Combination Therapy with a TLR7 Agonist and a BRD4 Inhibitor Suppresses Tumor Growth via Enhanced Immunomodulation
Abstract
1. Introduction
2. Results
2.1. SZU-101 Stimulated TLR7 Signaling to Prime Immune Responses In Vitro
2.2. JQ-1 Displayed Growth Inhibition on Tumor Cells In Vitro
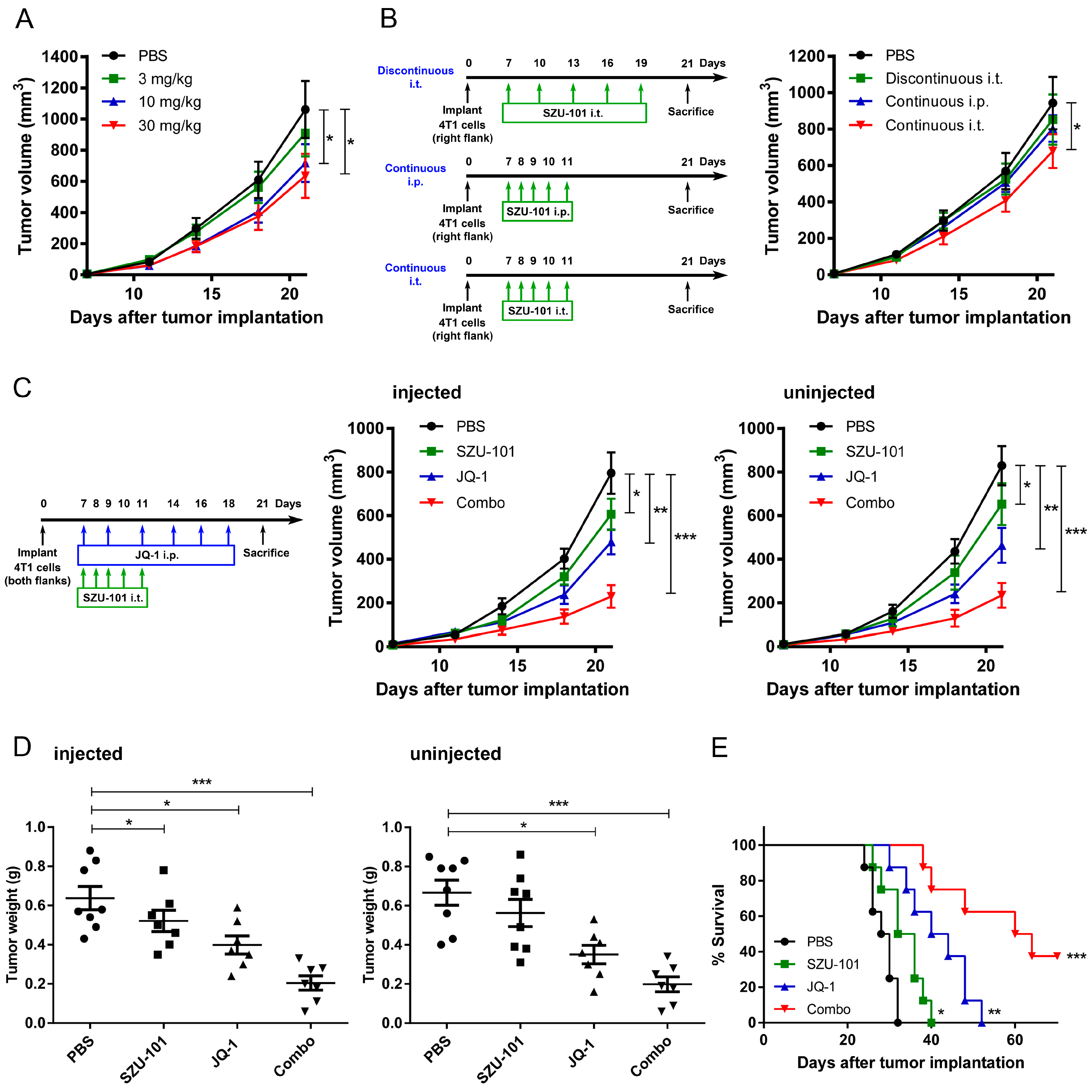
2.3. Combination Administration of SZU-101 and JQ-1 Inhibited Tumor Growth at Both Injected and Uninjected Sites
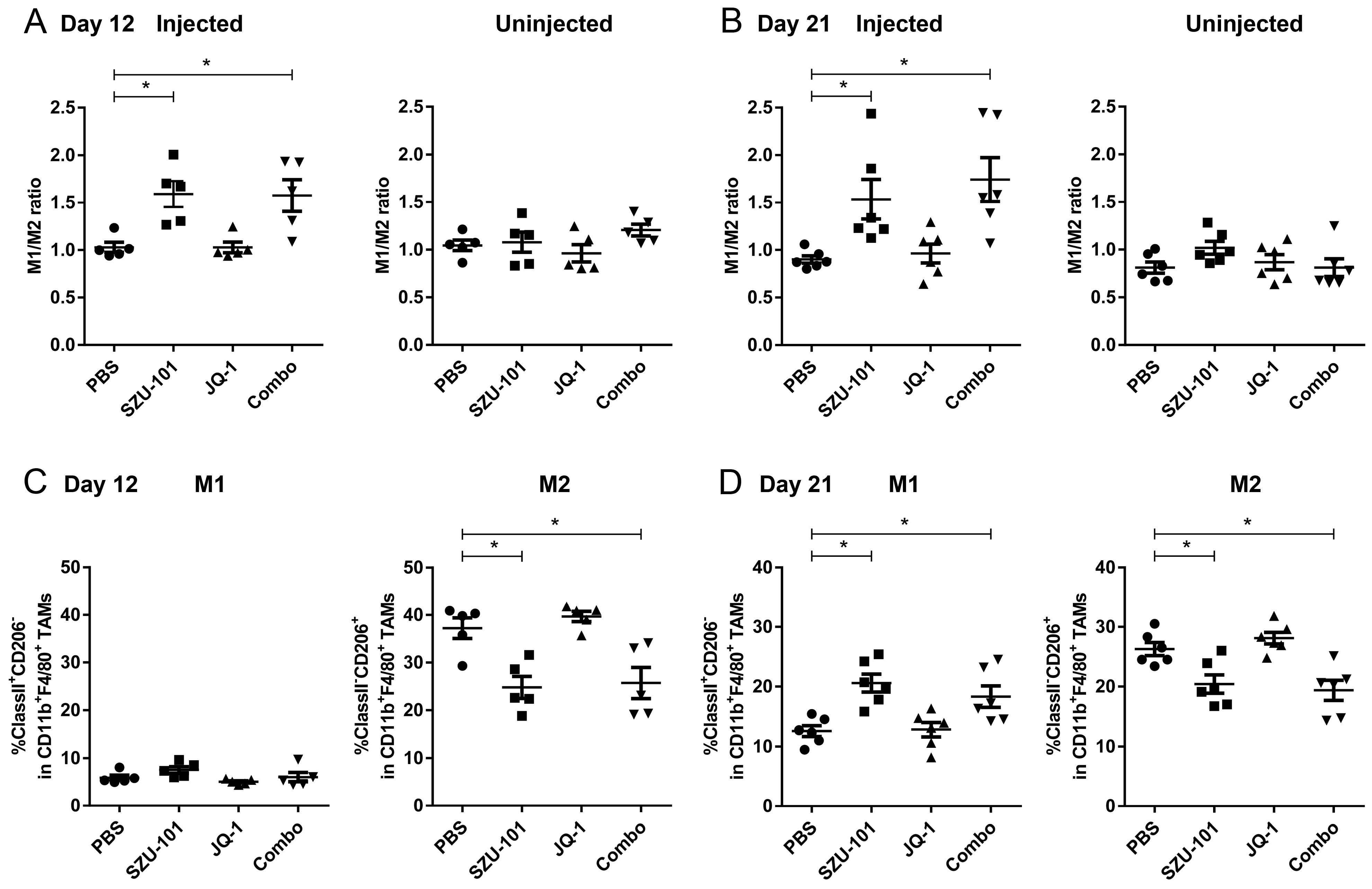
2.4. Combination Administration of SZU-101 and JQ-1 Increased M1/M2 Ratio in TAMs
2.5. Combination Administration of SZU-101 and JQ-1 Suppressed PD-L1 Expression in Tumor Cells
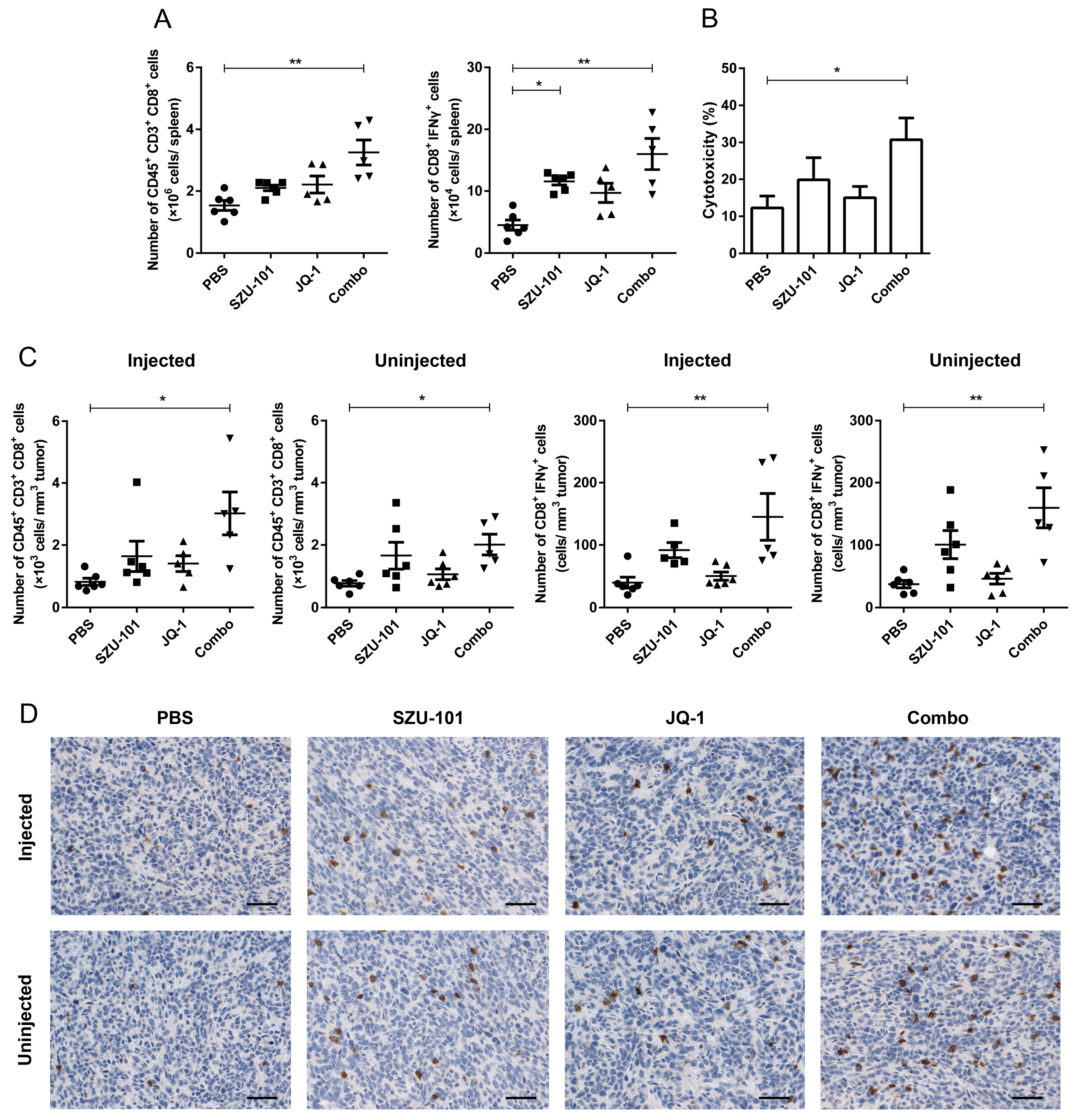
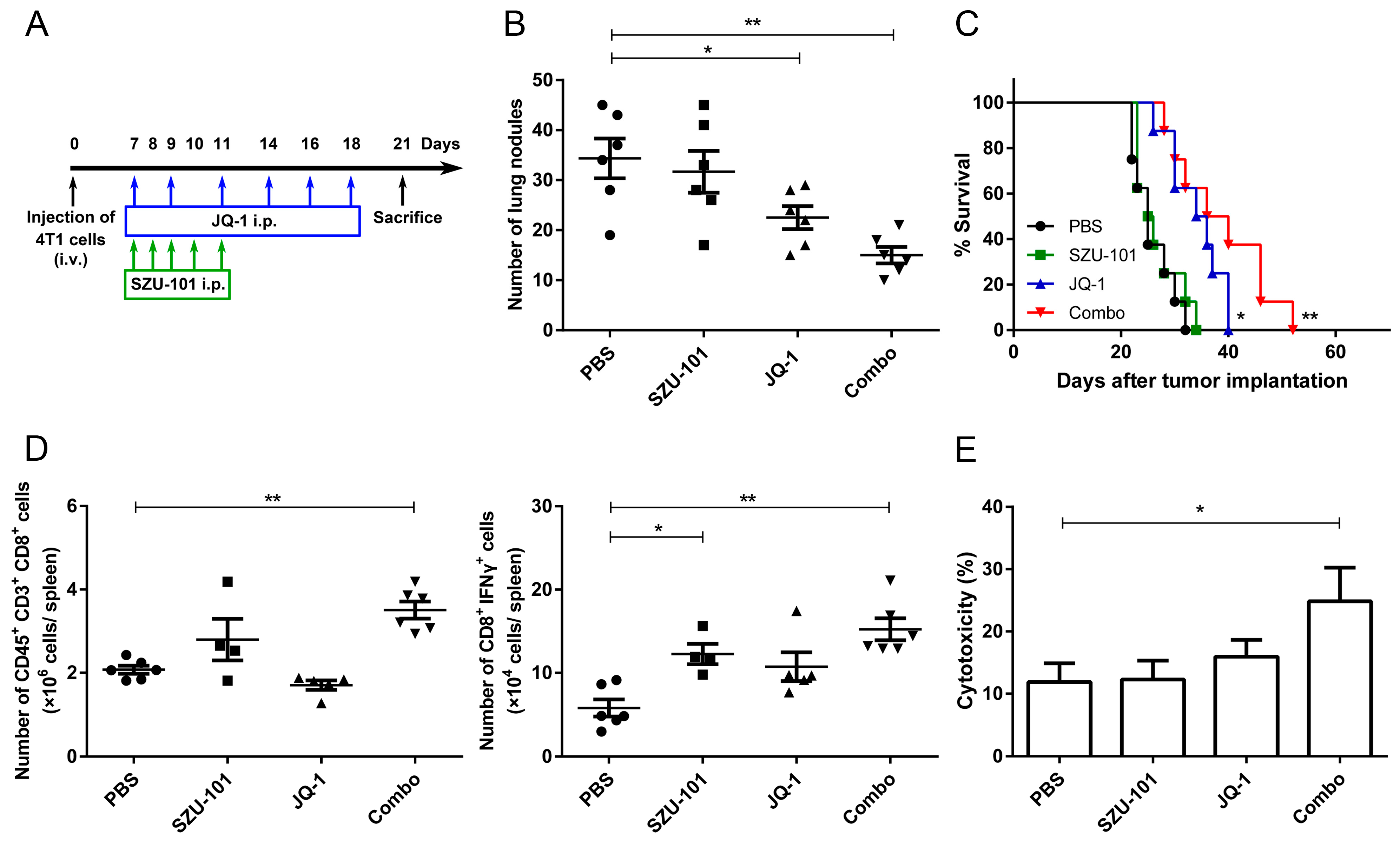
2.6. Combination Administration of SZU-101 and JQ-1 Increased CD8+ T Cells in Spleens and TILs
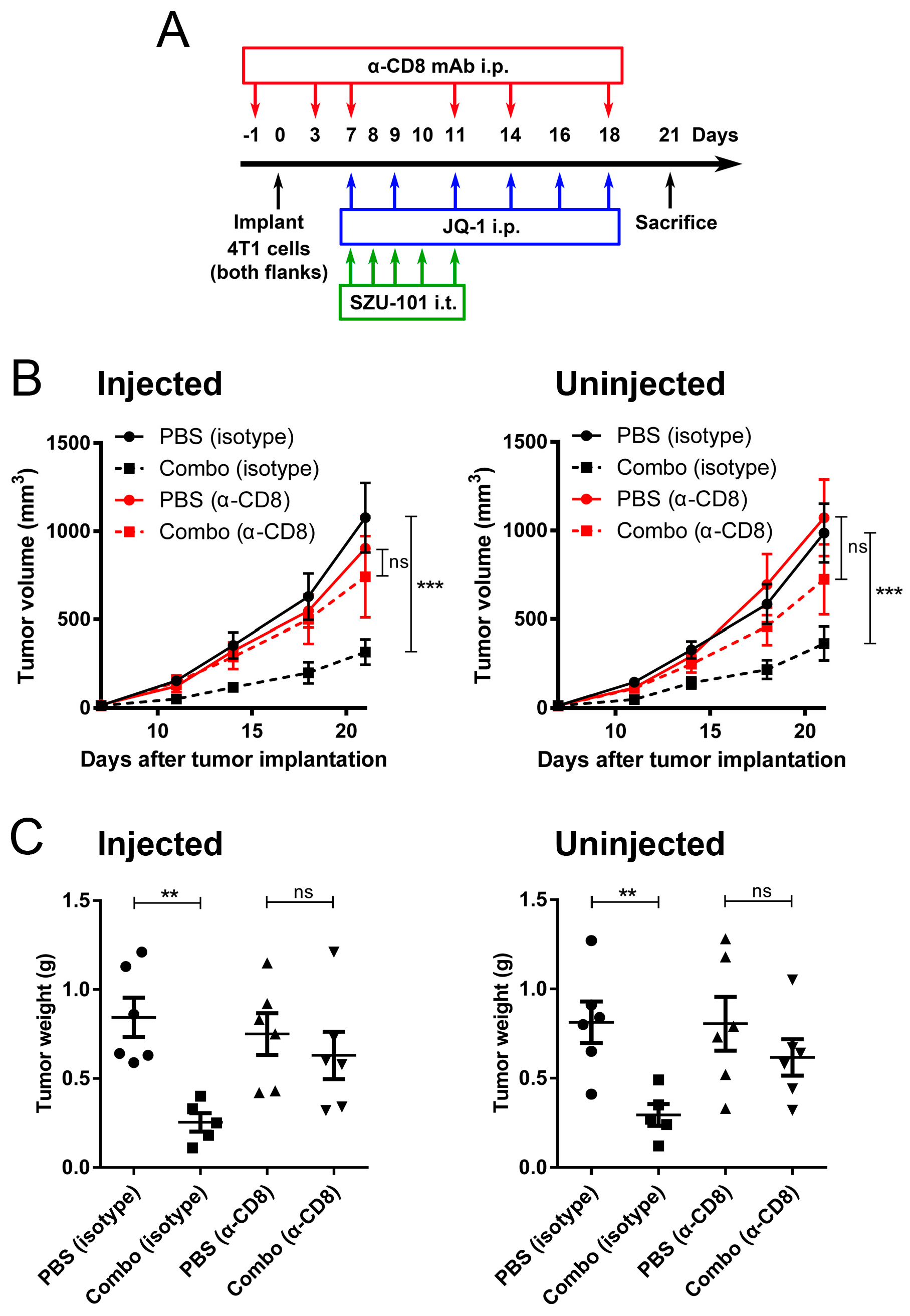
2.7. Depletion of CD8+ Cells Abrogated the Antitumor Effects of Combination Administration of SZU-101 and JQ-1
2.8. Combination Administration of SZU-101 and JQ-1 Inhibited Tumor Metastasis
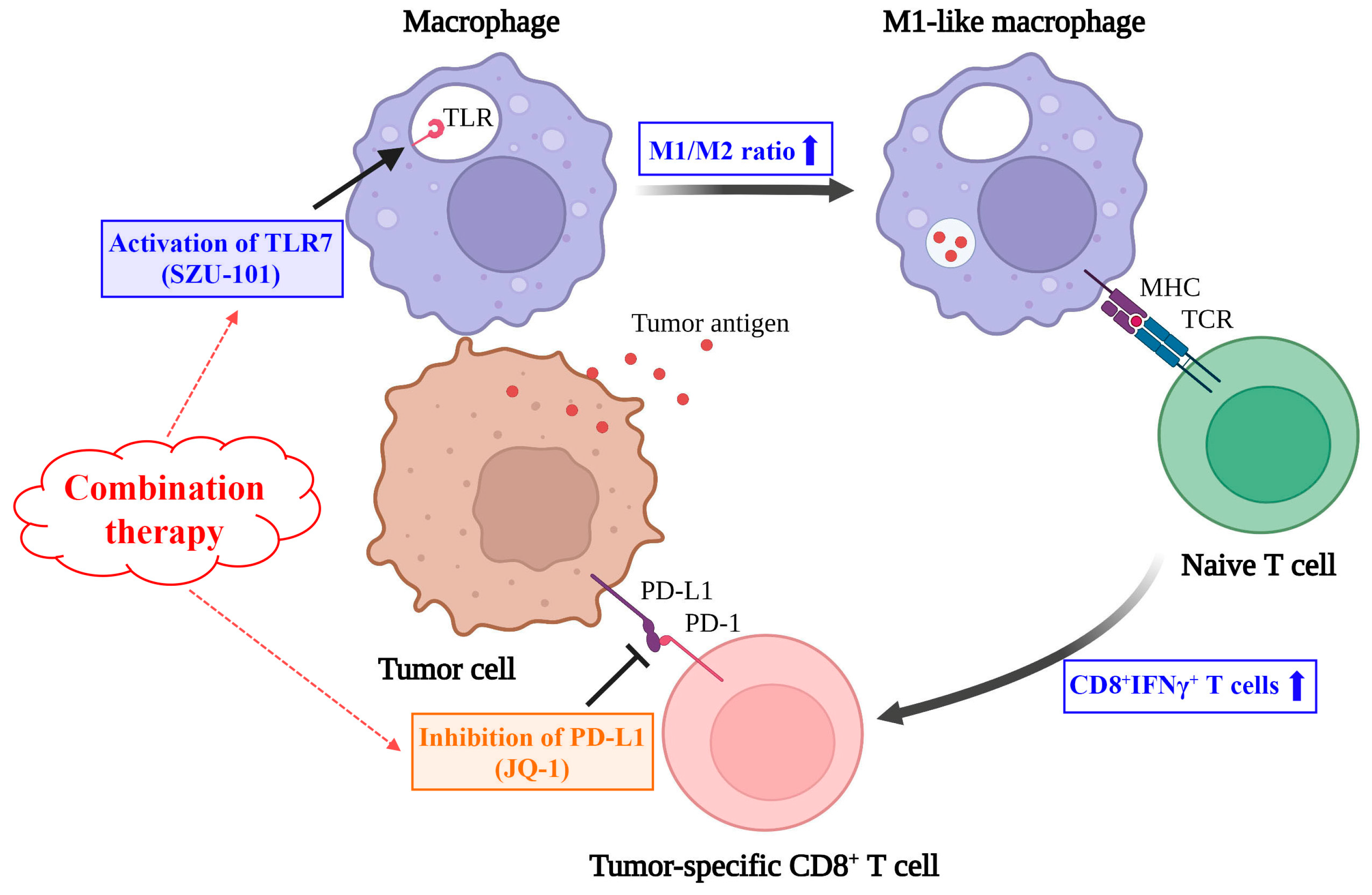
3. Discussion
4. Materials and Methods
4.1. Animals, Reagents and Cell Lines
4.2. Tumor Implantation Models
4.3. Lung Metastasis Models
4.4. Flow Cytometric Analysis
4.5. Histologic Analysis
4.6. CTL Analysis
4.7. CD8+ Cell Depletion In Vivo
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mokhtari, Y.; Pourbagheri-Sigaroodi, A.; Zafari, P.; Bagheri, N.; Ghaffari, S.H.; Bashash, D. Toll-like receptors (TLRs): An old family of immune receptors with a new face in cancer pathogenesis. J. Cell. Mol. Med. 2021, 25, 639–651. [Google Scholar] [CrossRef]
- Trinchieri, G.; Sher, A. Cooperation of Toll-like receptor signals in innate immune defence. Nat. Rev. Immunol. 2007, 7, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Hosoya, T.; Sato-Kaneko, F.; Ahmadi, A.; Yao, S.; Lao, F.; Kitaura, K.; Matsutani, T.; Carson, D.A.; Hayashi, T. Induction of oligoclonal CD8 T cell responses against pulmonary metastatic cancer by a phospholipid-conjugated TLR7 agonist. Proc. Natl. Acad. Sci. USA 2018, 115, E6836–E6844. [Google Scholar] [CrossRef] [PubMed]
- Michaelis, K.A.; Norgard, M.A.; Zhu, X.; Levasseur, P.R.; Sivagnanam, S.; Liudahl, S.M.; Burfeind, K.G.; Olson, B.; Pelz, K.R.; Angeles Ramos, D.M.; et al. The TLR7/8 agonist R848 remodels tumor and host responses to promote survival in pancreatic cancer. Nat. Commun. 2019, 10, 4682. [Google Scholar] [CrossRef] [PubMed]
- Papadavid, E.; Stratigos, A.J.; Falagas, M.E. Imiquimod: An immune response modifier in the treatment of precancerous skin lesions and skin cancer. Expert Opin. Pharmacother. 2007, 8, 1743–1755. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tang, L.; Gao, N.; Diao, Y.; Zhong, J.; Deng, Y.; Wang, Z.; Jin, G.; Wang, X. Synthetic MUC1 breast cancer vaccine containing a Toll-like receptor 7 agonist exerts antitumor effects. Oncol. Lett. 2020, 20, 2369–2377. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, Y.; Diao, Y.; Gao, N.; Wan, Y.; Zhong, J.; Zheng, H.; Wang, Z.; Jin, G. Gastric cancer vaccines synthesized using a TLR7 agonist and their synergistic antitumor effects with 5-fluorouracil. J. Transl. Med. 2018, 16, 120. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yu, B.; Cao, B.; Zhou, J.; Deng, Y.; Wang, Z.; Jin, G. A chemical conjugation of JQ-1 and a TLR7 agonist induces tumoricidal effects in a murine model of melanoma via enhanced immunomodulation. Int. J. Cancer 2021, 148, 437–447. [Google Scholar] [CrossRef]
- Ren, S.; Wang, X.; Jin, G. Conjugate of ibrutinib with a TLR7 agonist suppresses melanoma progression and enhances antitumor immunity. Int. J. Biol. Sci. 2022, 18, 166–179. [Google Scholar] [CrossRef]
- Marazzi, I.; Greenbaum, B.D.; Low, D.H.P.; Guccione, E. Chromatin dependencies in cancer and inflammation. Nat. Rev. Mol. Cell Biol. 2018, 19, 245–261. [Google Scholar] [CrossRef]
- White, M.E.; Fenger, J.M.; Carson, W.E., 3rd. Emerging roles of and therapeutic strategies targeting BRD4 in cancer. Cell. Immunol. 2019, 337, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Su, Z.Y.; Wang, L.; Shu, L.; Yang, Y.; Guo, Y.; Pung, D.; Bountra, C.; Kong, A.N. Epigenetic blockade of neoplastic transformation by bromodomain and extra-terminal (BET) domain protein inhibitor JQ-1. Biochem. Pharmacol. 2016, 117, 35–45. [Google Scholar] [CrossRef]
- Li, N.; Yang, L.; Qi, X.K.; Lin, Y.X.; Xie, X.; He, G.P.; Feng, Q.S.; Liu, L.R.; Xie, X.; Zeng, Y.X.; et al. BET bromodomain inhibitor JQ1 preferentially suppresses EBV-positive nasopharyngeal carcinoma cells partially through repressing c-Myc. Cell Death Dis. 2018, 9, 761. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Bengsch, F.; Svoronos, N.; Rutkowski, M.R.; Bitler, B.G.; Allegrezza, M.J.; Yokoyama, Y.; Kossenkov, A.V.; Bradner, J.E.; Conejo-Garcia, J.R.; et al. BET Bromodomain Inhibition Promotes Anti-tumor Immunity by Suppressing PD-L1 Expression. Cell Rep. 2016, 16, 2829–2837. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.U.; Katavolos, P.; Palanisamy, G.; Katewa, A.; Sioson, C.; Corpuz, J.; Pang, J.; DeMent, K.; Choo, E.; Ghilardi, N.; et al. Nonselective inhibition of the epigenetic transcriptional regulator BET induces marked lymphoid and hematopoietic toxicity in mice. Toxicol. Appl. Pharmacol. 2016, 300, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Zhang, Q.; Fan, Y.; Li, J.; Zhang, J.; Wang, W.; Fan, J.; Guo, Y.; Liu, S.; Hao, D.; et al. MYC inhibition reprograms tumor immune microenvironment by recruiting T lymphocytes and activating the CD40/CD40L system in osteosarcoma. Cell Death Discov. 2022, 8, 117. [Google Scholar] [CrossRef] [PubMed]
- Pervaiz, M.; Mishra, P.; Gunther, S. Bromodomain Drug Discovery—The Past, the Present, and the Future. Chem. Rec. 2018, 18, 1808–1817. [Google Scholar] [CrossRef] [PubMed]
- Naimi, A.; Mohammed, R.N.; Raji, A.; Chupradit, S.; Yumashev, A.V.; Suksatan, W.; Shalaby, M.N.; Thangavelu, L.; Kamrava, S.; Shomali, N.; et al. Tumor immunotherapies by immune checkpoint inhibitors (ICIs); the pros and cons. Cell Commun. Signal. CCS 2022, 20, 44. [Google Scholar] [CrossRef]
- Hamid, O.; Molinero, L.; Bolen, C.R.; Sosman, J.A.; Munoz-Couselo, E.; Kluger, H.M.; McDermott, D.F.; Powderly, J.D.; Sarkar, I.; Ballinger, M.; et al. Safety, Clinical Activity, and Biological Correlates of Response in Patients with Metastatic Melanoma: Results from a Phase I Trial of Atezolizumab. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2019, 25, 6061–6072. [Google Scholar] [CrossRef]
- Geoerger, B.; Kang, H.J.; Yalon-Oren, M.; Marshall, L.V.; Vezina, C.; Pappo, A.; Laetsch, T.W.; Petrilli, A.S.; Ebinger, M.; Toporski, J.; et al. Pembrolizumab in paediatric patients with advanced melanoma or a PD-L1-positive, advanced, relapsed, or refractory solid tumour or lymphoma (KEYNOTE-051): Interim analysis of an open-label, single-arm, phase 1-2 trial. Lancet Oncol. 2020, 21, 121–133. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Hsu, J.M.; Yang, W.H.; Hung, M.C. Mechanisms regulating PD-L1 expression in cancers and associated opportunities for novel small-molecule therapeutics. Nat. Rev. Clin. Oncol. 2022, 19, 287–305. [Google Scholar] [CrossRef] [PubMed]
- Sarnik, J.; Poplawski, T.; Tokarz, P. BET Proteins as Attractive Targets for Cancer Therapeutics. Int. J. Mol. Sci. 2021, 22, 11102. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, Q.; Xie, J.; Zhong, Z.; Deng, C. Enzyme-responsive micellar JQ1 induces enhanced BET protein inhibition and immunotherapy of malignant tumors. Biomater. Sci. 2021, 9, 6915–6926. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, Z.; Wang, Z.; Wang, S.; Chen, Z.; Li, Z.; Zhang, M.; Zou, J.; Dong, B.; Gao, J.; et al. Targeting c-Myc: JQ1 as a promising option for c-Myc-amplified esophageal squamous cell carcinoma. Cancer Lett. 2018, 419, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Iribarren, K.; Bloy, N.; Buque, A.; Cremer, I.; Eggermont, A.; Fridman, W.H.; Fucikova, J.; Galon, J.; Spisek, R.; Zitvogel, L.; et al. Trial Watch: Immunostimulation with Toll-like receptor agonists in cancer therapy. Oncoimmunology 2016, 5, e1088631. [Google Scholar] [CrossRef] [PubMed]
- Fidock, M.D.; Souberbielle, B.E.; Laxton, C.; Rawal, J.; Delpuech-Adams, O.; Corey, T.P.; Colman, P.; Kumar, V.; Cheng, J.B.; Wright, K.; et al. The innate immune response, clinical outcomes, and ex vivo HCV antiviral efficacy of a TLR7 agonist (PF-4878691). Clin. Pharmacol. Ther. 2011, 89, 821–829. [Google Scholar] [CrossRef]
- Bourquin, C.; Hotz, C.; Noerenberg, D.; Voelkl, A.; Heidegger, S.; Roetzer, L.C.; Storch, B.; Sandholzer, N.; Wurzenberger, C.; Anz, D.; et al. Systemic cancer therapy with a small molecule agonist of toll-like receptor 7 can be improved by circumventing TLR tolerance. Cancer Res. 2011, 71, 5123–5133. [Google Scholar] [CrossRef]
- Biswas, S.K.; Mantovani, A. Macrophage plasticity and interaction with lymphocyte subsets: Cancer as a paradigm. Nat. Immunol. 2010, 11, 889–896. [Google Scholar] [CrossRef]
- Wu, K.; Lin, K.; Li, X.; Yuan, X.; Xu, P.; Ni, P.; Xu, D. Redefining Tumor-Associated Macrophage Subpopulations and Functions in the Tumor Microenvironment. Front. Immunol. 2020, 11, 1731. [Google Scholar] [CrossRef]
- Schultze, J.L.; Schmidt, S.V. Molecular features of macrophage activation. Semin. Immunol. 2015, 27, 416–423. [Google Scholar] [CrossRef]
- Griess, B.; Mir, S.; Datta, K.; Teoh-Fitzgerald, M. Scavenging reactive oxygen species selectively inhibits M2 macrophage polarization and their pro-tumorigenic function in part, via Stat3 suppression. Free Radic. Biol. Med. 2020, 147, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Jayasingam, S.D.; Citartan, M.; Thang, T.H.; Mat Zin, A.A.; Ang, K.C.; Ch’ng, E.S. Evaluating the Polarization of Tumor-Associated Macrophages into M1 and M2 Phenotypes in Human Cancer Tissue: Technicalities and Challenges in Routine Clinical Practice. Front. Oncol. 2019, 9, 1512. [Google Scholar] [CrossRef] [PubMed]
- Sato-Kaneko, F.; Yao, S.; Ahmadi, A.; Zhang, S.S.; Hosoya, T.; Kaneda, M.M.; Varner, J.A.; Pu, M.; Messer, K.S.; Guiducci, C.; et al. Combination immunotherapy with TLR agonists and checkpoint inhibitors suppresses head and neck cancer. JCI Insight 2017, 2, e93397. [Google Scholar] [CrossRef] [PubMed]
- Dolina, J.S.; Van Braeckel-Budimir, N.; Thomas, G.D.; Salek-Ardakani, S. CD8(+) T Cell Exhaustion in Cancer. Front. Immunol. 2021, 12, 715234. [Google Scholar] [CrossRef]
- Pan, Y.; Yu, Y.; Wang, X.; Zhang, T. Tumor-Associated Macrophages in Tumor Immunity. Front. Immunol. 2020, 11, 583084. [Google Scholar] [CrossRef]
- Lambert, A.W.; Pattabiraman, D.R.; Weinberg, R.A. Emerging Biological Principles of Metastasis. Cell 2017, 168, 670–691. [Google Scholar] [CrossRef]
- Ostrand-Rosenberg, S.; Fenselau, C. Myeloid-Derived Suppressor Cells: Immune-Suppressive Cells That Impair Antitumor Immunity and Are Sculpted by Their Environment. J. Immunol. 2018, 200, 422–431. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.-S.; Wang, J.-X.; Jin, G.-Y.; Hu, M.-H.; Wang, X.-D. Combination Therapy with a TLR7 Agonist and a BRD4 Inhibitor Suppresses Tumor Growth via Enhanced Immunomodulation. Int. J. Mol. Sci. 2024, 25, 663. https://doi.org/10.3390/ijms25010663
Liu Y-S, Wang J-X, Jin G-Y, Hu M-H, Wang X-D. Combination Therapy with a TLR7 Agonist and a BRD4 Inhibitor Suppresses Tumor Growth via Enhanced Immunomodulation. International Journal of Molecular Sciences. 2024; 25(1):663. https://doi.org/10.3390/ijms25010663
Chicago/Turabian StyleLiu, Yong-Si, Jia-Xin Wang, Guang-Yi Jin, Ming-Hao Hu, and Xiao-Dong Wang. 2024. "Combination Therapy with a TLR7 Agonist and a BRD4 Inhibitor Suppresses Tumor Growth via Enhanced Immunomodulation" International Journal of Molecular Sciences 25, no. 1: 663. https://doi.org/10.3390/ijms25010663
APA StyleLiu, Y.-S., Wang, J.-X., Jin, G.-Y., Hu, M.-H., & Wang, X.-D. (2024). Combination Therapy with a TLR7 Agonist and a BRD4 Inhibitor Suppresses Tumor Growth via Enhanced Immunomodulation. International Journal of Molecular Sciences, 25(1), 663. https://doi.org/10.3390/ijms25010663





