Effectiveness of Cerium Oxide Nanoparticles in Non-Alcoholic Fatty Liver Disease Evolution Using In Vivo and In Vitro Studies: A Systematic Review
Abstract
:1. Introduction
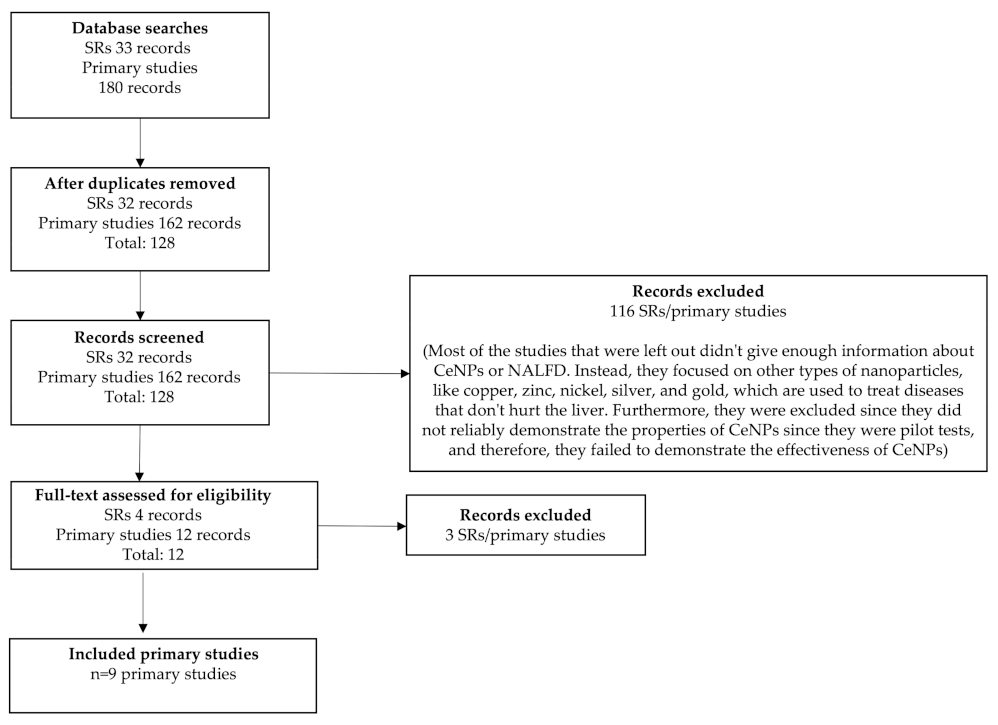
2. Materials and Methods
2.1. Search Strategy and Selection Criteria
Search Strategy
2.2. Identification of Relevant Studies
2.3. Types of Study and Design
2.4. Population
2.5. Quality Assessment/Risk of Bias
2.6. Data Extraction and Synthesis
3. Results
3.1. Summary of Included Studies
3.2. Evaluation of Quality
3.3. Correlation between NALFD and NPs
3.4. CeNP Characterization
3.5. Plasma Biomarkers and Cell Viability Assays
3.6. Oxidative Stress
3.7. Gene Expression
4. Discussion
4.1. Summary of Key Findings and Interpretation
4.2. Scope and Limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Levene, A.P.; Goldin, R.D. The epidemiology, pathogenesis and histopathology of fatty liver disease. Histopathology 2012, 61, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Vernon, G.; Baranova, A.; Younossi, Z.M. Systematic review: The epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment. Pharmacol. Ther. 2011, 34, 274–285. [Google Scholar] [CrossRef] [PubMed]
- Ertle, J.; Dechêne, A.; Sowa, J.P.; Penndorf, V.; Herzer, K.; Kaiser, G.; Schlaak, J.F.; Gerken, G.; Syn, W.K.; Canbay, A. Non-alcoholic fatty liver disease progress to hepatocellular carcinoma in the absence of apparent cirrosis. Int. J. Cancer. 2011, 128, 2436–2443. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.; Anstee, Q.M.; Marietti, M.; Hardy, T.; Henry, L.; Eslam, M.; George, J.; Bugianesi, E. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 11–20. [Google Scholar] [CrossRef]
- Ipsen, D.H.; Tveden-Nyborg, P.; Lykkesfeldt, J. Dyslipidemia: Obese or not obese-that is not the question. Curr. Obes. Rep. 2016, 5, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Hojland Ipsen, D.; Tveden-Nyborg, P.; Lykkesfeldt, J. Normal weight dyslipidemia: Is it all about the liver? Obesity 2016, 24, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Dixon, J.B.; Bhathal, P.S.; O’Brien, P.E. Nonalcoholic fatty liver disease: Predictors of nonalcoholic steatohepatitis and liver fibrosis in the severely obese. Gastroenterology 2001, 121, 91–100. [Google Scholar] [CrossRef]
- Adams, L.A.; Lymp, J.F.; Sauver, J.; Sanderson, S.O.; Lindor, K.D.; Feldstein, A.; Angulo, P. The natural history of nonalcoholic fatty liver disease: A population-based cohort study. Gastroenterology 2005, 129, 113–121. [Google Scholar] [CrossRef]
- Caldwell, S.H.; Chang, C.Y.; Nakamoto, R.K.; Krugner-Higby, L. Mitochondria in nonalcoholic fatty liver disease. Clin. Liver. Dis. 2004, 8, 595–617. [Google Scholar] [CrossRef]
- Angulo, P. Nonalcoholic fatty liver disease. N. Engl. J. Med. 2002, 346, 1221–1231. [Google Scholar] [CrossRef]
- Farrell, G.C. Non-alcoholic steatohepatitis: What is it, and why is it important in the Asia-Pacific region? J. Gastroenterol. Hepatol. 2003, 18, 124–138. [Google Scholar] [CrossRef] [PubMed]
- Sunny, N.E.; Bril, F.; Cusi, K. Mitochondrial Adaptation in Nonalcoholic Fatty Liver Disease: Novel Mechanisms and Treatment Strategies. Endocrinol. Metab. 2017, 28, 250–260. [Google Scholar] [CrossRef] [PubMed]
- Marra, F.; Svegliati-Baroni, G. Lipotoxicity and the gut-liver axis in NASH pathogenesis. J. Hepatol. 2018, 68, 280–295. [Google Scholar] [CrossRef]
- Malhi, H.; Bronk, S.F.; Werneburg, N.W.; Gores, G.J. Free fatty acids induce JNK-dependent hepatocyte lipoapoptosis. J. Biol. Chem. 2006, 281, 12093–12101. [Google Scholar] [CrossRef] [PubMed]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef] [PubMed]
- Newsholme, P.; Cruzat, V.F.; Keane, K.N.; Carlessi, R.; de Bittencourt, P.I., Jr. Molecular mechanisms of ROS production and oxidative stress in diabetes. Biochem. J. 2016, 473, 4527–4550. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Thorling, C.A.; Liang, X.; Bridle, K.R.; Grice, J.E.; Zhu, Y.; Crawford, D.H.G.; Xu, Z.P.; Liu, X.; Roberts, M.S. Diagnostic imaging and therapeutic application of nanoparticles targeting the liver. J. Mater. Chem. B 2015, 3, 939–958. [Google Scholar] [CrossRef]
- Poilil Surendran, S.; George Thomas, R.; Moon, M.J.; Jeong, Y.Y. Nanoparticles for the treatment of liver fibrosis. Int. J. Nanomed. 2017, 12, 6997–7006. [Google Scholar] [CrossRef]
- Kang, J.H.; Toita, R.; Murata, M. Liver cell-targeted delivery of therapeutic molecules. Crit. Rev. Biotechnol. 2016, 36, 132–143. [Google Scholar] [CrossRef]
- Böttger, R.; Pauli, G.; Chao, P.H.; Al Fayez, N.; Hohenwarter, L.; Li, S.D. Lipid-based nanoparticle technologies for liver targeting. Adv. Drug Deliv. Rev. 2020, 154–155, 79–101. [Google Scholar] [CrossRef]
- Xu, C.; Qu, X. Cerium oxide nanoparticle: A remarkably versatile rare earth nanomaterial for biological applications. NPG Asia Mater. 2014, 6, e90. [Google Scholar] [CrossRef]
- Dowding, J.M.; Seal, S.; Self, W.T. Cerium oxide nanoparticles accelerate the decay of peroxynitrite (ONOO−). Drug Deliv. Transl. Res. 2013, 3, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Caputo, F.; De Nicola, M.; Ghibelli, L. Pharmacological potential of bioactive engineered nanomaterials. Biochem. Pharmacol. 2014, 92, 112–130. [Google Scholar] [CrossRef] [PubMed]
- Olmedo, D.G.; Tasat, D.R.; Evelson, P.; Guglielmotti, M.B.; Cabrini, R.L. Biological response of tissues with macrophagic activity to titanium dioxide. J. Biomed. Mater. Res. A 2008, 84, 1087–1093. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Appendix F Quality Appraisal Checklist—Quantitative Intervention Studies. In Methods for the Development of NICE Public Health Guidance; National Institute for Health and Care Excellence: London, UK, 2012; Available online: https://www.nice.org.uk/process/pmg4/chapter/about-this-document (accessed on 1 September 2023).
- Carvajal, S.; Perramón, M.; Casals, G.; Oró, D.; Ribera, J.; Morales-Ruiz, M.; Casals, E.; Casado, P.; Melgar-Lesmes, P.; Fernández-Varo, G.; et al. Cerium Oxide Nanoparticles Protect against Oxidant Injury and Interfere with Oxidative Mediated Kinase Signaling in Human-Derived Hepatocytes. Int. J. Mol. Sci. 2019, 20, 5959. [Google Scholar] [CrossRef] [PubMed]
- Carvajal, S.; Perramón, M.; Oró, D.; Casals, G.; Fernández-Varo, G.; Casals, G.; Parra, M.; González de la Presa, B.; Ribera, J.; Pastor, O.; et al. Cerium oxide nanoparticles display antilipogenic effect in rats with non-alcoholic fatty liver diseas. Sci. Rep. 2019, 9, 12848. [Google Scholar] [CrossRef] [PubMed]
- Parra-Robert, M.; Casals, E.; Massana, N.; Zeng, M.; Perramón, M.; Fernández-Varo, G.; Morales-Ruiz, M.; Puntes, V.; Jiménez, W.; Casals, G. Beyond the Scavenging of Reactive Oxygen Species (ROS): Direct Effect of Cerium Oxide Nanoparticles in Reducing Fatty Acids Content in an In Vitro Model of Hepatocellular Steatosis. Biomolecules 2019, 9, 425. [Google Scholar] [CrossRef]
- Godugu, C.; Khurana, A.; Saifi, M.A. Rare earth cerium oxide nanoparticles attenuated liver fibrosis in bile duct ligation mice model. J. Trace Elem. Med. Biol. 2023, 75, 127102. [Google Scholar] [CrossRef]
- Abbasi, E.; Vafaei, S.A.; Naseri, N.; Darini, A.; Azandaryani, M.T.; Ara, F.K.; Mirzaei, F. Protective effects of cerium oxide nanoparticles in non-alcoholic fatty liver disease (NAFLD) and carbon tetrachloride-induced liver damage in rats: Study on intestine and liver. Metabol. Open 2021, 12, 100151. [Google Scholar] [CrossRef]
- Oró, D.; Yudina, T.; Fernández-Varo, G.; Casals, E.; Reichenbach, V.; Casals, G.; González de la Presa, B.; Sandalinas, S.; Carvajal, S.; Puntes, V.; et al. Cerium oxide nanoparticles reduce steatosis, portal hypertension and display anti-inflammatory properties in rats with liver fibrosis. J. Hepatol. 2016, 64, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Boey, A.; Leong, S.Q.; Bhave, S.; Ho, H.K. Cerium Oxide Nanoparticles Alleviate Hepatic Fibrosis Phenotypes In Vitro. Int. J. Mol. Sci. 2021, 22, 11777. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Varo, G.; Perramón, M.; Carvajal, S.; Oró, D.; Casals, E.; Boix, L.; Oller, L.; Macías-Muñoz, L.; Marfà, S.; Casals, G.; et al. Bespoken Nanoceria: An Effective Treatment in Experimental Hepatocellular Carcinoma. Hepatology 2020, 72, 1267–1282. [Google Scholar] [CrossRef] [PubMed]
- Kobyliak, N.; Abenavoli, L.; Falalyeyeva, T.; Virchenko, O.; Natalia, B.; Beregova, T.; Bodnar, P.; Spivak, M. Prevention of NAFLD development in rats with obesity via the improvement of pro/antioxidant state by cerium dioxide nanoparticles. Clujul Med. 2016, 89, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Thanh, N.T.; Maclean, N.; Mahiddine, S. Mechanisms of nucleation and growth of nanoparticles in solution. Chem. Rev. 2014, 114, 7610–7630. [Google Scholar] [CrossRef] [PubMed]
- Cimini, A.; D’Angelo, B.; Das, S.; Gentile, R.; Benedetti, E.; Singh, V.; Monaco, A.M.; Santucci, S.; Seal, S. Antibody-conjugated PEGylated cerium oxide nanoparticles for specific targeting of Aβ aggregates modulate neuronal survival pathways. Acta Biomater. 2012, 8, 2056–2067. [Google Scholar] [CrossRef] [PubMed]
- Estevez, A.; Pritchard, S.; Harper, K.; Aston, J.; Lynch, A.; Lucky, J.; Ludington, J.; Chatani, P.; Mosenthal, W.; Leiter, J. Neuroprotective mechanisms of cerium oxide nanoparticles in a mouse hippocampal brain slice model of ischemia. Free Radic. Biol. Med. 2011, 51, 1155–1163. [Google Scholar] [CrossRef] [PubMed]
- Heckman, K.L.; DeCoteau, W.; Estevez, A.; Reed, K.J.; Costanzo, W.; Sanford, D.; Leiter, J.C.; Clauss, J.; Knapp, K.; Gomez, C.; et al. Custom cerium oxide nanoparticles protect against a free radical mediated autoimmune degenerative disease in the brain. ACS Nano 2013, 7, 10582–10596. [Google Scholar] [CrossRef]
- Sangomla, S.; Saifi, M.A.; Khurana, A.; Godugu, C. Nanoceria ameliorates doxorubicin induced cardiotoxicity: Possible mitigation via reduction of oxidative stress and inflammation. J. Trace Elem. Med. Biol. 2018, 47, 53–62. [Google Scholar] [CrossRef]
- Niu, J.; Azfer, A.; Rogers, L.M.; Wang, X.; Kolattukudy, P.E. Cardioprotective effects of cerium oxide nanoparticles in a transgenic murine model of cardiomyopathy. Cardiovasc. Res. 2007, 73, 549–559. [Google Scholar] [CrossRef]
- Pourkhalili, N.; Hosseini, A.; Nili-Ahmadabadi, A.; Rahimifard, M.; Navaei-Nigjeh, M.; Hassani, S.; Baeeri, M.; Abdollahi, M. Improvement of isolated rat pancreatic islets function by combination of cerium oxide nanoparticles/sodium selenite through reduction of oxidative stress. Toxicol. Mech. Methods 2012, 22, 476–482. [Google Scholar] [CrossRef]
- Khurana, A.; Saifi, M.A.; Godugu, C. Nanoceria Ameliorates Fibrosis, Inflammation, and Cellular Stress in Experimental Chronic Pancreatitis. ACS Biomater. Sci. Eng. 2023, 9, 1030–1042. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Song, M.Y.; Park, J.D.; Song, K.S.; Ryu, H.R.; Chung, Y.H.; Chang, H.K.; Lee, J.H.; Oh, K.H.; Kelman, B.J.; et al. Subchronic oral toxicity of silver nanoparticles. Part Fibre Toxicol. 2010, 7, 20. [Google Scholar] [CrossRef] [PubMed]
- Comenge, J.; Sotelo, C.; Romero, F.; Gallego, O.; Barnadas, A.; Parada, T.G.; Domínguez, F.; Puntes, V.F. Detoxifying antitumoral drugs via nanoconjugation: The case of gold nanoparticles and cisplatin. PLoS ONE 2012, 7, e47562. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.N.; Poon, W.; Tavares, A.J.; McGilvray, I.D.; Chan, W.C.W. Nanoparticle-liver interactions: Cellular uptake and hepatobiliary elimination. J. Control Release 2016, 240, 332–348. [Google Scholar] [CrossRef]
- Lu, X.; Tian, Y.; Zhao, Q.; Jin, T.; Xiao, S.; Fan, X. Integrated metabonomics analysis of the size-response relationship of silica nanoparticles-induced toxicity in mice. Nanotechnology 2011, 22, 055101. [Google Scholar] [CrossRef] [PubMed]
- Nemmar, A.; Yuvaraju, P.; Beegam, S.; Yasin, J.; Kazzam, E.E.; Ali, B.H. Oxidative stress, inflammation, and DNA damage in multiple organs of mice acutely exposed to amorphous silica nanoparticles. Int. J. Nanomed. 2016, 11, 919–928. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhang, Y.; Li, Y.; Guo, C.; Fan, Z.; Li, Y.; Yang, M.; Zhou, X.; Sun, Z.; Wang, J. Integrative proteomics and metabolomics approach to elucidate metabolic dysfunction induced by silica nanoparticles in hepatocytes. J. Hazard Mater. 2022, 434, 128820. [Google Scholar] [CrossRef] [PubMed]
- Isoda, K.; Tetsuka, E.; Shimizu, Y.; Saitoh, K.; Ishida, I.; Tezuka, M. Liver injury induced by thirty- and fifty-nanometer-diameter silica nanoparticles. Biol. Pharm. Bull. 2013, 36, 370–375. [Google Scholar] [CrossRef]
- Zhuravskii, S.; Yukina, G.; Kulikova, O.; Panevin, A.; Tomson, V.; Korolev, D.; Galagudza, M. Mast cell accumulation precedes tissue fibrosis induced by intravenously administered amorphous silica nanoparticles. Toxicol. Mech. Methods 2016, 26, 260–269. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Desouky, E.M.; Hozayen, W.G.; Bin-Jumah, M.; El-Nahass, E.S.; Soliman, H.A.; Farghali, A.A. Mesoporous silica nanoparticles trigger liver and kidney injury and fibrosis via altering TLR4/NF-κB, JAK2/STAT3 and Nrf2/HO-1 signaling in rats. Biomolecules 2019, 9, 528. [Google Scholar] [CrossRef] [PubMed]
- Palomer, X.; Salvadó, L.; Barroso, E.; Vázquez-Carrera, M. An overview of the crosstalk between inflammatory processes and metabolic dysregulation during diabetic cardiomyopathy. Int. J. Cardiol. 2013, 168, 3160–3172. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Rosso, L.G.; Huang, P.; Wang, Z.; Xu, Y.; Yao, X.; Bao, M.; Yan, J.; Song, H.; Wang, G. Liver Med23 ablation improves glucose and lipid metabolism through modulating FOXO1 activity. Cell Res. 2014, 24, 1250–1265. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ma, Z.; Jiang, S.; Hu, W.; Li, T.; Di, S.; Wang, D.; Yang, Y. A global perspective on FOXO1 in lipid metabolism and lipid-related diseases. Prog. Lipid Res. 2017, 66, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Wicklow, B.; Wittmeier, K.; Jong, G.W.; McGavock, J.; Robert, M.; Duhamel, T.; Dolinsky, V.W. Proposed trial: Safety and efficacy of resveratrol for the treatment of non-alcoholic fatty liver disease (NAFLD) and associated insulin resistance in adolescents who are overweight or obese adolescents—Rationale and protocol. Biochem. Cell. Biol. 2015, 93, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Mykhalchyshyn, G.; Kobyliak, N.; Bodnar, P. Diagnostic accuracy of acyl-ghrelin and it association with non-alcoholic fatty liver disease in type 2 diabetic patients. J. Diabetes Metab. Disord. 2015, 14, 44. [Google Scholar] [CrossRef] [PubMed]
- Rocca, A.; Moscato, S.; Ronca, F.; Nitti, S.; Mattoli, V.; Giorgi, M.; Ciofani, G. Pilot in vivo investigation of cerium oxide nanoparticles as a novel anti-obesity pharmaceutical formulation. Nanomedicine 2015, 11, 1725–1734. [Google Scholar] [CrossRef] [PubMed]
- Kobyliak, N.; Virchenko, O.; Falalyeyeva, T.; Kondro, M.; Beregova, T.; Bodnar, P.; Shcherbakov, O.; Bubnov, R.; Caprnda, M.; Delev, D.; et al. Cerium dioxide nanoparticles possess anti-inflammatory properties in the conditions of the obesity-associated NAFLD in rats. Biomed. Pharmacother. 2017, 90, 608–614. [Google Scholar] [CrossRef]
- Wasef, L.; Nassar, A.M.K.; El-Sayed, Y.S.; Samak, D.; Noreldin, A.; Elshony, N.; Saleh, H.; Elewa, Y.H.A.; Hassan, S.M.A.; Saati, A.A.; et al. The potential ameliorative impacts of cerium oxide nanoparticles against fipronil-induced hepatic steatosis. Sci. Rep. 2021, 11, 1310. [Google Scholar] [CrossRef]
- Kobyliak, N.M.; Falalyeyeva, T.M.; Kuryk, O.G.; Beregova, T.V.; Bodnar, P.M.; Zholobak, N.M.; Shcherbakov, O.B.; Bubnov, R.V.; Spivak, M.Y. Antioxidative effects of cerium dioxide nanoparticles ameliorate age-related male infertility: Optimistic results in rats and the review of clinical clues for integrative concept of men health and fertility. EPMA J. 2015, 6, 12. [Google Scholar] [CrossRef]
- Hosseini, M.; Mozafari, M. Cerium Oxide Nanoparticles: Recent Advances in Tissue Engineering. Materials 2020, 13, 3072. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Sohail Raja, N.I.; Asad, M.J.; Mashwani, Z.U. Antioxidant and hypoglycemic potential of phytogenic cerium oxide nanoparticles. Sci. Rep. 2023, 13, 4514. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Gao, L.; Hou, Q.; Wu, P.; Zhou, Y.; Ding, Z. Enhanced Oxygen Storage Capacity of Porous CeO2 by Rare Earth Doping. Molecules 2023, 28, 6005. [Google Scholar] [CrossRef] [PubMed]
- Banavar, S.; Deshpande, A.; Sur, S.; Andreescu, S. Ceria nanoparticle theranostics: Harnessing antioxidant properties in biomedicine and beyond. J. Phys. Mater. 2021, 4, 042003. [Google Scholar] [CrossRef]
- Fujii, J.; Homma, T.; Osaki, T. Superoxide Radicals in the Execution of Cell Death. Antioxidants 2022, 11, 501. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Sun, W.; Balaz, M.; He, A.; Klug, M.; Wieland, S.; Caiazzo, R.; Raverdy, V.; Pattou, F.; Lefebvre, P.; et al. Peroxisomal β-oxidation acts as a sensor for intracellular fatty acids and regulates lipolysis. Nat. Metab. 2021, 3, 1648–1661. [Google Scholar] [CrossRef]
- Cichoz-Lach, H.; Michalak, A. Oxidative stress as a crucial factor in liver diseases. World J. Gastroenterol. 2014, 20, 8082–8091. [Google Scholar] [CrossRef]
- Sandoval, C.; Mella, L.; Godoy, K.; Adeli, K.; Farías, J. β-Carotene Increases Activity of Cytochrome P450 2E1 during Ethanol Consumption. Antioxidants 2022, 11, 1033. [Google Scholar] [CrossRef]
- Carrasco, C.; Carrasco, C.; Souza-Mello, V.; Sandoval, C. Effectiveness of antioxidant treatments on cytochrome P450 2E1 (CYP2E1) activity after alcohol exposure in humans and in vitro models: A systematic review. Int. J. Food Prop. 2021, 24, 1300–1317. [Google Scholar] [CrossRef]
- Sandoval, C.; Vásquez, B.; Vasconcellos, A.; Souza-Mello, V.; Adeli, K.; Mandarim-de-Lacerda, C.; del Sol, M. Oral supplementation of b-carotene benefits the hepatic structure and metabolism in mice exposed to chronic ethanol consumption. Sains Malays. 2022, 51, 285–296. [Google Scholar] [CrossRef]
- Sandoval, C.; Vásquez, B.; Souza-Mello, V.; Adeli, K.; Mandarim-de-Lacerda, C.; del Sol, M. Morphoquantitative Effects of Oral β-carotene Supplementation on Liver of C57BL/6 Mice Exposed to Ethanol Consumption. Int. J. Clin. Exp. Pathol. 2019, 12, 1713–1722. [Google Scholar]
- Sandoval, C.; Farías, J.; Zamorano, M.; Herrera, C. Vitamin Supplements as a Nutritional Strategy against Chronic Alcohol Consumption? An Updated Review. Antioxidants 2022, 11, 564. [Google Scholar] [CrossRef] [PubMed]
- Ore, A.; Akinloye, O.A. Oxidative stress and antioxidant biomarkers in clinical and experimental models of non-alcoholic fatty liver disease. Medicina 2019, 55, 26. [Google Scholar] [CrossRef] [PubMed]
- Abe, Y.; Hines, I.N.; Zibari, G.; Pavlick, K.; Gray, L.; Kitagawa, Y.; Grisham, M.B. Mouse model of liver ischemia and reperfusion injury: Method for studying reactive oxygen and nitrogen metabolites in vivo. Free Radic. Biol. Med. 2009, 46, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Al-Asmari, A.K.; Khan, A.Q.; Al-Masri, N. Mitigation of 5-fluorouracil-induced liver damage in rats by Vitamin C via targeting redox-sensitive transcription factors. Hum. Exp. Toxicol. 2016, 35, 1203–1213. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q. Role of Nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef]
- Khan, R.S.; Bril, F.; Cusi, K.; Newsome, P.N. Modulation of Insulin Resistance in Nonalcoholic Fatty Liver Disease. Hepatology 2019, 70, 711–724. [Google Scholar] [CrossRef]
- McGeehan, G.M.; Becherer, J.D.; Bast, R.C., Jr.; Boyer, C.M.; Champion, B.; Connolly, K.M.; Conway, J.G.; Furdon, P.; Karp, S.; Kidao, S.; et al. Regulation of Tumour Necrosis Factor-Alpha Processing by a Metalloproteinase Inhibitor. Nature 1994, 370, 558–561. [Google Scholar] [CrossRef]
- Loman, B.R.; Hernández-Saavedra, D.; An, R.; Rector, R.S. Prebiotic and Probiotic Treatment of Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Nutr. Rev. 2018, 76, 822–839. [Google Scholar] [CrossRef]
- Seo, Y.Y.; Cho, Y.K.; Bae, J.C.; Seo, M.H.; Park, S.E.; Rhee, E.J.; Park, C.Y.; Oh, K.W.; Park, S.W.; Lee, W.Y. Tumor Necrosis Factor-Alpha as a Predictor for the Development of Nonalcoholic Fatty Liver Disease: A 4-Year Follow-up Study. Endocrinol. Metab. 2013, 28, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Ajmal, M.R.; Yaccha, M.; Malik, M.A.; Rabbani, M.U.; Ahmad, I.; Isalm, N.; Abdali, N. Prevalence of Nonalcoholic Fatty Liver Disease (NAFLD) in Patients of Cardiovascular Diseases and its Association With hs-CRP and TNF-α. Indian Heart J. 2014, 66, 574–579. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Tsukamoto, H. Inflammation in Alcoholic and Nonalcoholic Fatty Liver Disease: Friend or Foe? Gastroenterology 2016, 150, 1704–1709. [Google Scholar] [CrossRef] [PubMed]
- Schwabe, R.F.; Brenner, D.A. Mechanisms of Liver Injury. I. TNF-alpha-induced liver injury: Role of IKK, JNK, and ROS pathways. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 290, G583–G589. [Google Scholar] [CrossRef] [PubMed]
- Orfila, C.; Lepert, J.C.; Alric, L.; Carrera, G.; Beraud, M.; Vinel, J.P.; Pipy, B. Expression of TNF-alpha and immunohistochemical distribution of hepatic macrophage surface markers in carbon tetrachloride-induced chronic liver injury in rats. Histochem. J. 1999, 31, 677–685. [Google Scholar] [CrossRef]
| References | Country | Population, Setting | Details of intervention | Investigated Outcomes | Study Aims | Main Results |
|---|---|---|---|---|---|---|
| [27] | ES | HepG2 human hepatocytes derived from a liver hepatocellular carcinoma were used. | CeNP synthesis: CeNPs were synthesized via the chemical precipitation of Ce(NO3)3 in a basic aqueous solution. Cell culture: HepG2 cells were grown to confluence. Then, cells were switched to serum-free medium. For cell stimulation and treatment, the old medium was replaced with medium containing 1.5 mM H2O2 and CeNPs (10 μg/mL) or vehicle (TMAOH, 0.17 mM), respectively. Cell viability analysis: Cell viability was assessed using the MTS technique. The absorbance was measured at 492 nm. ROS measurement: ROS was assessed via flow cytometry using DCF-DA at 490/520 nm. Gene expression: Total RNA from cultured cells was extracted. Then, cDNA synthesis was carried out, and gene expression testing was performed. Relative quantification was calculated using the CT, which is inversely related to the abundance of mRNA transcripts in the initial sample. The relative quantity of the product was expressed as fold induction of the target gene compared with the reference gene according to the formula 2−∆∆CT. Phosphoproteomic analysis: Peptide identification was performed by matching the MS/MS data to the SwissProt database. | CeNP characterization; cell viability analysis, ROS measurement, gene expression, and phosphoproteomic analysis. | The purpose of this study was to determine if CeNPs can inhibit the oxidative damage in HepG2 cells and to discover the processes involved in this phenomenon. | CeNP characterization: NPs had a spherical morphology and were ~4 nm in diameter. Cell viability analysis: decreased oxidation in HepG2 cells and increased cell viability were found after HepG2 cells were co-incubated with 10 μg/mL of CeNPs. ROS measurement: CeNP treatment reduces ROS accumulation and the associated cell death induced by H2O2 and LPS in HepG2 cells. Gene expression: CeNPs significantly decreased the expression of two genes with peroxidase activity (MPO, PTGS1). CeNPs exerted a specific inhibitory effect on iNOS expression. Phosphoproteomic analysis: CeNPs reversed the ability of H2O2 to induce the phosphorylation of mTOR substrates like 4EBP1 and PRAS40. CeNPs also dephosphorylated TERF2 and ARID1A, which are major therapeutic targets in hepatocellular carcinoma. |
| [28] | ES | Male Wistar rats (n = 20) were used. | CeNP synthesis and characterization: CeNPs (4 nm) were synthesized via the chemical precipitation of Ce(NO3)3 in a basic aqueous solution. Animal model: Fifteen rats were fed with MCDD diet and five with standard chow. After 6 weeks of MCDD, the rats were euthanized through isofluorane overdose. Then, livers were obtained, frozen in dry ice, and stored at −80 °C or fixed in 10% buffered formalin for H&E and immunostaining analysis. Serum samples were also obtained and kept at −20 °C until further analysis. CeNP administration: CeNPs or vehicle were diluted in saline solution and given as a bolus (500 μL) through the tail vein. Histological examination: H&E, Sirius Red F3B, and IHQ (CD68) were used for fibrosis analysis. Hepatic lipid peroxidation: MDA concentration in the livers of the rats was measured. Hepatic lipid profiling and gene expression: Identification of the FAME in the sample extracts was achieved via mass spectrum and GC retention time comparison with reference standards. Gene expression: Total RNA from cultured cells was extracted. Then, cDNA synthesis was carried out, and gene expression testing was performed. Relative quantification was calculated using the CT, which is inversely related to the abundance of mRNA transcripts in the initial sample. The relative quantity of the product was expressed as fold induction of the target gene compared with the reference gene according to the formula 2−∆∆CT. | CeNP characterization, body weight, liver to body weight ratio and serum biochemical parameters, histological examination, hepatic lipid peroxidation, hepatic lipid profiling, and gene expression. | To elucidate whether CeNPs display beneficial effects in an experimental model of NAFLD. | CeNP characterization: NPs had a spherical morphology and were ~4 nm in diameter. Body weight, liver to body weight ratio: MCDD animals showed significantly decreased body weight and increased liver to body weight ratio than control rats. Serum biochemical parameters: MCDD displayed increased activity of transaminases, hypocholesterolemia, and hyperbilirubinemia and significantly decreased levels of circulatory triglycerides. Histological examination: Macrovesicular steatosis was observed in the MCDD + vehicle and MCDD + CeNPs groups as single large fat intra cytoplasmatic droplets displacing the nucleus, but was significantly less pronounced in MCDD rats receiving CeNPs. Hepatic lipid peroxidation: The level of MDA in the liver of the MCDD rats treated with CeNPs was significantly lower than in those animals receiving vehicle. Hepatic lipid profiling: Administration of CeNPs markedly altered the lipogenic activity in MCDD animals, as indicated by a 26% and 33% decrease in the liver content of total TG and CE, respectively. Gene expression: CeNPs exerted a significant inhibitory effect on the expression of six genes related to antioxidant metabolism (Epx, Gpx7, Gstp1, Prdx2, Prdx4, and Vimp) and four genes related to ROS metabolism (Aox1, Ccl5, Hmox1, and Ncf1). |
| [29] | ES | HepG2 cells were used. | CeNP synthesis: NPs were synthesized in aqueous media via the chemical precipitation of Ce(NO3)3 under basic conditions. CeNP characterization: NPs were characterized using TEM, Z-sizer, and X-ray powder diffraction. Cell culture: HepG2 cells were grown to confluence. Then, cells were switched to serum-free DMEM. For cell stimulation and treatment, the old medium was replaced with medium containing 1.5 mM CeNPs. Cell viability analysis: Cell viability was assessed using the MTS technique. The absorbance was measured at 490 nm. ROS measurement: ROS were assessed via flow cytometry using 2′,7′-dichlorofluorescin diacetate (DCF-DA) at 485 nm. Induction of steatosis: Oxysterols were measured using GC–MS. Total FA measurements: Identification of the FAME in the sample extracts was achieved via mass spectrum and GC retention time comparison with reference standards. | CeNP characterization, cell viability, ROS measurement, cholesterol and oxysterols content, and total FA measurements. | To test the hypothesis of whether CeNPs can directly reduce FA accumulation in the absence of other stimuli. | CeNP characterization: Z-potential of CeNPs was +40 mV at pH = 6. NPs had an average of 4 ± 1 size. Cell viability analysis: CeNP treatment improved cell viability from 85.8 ± 1.1% in the H2O2 condition to 99.6 ± 1.8% in cells exposed to H2O2 and NPs. ROS measurement: ROS production was inhibited in the presence of CeNPs (H2O2 125.4 ± 3.3% and H2O2 with CeNPs 109.8 ± 3.8%, p = 0.006). These results indicate that CeNPs protect from the oxidative stress induced by H2O2. Cholesterol and oxysterols content: No significant differences in cholesterol content were observed between cells, but treatment with CeNPs reduced the concentration of oxysterols. Total FA measurements: Treatment with CeNPs of cells exposed to OA and PA produced a significant reduction in total saturated FAs (p < 0.05). |
| [30] | ES | Male C57BL/6J mice (n = 32) were used. | Characterization of NC: The average particle size, poly dispersity index, and zeta potential of NC were evaluated through DLS. NC size was characterized using TEM. The functional groups were analyzed using FTIR. The crystalline phase composition of particles was validated using an X-ray diffractometer. Experimental design: The mice were randomly divided into four groups (n = 8): 1. sham control; 2. BDL (disease) control (BDL operated and received daily normal saline, i.p.); 3. NC low dose (BDL operated and treated with 0.5 mg/kg NC, i.p. daily for two weeks); and 4. NC high dose (BDL operated and treated with 2 mg/kg NC, i.p. daily for two weeks. For treatment, an NC suspension was prepared in sterile saline and probe-sonicated prior to administration. Measurement of plasma markers of liver injury: The levels of AST, ALT, ALP, and bilirubin were determined. Estimation of hydroxyproline and oxidative–nitrosative stress parameters: The hydroxyproline levels act as a marker of collagen content in the liver tissues, and they were measured via tissue homogenization in PBS followed by acid digestion with 6 N HCl in an autoclave. The levels of nitrosative stress (MDA, nitrite, GSH, catalase, SOD) were measured. Assessment of inflammatory cytokines: The levels of IL-1β, IL-6, IL-17, TNF-α, and TGF-β1 in liver tissues were estimated via the ELISA method at 450 and 570 nm. Histological examination: H&E, Sirius Red F3B, and IHQ (α-SMA and COL-1) testing were performed for fibrosis analysis. Western blotting: The separated proteins were transferred to nitrocellulose membrane and incubated with the primary (TIMP1, Snail, α-SMA, LOXL-2, N-cadherin, and fibronectin) and the secondary antibodies. | Characterization of NC; measurement of plasma markers of liver injury; estimation of hydroxyproline and oxidative-nitrosative stress parameters; assessment of inflammatory cytokines; histological examination and Western blotting. | To evaluate the hepatoprotective and anti-fibrotic effects of NC against BDL induced liver injury. | Characterization of NC: The average size of the particles was 120 ± 7.5 nm, PDI value 0.27, and the zeta potential −25 mV, as observed using the zeta meter. TEM imaging showed crystalline nanoparticles. Measurement of plasma markers of liver injury: The levels of AST, ALT, ALP, and bilirubin were significantly reduced in groups treated with NC (p < 0.05 for AST, ALT, ALP, and bilirubin). Estimation of hydroxyproline and oxidative–nitrosative stress parameters: The levels of hydroxyproline were significantly reduced by the pharmacological intervention with NC (p < 0.001). The tissue nitrite levels (p < 0.001) and lipid peroxidation levels (p < 0.01) were significantly reduced in groups treated with NC. Assessment of inflammatory cytokines: Levels of IL-1β, IL-6, IL-17, TNF-α, and TGF-β1 in liver tissues were significantly reduced in groups treated with NC (p < 0.001). Histological examination: H&E staining showed infiltration by inflammatory cells, whereas the signs of inflammation were markedly decreased by the NC intervention. In addition, the Sirius Red F3B staining indicated reduced collagen deposition in the central and portal vein area as well as the septal regions in groups treated with NC. Western blotting: The expression of TIMP1, Snail, α-SMA, LOXL-2, N-cadherin, and fibronectin were significantly decreased in the livers of animals treated with NC. |
| [31] | US | Male Wistar rats were used. | The classification of animals was as follows: 1. Rats were administered a solution of normal saline. 2. Rats were exposed to carbon tetrachloride (CCl4). 3. Rats in the CCl4 group were treated with nanoparticles (NPs). 4. Rats were provided with a standard chow diet. 5. Rats with non-alcoholic fatty liver disease (NAFLD) were established. 6. Rats in the NAFLD group were treated with nanoparticles. The levels of oxidative stress indicators were assessed in both the liver and gut. The levels of TNF-α were quantified using the enzyme-linked immunosorbent assay (ELISA) technique. The histopathological alterations in the liver and gut were assessed using light microscopy. | Serum levels of enzymes, protein estimation, lipid peroxidation, total antioxidant activity, total oxidative status, glutathione and TNF-α levels, and histological examination. | To examine the protective effects of the CeNPs in two models of liver injury, NAFLD and CCl4-induced liver fibrosis, in rats. | Body weight: Reduced body weight in the NAFLD group treated with NPs was found. Blood chemical markers: High levels of liver enzymes (ALP, AST, and ALT) induced by HFD and CCl4 were alleviated markedly in the NP treatment rats. NP administration significantly reduced cholesterol and triglyceride levels. Lipid peroxidation: CeNPs administration increased GSH concentration in the liver tissues of the hepatotoxic rats.. Oxidative status: Treatment with CeNPs significantly increased TAC concentrations and reduced MDA levels as compared with the NAFLD and CCl4 group (p < 0.01). Glutathione and TNF-α levels: Lower levels of TNF-α were found in CeNP-treated groups. Histological examination: Administration of CeNPs significantly alleviated the cholangiocyte hyperplasia, hepatic fibrosis, vacuolization, and hepatocellular hydropic degeneration. |
| [32] | ES | Male Wistar rats were used. | Systemic and hepatic effects of nanoparticles were assessed in CCl4-treated rats receiving CeNPs (0.1 mg/kg bodyweight) or vehicle twice weekly for two weeks, and CCl4 treatment was continued for eight additional weeks. NPs were dispersed in saline solution and intravenously given as a bolus (500 μL) through the tail vein. Mean arterial pressure and PP were assessed and serum samples obtained to measure standard hepatic and renal function tests. Animals were euthanized on days 1, 21, 42, and 56 after the last administration of CeNPs and organs dissected and kept at 80 °C for further analysis. Organ and subcellular distribution of NPs were assessed using mass spectrometry and transmission electron microscopy. Liver samples were obtained to evaluate steatosis, α-SMA expression, macrophage infiltration, apoptosis, and mRNA expression of oxidative-stress-, inflammatory-, or vasoactive-related genes. | Characterization of NPs, organ distribution of Ce in CCl4-treated rats, subcellular location of CeNPs in the liver of CCl4-treated rats, histological examination, measurement of portal pressure and the levels of liver damage markers, measurement of α-SMA expression, activated caspase-3 and apoptosis markers, and gene expression. | To determine whether CeNPs display hepatoprotective properties in experimental chronic liver disease. | Characterization of NPs: TEM analysis of CeNPs revealed that the particles had a spherical morphology and were predominantly in the size range of 4–20 nm. Subcellular location of CeNPs in the liver of CCl4-treated rats: CeNPs were present in the form of agglomerates of different sizes in the intracellular space of the liver parenchyma and in intracellular single-membrane organelles as lysosomes. Histological examination: Liver biopsies obtained from CCl4-treated rats had a finely granulated surface macroscopically. Based on Sirius Red analysis, no significant differences in hepatic collagen content were found between CCl4-treated rats receiving CeNPs or vehicle. However, the morphometric measurement of fat revealed an almost 50% reduction in total steatosis in fibrotic rats receiving CeNPs than those receiving vehicle. Measurement of portal pressure and the circulating levels of liver injury biomarkers: CeNPs decreased portal pressure and the circulating levels of liver injury biomarkers (albumin, total protein, total bilirubin, AST, GGT, and ALT levels) in CCl4-treated rats. Measurement of α-SMA expression: The percentage of α-SMA was significantly reduced in rats receiving CeNPs as compared to fibrotic animals receiving vehicle. In addition, CeNP administration was associated with a significant reduction in the number of CD68-positive cells. Activated caspase-3 and apoptosis markers: The number of TUNEL-positive cells significantly decreased in animals receiving CeNPs compared with the vehicle group. Also, CeNPs significantly reduced activated caspase-3 expression in the hepatic tissue of fibrotic rats. Gene expression: CeNP administration was accompanied by diminution of genes related to inflammation (IL-1β, TNF-α, iNOS, and COX-2). In addition, CeNP administration was also associated with decreased expression of ET-1. CeNP treatment also reduced expression of PPARγ. Finally, CeNPs significantly reduced hepatic macrophages’ M1 abundance (genes TNF-α and iNOS). |
| [33] | SG | Human hepatic stellate cells LX2 were used. | NP synthesis: NP powder was weighed, dissolved in ultrapure water, and dispersed using probe sonication. CeNPs were first characterized in terms of their size and shape using transmission electron microscopy. Cell culture: LX2 cells were maintained in Dulbecco’s modified Eagle’s medium and activated using 2 ng/mL TGF-β in 1% FBS DMEM. Characterization of NPs: The hydrodynamic size and zeta potential were evaluated using DLS. Cell viability analysis: Cell viability was assessed using the MTS technique. The absorbance was measured at 490 nm. Cell Imaging and cell migration: A total of 200,000 cells were seeded in well plates before being treated with 2 ng/mL TGF-β and appropriate NPs. After the treatment period, cells were then stained with 1 μg/mL CellTracker Orange CMRA Dye and next stained with 2 μg/mL Hoechst 33342 stain. Oxidative stress measurement: Cells were analyzed on a flow cytometer. A total of 20,000 events per sample were used to calculate mean fluorescence intensity per cell and ultimately ROS accumulation per sample and normalized to TGF-β controls. Nrf2 activity assay: The ARE Reporter Kit was used to study total Nrf2 activity according to the manufacturer’s instructions. Immunoblots: The separated proteins were transferred to nitrocellulose membrane and incubated with the primary (anti-GAPDH; anti-phos-Smad2/3; anti-Smad2/3; anti-Smad4; anti-collagen I; anti-Smooth Muscle Actin; anti-microtubule-associated proteins 1A/1B light chain 3B; anti-p62; and anti-NQO1) and the secondary antibodies. Gene expression: Total RNA from cultured cells was extracted. Then, cDNA synthesis was carried out, and gene expression was performed. Relative quantification was calculated using the CT, which is inversely related to the abundance of mRNA transcripts in the initial sample. The relative quantity of the product was expressed as fold induction of the target gene compared with the reference gene according to the formula 2−∆∆CT. Caspase 3/7 activity assay: Caspase 3/7 activity was determined using the Caspase 3/7-GLO assay kit. | Characterization of NPs, cell viability analysis, cell imaging and cell migration, oxidative stress measurement, Nrf2 activity assay, immunoblots, gene expression, and caspase 3/7 activity assay. | To investigate liver fibrosis in the human cultured HSC cell line LX2 to confirm if CeNP treatment was able to reduce fibrosis symptoms in vitro. | Characterization of NPs: The hydrodynamic diameter of the CeNPs in water and DMEM was approximately 120 nm and 160 nm, respectively. Cell viability analysis: Cell viability was not significantly different between groups. Cell Imaging and cell migration: The morphology of NP/TGF-β-treated cells showed no obvious difference compared to TGF-β-activated cells. Oxidative stress measurement: A total reduction in ROS levels of 113.0% for 500 μM CeNPs was observed when quiescent cell ROS levels were used as a baseline for comparison. Nrf2 activity assay: CeNP-treated cells showed a marked dose-dependent reduction in Nrf2 activity compared to TGF-β-activated controls. Immunoblots: TGF-β-activated LX2 cells with CeNPs significantly reduced Col-I and α-SMA protein expression. Reduced Smad4 expression was found in CeNP-treated cells. Gene expression: TGF-β-activated LX2 cells with CeNPs significantly reduced Col-I and α-SMA expression. CeNPs downregulated TIMP2 and N-cad, and upregulated MMP1 and E-cad expression in LX2 cells. Caspase 3/7 activity assay: There were no significant differences in caspase 3 activity between TGF-β-treated control cells and both 100 μM and 500 μM CeNP-treated cells. |
| [34] | ES | Male Wistar rats (n = 118) were used. | NP synthesis and characterization: CeNPs of 4–5 nm were synthesized via the chemical precipitation of Ce(NO3)3 in a basic aqueous solution. Animal model: In total, 118 male Wistar rats were used. HCC was chemically induced in 110 rats. CeNPs or vehicle (saline solution containing TMAOH ammonium salts 0.8 mM) were dispersed in saline solution and intravenously given as a bolus (500 μL) through the tail vein, twice a week for two consecutive weeks starting at the sixteenth week after beginning DEN (50 mg/kg body weight) administration. Serum samples were also obtained and kept at −20 °C until further analysis. Liver samples were obtained to evaluate steatosis, apoptosis, and mRNA expression of P-ERK1/2. Histological examination: IHQ (Ki67) was used for fibrosis analysis. Western blotting: The separated proteins were transferred to nitrocellulose membrane and incubated with the primary (P-ERK1/2) and the secondary antibodies. | NP characterization, biochemical levels of AFP, collagen content and cellular apoptosis measurement, gene expression, and histological examination. | To elucidate the potential of CeNPs as therapeutic agents in HCC. | NP characterization: NPs had a spherical morphology and were predominantly within the size range of 4–20 nm. The colloidal stability is mediated by electrostatic repulsion (zeta potential + 43.0 ± 1.3 mV, conductivity 0.303 ± 0.006 mS/cm, and pH 4.3). Biochemical levels: CeNPs did significantly reduce the AFP circulating levels. Collagen content and cellular apoptosis measurement: No significant differences in hepatic collagen content were noted between treated and non-treated rats with HCC. The TUNEL assay showed a significant increase in positive cells in the liver sections of DEN-injured rats treated with CeNPs. A significantly increased protein expression of activated caspase-3 in HCC rats treated with CeNPs was found. Gene expression: Administration of CeNPs significantly down-regulated M1 genes involved in proinflammatory function. Histological examination: The cell proliferation rate, measured as the percent of Ki67-positive hepatocyte nuclei, was markedly lower in CeNP-treated rats. Western blotting: CeNP treatment resulted in a significant reduction in P-ERK1/2. |
| [35] | UA | White male Wistar rats (n = 30) were used. | Study design: Rats were divided into three groups: control, MSG, and MSG + CeNPs groups. Newborn rats of the control group were injected with saline (control). The MSG and MSG + CeNPs groups were subcutaneously injected with MSG (4 mg/g; 8 μL/g volume) on the 2nd and 10th day of life. At the age of 1 month, rats of group II were administered water in a volume of 2.9 mL/kg orally, and the MSG + CeNPs group was treated with 1 mM solution of CeNPs (1 mg/kg orally). The treatments were given intermittently in two-week courses alternated with two-week breaks for 3 months. During the experiment, rats aged between one and four months were fed with standard laboratory chow and tap water ad libitum. Four-month-old rats were sacrificed, and the liver was removed for histological and biochemical analysis. | Histological analysis, biochemical measurement of lipid peroxidation, and antioxidant systems activity. | To investigate the influence of CeNPs on lipid peroxidation and antioxidant enzyme activity in rats with experimentally induced NAFLD. | Histological analysis: There was a significantly lower total score (1.3 ± 0.26 vs. 3.6 ± 0.34, p < 0.001), degree of steatosis (1.1 ± 0.18 vs. 2.1 ± 0.18, p < 0.001), manifestation of lobular inflammation (0.2 ± 0.13 vs. 1.2 ± 0.2, p < 0.001) and ballooning degeneration (0.0 ± 0.0 vs. 0.3 ± 0.15, p = 0.034) due to NAS in the CeNPs group as compared to the MSG group. Biochemical measurement of lipid peroxidation and antioxidant systems activity: Short-term periodic oral administration of CeNPs significantly decreased the lipid peroxidation in liver tissue, namely reducing the DC content by 27% (p < 0.05), TBA-products by 43% (p < 0.05), and Schiff bases by 21% (p < 0.05). Treatment with CeNPs led to the restoration of SOD activity to the control values and decrease in excessive catalase activity by 22.1% (p < 0.05) compared to the MSG group. |
| Reference | Study Design | Population | Method of Allocation to Intervention (or Comparison) | Outcomes | Analyses | Summary | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | 27 | ||
| [27] | Experimental study | ++ | ++ | ++ | ++ | ++ | NR | NR | ++ | ++ | ++ | ++ | NA | NA | ++ | ++ | ++ | ++ | + | ++ | ++ | NR | ++ | + | ++ | ++ | ++ | ++ |
| [28] | Experimental study | + | ++ | ++ | + | ++ | NR | + | + | + | ++ | ++ | NA | NA | ++ | ++ | ++ | ++ | + | + | + | NR | ++ | + | ++ | + | ++ | ++ |
| [29] | Experimental study | ++ | ++ | ++ | - | ++ | ++ | ++ | ++ | ++ | NA | NA | NA | NA | ++ | ++ | ++ | ++ | ++ | ++ | + | NR | ++ | ++ | ++ | + | ++ | ++ |
| [30] | Experimental study | ++ | ++ | ++ | + | ++ | + | + | + | + | + | ++ | NA | NA | ++ | ++ | ++ | ++ | + | + | ++ | NR | + | ++ | ++ | + | ++ | ++ |
| [31] | Experimental study | ++ | + | ++ | ++ | ++ | ++ | ++ | + | ++ | - | ++ | NA | NA | ++ | ++ | ++ | ++ | + | ++ | + | NR | NR | ++ | ++ | ++ | ++ | ++ |
| [32] | Experimental study | ++ | ++ | ++ | ++ | ++ | ++ | ++ | + | ++ | NA | ++ | NA | NA | ++ | ++ | ++ | ++ | + | + | ++ | NR | + | NR | ++ | ++ | ++ | ++ |
| [33] | Experimental study | ++ | ++ | ++ | - | ++ | + | + | + | + | NA | ++ | NA | NA | ++ | ++ | ++ | ++ | ++ | + | + | NR | + | NA | ++ | + | ++ | ++ |
| [34] | Experimental study | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | + | NA | NA | ++ | ++ | ++ | ++ | ++ | ++ | ++ | NR | + | + | + | ++ | + | + |
| [35] | Experimental study | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | NR | ++ | NA | NA | ++ | ++ | ++ | ++ | ++ | + | ++ | NR | ++ | ++ | ++ | ++ | ++ | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sandoval, C.; Reyes, C.; Rosas, P.; Godoy, K.; Souza-Mello, V.; Farías, J. Effectiveness of Cerium Oxide Nanoparticles in Non-Alcoholic Fatty Liver Disease Evolution Using In Vivo and In Vitro Studies: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 15728. https://doi.org/10.3390/ijms242115728
Sandoval C, Reyes C, Rosas P, Godoy K, Souza-Mello V, Farías J. Effectiveness of Cerium Oxide Nanoparticles in Non-Alcoholic Fatty Liver Disease Evolution Using In Vivo and In Vitro Studies: A Systematic Review. International Journal of Molecular Sciences. 2023; 24(21):15728. https://doi.org/10.3390/ijms242115728
Chicago/Turabian StyleSandoval, Cristian, Carolina Reyes, Pamela Rosas, Karina Godoy, Vanessa Souza-Mello, and Jorge Farías. 2023. "Effectiveness of Cerium Oxide Nanoparticles in Non-Alcoholic Fatty Liver Disease Evolution Using In Vivo and In Vitro Studies: A Systematic Review" International Journal of Molecular Sciences 24, no. 21: 15728. https://doi.org/10.3390/ijms242115728
APA StyleSandoval, C., Reyes, C., Rosas, P., Godoy, K., Souza-Mello, V., & Farías, J. (2023). Effectiveness of Cerium Oxide Nanoparticles in Non-Alcoholic Fatty Liver Disease Evolution Using In Vivo and In Vitro Studies: A Systematic Review. International Journal of Molecular Sciences, 24(21), 15728. https://doi.org/10.3390/ijms242115728








