Anti-Atopic Dermatitis Effect of TPS240, a Novel Therapeutic Peptide, via Suppression of NF-κB and STAT3 Activation
Abstract
1. Introduction
2. Results
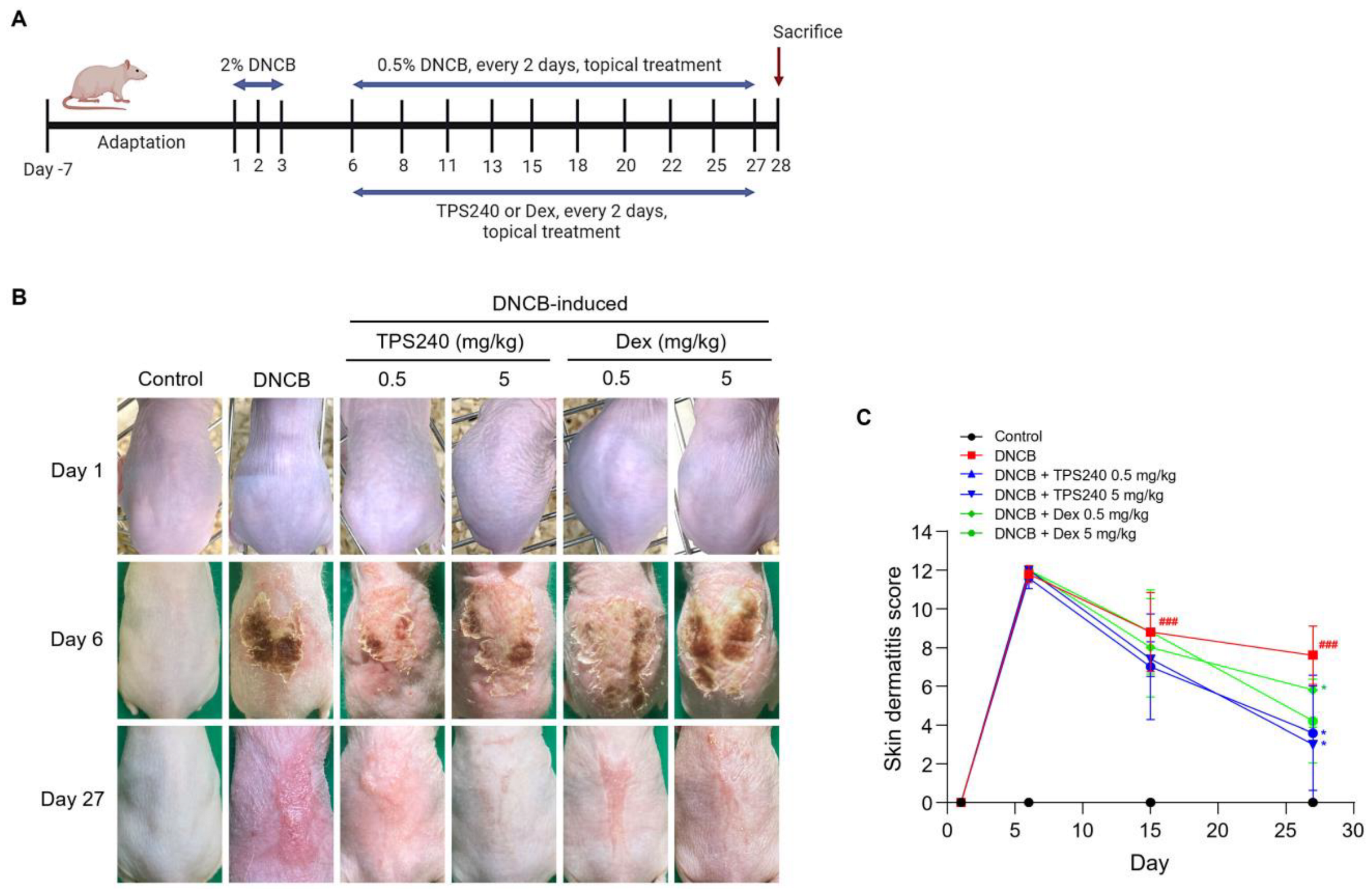
2.1. TPS240 Alleviates AD Symptoms in a DNCB-Induced Mouse Model
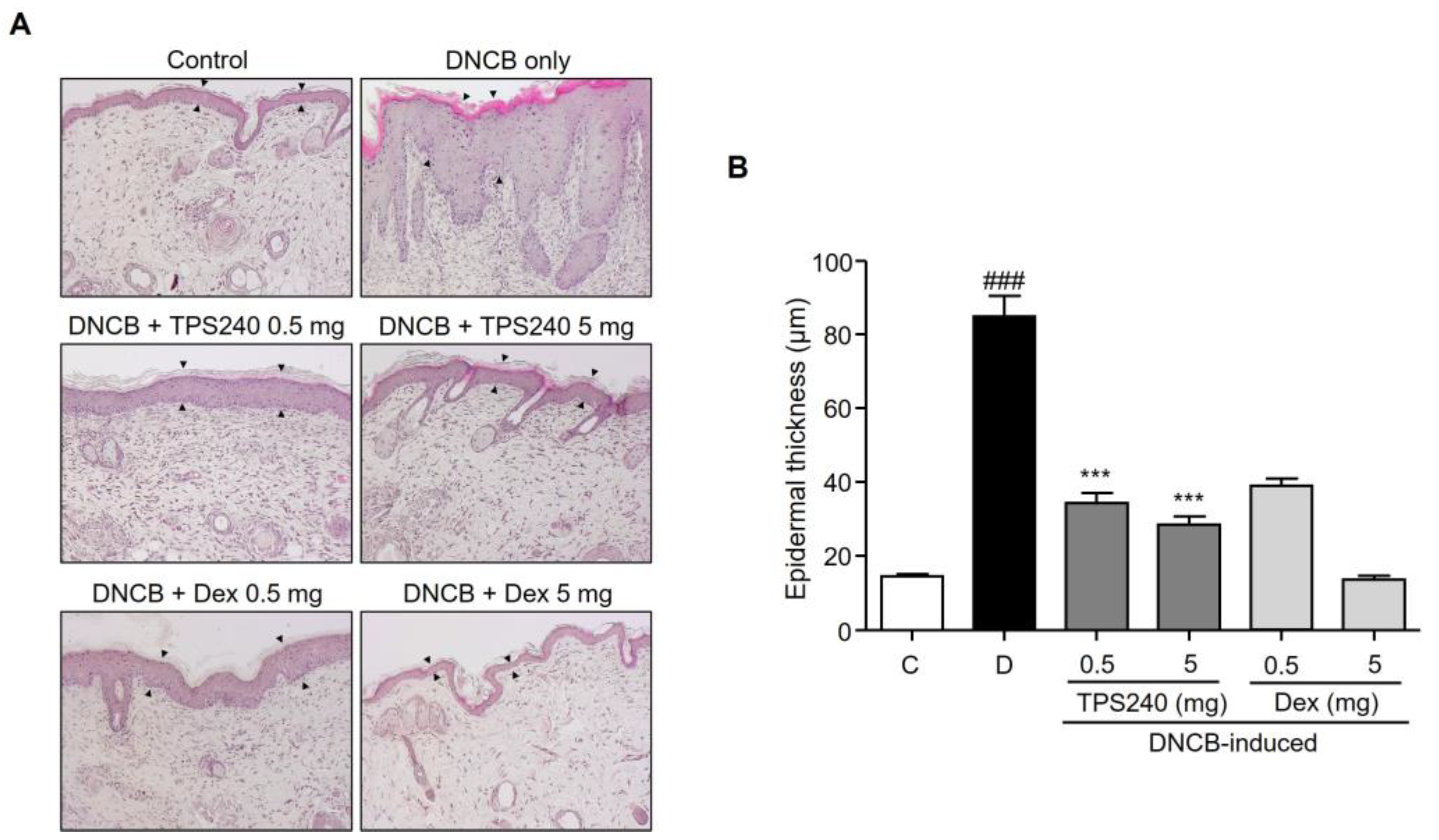
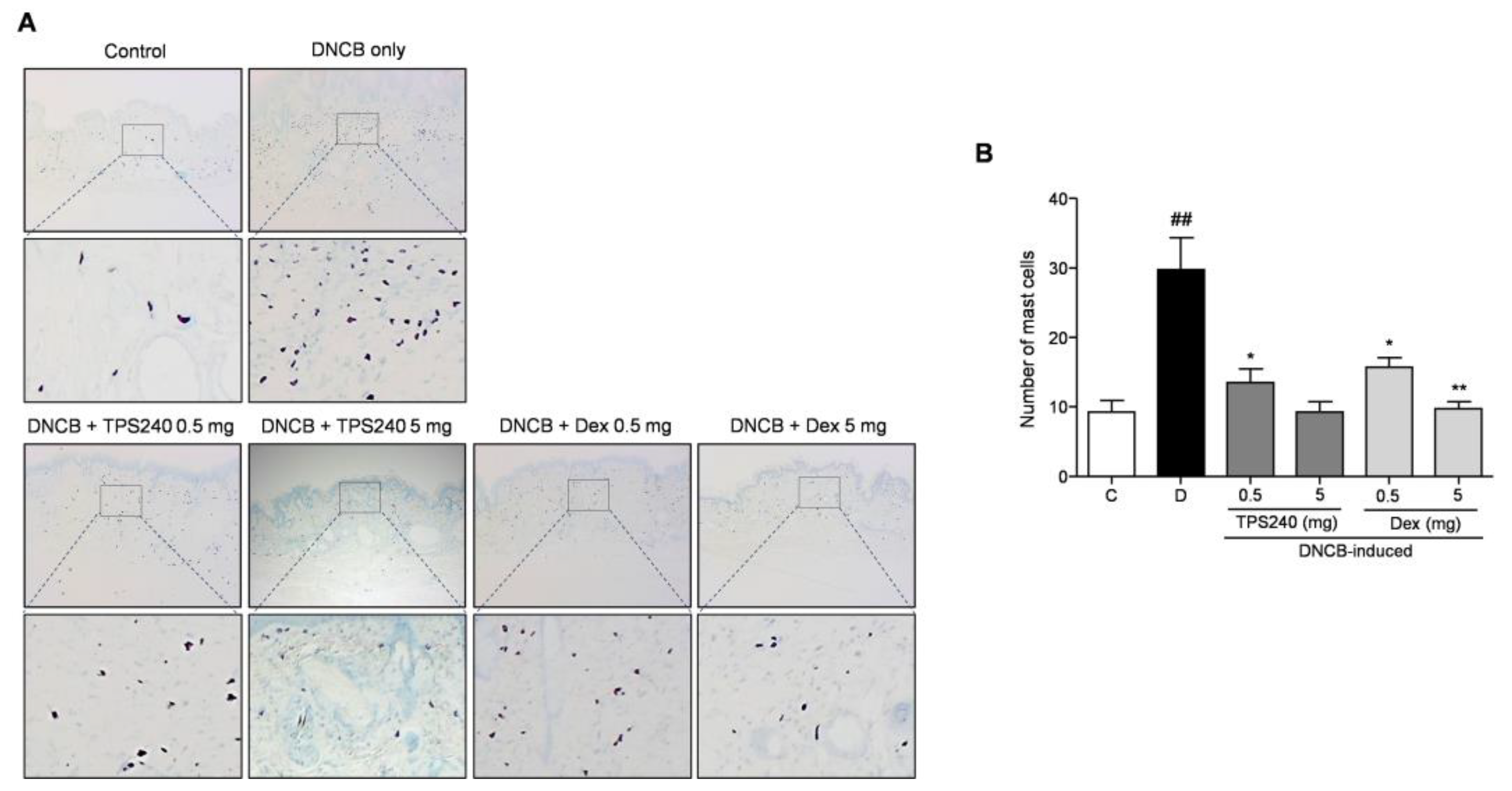
2.2. TPS240 Inhibits Epidermal Hyperplasia and Mast Cell Infiltration in DNCB-Induced AD-Like Skin Lesions
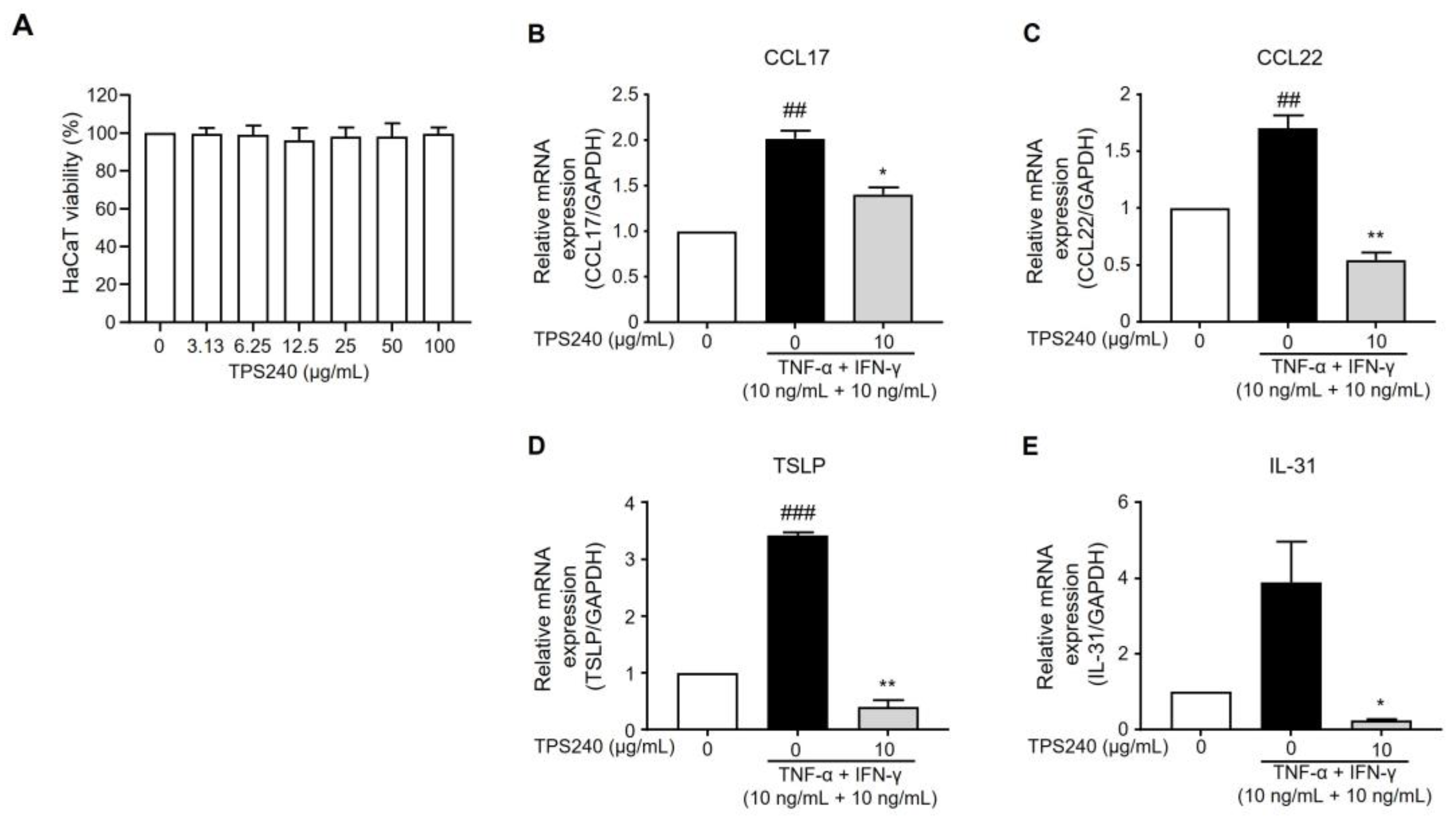
2.3. TPS240 Inhibits the Expression of Th2 Responsive Chemokines in TNF-α/IFN-γ-Stimulated HaCaT Cells
2.4. TPS240 Reduces the Expression of Pruritus-Related Cytokines in TNF-α/IFN-γ-Stimulated HaCaT Cells
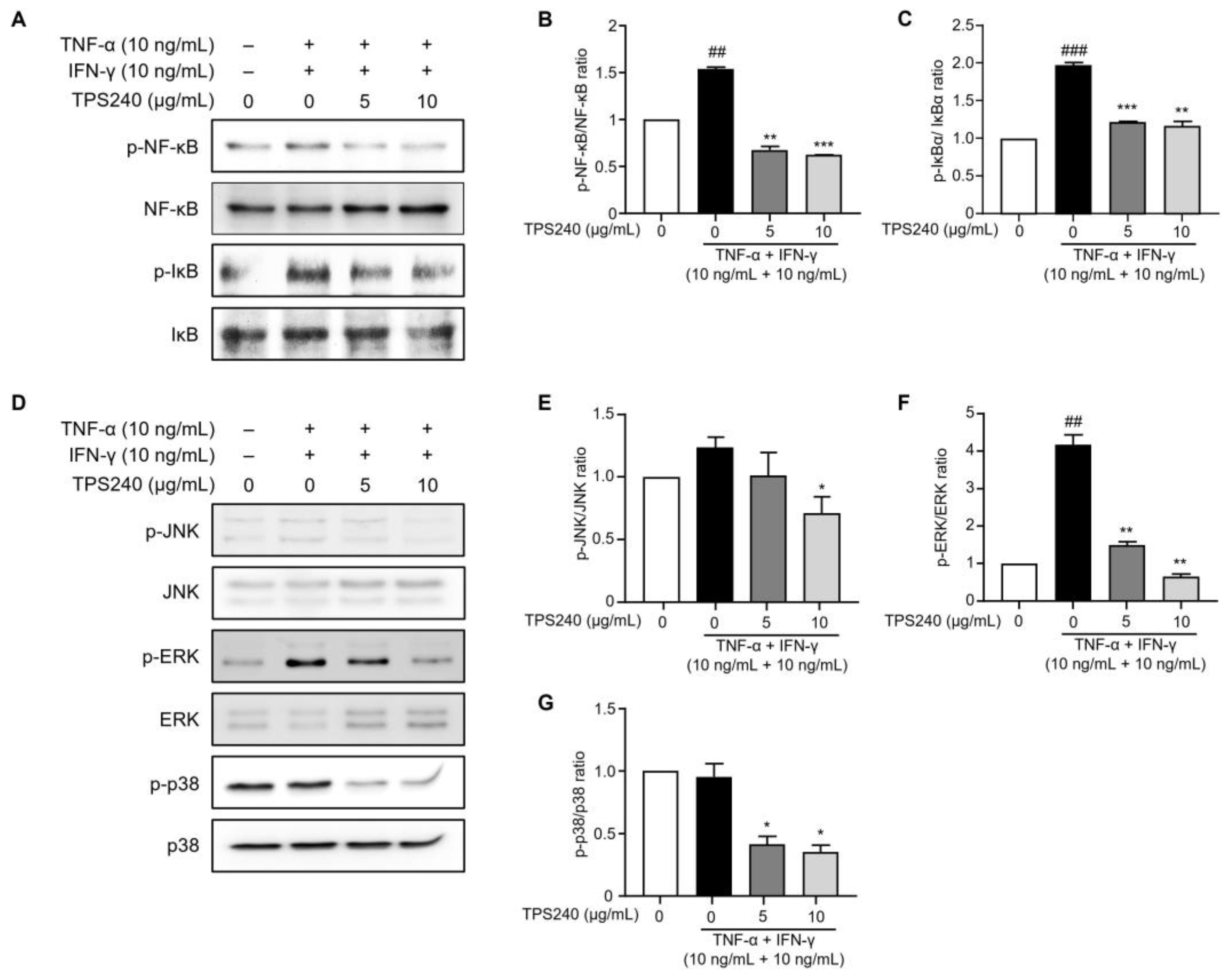
2.5. TPS240 Inhibits the Activation of NF-κB in TNF-α/IFN-γ-Stimulated HaCaT Cells
2.6. TPS240 Inhibits the Activation of JNK, ERK, p38 in TNF-α/IFN-γ-Stimulated HaCaT Cells
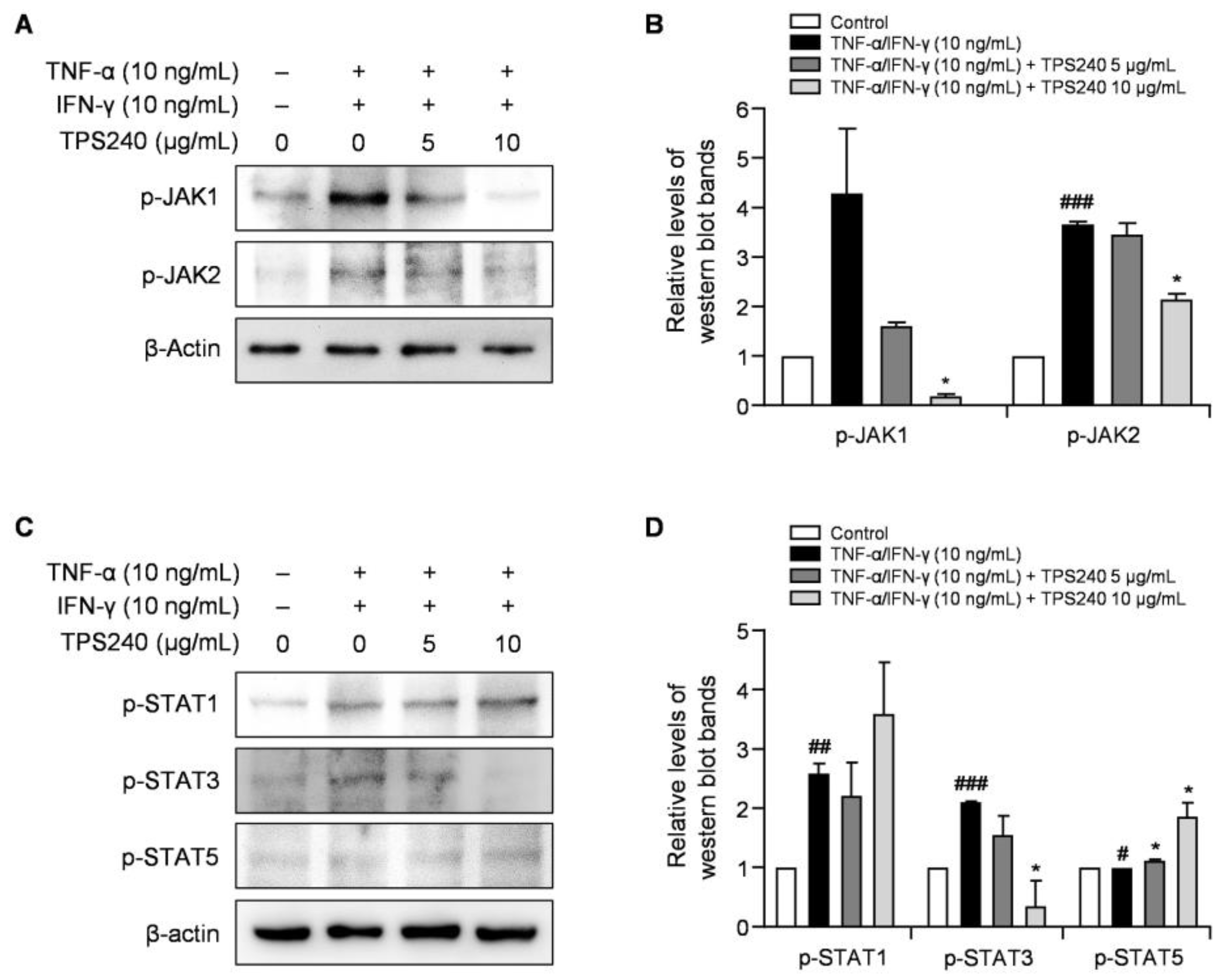
2.7. TPS240 Inhibits the Activation of the JAK1/STAT3 Pathway in TNF-α/IFN-γ-Stimulated HaCaT Cells
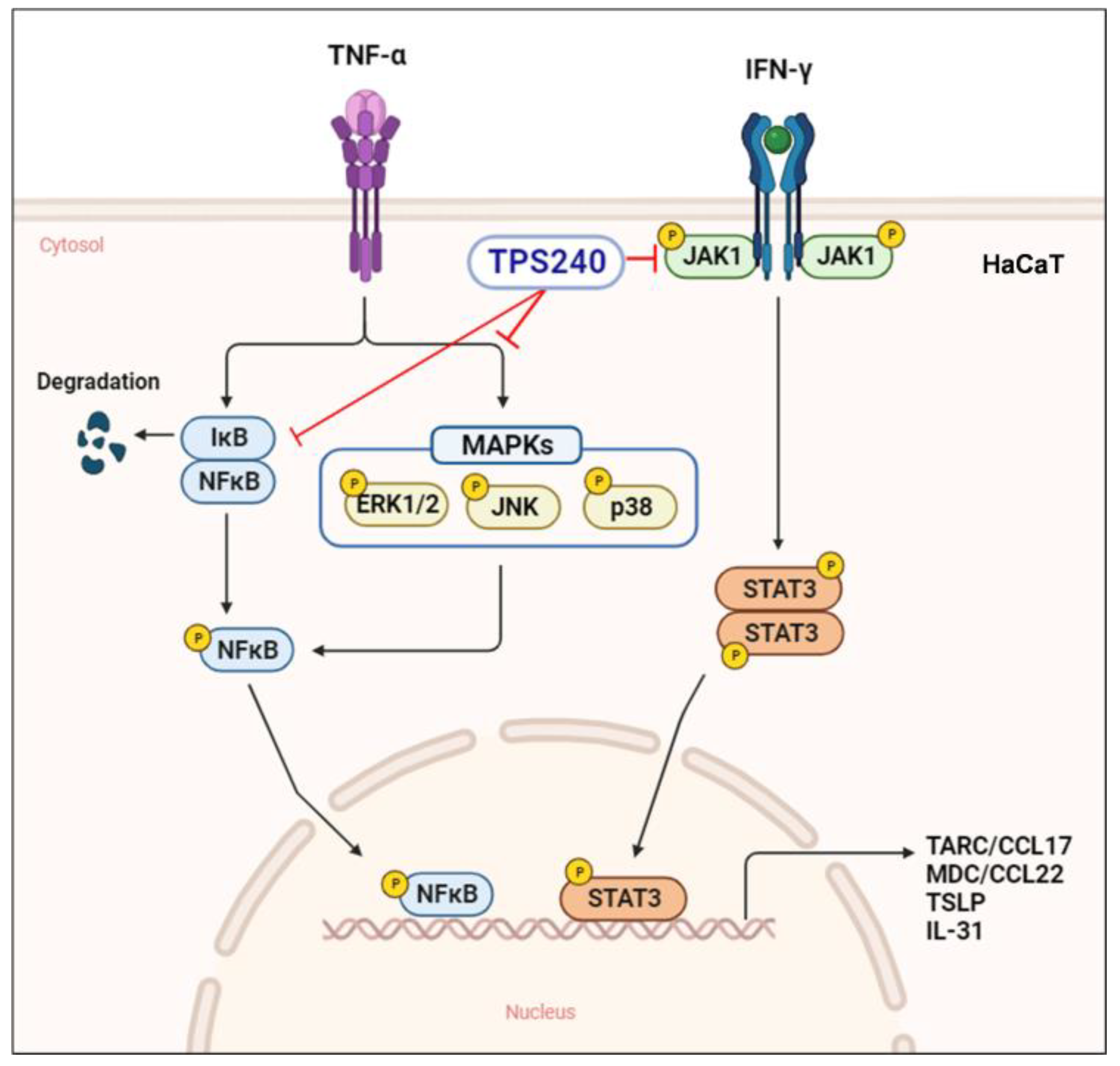
3. Discussion
4. Materials and Methods
4.1. Peptide Synthesis
4.2. Animals
4.3. Establishment of a DNCB-Induced AD-Like Mouse Model
4.4. Evaluation of Severity of Dermatitis
4.5. Histological Analysis
4.6. Cell Culture
4.7. Cell Viability Assay
4.8. Real-Time Quantitative PCR (RT-qPCR) Analysis
4.9. Western Blot Analysis
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gilaberte, Y.; Perez-Gilaberte, J.B.; Poblador-Plou, B.; Bliek-Bueno, K.; Gimeno-Miguel, A.; Prados-Torres, A. Prevalence and Comorbidity of Atopic Dermatitis in Children: A Large-Scale Population Study Based on Real-World Data. J. Clin. Med. 2020, 9, 1632. [Google Scholar] [CrossRef] [PubMed]
- Langan, S.M.; Irvine, A.D.; Weidinger, S. Atopic dermatitis. Lancet 2020, 396, 345–360. [Google Scholar] [CrossRef]
- Goh, M.S.; Yun, J.S.; Su, J.C. Management of atopic dermatitis: A narrative review. Med. J. Aust. 2022, 216, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Wollenberg, A.; Werfel, T.; Ring, J.; Ott, H.; Gieler, U.; Weidinger, S. Atopic Dermatitis in Children and Adults-Diagnosis and Treatment. Dtsch. Arztebl. Int. 2023, 120, 224–234. [Google Scholar] [CrossRef]
- Chiricozzi, A.; Maurelli, M.; Calabrese, L.; Peris, K.; Girolomoni, G. Overview of Atopic Dermatitis in Different Ethnic Groups. J. Clin. Med. 2023, 12, 2701. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.H.; Kim, S.H.; Kwon, O.K.; Kim, J.H.; Oh, S.R.; Han, S.B.; Park, J.W.; Ahn, K.S. Purpurin suppresses atopic dermatitis via TNF-alpha/IFN-gamma-induced inflammation in HaCaT cells. Int. J. Immunopathol. Pharmacol. 2022, 36, 3946320221111135. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.; Lee, T.H.; Oh, H.J.; Kim, H.; Son, Y.; Lee, E.H.; Kim, J. Inhibitory effect of 5,6-dihydroergosteol-glucoside on atopic dermatitis-like skin lesions via suppression of NF-kappaB and STAT activation. J. Dermatol. Sci. 2015, 79, 252–261. [Google Scholar] [CrossRef]
- Nygaard, U.; Hvid, M.; Johansen, C.; Buchner, M.; Folster-Holst, R.; Deleuran, M.; Vestergaard, C. TSLP, IL-31, IL-33 and sST2 are new biomarkers in endophenotypic profiling of adult and childhood atopic dermatitis. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 1930–1938. [Google Scholar] [CrossRef]
- Guttman-Yassky, E.; Waldman, A.; Ahluwalia, J.; Ong, P.Y.; Eichenfield, L.F. Atopic dermatitis: Pathogenesis. Semin. Cutan. Med. Surg. 2017, 36, 100–103. [Google Scholar] [CrossRef]
- Yang, X.; Kambe, N.; Takimoto-Ito, R.; Kabashima, K. Advances in the pathophysiology of atopic dermatitis revealed by novel therapeutics and clinical trials. Pharmacol. Ther. 2021, 224, 107830. [Google Scholar] [CrossRef]
- Seegraber, M.; Srour, J.; Walter, A.; Knop, M.; Wollenberg, A. Dupilumab for treatment of atopic dermatitis. Expert. Rev. Clin. Pharmacol. 2018, 11, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Wollenberg, A.; Blauvelt, A.; Guttman-Yassky, E.; Worm, M.; Lynde, C.; Lacour, J.P.; Spelman, L.; Katoh, N.; Saeki, H.; Poulin, Y.; et al. Tralokinumab for moderate-to-severe atopic dermatitis: Results from two 52-week, randomized, double-blind, multicentre, placebo-controlled phase III trials (ECZTRA 1 and ECZTRA 2). Br. J. Dermatol. 2021, 184, 437–449. [Google Scholar] [CrossRef] [PubMed]
- Bieber, T.; Simpson, E.L.; Silverberg, J.I.; Thaci, D.; Paul, C.; Pink, A.E.; Kataoka, Y.; Chu, C.Y.; DiBonaventura, M.; Rojo, R.; et al. Abrocitinib versus Placebo or Dupilumab for Atopic Dermatitis. N. Engl. J. Med. 2021, 384, 1101–1112. [Google Scholar] [CrossRef]
- La Manna, S.; Di Natale, C.; Florio, D.; Marasco, D. Peptides as Therapeutic Agents for Inflammatory-Related Diseases. Int. J. Mol. Sci. 2018, 19, 2714. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, N.; Zhang, W.; Cheng, X.; Yan, Z.; Shao, G.; Wang, X.; Wang, R.; Fu, C. Therapeutic peptides: Current applications and future directions. Signal Transduct. Target. Ther. 2022, 7, 48. [Google Scholar] [CrossRef]
- Lee, S.; Kim, M.S.; Jung, S.J.; Kim, D.; Park, H.J.; Cho, D. ERK activating peptide, AES16-2M promotes wound healing through accelerating migration of keratinocytes. Sci. Rep. 2018, 8, 14398. [Google Scholar] [CrossRef]
- Kim, M.S.; Song, J.; Park, S.; Kim, T.S.; Park, H.J.; Cho, D. The Wound Healing Peptide, AES16-2M, Ameliorates Atopic Dermatitis In Vivo. Molecules 2021, 26, 1168. [Google Scholar] [CrossRef]
- Kiatsurayanon, C.; Niyonsaba, F.; Smithrithee, R.; Akiyama, T.; Ushio, H.; Hara, M.; Okumura, K.; Ikeda, S.; Ogawa, H. Host defense (Antimicrobial) peptide, human beta-defensin-3, improves the function of the epithelial tight-junction barrier in human keratinocytes. J. Investig. Dermatol. 2014, 134, 2163–2173. [Google Scholar] [CrossRef]
- Hakuta, A.; Yamaguchi, Y.; Okawa, T.; Yamamoto, S.; Sakai, Y.; Aihara, M. Anti-inflammatory effect of collagen tripeptide in atopic dermatitis. J. Dermatol. Sci. 2017, 88, 357–364. [Google Scholar] [CrossRef]
- Hwang-Bo, J.; Veerappan, K.; Moon, H.; Lee, T.H.; Lee, K.W.; Park, J.; Chung, H. Parnassin, a Novel Therapeutic Peptide, Alleviates Skin Lesions in a DNCB-Induced Atopic Dermatitis Mouse Model. Biomedicines 2023, 11, 1389. [Google Scholar] [CrossRef]
- Edwards, I.A.; Elliott, A.G.; Kavanagh, A.M.; Zuegg, J.; Blaskovich, M.A.; Cooper, M.A. Contribution of Amphipathicity and Hydrophobicity to the Antimicrobial Activity and Cytotoxicity of β-Hairpin Peptides. ACS Infect. Dis. 2016, 2, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Zhang, M.; Lai, R.; Zhang, Z. Chemical modifications to increase the therapeutic potential of antimicrobial peptides. Peptides 2021, 146, 170666. [Google Scholar] [CrossRef] [PubMed]
- Horikawa, T.; Nakayama, T.; Hikita, I.; Yamada, H.; Fujisawa, R.; Bito, T.; Harada, S.; Fukunaga, A.; Chantry, D.; Gray, P.W.; et al. IFN-gamma-inducible expression of thymus and activation-regulated chemokine/CCL17 and macrophage-derived chemokine/CCL22 in epidermal keratinocytes and their roles in atopic dermatitis. Int. Immunol. 2002, 14, 767–773. [Google Scholar] [CrossRef]
- Saeki, H.; Tamaki, K. Thymus and activation regulated chemokine (TARC)/CCL17 and skin diseases. J. Dermatol. Sci. 2006, 43, 75–84. [Google Scholar] [CrossRef]
- Oyoshi, M.K.; Larson, R.P.; Ziegler, S.F.; Geha, R.S. Mechanical injury polarizes skin dendritic cells to elicit a T(H)2 response by inducing cutaneous thymic stromal lymphopoietin expression. J. Allergy Clin. Immunol. 2010, 126, 976–984. [Google Scholar] [CrossRef]
- Klonowska, J.; Glen, J.; Nowicki, R.J.; Trzeciak, M. New Cytokines in the Pathogenesis of Atopic Dermatitis-New Therapeutic Targets. Int. J. Mol. Sci. 2018, 19, 3086. [Google Scholar] [CrossRef] [PubMed]
- Kato, A.; Fujii, E.; Watanabe, T.; Takashima, Y.; Matsushita, H.; Furuhashi, T.; Morita, A. Distribution of IL-31 and its receptor expressing cells in skin of atopic dermatitis. J. Dermatol. Sci. 2014, 74, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Huang, I.H.; Chung, W.H.; Wu, P.C.; Chen, C.B. JAK-STAT signaling pathway in the pathogenesis of atopic dermatitis: An updated review. Front. Immunol. 2022, 13, 1068260. [Google Scholar] [CrossRef]
- David Boothe, W.; Tarbox, J.A.; Tarbox, M.B. Atopic Dermatitis: Pathophysiology. Adv. Exp. Med. Biol. 2017, 1027, 21–37. [Google Scholar] [CrossRef]
- Elias, P.M.; Steinhoff, M. “Outside-to-inside” (and now back to “outside”) pathogenic mechanisms in atopic dermatitis. J. Investig. Dermatol. 2008, 128, 1067–1070. [Google Scholar] [CrossRef]
- Kong, L.; Liu, J.; Wang, J.; Luo, Q.; Zhang, H.; Liu, B.; Xu, F.; Pang, Q.; Liu, Y.; Dong, J. Icariin inhibits TNF-alpha/IFN-gamma induced inflammatory response via inhibition of the substance P and p38-MAPK signaling pathway in human keratinocytes. Int. Immunopharmacol. 2015, 29, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, A.B.; Chamian, F.; Masud, S.; Cardinale, I.; Abello, M.V.; Lowes, M.A.; Chen, F.; Magliocco, M.; Krueger, J.G. TNF inhibition rapidly down-regulates multiple proinflammatory pathways in psoriasis plaques. J. Immunol. 2005, 175, 2721–2729. [Google Scholar] [CrossRef]
- Mehta, N.N.; Teague, H.L.; Swindell, W.R.; Baumer, Y.; Ward, N.L.; Xing, X.; Baugous, B.; Johnston, A.; Joshi, A.A.; Silverman, J.; et al. IFN-gamma and TNF-alpha synergism may provide a link between psoriasis and inflammatory atherogenesis. Sci. Rep. 2017, 7, 13831. [Google Scholar] [CrossRef] [PubMed]
- Vestergaard, C.; Bang, K.; Gesser, B.; Yoneyama, H.; Matsushima, K.; Larsen, C.G. A Th2 chemokine, TARC, produced by keratinocytes may recruit CLA+CCR4+ lymphocytes into lesional atopic dermatitis skin. J. Investig. Dermatol. 2000, 115, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.; Lopez, J.M. Understanding MAPK Signaling Pathways in Apoptosis. Int. J. Mol. Sci. 2020, 21, 2346. [Google Scholar] [CrossRef]
- Yano, C.; Saeki, H.; Komine, M.; Kagami, S.; Tsunemi, Y.; Ohtsuki, M.; Nakagawa, H. Mechanism of Macrophage-Derived Chemokine/CCL22 Production by HaCaT Keratinocytes. Ann. Dermatol. 2015, 27, 152–156. [Google Scholar] [CrossRef]
- Seif, F.; Khoshmirsafa, M.; Aazami, H.; Mohsenzadegan, M.; Sedighi, G.; Bahar, M. The role of JAK-STAT signaling pathway and its regulators in the fate of T helper cells. Cell Commun. Signal 2017, 15, 23. [Google Scholar] [CrossRef]
- Kwon, D.J.; Bae, Y.S.; Ju, S.M.; Goh, A.R.; Youn, G.S.; Choi, S.Y.; Park, J. Casuarinin suppresses TARC/CCL17 and MDC/CCL22 production via blockade of NF-kappaB and STAT1 activation in HaCaT cells. Biochem. Biophys. Res. Commun. 2012, 417, 1254–1259. [Google Scholar] [CrossRef]

| Genes | Primer Sequences | |
|---|---|---|
| CCL17 | Forward | 5′-ACTGCTCCAGGGATGCCATCGTTTTT-3′ |
| Reverse | 5′- ACAAGGGGATGGATCTCCCTCACTG-3′ | |
| CCL22 | Forward | 5′-AGGACAGAGCATGGCTCGCCTACAGA-3′ |
| Reverse | 5′-TAATGGCAGGGAGGTAGGGCTCCTGA-3′ | |
| TSLP | Forward | 5′-GGGGCTAAACCATGACAGAA-3′ |
| Reverse | 5′-GTTTGGCTGAAGGCTTGTTC-3′ | |
| IL-31 | Forward | 5′-CGACGTCTGTGCTCTTTCTG-3′ |
| Reverse | 5′-AGCATCTTCGAGAGGGACTG-3′ | |
| GAPDH | Forward | 5′-GACCCTCGAAATCCCATCACAG-3′ |
| Reverse | 5′-GTGCGAACTTCCACGGTGTGTT-3′ | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, D.; Hwang-Bo, J.; Veerappan, K.; Moon, H.; Park, J.; Chung, H. Anti-Atopic Dermatitis Effect of TPS240, a Novel Therapeutic Peptide, via Suppression of NF-κB and STAT3 Activation. Int. J. Mol. Sci. 2023, 24, 15814. https://doi.org/10.3390/ijms242115814
Lee D, Hwang-Bo J, Veerappan K, Moon H, Park J, Chung H. Anti-Atopic Dermatitis Effect of TPS240, a Novel Therapeutic Peptide, via Suppression of NF-κB and STAT3 Activation. International Journal of Molecular Sciences. 2023; 24(21):15814. https://doi.org/10.3390/ijms242115814
Chicago/Turabian StyleLee, Dongwoo, Jeon Hwang-Bo, Karpagam Veerappan, Hyunhye Moon, Junhyung Park, and Hoyong Chung. 2023. "Anti-Atopic Dermatitis Effect of TPS240, a Novel Therapeutic Peptide, via Suppression of NF-κB and STAT3 Activation" International Journal of Molecular Sciences 24, no. 21: 15814. https://doi.org/10.3390/ijms242115814
APA StyleLee, D., Hwang-Bo, J., Veerappan, K., Moon, H., Park, J., & Chung, H. (2023). Anti-Atopic Dermatitis Effect of TPS240, a Novel Therapeutic Peptide, via Suppression of NF-κB and STAT3 Activation. International Journal of Molecular Sciences, 24(21), 15814. https://doi.org/10.3390/ijms242115814






