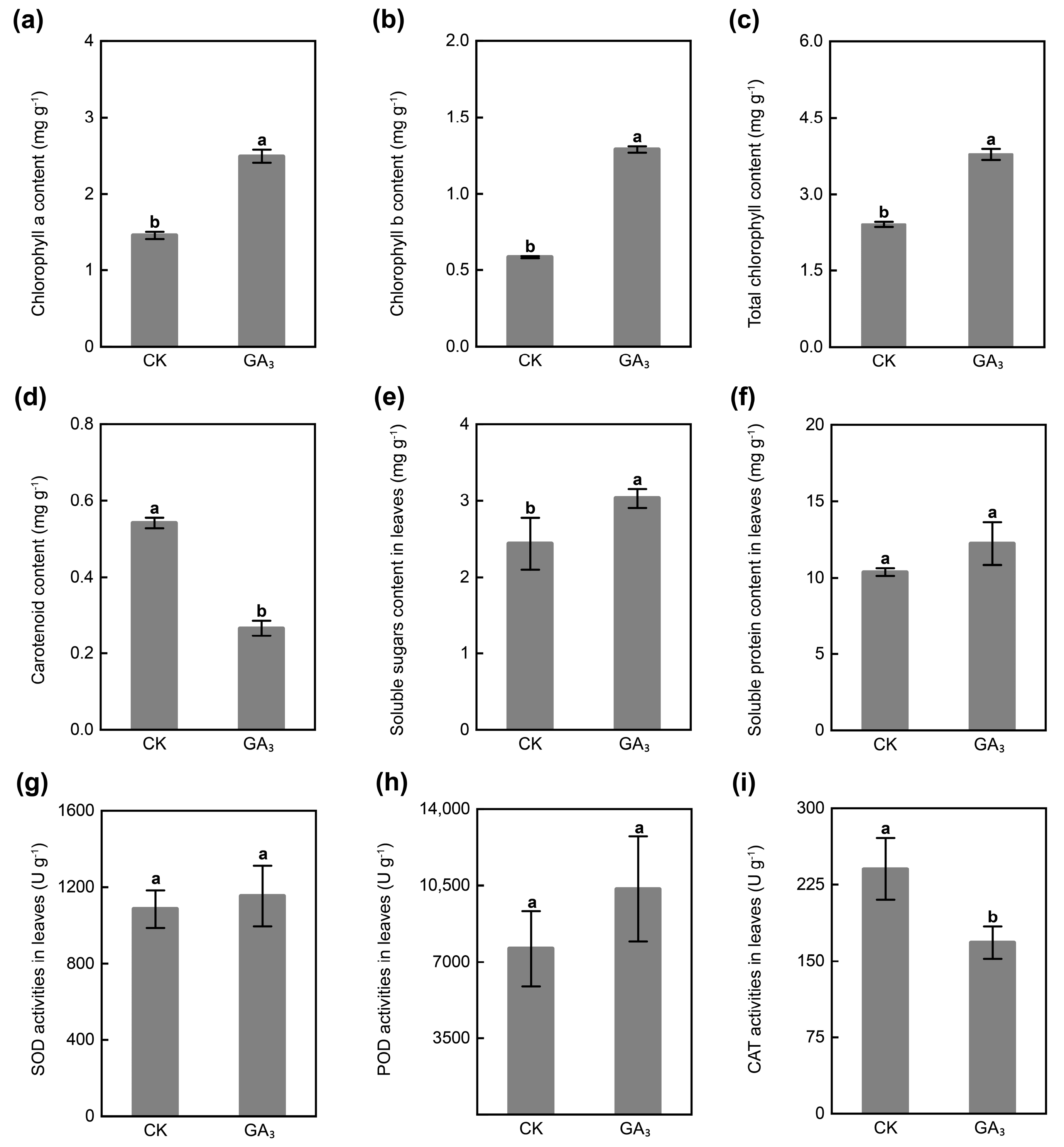
2.2. GA3 Regulates Physicochemical Property
2.3. Correlation Analysis of Growth Index with Physiological Index
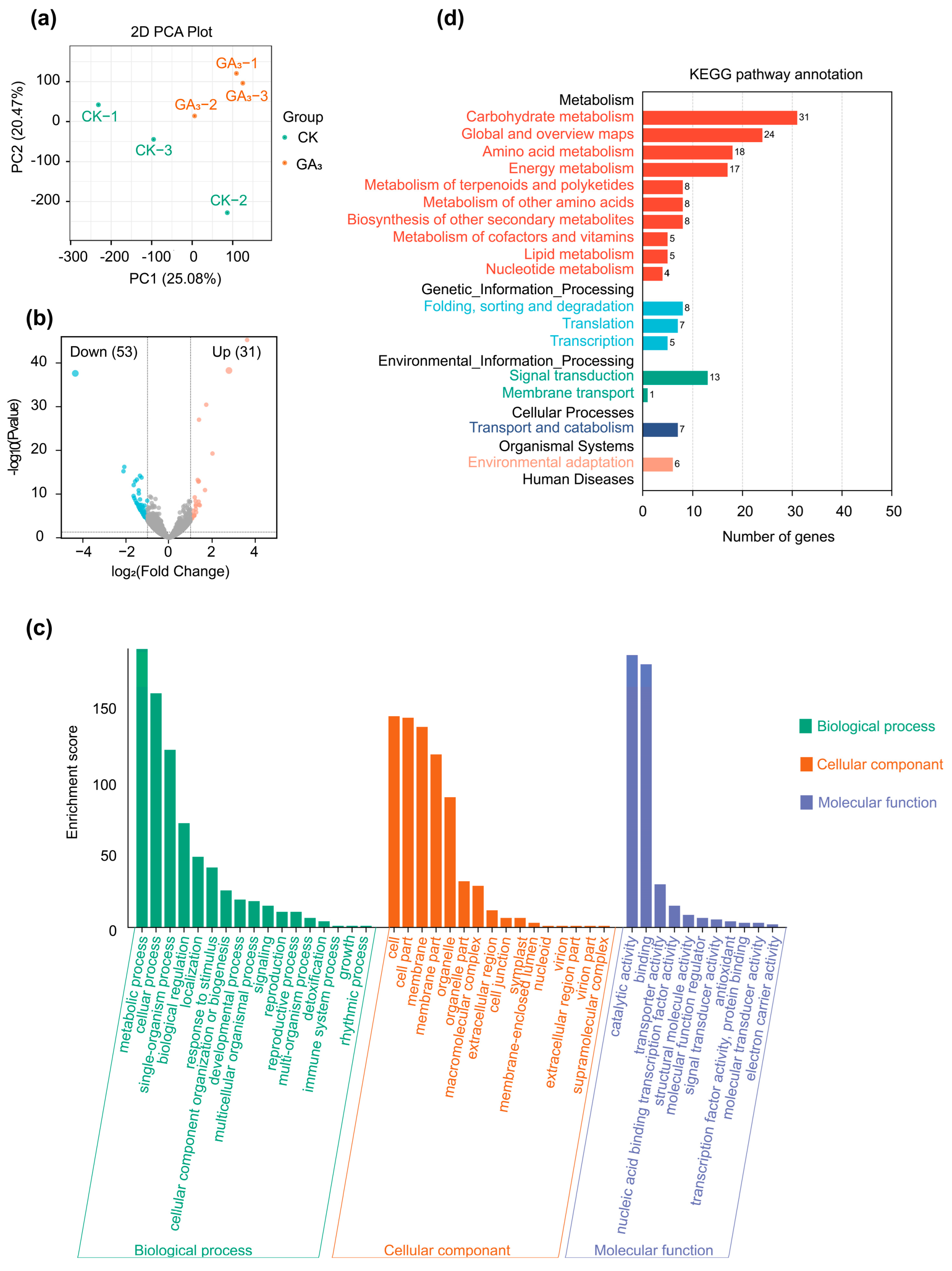
2.4. Transcriptome Analysis in Leaves
2.5. Differentially Expressed Genes (DEGs) Analysis
2.6. Analysis of DEGs during Photosynthesis and Carbon Metabolism
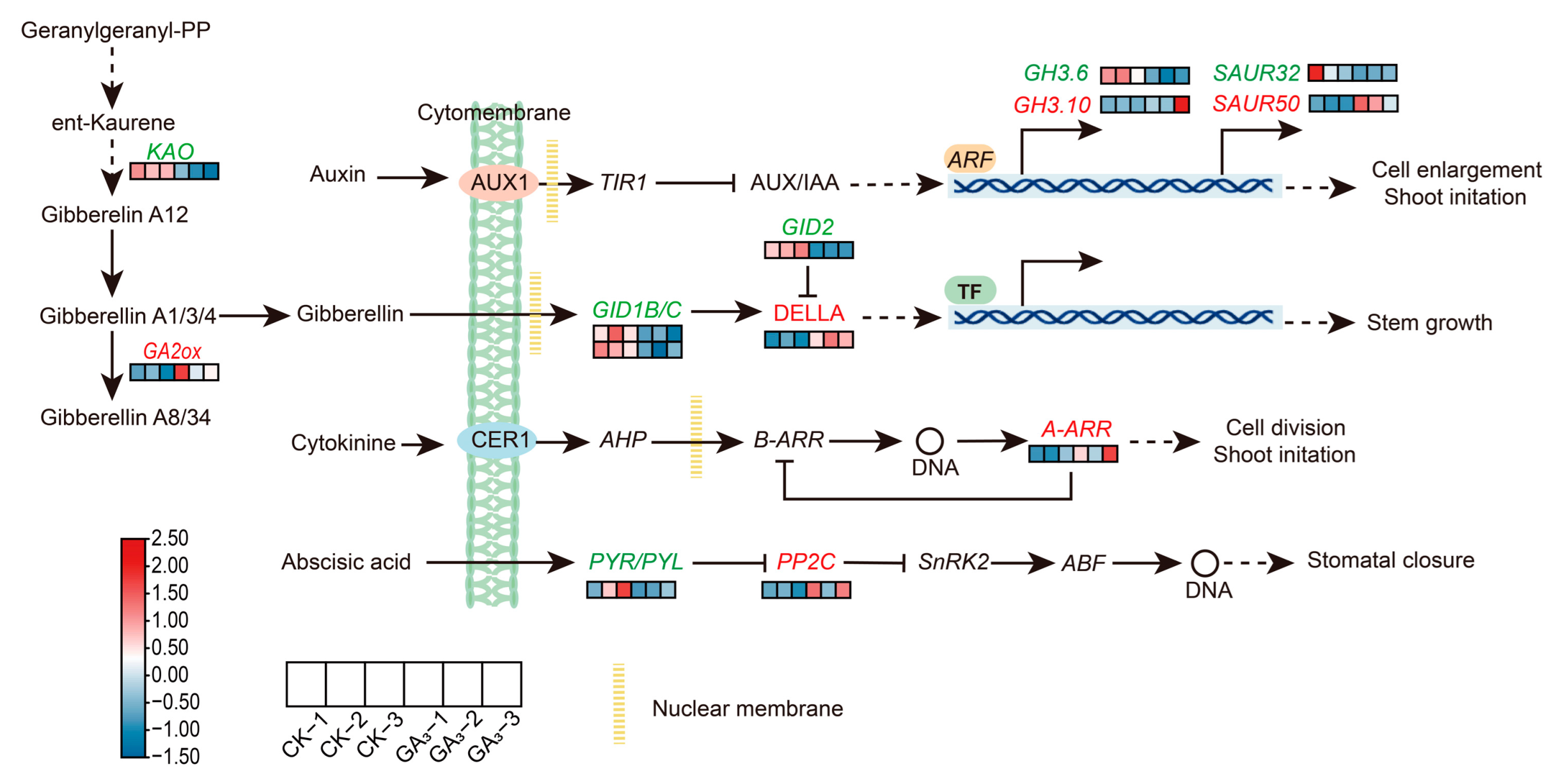
2.7. Analysis of DEGs during Hormone Signal Pathway
2.8. Differentially Accumulated Metabolites (DAMs) Analysis
2.9. DAMs during Flavonoids Synthesis Pathway
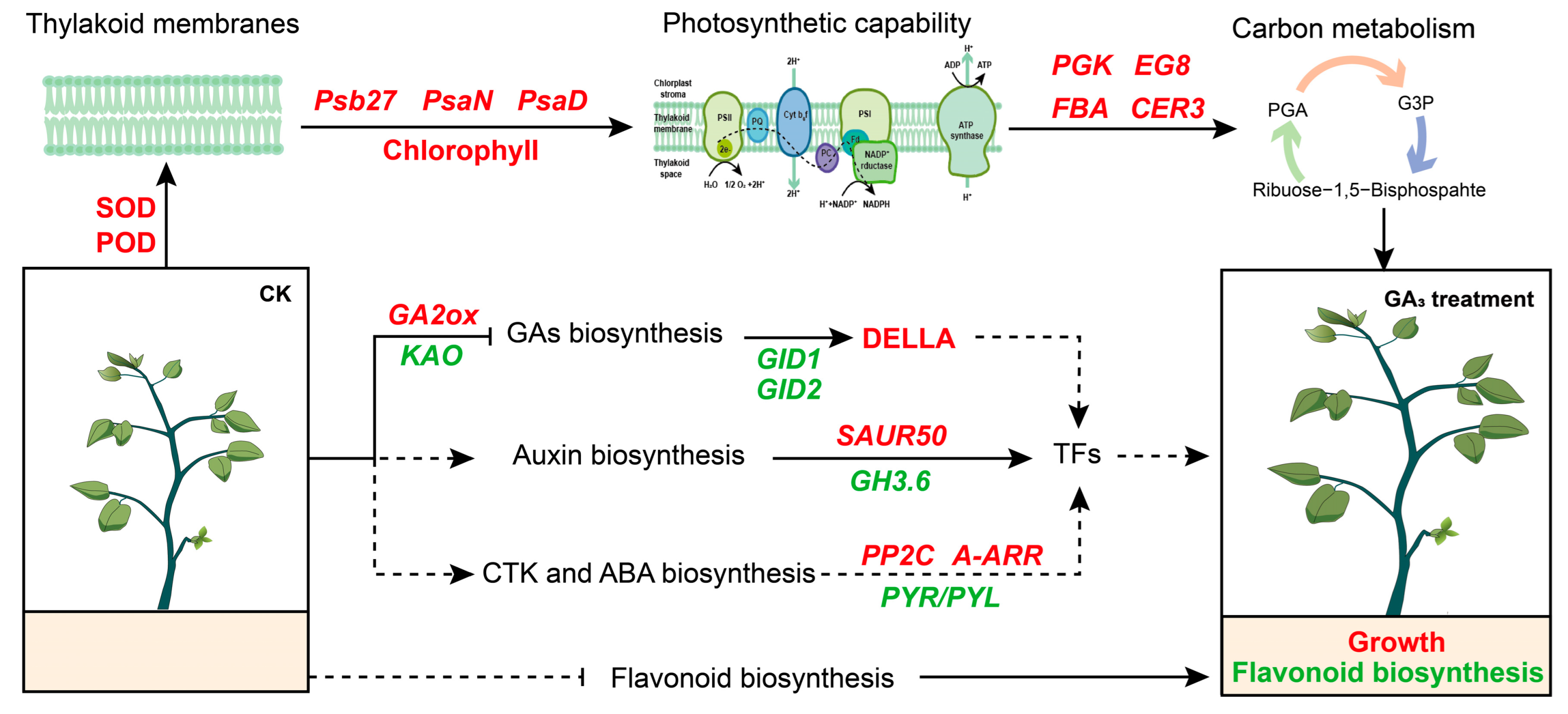
3. Discussion
4. Materials and Methods
4.1. Seedling Culture and Treatment
4.2. Growth Index Determination
4.3. Pigment Content Measurement
4.4. Soluble Sugar and Protein Content Determination
4.5. Antioxidase Activity Detection
4.6. Transcriptome Sequencing and Quality Control
4.7. Transcriptome Data Analysis
4.8. Gene Annotation and Pathway Analysis
4.9. Metabolite Analysis
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anfang, M.; Shani, E. Transport Mechanisms of Plant Hormones. Curr. Opin. Plant Biol. 2021, 63, 102055. [Google Scholar] [CrossRef]
- Yuan, H.; Zhao, L.; Guo, W.; Yu, Y.; Tao, L.; Zhang, L.; Song, X.; Huang, W.; Cheng, L.; Chen, J.; et al. Exogenous Application of Phytohormones Promotes Growth and Regulates Expression of Wood Formation-Related Genes in Populus simonii × P. nigra. Int. J. Mol. Sci. 2019, 20, 792. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Chu, C. Gibberellin Metabolism and Signaling: Targets for Improving Agronomic Performance of Crops. Plant Cell Physiol. 2020, 61, 1902–1911. [Google Scholar] [CrossRef] [PubMed]
- Binenbaum, J.; Weinstain, R.; Shani, E. Gibberellin Localization and Transport in Plants. Trends Plant Sci. 2018, 23, 410–421. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Tian, J.; Guo, C.; Luo, S.; Li, J. Interaction of Gibberellin and Other Hormones in Almond Anthers: Phenotypic and Physiological Changes and Transcriptomic Reprogramming. Hortic. Res. 2021, 8, 94. [Google Scholar] [CrossRef]
- Shah, S.H.; Islam, S.; Mohammad, F.; Siddiqui, M.H. Gibberellic Acid: A Versatile Regulator of Plant Growth, Development and Stress Responses. J. Plant Growth Regul. 2023, 42, 7352–7373. [Google Scholar] [CrossRef]
- Li, L.; Wang, J.; Chen, J.; Wang, Z.; Qaseem, M.F.; Li, H.; Wu, A. Physiological and Transcriptomic Responses of Growth in Neolamarckia cadamba Stimulated by Exogenous Gibberellins. Int. J. Mol. Sci. 2022, 23, 11842. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Li, L.; Wang, S.; Yu, N.; Shan, H.; Shi, Z.; Li, F.; Zhong, X. Effect of Gibberellic Acid on Photosynthesis and Oxidative Stress Response in Maize Under Weak Light Conditions. Front. Plant Sci. 2023, 14, 1128780. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, L.; Wang, J.; Liang, D.; Xia, H.; Lv, X.; Deng, Q.; Wang, X.; Luo, X.; Liao, M.; et al. Gibberellic Acid Promotes Selenium Accumulation in Cyphomandra betacea Under Selenium Stress. Front. Plant Sci. 2022, 13, 968768. [Google Scholar] [CrossRef]
- Du, G.; Zhang, H.; Yang, Y.; Zhao, Y.; Tang, K.; Liu, F. Effects of Gibberellin Pre-Treatment on Seed Germination and Seedling Physiology Characteristics in Industrial Hemp under Drought Stress Condition. Life 2022, 12, 1907. [Google Scholar] [CrossRef]
- Rady, M.M.; Boriek, S.H.K.; Abd El-Mageed, T.A.; Seif El-Yazal, M.A.; Ali, E.F.; Hassan, F.A.S.; Abdelkhalik, A. Exogenous Gibberellic Acid or Dilute Bee Honey Boosts Drought Stress Tolerance in Vicia faba by Rebalancing Osmoprotectants, Antioxidants, Nutrients, and Phytohormones. Plants 2021, 10, 748. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, H.; Chen, Y.; Yang, W.; Li, M.; Sun, B.; Song, H.; Tang, W.; Zhang, Y.; Gong, R. Joint Metabolome and Transcriptome Analysis of the Effects of Exogenous GA(3) on Endogenous Hormones in Sweet Cherry and Mining of Potential Regulatory Genes. Front. Plant Sci. 2022, 13, 1041068. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhou, T.; Zhang, C.; Zheng, W.; Li, J.; Jiang, W.; Xiao, C.; Wei, D.; Yang, C.; Xu, R.; et al. Gibberellin Disturbs the Balance of Endogenesis Hormones and Inhibits Adventitious Root Development of Pseudostellaria heterophylla through Regulating Gene Expression Related to Hormone Synthesis. Saudi J. Biol. Sci. 2021, 28, 135–147. [Google Scholar] [CrossRef]
- Gantait, S.; Sinniah, U.R.; Ali, M.N.; Sahu, N.C. Gibberellins—A Multifaceted Hormone in Plant Growth Regulatory Network. Curr. Protein Pept. Sci. 2015, 16, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Yuxi, Z.; Yanchao, Y.; Zejun, L.; Tao, Z.; Feng, L.; Chunying, L.; Shupeng, G. GA(3) is Superior to GA(4) in Promoting Bud Endodormancy Release in Tree Peony (Paeonia suffruticosa) and Their Potential Working Mechanism. BMC Plant Biol. 2021, 21, 323. [Google Scholar] [CrossRef]
- Tejeda-Sartorius, O.; Soto-Hernández, R.M.; San Miguel-Chávez, R.; Trejo-Téllez, L.I.; Caamal-Velázquez, H. Endogenous Hormone Profile and Sugars Display Differential Distribution in Leaves and Pseudobulbs of Laelia anceps Plants Induced and Non-Induced to Flowering by Exogenous Gibberellic Acid. Plants 2022, 11, 845. [Google Scholar] [CrossRef]
- Ci, J.; Wang, X.; Wang, Q.; Zhao, F.; Yang, W.; Cui, X.; Jiang, L.; Ren, X.; Yang, W. Genome-Wide Analysis of Gibberellin-Dioxygenases Gene Family and Their Responses to GA Applications in Maize. PLoS ONE 2021, 16, e0250349. [Google Scholar] [CrossRef]
- Sheng, Z.L.; Xuan, X.X.; Wang, F.; Sadeghnezhad, E.; Gong, P.J.; Xiao, Y.K.; Dong, T.Y.; Zhang, P.A.; Wang, X.C.; Fang, J.G.; et al. Identification and Characterization of AUXIN Response Factor Gene Family Reveals Their Regulatory Network to Respond the Multi-Hormones Crosstalk during GA-Induced Grape Parthenocarpic Berry. Int. J. Mol. Sci. 2022, 23, 11108. [Google Scholar] [CrossRef]
- Jie, H.; Ma, Y.; Xie, D.Y.; Jie, Y. Transcriptional and Metabolic Characterization of Feeding Ramie Growth Enhanced by a Combined Application of Gibberellin and Ethrel. Int. J. Mol. Sci. 2022, 23, 12025. [Google Scholar] [CrossRef]
- Jie, H.; Zhao, L.; Ma, Y.; Rasheed, A.; Jie, Y. Integrated Transcriptome and Metabolome Analysis Reveal That Exogenous Gibberellin Application Regulates Lignin Synthesis in Ramie. Agronomy 2023, 13, 1450. [Google Scholar] [CrossRef]
- Sun, H.; Cui, H.; Zhang, J.; Kang, J.; Wang, Z.; Li, M.; Yi, F.; Yang, Q.; Long, R. Gibberellins Inhibit Flavonoid Biosynthesis and Promote Nitrogen Metabolism in Medicago truncatula. Int. J. Mol. Sci. 2021, 22, 9291. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.C.; Wu, J.; Guan, M.L.; Zhao, C.H.; Geng, P.; Zhao, Q. Arabidopsis MYB4 Plays Dual Roles in Flavonoid Biosynthesis. Plant J. Cell Mol. Biol. 2020, 101, 637–652. [Google Scholar] [CrossRef] [PubMed]
- Premathilake, A.T.; Ni, J.; Bai, S.; Tao, R.; Ahmad, M.; Teng, Y. R2R3-MYB Transcription Factor PpMYB17 Positively Regulates Flavonoid Biosynthesis in Pear Fruit. Planta 2020, 252, 59. [Google Scholar] [CrossRef] [PubMed]
- Naik, J.; Misra, P.; Trivedi, P.K.; Pandey, A. Molecular Components Associated with the Regulation of Flavonoid Biosynthesis. Plant Sci. Int. J. Exp. Plant Biol. 2022, 317, 111196. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, H.; Tian, S.; Hao, W.; Chen, K.; Du, L. The R2R3MYB Transcription Factors MaMYBF and MaMYB1 Regulate Flavonoid Biosynthesis in Grape Hyacinth. Plant Physiol. Biochem. PPB 2023, 194, 85–95. [Google Scholar] [CrossRef]
- Si, Y.; Li, X.; Guo, T.; Wei, W.; Zhang, J.; Jia, A.; Wang, Y.; Zhao, A.; Chang, J.; Feng, S. Isolation and Characterization of Phellodendronoside A, A New Isoquinoline Alkaloid Glycoside with Anti-inflammatory Activity from Phellodendron chinense Schneid. Fitoterapia 2021, 154, 105021. [Google Scholar] [CrossRef]
- Wang, N.; Xu, P.; Yao, W.; Zhang, J.; Liu, S.; Wang, Y.; Zhang, Y. Structural Elucidation and Anti-diabetic Osteoporotic Activity of An Arabinogalactan from Phellodendron chinense Schneid. Carbohydr. Polym. 2021, 271, 118438. [Google Scholar] [CrossRef]
- Cheng, H.; Liu, J.; Tan, Y.; Feng, W.; Peng, C. Interactions Between Gut Microbiota and Berberine, a Necessary Procedure to Understand the Mechanisms of Berberine. J. Pharm. Anal. 2022, 12, 541–555. [Google Scholar] [CrossRef]
- Du, Q.; Meng, X.; Wang, S. A Comprehensive Review on the Chemical Properties, Plant Sources, Pharmacological Activities, Pharmacokinetic and Toxicological Characteristics of Tetrahydropalmatine. Front. Pharmacol. 2022, 13, 890078. [Google Scholar] [CrossRef]
- Liu, J.; Wu, Y.; Dong, G.; Zhu, G.; Zhou, G. Progress of Research on the Physiology and Molecular Regulation of Sorghum Growth under Salt Stress by Gibberellin. Int. J. Mol. Sci. 2023, 24, 6777. [Google Scholar] [CrossRef]
- Kosakivska, I.V.; Vedenicheva, N.P.; Babenko, L.M.; Voytenko, L.V.; Romanenko, K.O.; Vasyuk, V.A. Exogenous Phytohormones in the Regulation of Growth and Development of Cereals Under Abiotic Stresses. Mol. Biol. Rep. 2022, 49, 617–628. [Google Scholar] [CrossRef]
- Di, T.; Zhao, L.; Chen, H.; Qian, W.; Wang, P.; Zhang, X.; Xia, T. Transcriptomic and Metabolic Insights into the Distinctive Effects of Exogenous Melatonin and Gibberellin on Terpenoid Synthesis and Plant Hormone Signal Transduction Pathway in Camellia sinensis. J. Agric. Food Chem. 2019, 67, 4689–4699. [Google Scholar] [CrossRef]
- Keawmanee, N.; Ma, G.; Zhang, L.; Yahata, M.; Murakami, K.; Yamamoto, M.; Kojima, N.; Kato, M. Exogenous Gibberellin Induced Regreening Through the Regulation of Chlorophyll and Carotenoid Metabolism in Valencia Oranges. Plant Physiol. Biochem. PPB 2022, 173, 14–24. [Google Scholar] [CrossRef]
- Du, K.; Wu, W.; Liao, T.; Yang, J.; Kang, X. Transcriptome Analysis Uncovering Regulatory Networks and Hub Genes of Populus Photosynthesis and Chlorophyll Content. Genomics 2022, 114, 110385. [Google Scholar] [CrossRef] [PubMed]
- Duan, W.; Lu, B.; Liu, L.; Meng, Y.; Ma, X.; Li, J.; Zhang, K.; Sun, H.; Zhang, Y.; Dong, H.; et al. Effects of Exogenous Melatonin on Root Physiology, Transcriptome and Metabolome of Cotton Seedlings under Salt Stress. Int. J. Mol. Sci. 2022, 23, 9456. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Tian, J.; Du, Q.; Chen, J.; Li, Y.; Yang, X.; Li, B.; Zhang, D. Association Genetics and Transcriptome Analysis Reveal a Gibberellin-Responsive Pathway Involved in Regulating Photosynthesis. J. Exp. Bot. 2016, 67, 3325–3338. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Du, Q.; Xie, J.; Zhou, D.; Chen, B.; Yang, H.; Zhang, D. Genetic Variation in Transcription Factors and Photosynthesis Light-Reaction Genes Regulates Photosynthetic Traits. Tree Physiol. 2018, 38, 1871–1885. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, T.; Fu, F.F.; El-Kassaby, Y.A.; Wang, G. Metabolome and Transcriptome Analyses Reveal the Regulatory Mechanisms of Photosynthesis in Developing Ginkgo biloba Leaves. Int. J. Mol. Sci. 2021, 22, 2601. [Google Scholar] [CrossRef]
- Chen, R.; Fan, Y.; Yan, H.; Zhou, H.; Zhou, Z.; Weng, M.; Huang, X.; Lakshmanan, P.; Li, Y.; Qiu, L.; et al. Enhanced Activity of Genes Associated with Photosynthesis, Phytohormone Metabolism and Cell Wall Synthesis Is Involved in Gibberellin-Mediated Sugarcane Internode Growth. Front. Genet. 2020, 11, 570094. [Google Scholar] [CrossRef]
- Liu, H.; Huang, R.Y.; Chen, J.; Gross, M.L.; Pakrasi, H.B. Psb27, A Transiently Associated Protein, Binds to the Chlorophyll Binding Protein CP43 in Photosystem II Assembly Intermediates. Proc. Natl. Acad. Sci. USA 2011, 108, 18536–18541. [Google Scholar] [CrossRef]
- Huang, G.; Xiao, Y.; Pi, X.; Zhao, L.; Zhu, Q.; Wang, W.; Kuang, T.; Han, G.; Sui, S.F.; Shen, J.R. Structural Insights into a Dimeric Psb27-Photosystem II Complex from a Cyanobacterium Thermosynechococcus vulcanus. Proc. Natl. Acad. Sci. USA 2021, 118, e2018053118. [Google Scholar] [CrossRef] [PubMed]
- Gisriel, C.J.; Brudvig, G.W. Comparison of PsbQ and Psb27 in Photosystem II Provides Insight into Their Roles. Photosynth. Res. 2022, 152, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ding, Y. Molecular Dynamics Simulation and Bioinformatics Study on Chloroplast Stromal Ridge Complex from Rice (Oryza sativa L.). BMC Bioinform. 2016, 17, 28. [Google Scholar] [CrossRef]
- Pan, X.; Ma, J.; Su, X.; Cao, P.; Chang, W.; Liu, Z.; Zhang, X.; Li, M. Structure of the Maize Photosystem I Supercomplex with Light-Harvesting Complexes I and II. Science 2018, 360, 1109–1113. [Google Scholar] [CrossRef]
- Jagtap, A.B.; Yadav, I.S.; Vikal, Y.; Praba, U.P.; Kaur, N.; Gill, A.S.; Johal, G.S. Transcriptional Dynamics of Maize Leaves, Pollens and Ovules to Gain Insights into Heat Stress-Related Responses. Front. Plant Sci. 2023, 14, 1117136. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Shen, L.; Wang, W.; Mao, Z.; Yi, X.; Kuang, T.; Shen, J.R.; Zhang, X.; Han, G. Structure of Photosystem I-LHCI-LHCII from the Green Alga Chlamydomonas Reinhardtii in State 2. Nat. Commun. 2021, 12, 1100. [Google Scholar] [CrossRef] [PubMed]
- Li, S.M.; Zheng, H.X.; Zhang, X.S.; Sui, N. Cytokinins as Central Regulators During Plant Growth and Stress Response. Plant Cell Rep. 2021, 40, 271–282. [Google Scholar] [CrossRef]
- Wu, K.; Xu, H.; Gao, X.; Fu, X. New Insights into Gibberellin Signaling in Regulating Plant Growth-Metabolic Coordination. Curr. Opin. Plant Biol. 2021, 63, 102074. [Google Scholar] [CrossRef]
- Ritonga, F.N.; Zhou, D.; Zhang, Y.; Song, R.; Li, C.; Li, J.; Gao, J. The Roles of Gibberellins in Regulating Leaf Development. Plants 2023, 12, 1243. [Google Scholar] [CrossRef]
- Salazar-Cerezo, S.; Martínez-Montiel, N.; García-Sánchez, J.; Pérez, Y.T.R.; Martínez-Contreras, R.D. Gibberellin Biosynthesis and Metabolism: A Convergent Route for Plants, Fungi and Bacteria. Microbiol. Res. 2018, 208, 85–98. [Google Scholar] [CrossRef]
- Xu, P.; Chen, H.; Li, T.; Xu, F.; Mao, Z.; Cao, X.; Miao, L.; Du, S.; Hua, J.; Zhao, J.; et al. Blue Light-Dependent Interactions of CRY1 with GID1 and DELLA Proteins Regulate Gibberellin Signaling and Photomorphogenesis in Arabidopsis. Plant Cell 2021, 33, 2375–2394. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Li, G.; Xu, J.; Ma, Q.; Qi, B.; Zhang, Y. Genome-Wide Analysis of Genes Involved in the GA Signal Transduction Pathway in ‘duli’ Pear (Pyrus betulifolia Bunge). Int. J. Mol. Sci. 2022, 23, 6570. [Google Scholar] [CrossRef] [PubMed]
- Di, D.W.; Sun, L.; Wang, M.; Wu, J.; Kronzucker, H.J.; Fang, S.; Chu, J.; Shi, W.; Li, G. WRKY46 Promotes Ammonium Tolerance in Arabidopsis by Repressing NUDX9 and Indole-3-cetic Acid-Conjugating Genes and by Inhibiting Ammonium Efflux in the Root Elongation Zone. New Phytol. 2021, 232, 190–207. [Google Scholar] [CrossRef] [PubMed]
- Jez, J.M. Connecting Primary and Specialized Metabolism: Amino Acid Conjugation of Phytohormones by GRETCHEN HAGEN 3 (GH3) Acyl Acid Amido Synthetases. Curr. Opin. Plant Biol. 2022, 66, 102194. [Google Scholar] [CrossRef]
- Wojtaczka, P.; Ciarkowska, A.; Starzynska, E.; Ostrowski, M. The GH3 Amidosynthetases Family and Their Role in Metabolic Crosstalk Modulation of Plant Signaling Compounds. Phytochemistry 2022, 194, 113039. [Google Scholar] [CrossRef]
- Ren, H.; Gray, W.M. SAUR Proteins as Effectors of Hormonal and Environmental Signals in Plant Growth. Mol. Plant 2015, 8, 1153–1164. [Google Scholar] [CrossRef]
- Stortenbeker, N.; Bemer, M. The SAUR Gene Family: The Plant’s Toolbox for Adaptation of Growth and Development. J. Exp. Bot. 2019, 70, 17–27. [Google Scholar] [CrossRef]
- Wang, J.J.; Sun, N.; Zhang, F.F.; Yu, R.B.; Chen, H.D.; Deng, X.W.; Wei, N. SAUR17 and SAUR50 Differentially Regulate PP2C-D1 during Apical Hook Development and Cotyledon Opening in Arabidopsis. Plant Cell 2020, 32, 3792–3811. [Google Scholar] [CrossRef]
- Ren, H.; Park, M.Y.; Spartz, A.K.; Wong, J.H.; Gray, W.M. A Subset of Plasma Membrane-Localized PP2C.D Phosphatases Negatively Regulate SAUR-Mediated Cell Expansion in Arabidopsis. PLoS Genet. 2018, 14, e1007455. [Google Scholar] [CrossRef]
- Acheampong, A.K.; Shanks, C.; Cheng, C.Y.; Schaller, G.E.; Dagdas, Y.; Kieber, J.J. EXO70D Isoforms Mediate Selective Autophagic Degradation of Type-A ARR Proteins to Regulate Cytokinin Sensitivity. Proc. Natl. Acad. Sci. USA 2020, 117, 27034–27043. [Google Scholar] [CrossRef]
- Chen, K.; Li, G.J.; Bressan, R.A.; Song, C.P.; Zhu, J.K.; Zhao, Y. Abscisic Acid Dynamics, Signaling, and Functions in Plants. J. Integr. Plant Biol. 2020, 62, 25–54. [Google Scholar] [CrossRef] [PubMed]
- Korek, M.; Marzec, M. Strigolactones and Abscisic Acid Interactions Affect Plant Development and Response to Abiotic Stresses. BMC Plant Biol. 2023, 23, 314. [Google Scholar] [CrossRef] [PubMed]
- Murcia, G.; Fontana, A.; Pontin, M.; Baraldi, R.; Bertazza, G.; Piccoli, P.N. ABA and GA(3) Regulate the Synthesis of Primary and Secondary Metabolites Related to Alleviation from Biotic and Abiotic Stresses in Grapevine. Phytochemistry 2017, 135, 34–52. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Liu, Y.Q.; Chen, H.W.; Yang, B.W.; Ge, M.S.; Zhang, Z.W. Effects of Gibberellin Applications before Flowering on the Phenotype, Ripening, and Flavonoid Compounds of Syrah grape berries. J. Sci. Food Agric. 2022, 102, 6100–6111. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Gao, H.; Zhang, D.; Shi, Y.; Zhang, T.; Shen, X.; Wu, L.; Xiang, L.; Chen, S. Transcriptome Analyses Revealed the Ultraviolet B Irradiation and Phytohormone Gibberellins Coordinately Promoted the Accumulation of Artemisinin in Artemisia annua L. Chin. Med. 2020, 15, 67. [Google Scholar] [CrossRef]
- Li, L.; Liu, Y.; Huang, Y.; Li, B.; Ma, W.; Wang, D.; Cao, X.; Wang, Z. Genome-Wide Identification of the TIFY Family in Salvia miltiorrhiza Reveals That SmJAZ3 Interacts with SmWD40-170, a Relevant Protein That Modulates Secondary Metabolism and Development. Front. Plant Sci. 2021, 12, 630424. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, W.; Chen, X.; Chen, L.; Lv, Z.; He, H.; Yan, W. Integrated Physiological and Transcriptomic Analysis Reveals Mechanism of Leaf in Phellodendron chinense Schneid Seedlings Response to Drought Stress. Ind. Crops Prod. 2023, 198, 116679. [Google Scholar] [CrossRef]
- He, X.; Wan, Z.; Jin, N.; Jin, L.; Zhang, G.; Lyu, J.; Liu, Z.; Luo, S.; Yu, J. Enhancement of Cucumber Resistance Under Salt Stress by 2, 4-epibrassinolide Lactones. Front. Plant Sci. 2022, 13, 1023178. [Google Scholar] [CrossRef]
- Mohammadi, M.; Tavakoli, A.; Pouryousef, M.; Mohseni Fard, E. Study the Effect of 24-epibrassinolide Application on the Cu/Zn-SOD Expression and Tolerance to Drought Stress in Common Bean. Physiol. Mol. Biol. Plants Int. J. Funct. Plant Biol. 2020, 26, 459–474. [Google Scholar] [CrossRef]
- Anjum, S.A.; Ashraf, U.; Tanveer, M.; Khan, I.; Hussain, S.; Shahzad, B.; Zohaib, A.; Abbas, F.; Saleem, M.F.; Ali, I.; et al. Drought Induced Changes in Growth, Osmolyte Accumulation and Antioxidant Metabolism of Three Maize Hybrids. Front. Plant Sci. 2017, 8, 69. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B (Methodol.) 2018, 57, 289–300. [Google Scholar] [CrossRef]



| Applications a | Treatments | Total Clean Reads b | Total Clean Bases (bp) | G-C (%) c | Q30 (%) d | Mapped Reads e | Mapped Ratio f (%) |
|---|---|---|---|---|---|---|---|
| CK-1 | 21,182,430 | 6,332,375,932 | 44.68 | 93.29 | 16,329,151 | 77.09 | |
| CK | CK-2 | 21,770,028 | 6,520,262,422 | 43.65 | 93.15 | 16,529,517 | 75.93 |
| CK-3 | 20,888,663 | 6,249,140,252 | 43.76 | 92.68 | 16,066,835 | 76.92 | |
| GA3-1 | 21,782,716 | 6,513,190,704 | 43.41 | 93.29 | 16,702,799 | 76.68 | |
| GA3 | GA3-2 | 20,993,405 | 6,287,678,294 | 43.70 | 92.25 | 15,738,212 | 74.97 |
| GA3-3 | 21,035,971 | 6,288,453,804 | 43.50 | 92.55 | 16,069,780 | 76.39 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Luo, S.; Jiao, J.; Yan, W.; Zeng, B.; He, H.; He, G. Integrated Transcriptomic and Metabolomic Analysis Reveals the Mechanism of Gibberellic Acid Regulates the Growth and Flavonoid Synthesis in Phellodendron chinense Schneid Seedlings. Int. J. Mol. Sci. 2023, 24, 16045. https://doi.org/10.3390/ijms242216045
Yang L, Luo S, Jiao J, Yan W, Zeng B, He H, He G. Integrated Transcriptomic and Metabolomic Analysis Reveals the Mechanism of Gibberellic Acid Regulates the Growth and Flavonoid Synthesis in Phellodendron chinense Schneid Seedlings. International Journal of Molecular Sciences. 2023; 24(22):16045. https://doi.org/10.3390/ijms242216045
Chicago/Turabian StyleYang, Lv, Shengwei Luo, Jing Jiao, Wende Yan, Baiquan Zeng, Hanjie He, and Gongxiu He. 2023. "Integrated Transcriptomic and Metabolomic Analysis Reveals the Mechanism of Gibberellic Acid Regulates the Growth and Flavonoid Synthesis in Phellodendron chinense Schneid Seedlings" International Journal of Molecular Sciences 24, no. 22: 16045. https://doi.org/10.3390/ijms242216045
APA StyleYang, L., Luo, S., Jiao, J., Yan, W., Zeng, B., He, H., & He, G. (2023). Integrated Transcriptomic and Metabolomic Analysis Reveals the Mechanism of Gibberellic Acid Regulates the Growth and Flavonoid Synthesis in Phellodendron chinense Schneid Seedlings. International Journal of Molecular Sciences, 24(22), 16045. https://doi.org/10.3390/ijms242216045






