Glucokinase Variant Proteins Are Resistant to Fasting-Induced Uridine Diphosphate Glucose-Dependent Degradation in Maturity-Onset Diabetes of the Young Type 2 Patients
Abstract
1. Introduction
2. Results
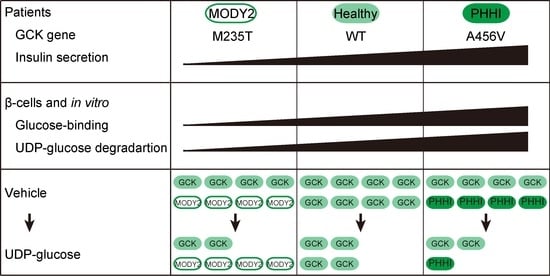
2.1. The Correlation between Low-Glucose-Dependent Insulin Secretion in MODY2 Patients and In Vitro
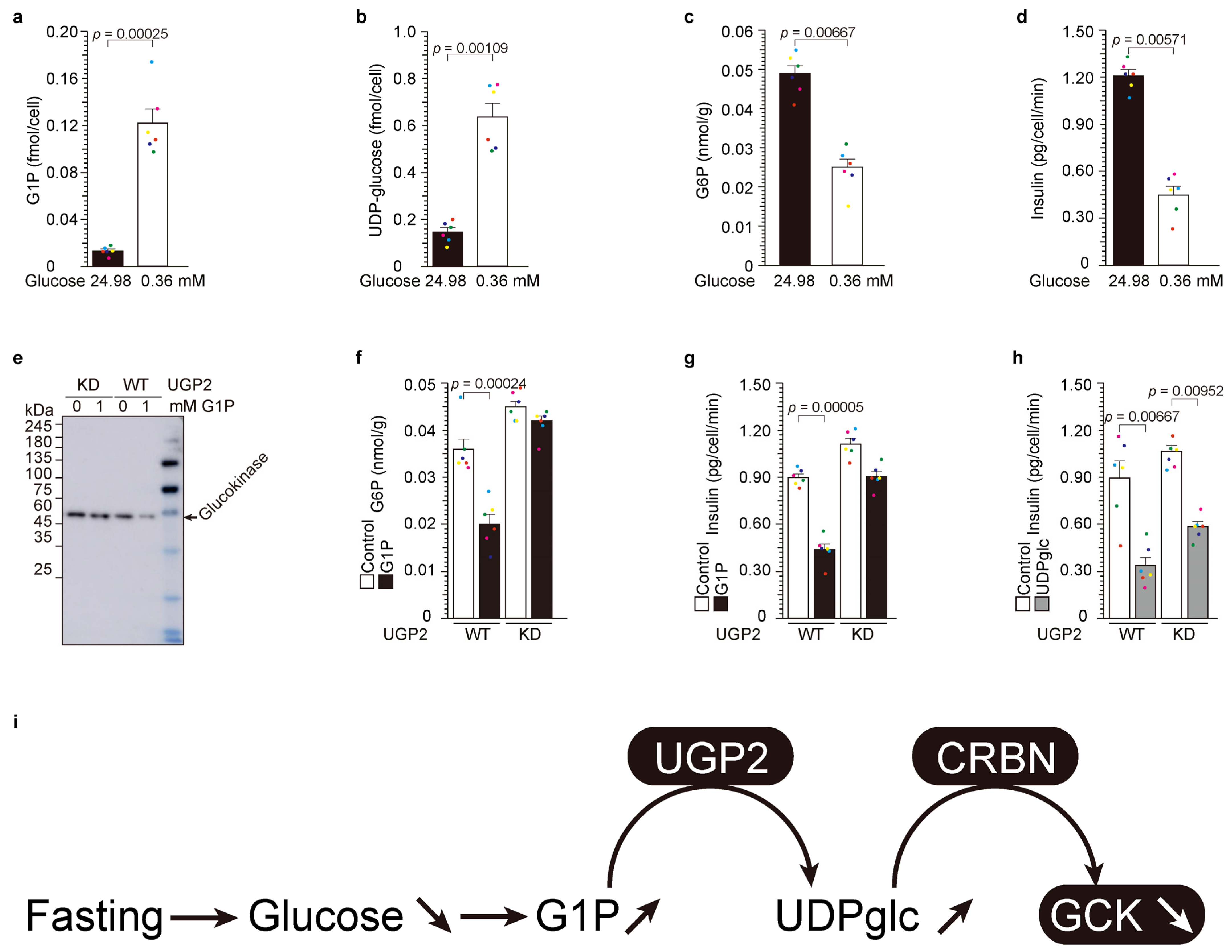
2.2. Upstream of UDP-Glucose Pyrophosphorylase 2 (UGP2): Low-Glucose-Induced UDP-Glucose-Dependent Glucokinase Ubiquitination and Degradation
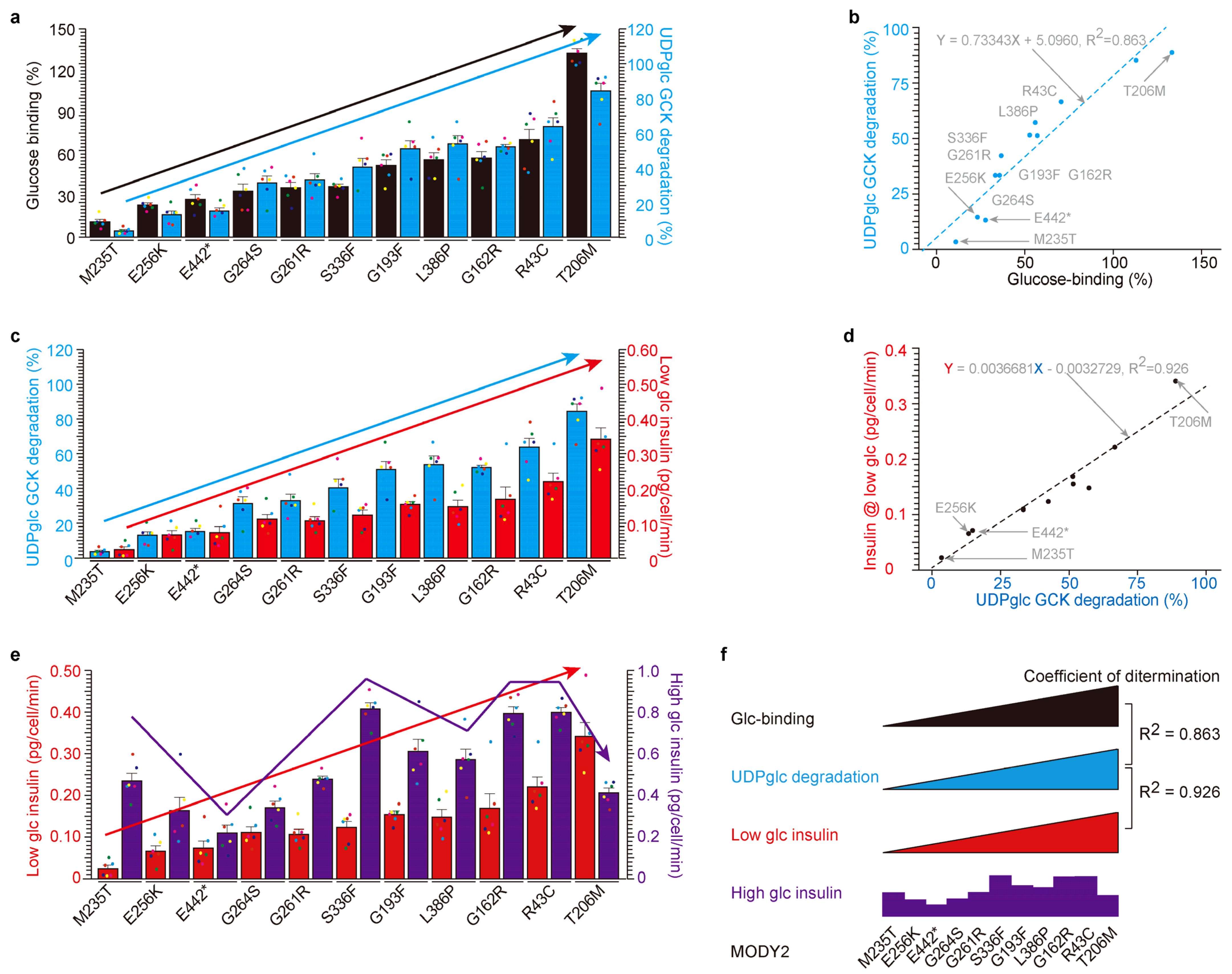
2.3. High Correlation among Glucose-Binding, UDP-Glucose-Dependent Degradation and Insulin Secretion under Low Glucose in MODY2 Glucokinase Variant Proteins In Vitro
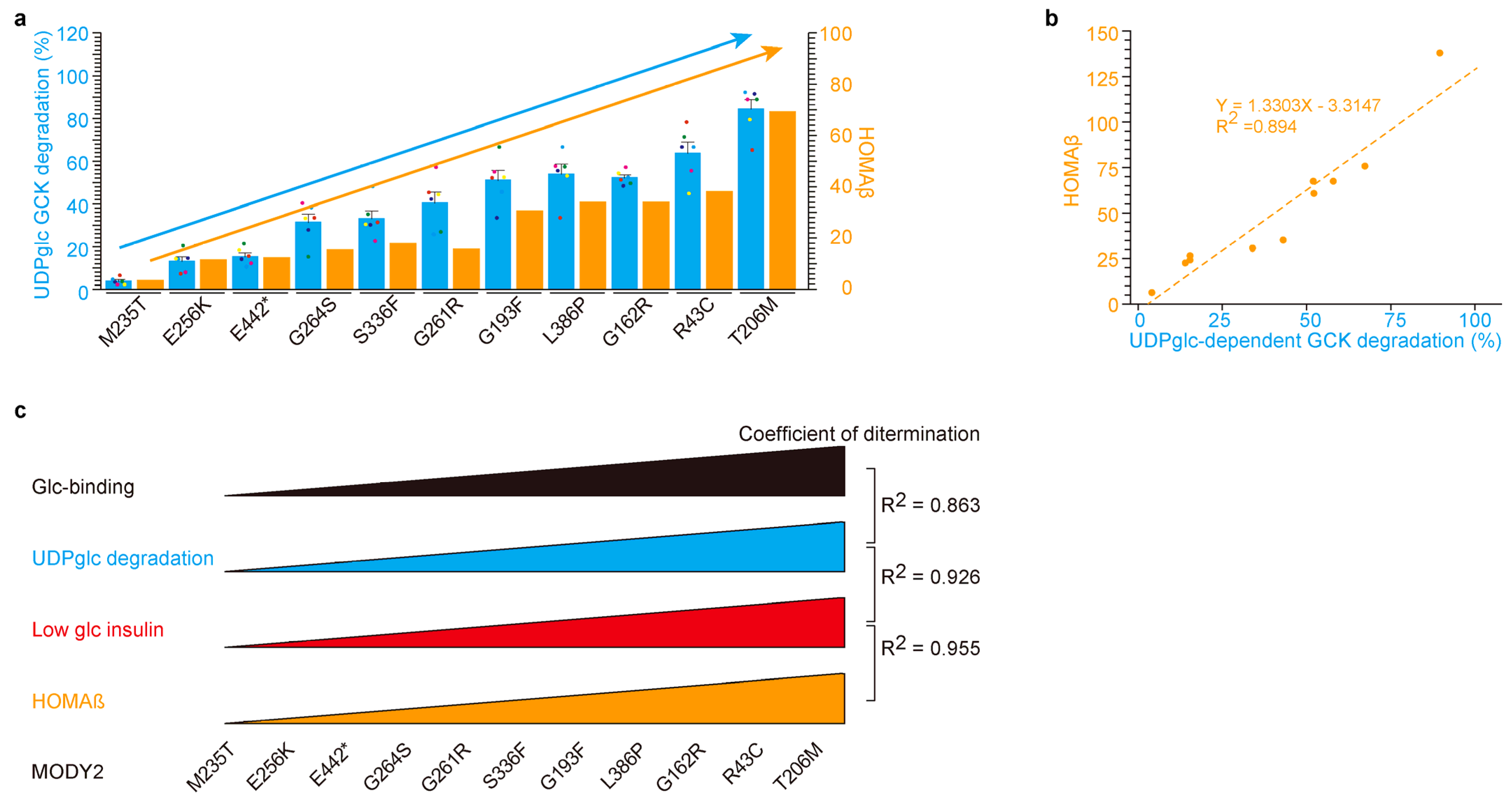
2.4. UDP-Glucose-Dependent MODY2 Glucokinase Variant Protein Degradation Was Involved in HOMAβ
3. Discussion
3.1. Glucose-Binding and UDP-Glucose-Dependent Degradation
3.2. Glucose-Binding Activity of Glucokinase Variants
3.3. UDP-Glucose as a Therapeutic Drug
4. Materials and Methods
4.1. Reagents
4.2. Cell Culture
4.3. Analysis of Insulin Secretion from Cells
4.4. Chemical Detection
4.5. Immunoprecipitation and Western Blot
4.6. Immunocytochemical Analysis
4.7. MODY Study
4.8. Ethical Approval
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cho, J.; Horikawa, Y.; Enya, M.; Takeda, J.; Imai, Y.; Imai, Y.; Handa, H.; Imai, T. L-Arginine prevents cereblon-mediated ubiquitination of glucokinase and stimulates glucose-6-phosphate production in pancreatic β-cells. Commun. Biol. 2020, 3, 497. [Google Scholar] [CrossRef] [PubMed]
- Sagen, J.V.; Odili, S.; Bjørkhaug, L.; Zelent, D.; Buettger, C.; Kwagh, J.; Stanley, C.; Dahl-Jørgensen, K.; de Beaufort, C.; Bell, G.I.; et al. From Clinicogenetic Studies of Maturity-Onset Diabetes of the Young to Unraveling Complex Mechanisms of Glucokinase Regulation. Diabetes 2006, 55, 1713–1722. [Google Scholar] [CrossRef] [PubMed]
- García-Herrero, C.M.; Galán, M.; Vincent, O.; Flández, B.; Gargallo, M.; Delgado-Alvarez, E.; Blázquez, E.; Navas, M.A. Functional analysis of human glucokinase gene mutations causing MODY2: Exploring the regulatory mechanisms of glucokinase activity. Diabetologia 2007, 50, 325–333. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shields, B.M.; Hicks, S.; Shepherd, M.H.; Colclough, K.; Hattersley, A.T.; Ellard, S. Maturity-onset diabetes of the young (MODY): How many cases are we missing? Diabetologia 2010, 53, 2504–2508. [Google Scholar] [CrossRef] [PubMed]
- Antosik, K.; Gnys, P.; De Franco, E.; Borowiec, M.; Mysliwiec, M.; Ellard, S.; Mlynarski, W. Single patient in GCK-MODY family successfully re-diagnosed into GCK-PNDM through targeted next-generation sequencing technology. Acta Diabetol. 2016, 53, 337–338. [Google Scholar] [CrossRef][Green Version]
- Horikawa, Y. Maturity-onset diabetes of the young as a model for elucidating the multifactorial origin of type 2 diabetes mellitus. J. Diabetes Investig. 2018, 9, 704–712. [Google Scholar] [CrossRef]
- Gutierrez-Nogués, A.; García-Herrero, C.-M.; Oriola, J.; Vincent, O.; Navas, M.-A. Functional characterization of MODY2 mutations in the nuclear export signal of glucokinase. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2018, 1864, 2385–2394. [Google Scholar] [CrossRef]
- Hulín, J.; Škopková, M.; Valkovičová, T.; Mikulajová, S.; Rosoľanková, M.; Papcun, P.; Gašperíková, D.; Staník, J. Clinical implications of the glucokinase impaired function—GCK MODY today. Physiol. Res. 2020, 69, 995–1011. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, S.; Wu, J.; Dong, J.; Liao, L. MODY2 in Asia: Analysis of GCK mutations and clinical characteristics. Endocr. Connect. 2020, 9, 471–478. [Google Scholar] [CrossRef]
- Christesen, H.B.; Jacobsen, B.B.; Odili, S.; Buettger, C.; Cuesta-Munoz, A.; Hansen, T.; Brusgaard, K.; Massa, O.; Magnuson, M.A.; Shiota, C.; et al. The Second Activating Glucokinase Mutation (A456V): Implications for glucose homeostasis and diabetes therapy. Diabetes 2002, 51, 1240–1246. [Google Scholar] [CrossRef]
- Christesen, H.B.T.; Tribble, N.D.; Molven, A.; Siddiqui, J.; Sandal, T.; Brusgaard, K.; Ellard, S.; Njølstad, P.R.; Alm, J.; Jacobsen, B.B.; et al. Activating glucokinase (GCK) mutations as a cause of medically responsive congenital hyperinsulinism: Prevalence in children and characterisation of a novel GCK mutation. Eur. J. Endocrinol. 2008, 159, 27–34. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pino, M.F.; Kim, K.-A.; Shelton, K.D.; Lindner, J.; Odili, S.; Li, C.; Collins, H.W.; Shiota, M.; Matschinsky, F.M.; Magnuson, M.A. Glucokinase Thermolability and Hepatic Regulatory Protein Binding Are Essential Factors for Predicting the Blood Glucose Phenotype of Missense Mutations. J. Biol. Chem. 2007, 282, 13906–13916. [Google Scholar] [CrossRef] [PubMed]
- Osbak, K.K.; Colclough, K.; Saint-Martin, C.; Beer, N.L.; Bellanné-Chantelot, C.; Ellard, S.; Gloyn, A.L. Update on mutations in glucokinase (GCK), which cause maturity-onset diabetes of the young, permanent neonatal diabetes, and hyperinsulinemic hypoglycemia. Hum. Mutat. 2009, 30, 1512–1526. [Google Scholar] [CrossRef] [PubMed]
- Vidal-Alabró, A.; Gómez-Valadés, A.G.; Méndez-Lucas, A.; Llorens, J.; Bartrons, R.; Bermúdez, J.; Perales, J.C. Liver GlucokinaseA456V Induces Potent Hypoglycemia without Dyslipidemia through a Paradoxical Induction of the Catalytic Subunit of Glucose-6-Phosphatase. Int. J. Endocrinol. 2011, 2011, 707928. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Chen, X.; Sun, X.; Wang, L.; Chen, S. The Glycolytic Switch in Tumors: How Many Players Are Involved? J. Cancer 2017, 8, 3430–3440. [Google Scholar] [CrossRef]
- Lopez-Girona, A.; Mendy, D.; Ito, T.A.; Miller, K.H.; Gandhi, A.K.; Kang, J.; Karasawa, S.; Carmel, G.; Jackson, P.E.; Abbasian, M.; et al. Cereblon is a direct protein target for immunomodulatory and antiproliferative activities of lenalidomide and pomalidomide. Leukemia 2012, 26, 2326–2335. [Google Scholar] [CrossRef]
- Fischer, E.S.; Böhm, K.; Lydeard, J.R.; Yang, H.; Stadler, M.B.; Cavadini, S.; Nagel, J.; Serluca, F.; Acker, V.; Lingaraju, G.M.; et al. Structure of the DDB1–CRBN E3 ubiquitin ligase in complex with thalidomide. Nature 2014, 512, 49–53. [Google Scholar] [CrossRef]
- Chen, Y.-A.; Peng, Y.-J.; Hu, M.-C.; Huang, J.-J.; Chien, Y.-C.; Wu, J.-T.; Chen, T.-Y.; Tang, C.-Y. The Cullin 4A/B-DDB1-Cereblon E3 Ubiquitin Ligase Complex Mediates the Degradation of CLC-1 Chloride Channels. Sci. Rep. 2015, 5, 10667. [Google Scholar] [CrossRef]
- Heim, C.; Pliatsika, D.; Mousavizadeh, F.; Bär, K.; Alvarez, B.H.; Giannis, A.; Hartmann, M.D. De-Novo Design of Cereblon (CRBN) Effectors Guided by Natural Hydrolysis Products of Thalidomide Derivatives. J. Med. Chem. 2019, 62, 6615–6629. [Google Scholar] [CrossRef]
- Fu, S.-J.; Hu, M.-C.; Peng, Y.-J.; Fang, H.-Y.; Hsiao, C.-T.; Chen, T.-Y.; Jeng, C.-J.; Tang, C.-Y. CUL4-DDB1-CRBN E3 Ubiquitin Ligase Regulates Proteostasis of ClC-2 Chloride Channels: Implication for Aldosteronism and Leukodystrophy. Cells 2020, 9, 1332. [Google Scholar] [CrossRef]
- Cho, J.; Miyagawa, A.; Yamaguchi, K.; Abe, W.; Tsugawa, Y.; Yamamura, H.; Imai, T. UDP-Glucose: A Cereblon-Dependent Glucokinase Protein Degrader. Int. J. Mol. Sci. 2022, 23, 9094. [Google Scholar] [CrossRef] [PubMed]
- Heim, C.; Spring, A.-K.; Kirchgäßner, S.; Schwarzer, D.; Hartmann, M.D. Identification and structural basis of C-terminal cyclic imides as natural degrons for cereblon. Biochem. Biophys. Res. Commun. 2022, 637, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.; Miyagawa, A.; Yamaguchi, K.; Abe, W.; Tsugawa, Y.; Yamamura, H.; Imai, T. UDP-glucose, cereblon-dependent proinsulin degrader. Sci. Rep. 2022, 12, 14568. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9418190/ (accessed on 28 October 2023). [CrossRef] [PubMed]
- Békés, M.; Langley, D.R.; Crews, C.M. PROTAC targeted protein degraders: The past is prologue. Nat. Rev. Drug Discov. 2022, 21, 181–200. [Google Scholar] [CrossRef] [PubMed]
- Barbetti, F.; Cobo-Vuilleumier, N.; Dionisi-Vici, C.; Toni, S.; Ciampalini, P.; Massa, O.; Rodriguez-Bada, P.; Colombo, C.; Lenzi, L.; Garcia-Gimeno, M.A.; et al. Opposite Clinical Phenotypes of Glucokinase Disease: Description of a Novel Activating Mutation and Contiguous Inactivating Mutations in Human Glucokinase (GCK) Gene. Mol. Endocrinol. 2009, 23, 1983–1989. [Google Scholar] [CrossRef]
- Galán, M.; Vincent, O.; Roncero, I.; Azriel, S.; Boix-Pallares, P.; Delgado-Alvarez, E.; Díaz-Cadórniga, F.; Blázquez, E.; Navas, M.-A. Effects of novel maturity-onset diabetes of the young (MODY)-associated mutations on glucokinase activity and protein stability. Biochem. J. 2005, 393, 389–396. [Google Scholar] [CrossRef]
- Sayed, S.; Langdon, D.R.; Odili, S.; Chen, P.; Buettger, C.; Schiffman, A.B.; Suchi, M.; Taub, R.; Grimsby, J.; Matschinsky, F.M.; et al. Extremes of Clinical and Enzymatic Phenotypes in Children With Hyperinsulinism Caused by Glucokinase Activating Mutations. Diabetes 2009, 58, 1419–1427. [Google Scholar] [CrossRef]
- Zelent, B.; Odili, S.; Buettger, C.; Zelent, D.K.; Chen, P.; Fenner, D.; Bass, J.; Stanley, C.; Laberge, M.; Vanderkooi, J.M.; et al. Mutational analysis of allosteric activation and inhibition of glucokinase. Biochem. J. 2011, 440, 203–215. [Google Scholar] [CrossRef]
- Ito, T.; Ando, H.; Suzuki, T.; Ogura, T.; Hotta, K.; Imamura, Y.; Yamaguchi, Y.; Handa, H. Identification of a Primary Target of Thalidomide Teratogenicity. Science 2010, 327, 1345–1350. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, J.; Horikawa, Y.; Oiwa, Y.; Hosomichi, K.; Yabe, D.; Imai, T. Glucokinase Variant Proteins Are Resistant to Fasting-Induced Uridine Diphosphate Glucose-Dependent Degradation in Maturity-Onset Diabetes of the Young Type 2 Patients. Int. J. Mol. Sci. 2023, 24, 15842. https://doi.org/10.3390/ijms242115842
Cho J, Horikawa Y, Oiwa Y, Hosomichi K, Yabe D, Imai T. Glucokinase Variant Proteins Are Resistant to Fasting-Induced Uridine Diphosphate Glucose-Dependent Degradation in Maturity-Onset Diabetes of the Young Type 2 Patients. International Journal of Molecular Sciences. 2023; 24(21):15842. https://doi.org/10.3390/ijms242115842
Chicago/Turabian StyleCho, Jaeyong, Yukio Horikawa, Yuki Oiwa, Kazuyoshi Hosomichi, Daisuke Yabe, and Takeshi Imai. 2023. "Glucokinase Variant Proteins Are Resistant to Fasting-Induced Uridine Diphosphate Glucose-Dependent Degradation in Maturity-Onset Diabetes of the Young Type 2 Patients" International Journal of Molecular Sciences 24, no. 21: 15842. https://doi.org/10.3390/ijms242115842
APA StyleCho, J., Horikawa, Y., Oiwa, Y., Hosomichi, K., Yabe, D., & Imai, T. (2023). Glucokinase Variant Proteins Are Resistant to Fasting-Induced Uridine Diphosphate Glucose-Dependent Degradation in Maturity-Onset Diabetes of the Young Type 2 Patients. International Journal of Molecular Sciences, 24(21), 15842. https://doi.org/10.3390/ijms242115842