Therapeutic Potential of 1,8-Dihydroanthraquinone Derivatives for Breast Cancer
Abstract
:1. Introduction
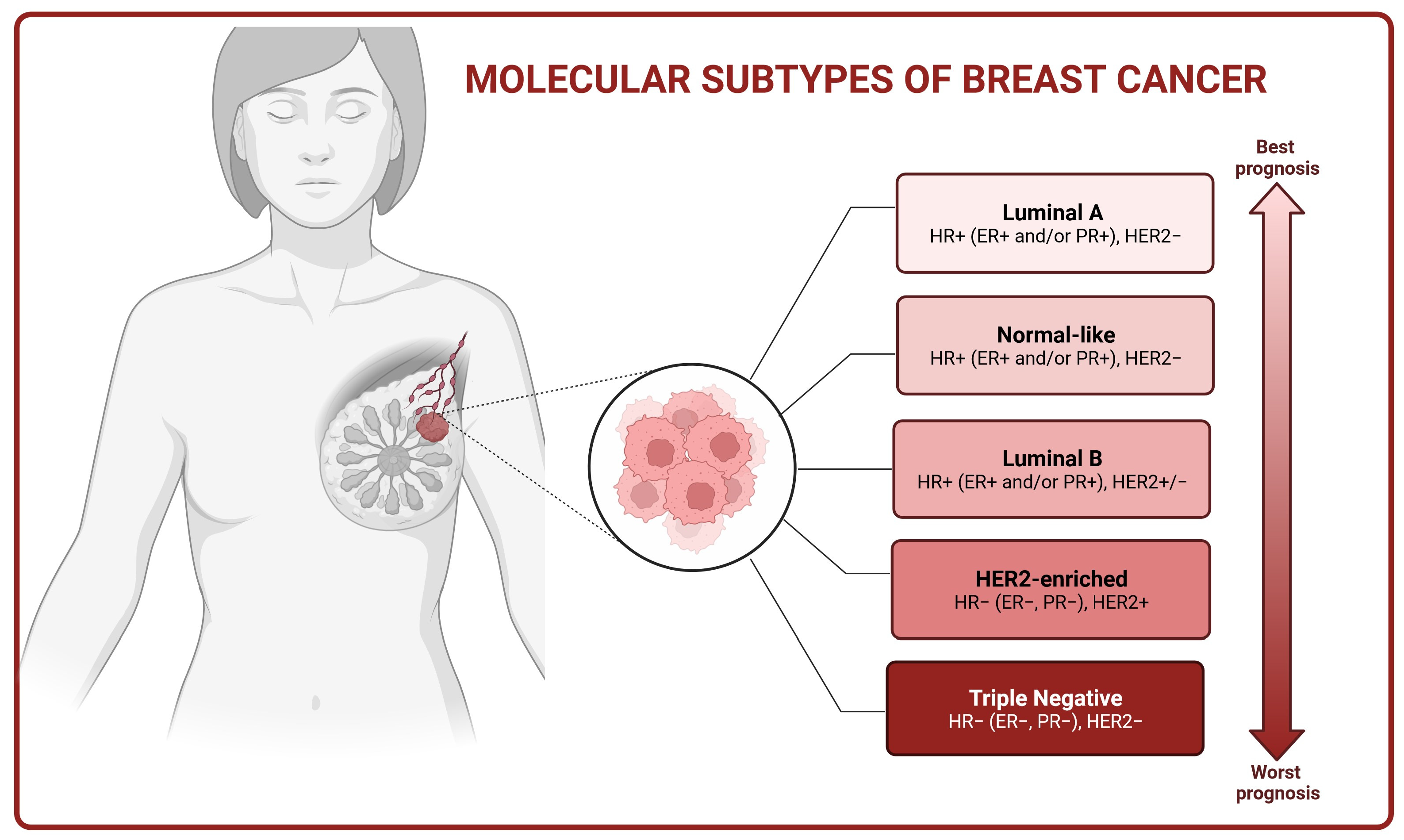
2. Molecular Basis of Breast Cancer
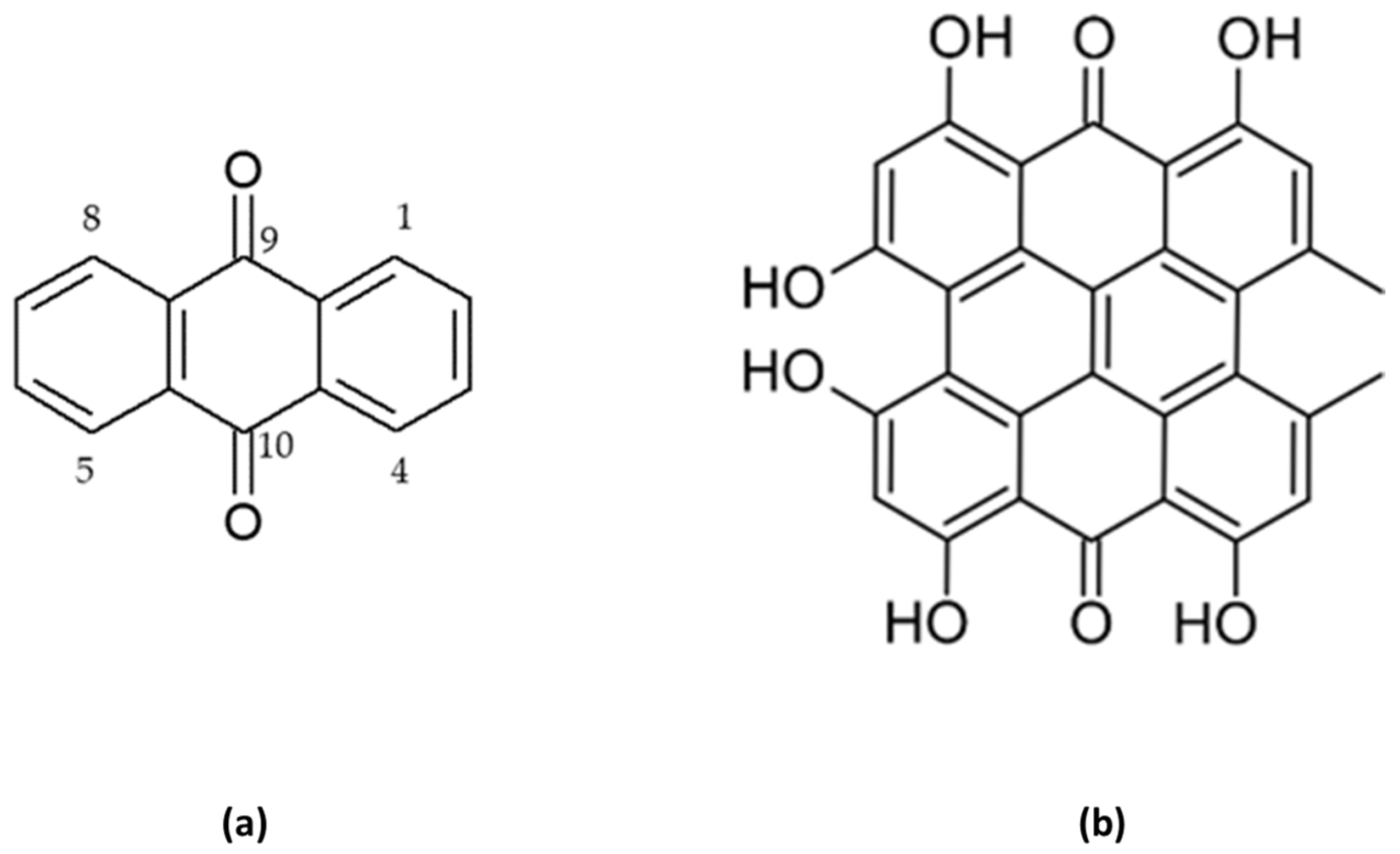
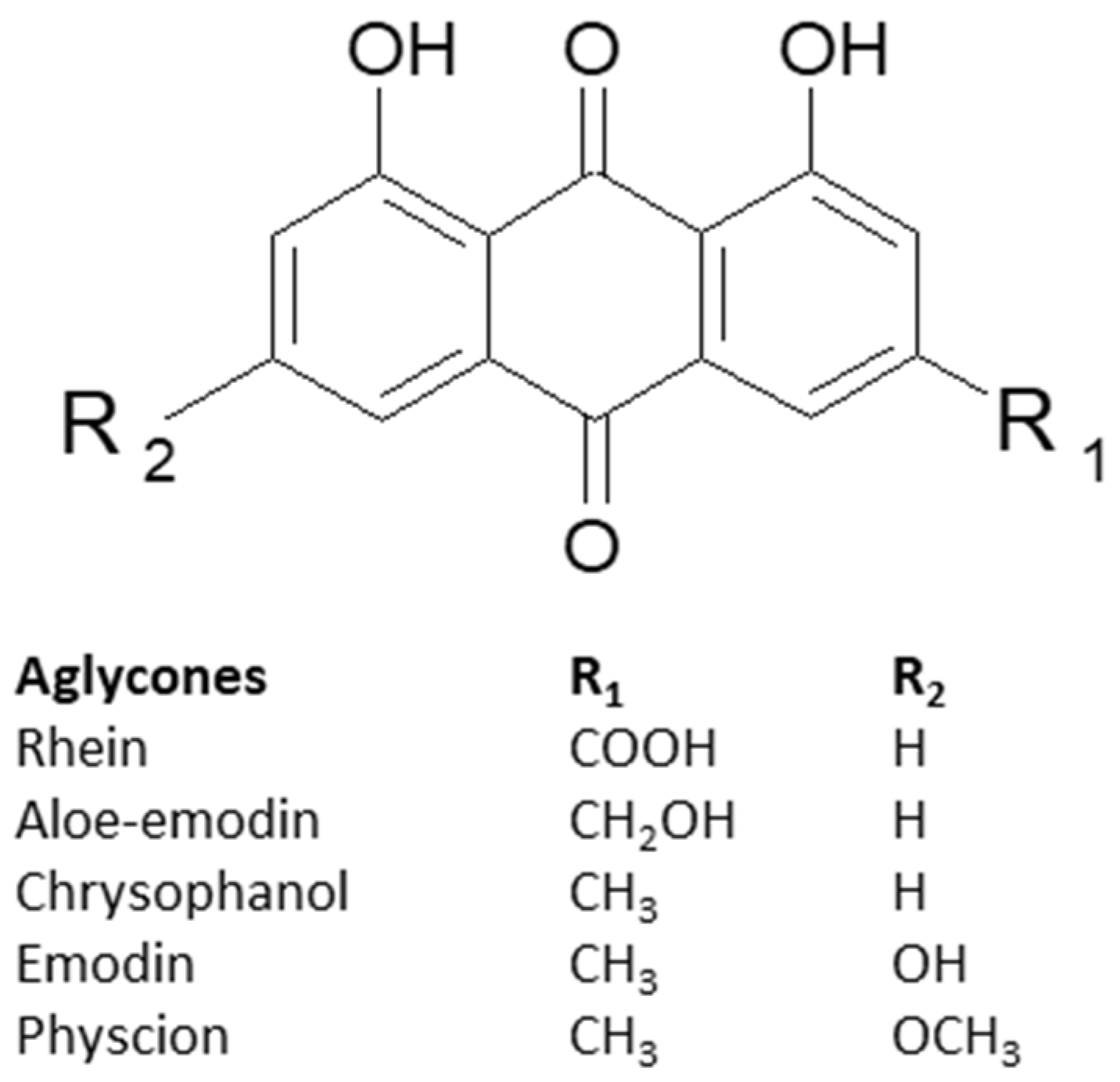
3. Characteristics of Anthracene Derivatives
4. Anticancer Activity of 1,8-Dihydroanthraquinone Derivatives in Breast Cancer In Vitro Models
4.1. Emodin
4.2. Aloe-Emodin
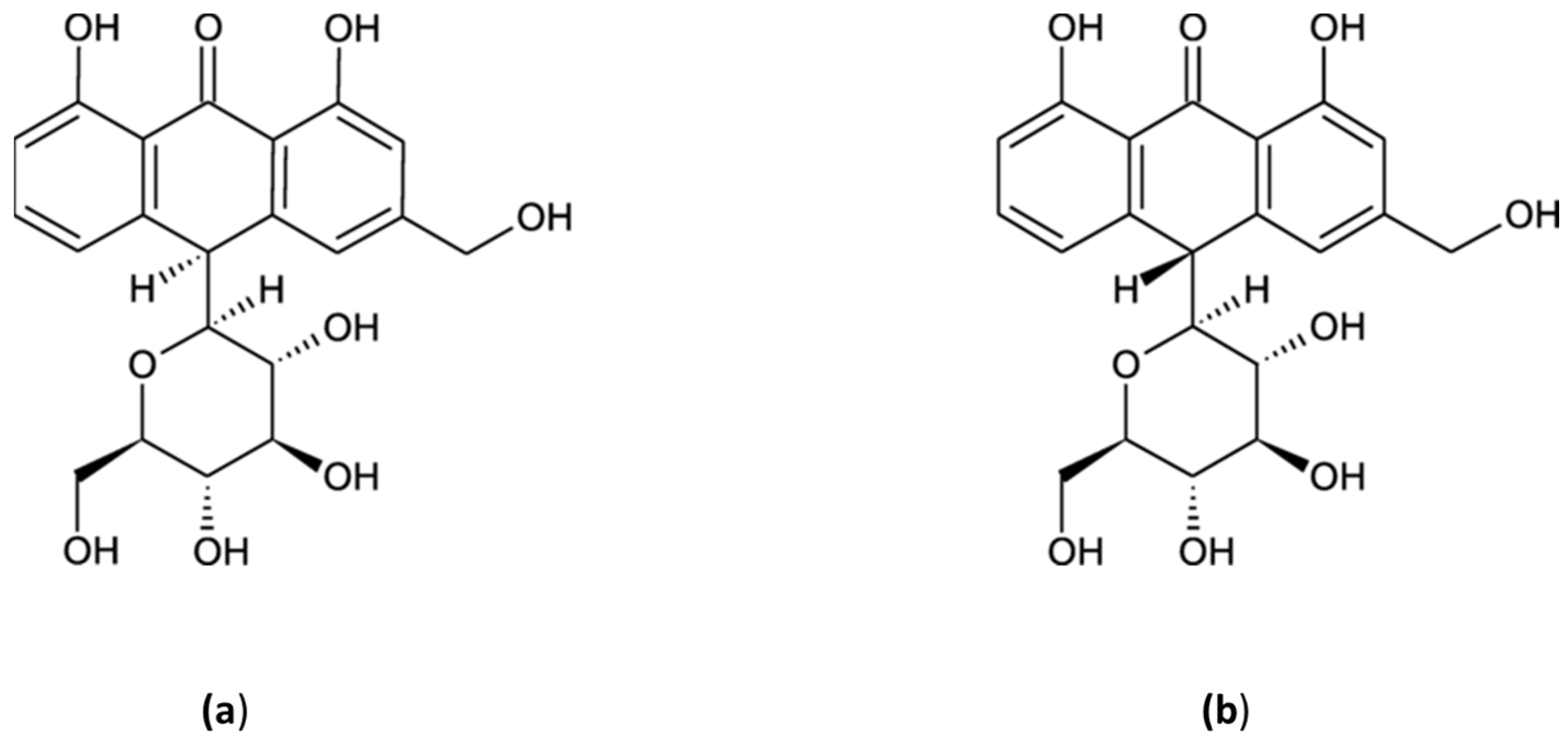
4.3. Aloin A & B
4.4. Physcion
4.5. Rhein
4.6. Chrysophanol
4.7. Hypericin
5. Anticancer Activity of 1,8-Dihydroanthraquinone Derivatives in Breast Cancer In Vivo Models
6. Sensitizing Properties of 1,8-Dihydroxyanthraquinones towards Known Drugs
7. Discussion
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roy, P.S.; Saikia, B.J. Cancer and cure: A critical analysis. Indian J. Cancer 2016, 53, 441–442. [Google Scholar] [CrossRef] [PubMed]
- Fabiani, R. Antitumoral Properties of Natural Products. Molecules 2020, 25, 650. [Google Scholar] [CrossRef]
- Arrousse, N.; Harras, M.F.; El Kadiri, S.; Haldhar, R.; Ichou, H.; Bousta, D.; Grafov, A.; Rais, Z.; Taleb, M. New anthraquinone drugs and their anticancer activities: Cytotoxicity, DFT, docking and ADMET properties. Results Chem. 2023, 6, 100996. [Google Scholar]
- Baviera, G.S.; Donate, P.M. Recent advances in the syntheses of anthracene derivatives. Beilstein J. Org. Chem. 17131 2021, 17, 2028–2050. [Google Scholar]
- Şeker Karatoprak, G.; Küpeli Akkol, E.; Yücel, Ç.; Bahadir Acikara, Ö.; Sobarzo-Sánchez, E. Advances in Understanding the Role of Aloe Emodin and Targeted Drug Delivery Systems in Cancer. Oxidative Med. Cell. Longev. 2022, 2022, 7928200. [Google Scholar]
- Montazeri Aliabadi, H.; Manda, A.; Sidgal, R.; Chung, C. Targeting Breast Cancer: The Familiar, the Emerging, and the Uncharted Territories. Biomolecules 2023, 13, 1306. [Google Scholar]
- Azadnajafabad, S.; Saeedi Moghaddam, S.; Mohammadi, E.; Delazar, S.; Rashedi, S.; Baradaran, H.R.; Mansourian, M. Patterns of better breast cancer care in countries with higher human development index and healthcare expenditure: Insights from GLOBOCAN 2020. Front. Public Health 2023, 11, 1137286. [Google Scholar]
- Wawruszak, A.; Halasa, M.; Okon, E.; Kukula-Koch, W.; Stepulak, A. Valproic Acid and Breast Cancer: State of the Art in 2021. Cancers 2021, 13, 3409. [Google Scholar]
- Uchida, N.; Suda, T.; Ishiguro, K. Effect of Chemotherapy for Luminal A Breast Cancer. Yonago Acta Med. 2013, 56, 51. [Google Scholar]
- Creighton, C.J. The molecular profile of luminal B breast cancer. Biol. Targets Ther. 2012, 6, 289–297. [Google Scholar] [CrossRef]
- Orrantia-Borunda, E.; Anchondo-Nuñez, P.; Acuña-Aguilar, L.E.; Gómez-Valles, F.O.; Ramírez-Valdespino, C.A. Subtypes of Breast Cancer. In Breast Cancer; Exon Publications: Brisbane, Australia, 2022; pp. 31–42. [Google Scholar]
- Li, Y.; Zhang, H.; Merkher, Y.; Chen, L.; Liu, N.; Leonov, S.; Chen, Y. Recent advances in therapeutic strategies for triple-negative breast cancer. J. Hematol. Oncol. 2022, 15, 121. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Dash, R. Natural Products for the Management and Prevention of Breast Cancer. Evid. Based. Complement. Alternat. Med. 2018, 2018, 8324696. [Google Scholar] [CrossRef] [PubMed]
- Thomson, R. Naturally Occurring Quinones. Available online: https://books.google.pl/books?hl=pl&lr=&id=iwFs2rpKOFgC&oi=fnd&pg=PP1&ots=E10gXwxkcL&sig=MxN3Epb0yXu_kfr-mF5weiKnjwg&redir_esc=y#v=onepage&q&f=false (accessed on 15 September 2023).
- Duval, J.; Pecher, V.; Poujol, M.; Lesellier, E. Research advances for the extraction, analysis and uses of anthraquinones: A review. Ind. Crops Prod. 2016, 94, 812–833. [Google Scholar] [CrossRef]
- Farmacognosia Da Planta Ao Medicamento|PDF|Biodiversidade|Biologia de Conservação. Available online: https://www.scribd.com/document/377628211/Farmacognosia-Da-Planta-Ao-Medicamento (accessed on 5 October 2023).
- Han, Y.S.; Van Der Heijden, R.; Verpoorte, R. Improved anthraquinone accumulation in cell cultures of Cinchona “Robusta” by feeding of biosynthetic precursors and inhibitors. Biotechnol. Lett. 2002, 24, 705–710. [Google Scholar] [CrossRef]
- Derksen, G.C.H.; Naayer, M.; van Beek, T.A.; Capelle, A.; Haaksman, I.K.; van Doren, H.A.; de Groot, Æ. Chemical and enzymatic hydrolysis of anthraquinone glycosides from madder roots. Phytochem. Anal. 2003, 14, 137–144. [Google Scholar] [CrossRef] [PubMed]
- de Morais, F.A.P.; Balbinot, R.B.; Bakoshi, A.B.K.; Lazarin-Bidoia, D.; da Silva Souza Campanholi, K.; da Silva Junior, R.C.; Gonçalves, R.S.; Ueda-Nakamura, T.; de Oliveira Silva, S.; Caetano, W.; et al. Advanced theranostic nanoplatforms for hypericin delivery in the cancer treatment. J. Photochem. Photobiol. B Biol. 2023, 247, 112782. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Chen, G.; Shi, P. Emodin-induced apoptosis in human breast cancer BCap-37 cells through the mitochondrial signaling pathway. Arch. Pharm. Res. 2008, 31, 742–748. [Google Scholar] [CrossRef]
- Sousa, S.; Brion, R.; Lintunen, M.; Kronqvist, P.; Sandholm, J.; Mönkkönen, J.; Kellokumpu-Lehtinen, P.L.; Lauttia, S.; Tynninen, O.; Joensuu, H.; et al. Human breast cancer cells educate macrophages toward the M2 activation status. Breast Cancer Res. 2015, 17, 101. [Google Scholar] [CrossRef]
- Yan, Y.; Su, X.; Liang, Y.; Zhang, J.; Shi, C.; Lu, Y.; Gu, L.; Fu, L. Emodin azide methyl anthraquinone derivative triggers mitochondrial-dependent cell apoptosis involving in caspase-8-mediated Bid cleavage. Mol. Cancer Ther. 2008, 7, 1688–1697. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, X.; Zhou, Q.; Lu, Y.; Zhang, H.; Chen, Q.; Zhao, M.; Su, S. Inhibitory effect of emodin on migration, invasion and metastasis of human breast cancer MDA-MB-231 cells in vitro and in vivo. Oncol. Rep. 2015, 33, 338–346. [Google Scholar] [CrossRef]
- Gupta, V.; Mun, G.H.; Choi, B.; Aseh, A.; Mildred, L.; Patel, A.; Zhang, Q.; Price, J.E.; Chang, D.; Robb, G.; et al. Repair and reconstruction of a resected tumor defect using a composite of tissue flap-nanotherapeutic-silk fibroin and chitosan scaffold. Ann. Biomed. Eng. 2011, 39, 2374–2387. [Google Scholar] [PubMed]
- Huang, Z.; Chen, G.; Shi, P. Effects of emodin on the gene expression profiling of human breast carcinoma cells. Cancer Detect. Prev. 2009, 32, 286–291. [Google Scholar] [PubMed]
- Zu, C.; Zhang, M.; Xue, H.; Cai, X.; Zhao, L.; He, A.; Qin, G.; Yang, C.; Zheng, X. Emodin induces apoptosis of human breast cancer cells by modulating the expression of apoptosis-related genes. Oncol. Lett. 2015, 10, 2919. [Google Scholar]
- Li, W.Y.; Chan, R.Y.K.; Yu, P.H.F.; Chan, S.W. Emodin induces cytotoxic effect in human breast carcinoma MCF-7 cell through modulating the expression of apoptosis-related genes. Pharm. Biol. 2013, 51, 1175–1181. [Google Scholar] [CrossRef]
- Zhang, L.; Lau, Y.K.; Xi, L.; Hong, R.L.; Kim, D.S.H.L.; Chen, C.F.; Hortobagyi, G.N.; Chang, C.J.; Hung, M.C. Tyrosine kinase inhibitors, emodin and its derivative repress HER-2/neu-induced cellular transformation and metastasis-associated properties. Oncogene 1998, 16, 2855–2863. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lau, Y.-K.; Xia, W.; Hortobagyi, G.N.; Hung, M.-C. Tyrosine Kinase Inhibitor Emodin Suppresses Growth of HER-2/neu-Overexpressing Breast Cancer Cells in Athymic Mice and Sensitizes These Cells to the Inhibitory Effect of Paclitaxel. Clin. Cancer Res. 1999, 5, 343–353. [Google Scholar] [PubMed]
- Shao-Chun, W.; Lisha, Z.; Hortobagyi, G.N.; Mien-Chie, H. Targeting HER2: Recent developments and future directions for breast cancer patients. Semin. Oncol. 2001, 28, 21–29. [Google Scholar]
- Ueno, N.; Kiyokawa, N.O.; Hung, M. Growth suppression of low HER-2/neu-expressing breast cancer cell line MDA-MB-435 by tyrosine kinase inhibitor emodin. Oncol. Rep. 1996, 3, 509–511. [Google Scholar] [CrossRef]
- Li, F.; Song, X.; Zhou, X.; Chen, L.; Zheng, J. Emodin attenuates high lipid-induced liver metastasis through the AKT and ERK pathways in vitro in breast cancer cells and in a mouse xenograft model. Heliyon 2023, 9, e17052. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, J.; Sheng, A.; Huang, S.; Tang, Y.; Ma, S.; Hong, G. Emodin Inhibits the Proliferation of MCF-7 Human Breast Cancer Cells Through Activation of Aryl Hydrocarbon Receptor (AhR). Front. Pharmacol. 2020, 11, 622046. [Google Scholar] [CrossRef]
- Sui, J.Q.; Xie, K.P.; Zou, W.; Xie, M.J. Emodin inhibits breast cancer cell proliferation through the ERα-MAPK/Akt-cyclin D1/Bcl-2 signaling pathway. Asian Pac. J. Cancer Prev. 2014, 15, 6247–6251. [Google Scholar] [PubMed]
- Huang, P.H.; Huang, C.Y.; Chen, M.C.; Lee, Y.T.; Yue, C.H.; Wang, H.Y.; Lin, H. Emodin and Aloe-Emodin Suppress Breast Cancer Cell Proliferation through ERα Inhibition. Evid. Based. Complement. Alternat. Med. 2013, 2013, 376123. [Google Scholar] [PubMed]
- Song, X.; Zhou, X.; Qin, Y.; Yang, J.; Wang, Y.; Sun, Z.; Yu, K.; Zhang, S.; Liu, S. Emodin inhibits epithelial-mesenchymal transition and metastasis of triple negative breast cancer via antagonism of CC-chemokine ligand 5 secreted from adipocytes. Int. J. Mol. Med. 2018, 42, 579–588. [Google Scholar] [PubMed]
- Yan, Y.Y.; Zheng, L.S.; Zhang, X.; Chen, L.K.; Singh, S.; Wang, F.; Zhang, J.Y.; Liang, Y.J.; Dai, C.L.; Gu, L.Q.; et al. Blockade of Her2/neu binding to Hsp90 by emodin azide methyl anthraquinone derivative induces proteasomal degradation of Her2/neu. Mol. Pharm. 2011, 8, 1687–1697. [Google Scholar] [PubMed]
- Jelassi, B.; Anchelin, M.; Chamouton, J.; Cayuela, M.L.; Clarysse, L.; Li, J.; Goré, J.; Jiang, L.H.; Roger, S. Anthraquinone emodin inhibits human cancer cell invasiveness by antagonizing P2X7 receptors. Carcinogenesis 2013, 34, 1487–1496. [Google Scholar] [PubMed]
- Lara, R.; Adinolfi, E.; Harwood, C.A.; Philpott, M.; Barden, J.A.; Di Virgilio, F.; McNulty, S. P2X7 in Cancer: From Molecular Mechanisms to Therapeutics. Front. Pharmacol. 2020, 11, 793. [Google Scholar]
- Iwanowycz, S.; Wang, J.; Altomare, D.; Hui, Y.; Fan, D. Emodin Bidirectionally Modulates Macrophage Polarization and Epigenetically Regulates Macrophage Memory. J. Biol. Chem. 2016, 291, 11491. [Google Scholar]
- Iwanowycz, S.; Wang, J.; Hodge, J.; Wang, Y.; Yu, F.; Fan, D. Emodin inhibits breast cancer growth by blocking the tumor-promoting feedforward loop between cancer cells and macrophages. Mol. Cancer Ther. 2016, 15, 1931. [Google Scholar]
- Jia, X.; Yu, F.; Wang, J.; Iwanowycz, S.; Saaoud, F.; Wang, Y.; Hu, J.; Wang, Q.; Fan, D. Emodin suppresses pulmonary metastasis of breast cancer accompanied with decreased macrophage recruitment and M2 polarization in the lungs. Breast Cancer Res. Treat. 2014, 148, 291–302. [Google Scholar]
- Liu, Q.; Hodge, J.; Wang, J.; Wang, Y.; Wang, L.; Singh, U.; Li, Y.; Yao, Y.; Wang, D.; Ai, W.; et al. Emodin reduces Breast Cancer Lung Metastasis by suppressing Macrophage-induced Breast Cancer Cell Epithelial-mesenchymal transition and Cancer Stem Cell formation. Theranostics 2020, 10, 8365–8381. [Google Scholar] [CrossRef]
- Hsu, H.C.; Liu, L.C.; Wang, H.Y.; Hung, C.M.; Lin, Y.C.; Ho, C.T.; Way, T. Der Stromal Fibroblasts from the Interface Zone of Triple Negative Breast Carcinomas Induced Epithelial-Mesenchymal Transition and its Inhibition by Emodin. PLoS ONE 2017, 12, e0164661. [Google Scholar]
- Kwak, H.J.; Park, M.J.; Park, C.M.; Moon, S.I.; Yoo, D.H.; Lee, H.C.; Lee, S.H.; Kim, M.S.; Lee, H.W.; Shin, W.S.; et al. Emodin inhibits vascular endothelial growth factor-A-induced angiogenesis by blocking receptor-2 (KDR/Flk-1) phosphorylation. Int. J. Cancer 2006, 118, 2711–2720. [Google Scholar] [PubMed]
- Ma, J.; Lu, H.; Wang, S.; Chen, B.; Liu, Z.; Ke, X.; Liu, T.; Fu, J. The anthraquinone derivative Emodin inhibits angiogenesis and metastasis through downregulating Runx2 activity in breast cancer. Int. J. Oncol. 2015, 46, 1619–1628. [Google Scholar]
- Gupta, G.P.; Massagué, J. Cancer metastasis: Building a framework. Cell 2006, 127, 679–695. [Google Scholar] [PubMed]
- Zou, G.; Zhang, X.; Wang, L.; Li, X.; Xie, T.; Zhao, J.; Yan, J.; Wang, L.; Ye, H.; Jiao, S.; et al. Herb-sourced emodin inhibits angiogenesis of breast cancer by targeting VEGFA transcription. Theranostics 2020, 10, 6839. [Google Scholar] [CrossRef]
- Fu, J.M.; Zhou, J.; Shi, J.; Xie, J.; Huang, L.; Yip, A.Y.S.; Loo, W.T.Y.; Chow, L.W.C.; Ng, E.L.Y. Emodin affects ERCC1 expression in breast cancer cells. J. Transl. Med. 2012, 10, 1–6. [Google Scholar] [CrossRef]
- Zu, C.; Qin, G.; Yang, C.; Liu, N.; He, A.; Zhang, M.; Zheng, X. Low dose Emodin induces tumor senescence for boosting breast cancer chemotherapy via silencing NRARP. Biochem. Biophys. Res. Commun. 2018, 505, 973–978. [Google Scholar] [CrossRef]
- Christowitz, C.; Davis, T.; Isaacs, A.; Van Niekerk, G.; Hattingh, S.; Engelbrecht, A.M. Mechanisms of doxorubicin-induced drug resistance and drug resistant tumour growth in a murine breast tumour model. BMC Cancer 2019, 19, 1–10. [Google Scholar]
- Wang, S.; Chen, T.; Chen, R.; Hu, Y.; Chen, M.; Wang, Y. Emodin loaded solid lipid nanoparticles: Preparation, characterization and antitumor activity studies. Int. J. Pharm. 2012, 430, 238–246. [Google Scholar] [CrossRef]
- Liu, H.; Zhuang, Y.; Wang, P.; Zou, T.; Lan, M.; Li, L.; Liu, F.; Cai, T.; Cai, Y. Polymeric Lipid Hybrid Nanoparticles as a Delivery System Enhance the Antitumor Effect of Emodin in Vitro and in Vivo. J. Pharm. Sci. 2021, 110, 2986–2996. [Google Scholar]
- Bhattacharjee, M.; Upadhyay, P.; Sarker, S.; Basu, A.; Das, S.; Ghosh, A.; Ghosh, S.; Adhikary, A. Combinatorial therapy of Thymoquinone and Emodin synergistically enhances apoptosis, attenuates cell migration and reduces stemness efficiently in breast cancer. Biochim. Biophys. Acta-Gen. Subj. 2020, 1864, 129695. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Tang, W.; Liu, J.J.; Gong, X.Q.; Kong, L.; Yao, X.M.; Jing, M.; Cai, F.Y.; Li, X.T.; Ju, R.J. Combination of targeted daunorubicin liposomes and targeted emodin liposomes for treatment of invasive breast cancer. J. Drug Target. 2020, 28, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Li, W.; Shi, H.; Xie, X.; Li, L.; Tang, H.; Wu, M.; Kong, Y.; Yang, L.; Gao, J.; et al. Synergistic effects of curcumin with emodin against the proliferation and invasion of breast cancer cells through upregulation of miR-34a. Mol. Cell. Biochem. 2013, 382, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Ponnusamy, L.; Kothandan, G.; Manoharan, R. Berberine and Emodin abrogates breast cancer growth and facilitates apoptosis through inactivation of SIK3-induced mTOR and Akt signaling pathway. Biochim. Biophys. Acta-Mol. Basis Dis. 2020, 1866, 165897. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Park, S.J.; Choi, S.; Kim, S.H.; Kang, K.S. In Vitro Estrogenic and Breast Cancer Inhibitory Activities of Chemical Constituents Isolated from Rheum undulatum L. Molecules 2018, 23, 1215. [Google Scholar] [CrossRef]
- He, Z.-H.; Huang, Y.-Q.; Weng, S.-F.; Tan, Y.-R.; He, T.-P.; Qin, Y.-M.; Liang, N.-C. Effect of Aloe Emodin on Invasion and Metastasis of High Metastatic Breast Cancer MDA-MB-231 Cells. Zhong Yao Cai 2013, 36, 1481–1485. [Google Scholar]
- Majumder, R.; Parida, P.; Paul, S.; Basak, P. In vitro and in silico study of Aloe vera leaf extract against human breast cancer. Nat. Prod. Res. 2020, 34, 2363–2366. [Google Scholar] [CrossRef]
- Freag, M.S.; Elnaggar, Y.S.R.; Abdelmonsif, D.A.; Abdallah, O.Y. Stealth, biocompatible monoolein-based lyotropic liquid crystalline nanoparticles for enhanced aloe-emodin delivery to breast cancer cells: In vitro and in vivo studies. Int. J. Nanomedicine 2016, 11, 4799–4818. [Google Scholar] [CrossRef]
- Chen, R.; Wang, S.; Zhang, J.; Chen, M.; Wang, Y. Aloe-emodin loaded solid lipid nanoparticles: Formulation design and in vitro anti-cancer study. Drug Deliv. 2015, 22, 666–674. [Google Scholar] [CrossRef]
- Majumder, R.; Das, C.K.; Banerjee, I.; Chandra Jena, B.; Mandal, A.; Das, P.; Pradhan, A.K.; Das, S.; Basak, P.; Das, S.K.; et al. Screening of the Prime bioactive compounds from Aloe vera as potential anti-proliferative agents targeting DNA. Comput. Biol. Med. 2022, 141, 105052. [Google Scholar] [CrossRef]
- Esmat, A.Y.; El-Gerzawy, S.M.; Rafaat, A. DNA ploidy and S phase fraction of breast and ovarian tumor cells treated with a natural anthracycline analog (aloin). Cancer Biol. Ther. 2005, 4, 115–119. [Google Scholar] [CrossRef]
- Beerman, H.; Kluin, P.M.; Hermans, J.; Van de Velde, C.J.H.; Cornelisse, C.J. Prognostic significance of DNA-ploidy in a series of 690 primary breast cancer patients. Int. J. Cancer 1990, 45, 34–39. [Google Scholar] [CrossRef]
- Järvinen, T.A.H.; Tanner, M.; Rantanen, V.; Bärlund, M.; Borg, Å.; Grénman, S.; Isola, J. Amplification and deletion of topoisomerase IIalpha associate with ErbB-2 amplification and affect sensitivity to topoisomerase II inhibitor doxorubicin in breast cancer. Am. J. Pathol. 2000, 156, 839–847. [Google Scholar] [CrossRef]
- Esmat, A.Y.; Tomasetto, C.; Rio, M.C. Cytotoxicity of a natural anthraquinone (Aloin) against human breast cancer cell lines with and without ErbB-2: Topoisomerase IIalpha coamplification. Cancer Biol. Ther. 2006, 5, 97–103. [Google Scholar] [CrossRef]
- Bonuccelli, G.; Sotgia, F.; Lisanti, M.P. Identification of natural products and FDA-approved drugs for targeting cancer stem cell (CSC) propagation. Aging 2022, 14, 9466–9483. [Google Scholar] [CrossRef]
- Zhang, L.; Dong, R.; Wang, Y.; Wang, L.; Zhou, T.; Jia, D.; Meng, Z. The anti-breast cancer property of physcion via oxidative stress-mediated mitochondrial apoptosis and immune response. Pharm. Biol. 2021, 59, 303–310. [Google Scholar] [CrossRef]
- Cao, X.H.; Zhao, S.S.; Liu, D.Y.; Wang, Z.; Niu, L.L.; Hou, L.H.; Wang, C.L. ROS-Ca2+ is associated with mitochondria permeability transition pore involved in surfactin-induced MCF-7 cells apoptosis. Chem. Biol. Interact. 2011, 190, 16–27. [Google Scholar] [CrossRef]
- Chong, S.J.F.; Low, I.C.C.; Pervaiz, S. Mitochondrial ROS and involvement of Bcl-2 as a mitochondrial ROS regulator. Mitochondrion 2014, 19, 39–48. [Google Scholar] [CrossRef]
- Kim, B.M.; Chung, H.W. Hypoxia/reoxygenation induces apoptosis through a ROS-mediated caspase-8/Bid/Bax pathway in human lymphocytes. Biochem. Biophys. Res. Commun. 2007, 363, 745–750. [Google Scholar] [CrossRef]
- Hong, J.-Y.; Chung, H.-J.; Bae, S.Y.; Trung, T.N.; Bae, K.; Lee, S.K. Induction of Cell Cycle Arrest and Apoptosis by Physcion, an Anthraquinone Isolated From Rhubarb (Rhizomes of Rheum tanguticum), in MDA-MB-231 Human Breast Cancer Cells. J. Cancer Prev. 2014, 19, 273–278. [Google Scholar] [CrossRef]
- Malumbres, M.; Barbacid, M. Cell cycle, CDKs and cancer: A changing paradigm. Nat. Rev. Cancer 2009, 9, 153–166. [Google Scholar] [PubMed]
- Li, X.; Liu, Y.; Zhao, Y.; Tian, W.; Zhai, L.; Pang, H.; Kang, J.; Hou, H.; Chen, Y.; Li, D. Rhein Derivative 4F Inhibits the Malignant Phenotype of Breast Cancer by Downregulating Rac1 Protein. Front. Pharmacol. 2020, 11, 754. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.; Li, J.; Su, Z.; Lan, F.; Li, Z.; Liang, D.; Wang, C.; Li, D.; Hou, H. Novel Anthraquinone Compounds Induce Cancer Cell Death through Paraptosis. ACS Med. Chem. Lett. 2019, 10, 732–736. [Google Scholar] [CrossRef] [PubMed]
- Henamayee, S.; Banik, K.; Sailo, B.L.; Shabnam, B.; Harsha, C.; Srilakshmi, S.; Naidu, V.G.M.; Baek, S.H.; Ahn, K.S.; Kunnumakkara, A.B. Therapeutic emergence of rhein as a potential anticancer drug: A review of its molecular targets and anticancer properties. Molecules 2020, 25, 2278. [Google Scholar]
- Park, S.; Lim, W.; Song, G. Chrysophanol selectively represses breast cancer cell growth by inducing reactive oxygen species production and endoplasmic reticulum stress via AKT and mitogen-activated protein kinase signal pathways. Toxicol. Appl. Pharmacol. 2018, 360, 201–211. [Google Scholar] [PubMed]
- Ni, C.H.; Chen, P.Y.; Lu, H.F.; Yang, J.S.; Huang, H.Y.; Wu, S.H.; Ip, S.W.; Wu, C.T.; Chiang, S.Y.; Lin, J.G.; et al. Chrysophanol-induced necrotic-like cell death through an impaired mitochondrial ATP synthesis in Hep3B human liver cancer cells. Arch. Pharm. Res. 2012, 35, 887–895. [Google Scholar]
- Ren, L.; Li, Z.; Dai, C.; Zhao, D.; Wang, Y.; Ma, C.; Liu, C. Chrysophanol inhibits proliferation and induces apoptosis through NF-κB/cyclin D1 and NF-κB/Bcl-2 signaling cascade in breast cancer cell lines. Mol. Med. Rep. 2018, 17, 4376–4382. [Google Scholar] [CrossRef]
- Su, S.; Wu, J.; Gao, Y.; Luo, Y.; Yang, D.; Wang, P. The pharmacological properties of chrysophanol, the recent advances. Biomed. Pharmacother. 2020, 125. [Google Scholar] [CrossRef]
- de Souza, M.V.F.; Shinobu-Mesquita, C.S.; Meirelles, L.E.F.; Mari, N.L.; César, G.B.; Gonçalves, R.S.; Caetano, W.; Damke, E.; Silva, V.R.S.; Damke, G.M.Z.F.; et al. Effects of hypericin encapsulated on Pluronic F127 photodynamic therapy against triple negative breast cancer. Asian Pac. J. Cancer Prev. 2022, 23, 1741–1751. [Google Scholar] [CrossRef]
- Damke, G.M.Z.F.; Damke, E.; de Souza Bonfim-Mendonça, P.; Ratti, B.A.; de Freitas Meirelles, L.E.; da Silva, V.R.S.; Gonçalves, R.S.; César, G.B.; de Oliveira Silva, S.; Caetano, W.; et al. Selective photodynamic effects on cervical cancer cells provided by P123 Pluronic®-based nanoparticles modulating hypericin delivery. Life Sci. 2020, 255, 117858. [Google Scholar]
- Diwu, Z.; William Lown, J. Photosensitization with anticancer agents 17. EPR studies of photodynamic action of hypericin: Formation of semiquinone radical and activated oxygen species on illumination. Free Radic. Biol. Med. 1993, 14, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Solár, P.; Ferenc, P.; Koval, J.; Mikeš, J.; Solárova, Z.; Hrčková, G.; Fulton, B.L.; Fedoročko, P. Photoactivated hypericin induces downregulation of HER2 gene expression. Radiat. Res. 2011, 175, 51–56. [Google Scholar] [PubMed]
- Kimáková, P.; Solár, P.; Fecková, B.; Sačková, V.; Solárová, Z.; Ilkovičová, L.; Kello, M. Photoactivated hypericin increases the expression of SOD-2 and makes MCF-7 cells resistant to photodynamic therapy. Biomed. Pharmacother. 2017, 85, 749–755. [Google Scholar] [PubMed]
- de Andrade, G.P.; Manieri, T.M.; Nunes, E.A.; Viana, G.M.; Cerchiaro, G.; Ribeiro, A.O. Comparative in vitro study of photodynamic activity of hypericin and hypericinates in MCF-7 cells. J. Photochem. Photobiol. B Biol. 2017, 175, 89–98. [Google Scholar]
- Freitas, V.M.; do Amaral, J.B.; Silva, T.A.; Santos, E.S.; Mangone, F.R.; Pinheiro, J.D.; Jaeger, R.G.; Nagai, M.A.; Machado-Santelli, G.M. Decreased expression of ADAMTS-1 in human breast tumors stimulates migration and invasion. Mol. Cancer 2013, 12, 1–15. [Google Scholar]
- Acar, M.; Ocak, Z.; Erdogan, K.; Cetin, E.N.; Hatipoglu, O.F.; Uyeturk, U.; Gunduz, E.; Gunduz, M. The Effects of Hypericin on ADAMTS and p53 Gene Expression in MCF-7 Breast Cancer Cells. J. BUON 2014, 19, 627–632. [Google Scholar]
- Stojanovic, G.; Dordevic, A.; Smelcerovic, A. Do other Hypericum species have medical potential as St. John’s wort (Hypericum perforatum)? Curr. Med. Chem. 2013, 20, 2273–2295. [Google Scholar] [CrossRef]
- Shemanko, C.S.; Cong, Y.; Forsyth, A. What Is Breast in the Bone? Int. J. Mol. Sci. 2016, 17, 1764. [Google Scholar]
- Ouyang, Z.; Guo, X.; Chen, X.; Liu, B.; Zhang, Q.; Yin, Z.; Zhai, Z.; Qu, X.; Liu, X.; Peng, D.; et al. Hypericin targets osteoclast and prevents breast cancer-induced bone metastasis via NFATc1 signaling pathway. Oncotarget 2018, 9, 1868. [Google Scholar]
- Ouyang, Z.; Zhai, Z.; Li, H.; Liu, X.; Qu, X.; Li, X.; Fan, Q.; Tang, T.; Qin, A.; Dai, K. Hypericin suppresses osteoclast formation and wear particle-induced osteolysis via modulating ERK signalling pathway. Biochem. Pharmacol. 2014, 90, 276–287. [Google Scholar]
- Shen, Z.; Shen, Z.; Li, J.; Qin, L. Rhein Augments Antiproliferative Effects of Atezolizumab Based on Breast Cancer (4T1) Regression. Planta Med. 2019, 85, 1143–1149. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhao, X.; Zhang, L.; Cheng, W. Emodin Interferes With AKT1-Mediated DNA Damage and Decreases Resistance of Breast Cancer Cells to Doxorubicin. Front. Oncol. 2021, 10, 588533. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Ren, G.; Cheng, Q. Inhibition of 6-Phosphogluconate Dehydrogenase Reverses Epirubicin Resistance through Metabolic Reprograming in Triple-Negative Breast Cancer Cells. Technol. Cancer Res. Treat. 2023, 22, 15330338231190736. [Google Scholar] [CrossRef] [PubMed]
| Botanical Family | Gender Name | Selected Species |
|---|---|---|
| Rhamnaceae | Rhamnus, Ventilago | Rhamnus purshiana, Rhamnus frangula, Rhamnus catartica, Ventilago maderaspatana |
| Fabaceae | Cassia | Cassia angustifolia, Cassia senna, Cassia obtusifolia |
| Polygonaceae | Rheum, Rumex | Rheum palmatum, Rheum officinale, Rheum rabarbarum Rumex conglumeratus, Rumex pulcher, Rumex alpinus, Rumex maritimus, Rumex obtusifolius |
| Liliaceae | Aloe, Bulbine | Aloe ferox, Aloe vera, Bulbine capitate |
| Guttiferae | Hypericum | Hypericum perforatum |
| Rubiaceae | Morinda, Rubia, Galium, Heterophyllea | Morinda citrifolia, Morinda officinalis Rubia tinctorum, Galium sinaicum, Galium verum, Heterophyllea pustulata |
| Compound | Cancer Type | Model | Dosing | Effect | References |
|---|---|---|---|---|---|
| Emodin | breast cancer MDA-MB-231 cells | nude BALB/c nu/nu mice fed a high-fat diet | 400 mg/kg, p.o., q.d. for 4 weeks | Regulation of body weight − cholesterol and fatty acids synthesis − fatty acids oxidation − Fasn, Sed1, Gpat1 gene expression | [32] |
| Physcion | breast cancer | 8 weeks, male BALB/c nude mice | 30 mg/kg/day i.p. for 2 weeks | + Bax and cleaved caspase-3, − Bcl-xL, Bcl-2, Nrf2, HO-1, SOD-1, and SOD-2 proteins expression in tumor tissue Immunomodulatory action | [69] |
| Rhein | breast cancer 4T1 cells | 6–8 weeks, female BALB/c mice | 10 mg/kg i.p. rhein every 3 days for a total of three times alone or with 10 mg/kg i.p. atezolizumab once every 2 days for a total of three times | With and without atezolizumab − tumor size (weight and volume) + CD8+ T cells production in the tumor and the spleen + serum levels of IL-6 and TNF-α | [94] |
| Type of Cancer | 1,8-Dihydroanthraquinone Derivatives | Chemotherapeutic | Effect | Ref. |
|---|---|---|---|---|
| Breast cancer | Rhein 10 mg/kg i.p. every 3 days, 3 times | Atezolizumab 10 mg/kg i.p. every 2 days, 3 times | − tumour size + CD8+ T cells production in the tumor and in the spleen + serum levels of IL-6 and TNF-α | [94] |
| Breast cancer | Emodin 110 µM | Doxorubicin 5 µM | Sensitisation to doxorubicin + apoptosis + DNA damage in cancer cells + γH2Ax expression − drug resistance to doxorubicin (PI3K-AKT pathway) | [95] |
| Breast cancer | Emodin 20 µM | 5-Fluorouracil 40 µM | Sensitization to 5-fluorouracyl + apoptosis + ROS generation +cyclin-dependant kinase inhibitors expression − E2F1 expression − notch-regulated ankyrin repeat protein expression | [50] |
| Breast cancer | Physcion 5 mg/kg, i.p. | Paclitaxel 5 mg/kg, i.p. | Inhibition of 6PGD by physcion that results in the sensitization of breast cancer cells to paclitaxel | [96] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okon, E.; Gaweł-Bęben, K.; Jarzab, A.; Koch, W.; Kukula-Koch, W.; Wawruszak, A. Therapeutic Potential of 1,8-Dihydroanthraquinone Derivatives for Breast Cancer. Int. J. Mol. Sci. 2023, 24, 15789. https://doi.org/10.3390/ijms242115789
Okon E, Gaweł-Bęben K, Jarzab A, Koch W, Kukula-Koch W, Wawruszak A. Therapeutic Potential of 1,8-Dihydroanthraquinone Derivatives for Breast Cancer. International Journal of Molecular Sciences. 2023; 24(21):15789. https://doi.org/10.3390/ijms242115789
Chicago/Turabian StyleOkon, Estera, Katarzyna Gaweł-Bęben, Agata Jarzab, Wojciech Koch, Wirginia Kukula-Koch, and Anna Wawruszak. 2023. "Therapeutic Potential of 1,8-Dihydroanthraquinone Derivatives for Breast Cancer" International Journal of Molecular Sciences 24, no. 21: 15789. https://doi.org/10.3390/ijms242115789
APA StyleOkon, E., Gaweł-Bęben, K., Jarzab, A., Koch, W., Kukula-Koch, W., & Wawruszak, A. (2023). Therapeutic Potential of 1,8-Dihydroanthraquinone Derivatives for Breast Cancer. International Journal of Molecular Sciences, 24(21), 15789. https://doi.org/10.3390/ijms242115789










