In Vitro Assessment of Cisplatin/Hyaluronan Complex for Loco-Regional Chemotherapy
Abstract
:1. Introduction
2. Results and Discussion
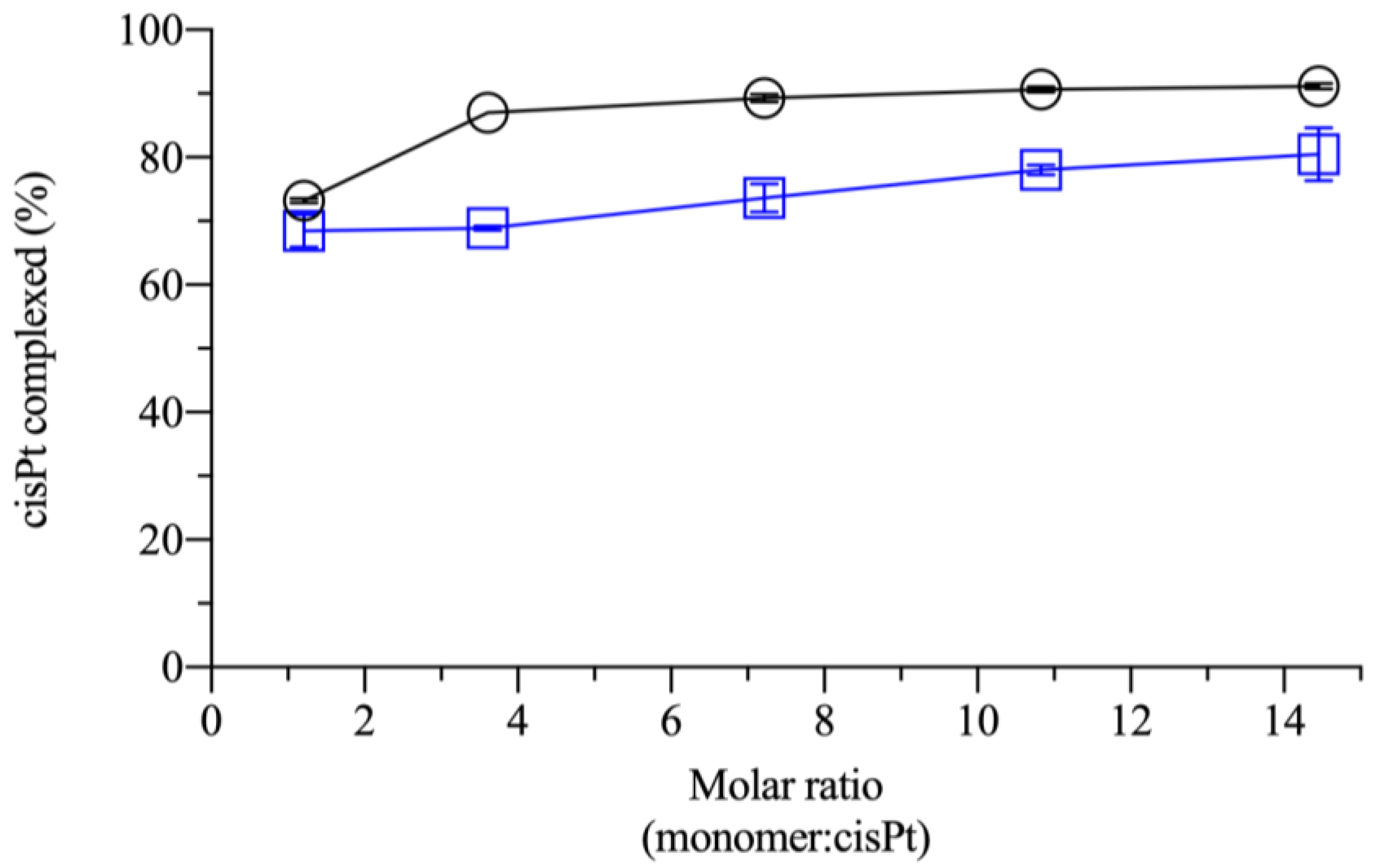
2.1. Effect of Monomer:Drug Molar Ratio and Polymer Molecular Weight in the Complexation
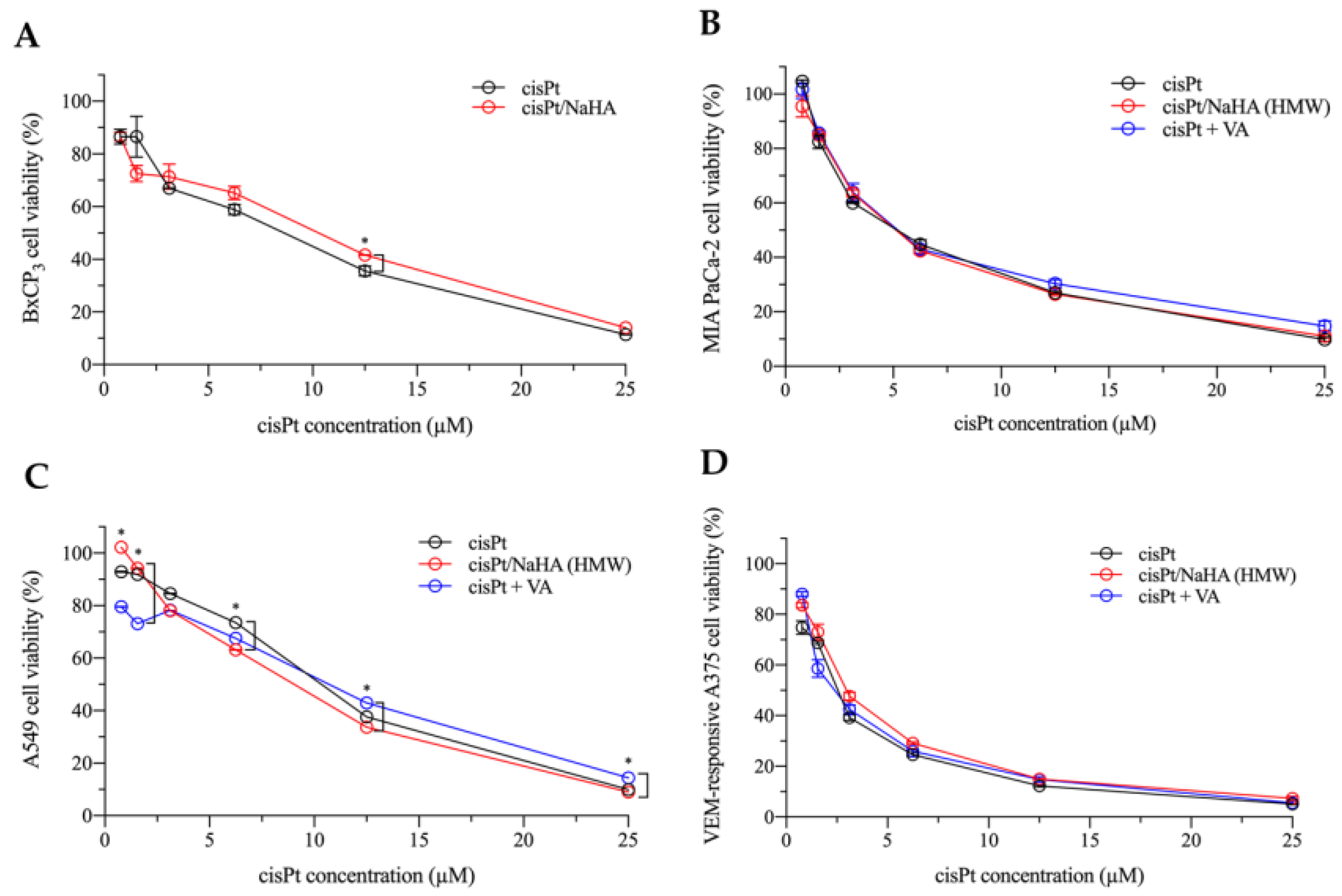
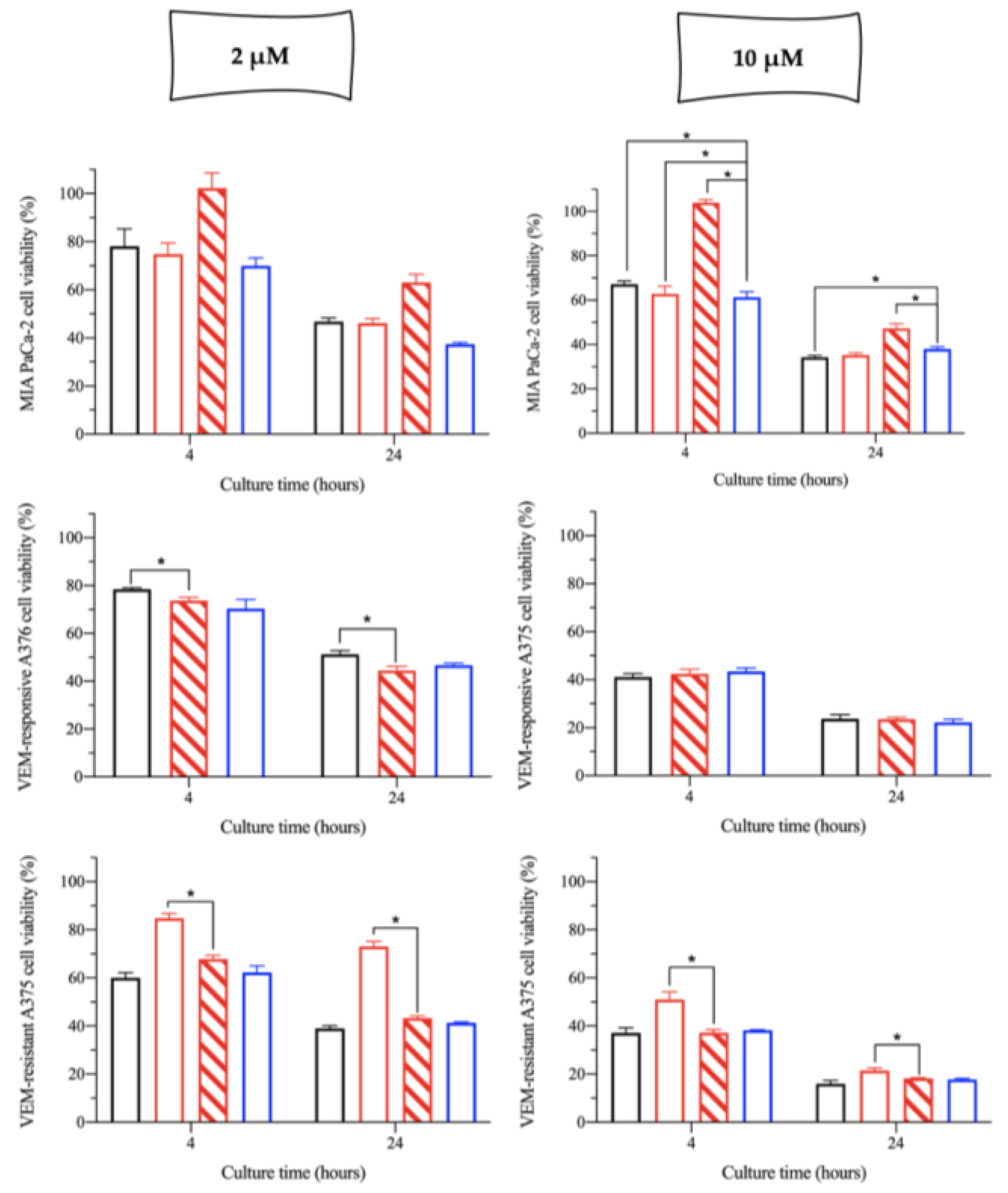
2.2. In Vitro Cytotoxicity of the cisPt/NaHA Complex
- cisplatin alone;
- cisPt complexed with high molecular weight hyaluronan;
- cisPt combined with valproic acid (VA).
- Was cisPt really complexed by hyaluronan in the three stock solutions as it was in the film-forming mixture and film applied in vivo?
- Did the dilution of the stock solution with the cell culture medium dissociate any complex formed?
- Did the polymer high molecular weight of hyaluronan affect cisPt availability at intracellular level, also considering the CD44 receptor presence?
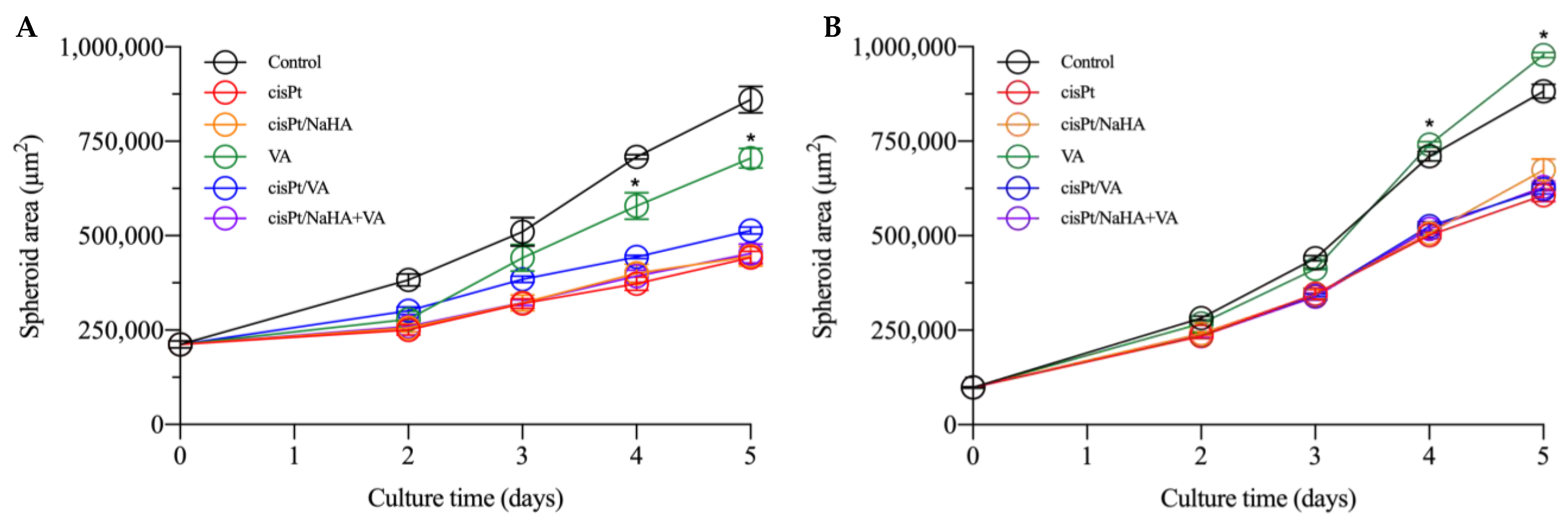
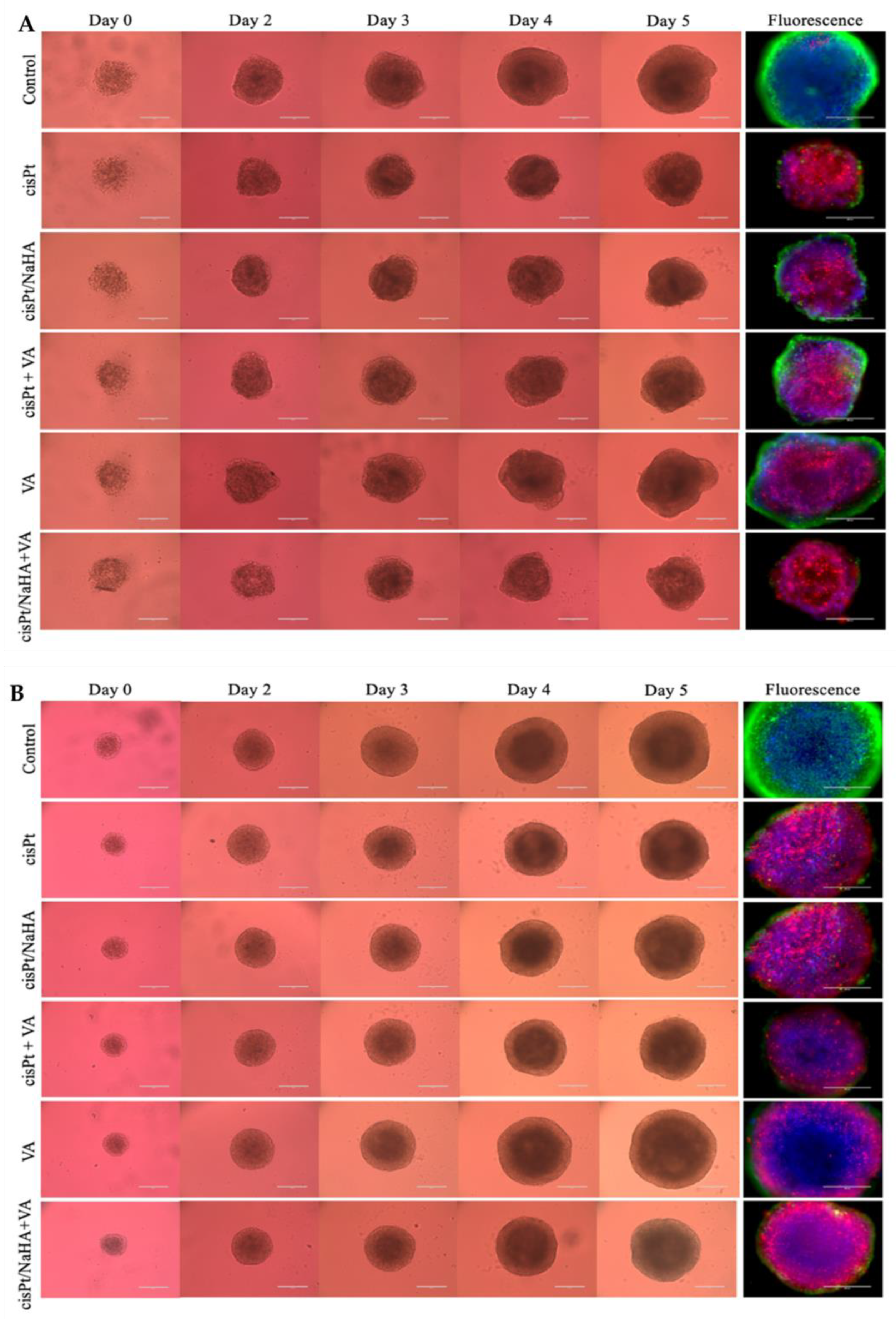
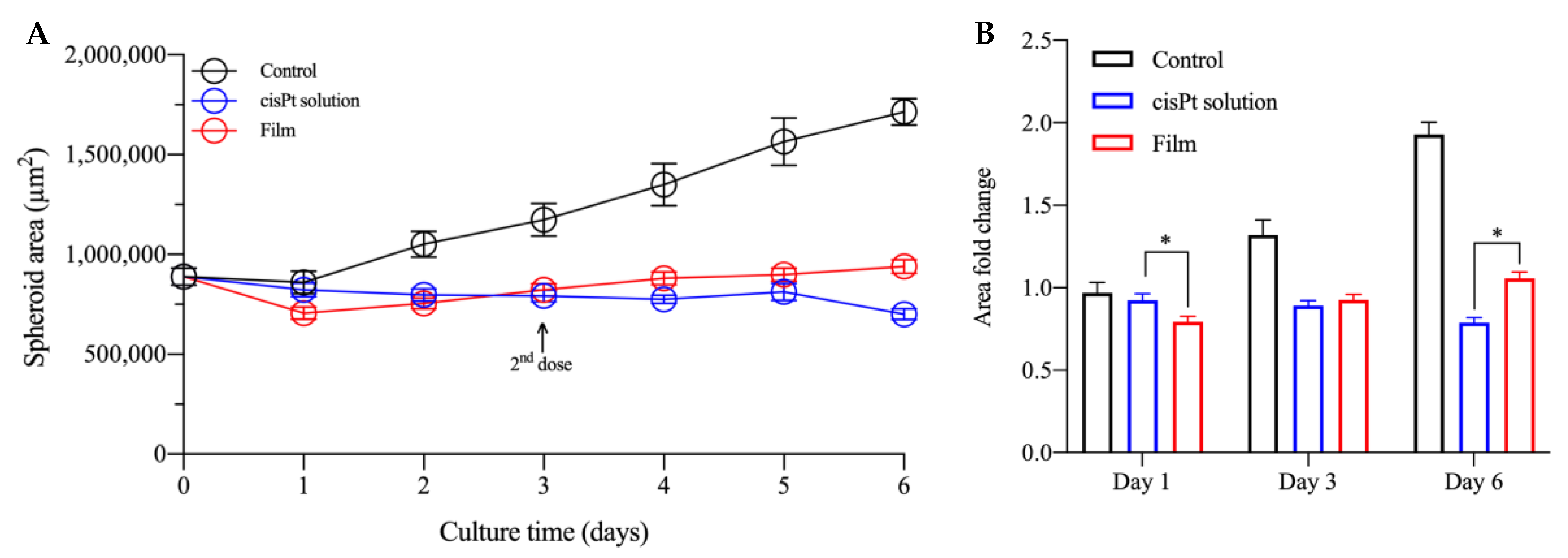
2.3. cisPt/NaHA Complex (Solution and Film) Slowed Down Spheroid Growth
2.3.1. Cytotoxic Activity Testing in VEM-Sensitive and Resistant A375 Spheroids
- cisplatin alone;
- cisPt complexed with high molecular weight hyaluronan;
- cisPt combined with valproic acid;
- valproic acid alone;
- cisPt/NaHA HMW complex combined with valproic acid.
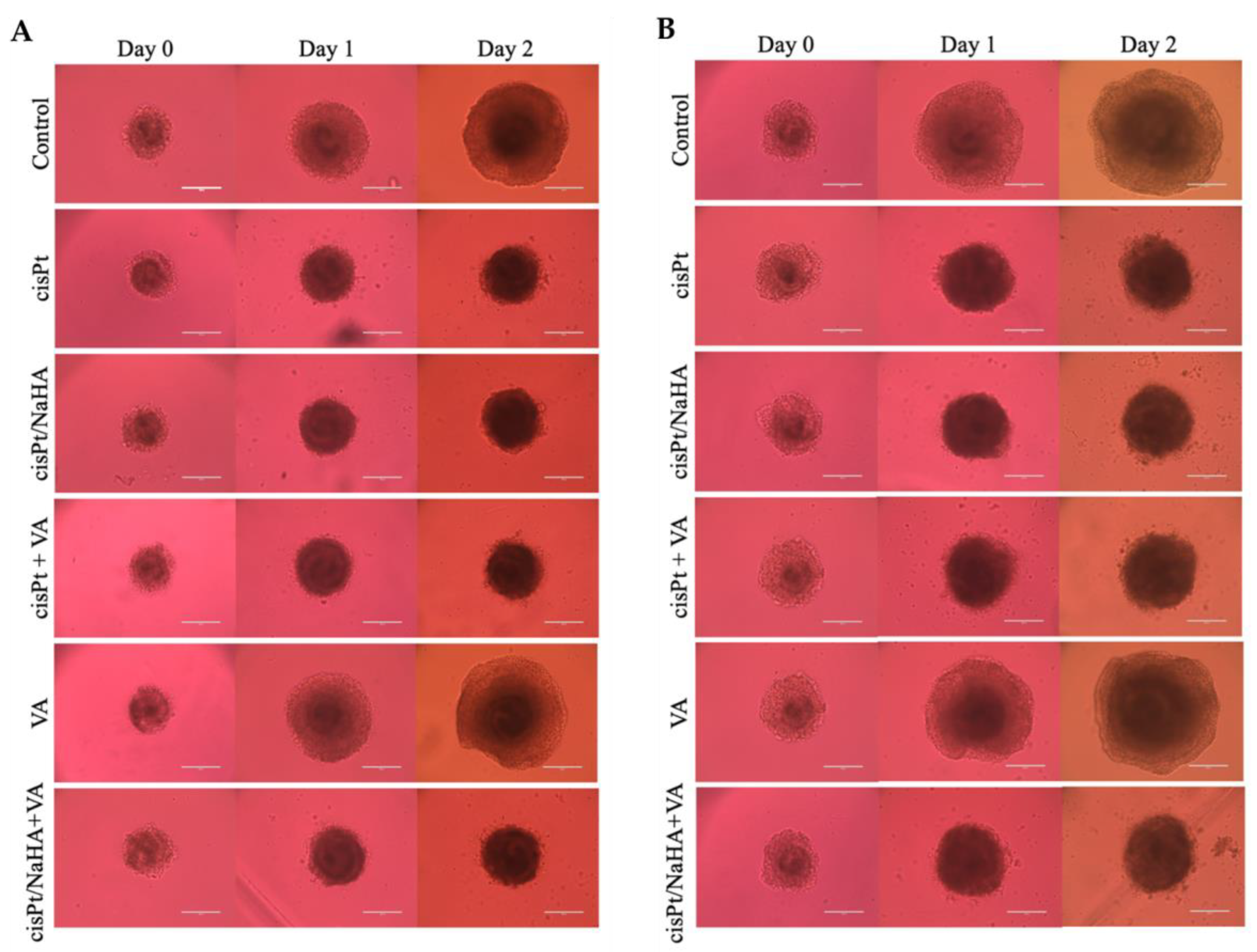
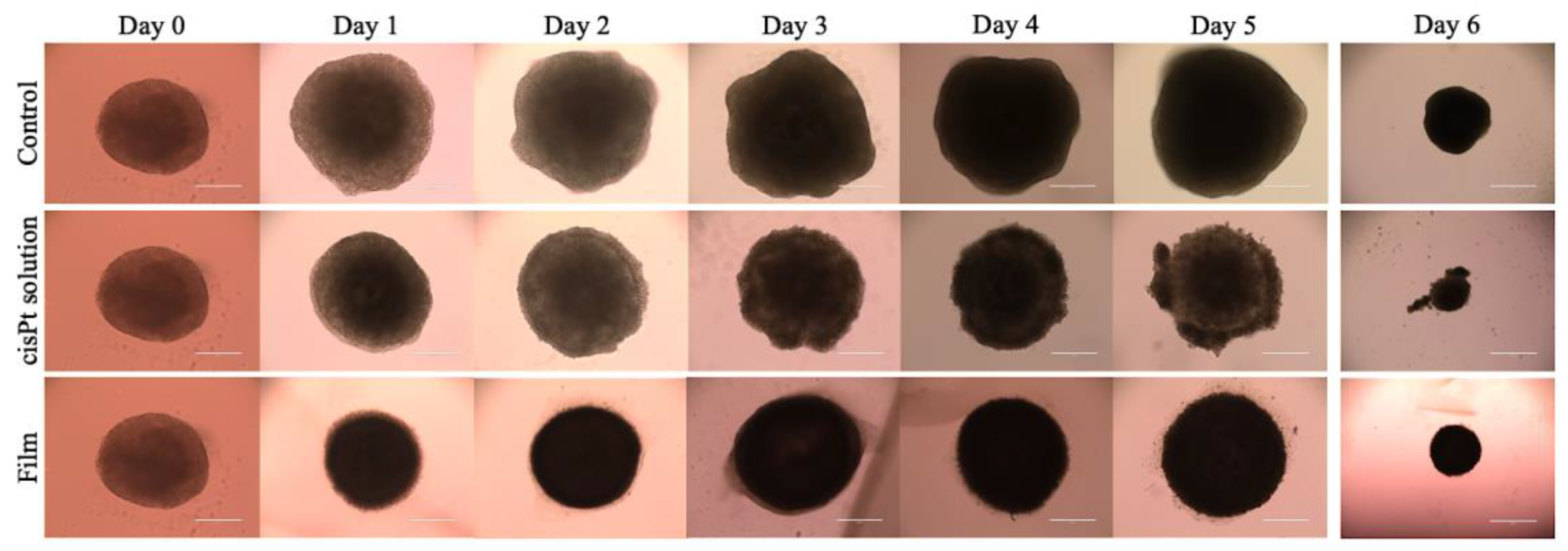
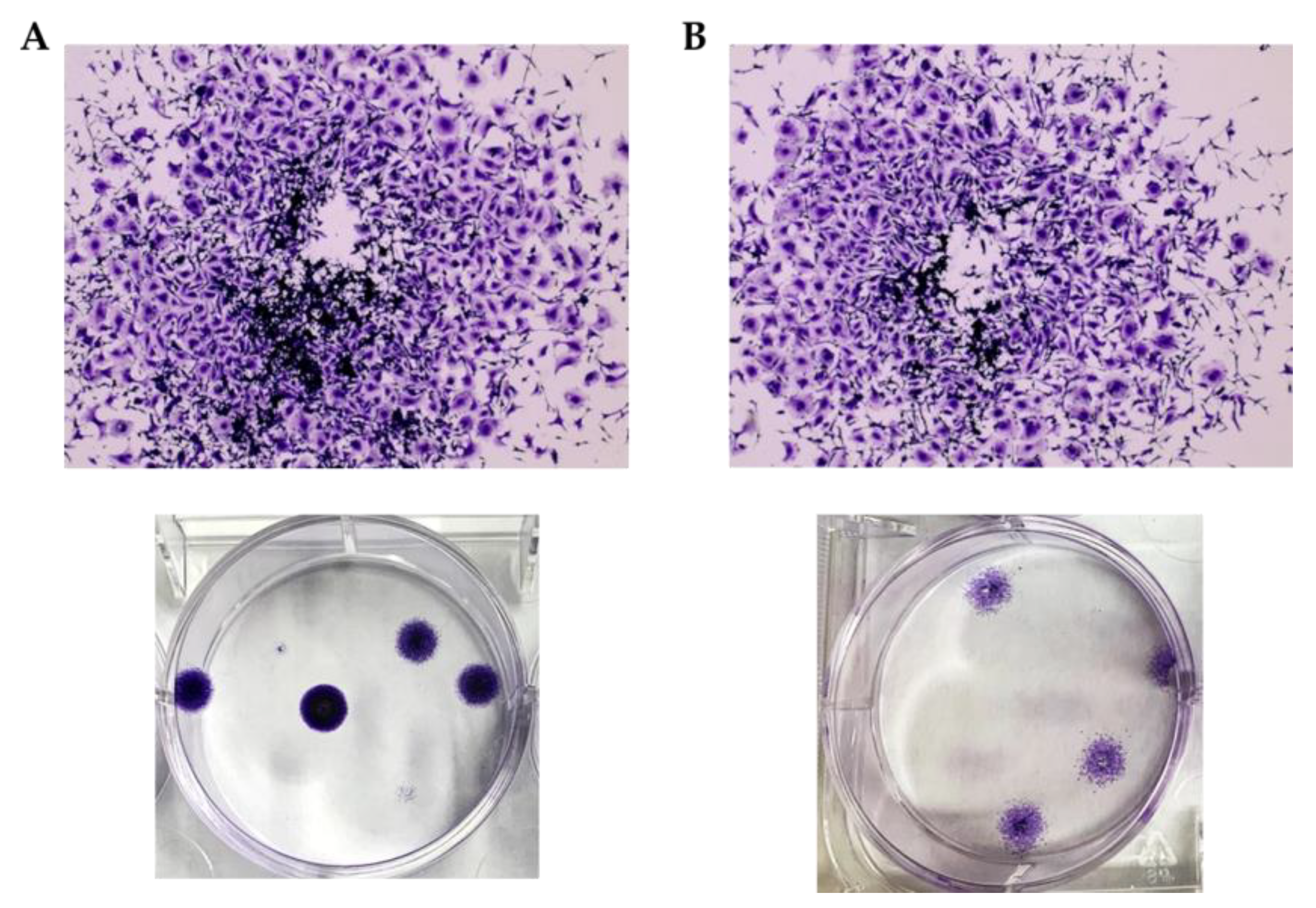
2.3.2. cisPt/NaHA Film Inhibited the Development of Spheroid-Derived New Cell Colonies
3. Materials and Methods
3.1. Materials
3.2. Complexation Efficiency between Cisplatin and Hyaluronan
3.3. HPLC Analysis
3.4. Experiments with 2D Cell Models
3.4.1. Preparation of cisPt and cisPt/NaHA Stock Solutions
3.4.2. Cell Culture
3.4.3. In Vitro Cytotoxicity Experiments
3.5. Film-Forming Mixture Preparation and Film Manufacturing
3.6. Formation and Treatment of 3D Multicellular Tumor Spheroids
3.6.1. Treatment with Drug Solutions
3.6.2. Treatment of Spheroids with the Film
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Agnihotri, T.G.; Gomte, S.S.; Jain, A. Emerging theranostics to combat cancer: A perspective on metal-based nanomaterials. Drug Dev. Ind. Pharm. 2022, 48, 48585–48601. [Google Scholar] [CrossRef]
- Ghosh, S. Cisplatin: The first metal based anticancer drug. Bioorg. Chem. 2019, 88, 102925. [Google Scholar] [CrossRef] [PubMed]
- Riley, R.S.; June, C.H.; Langer, R.; Mitchell, M.J. Delivery technologies for cancer immunotherapy. Nat. Rev. Drug Discov. 2019, 18, 175–196. [Google Scholar] [CrossRef]
- Dang, Y.; Guan, J. Nanoparticle-based drug delivery systems for cancer therapy. Smart Mater. Med. 2020, 1, 10–19. [Google Scholar] [CrossRef]
- Mu, W.; Chu, Q.; Liu, Y.; Zhang, N. A Review on Nano-Based Drug Delivery System for Cancer Chemoimmunotherapy. Nanomicro. Lett. 2020, 12, 142. [Google Scholar] [CrossRef]
- Patel, R.; Kuwar, U.; Dhote, N.; Alexander, A.; Nakhate, K.; Jain, P.; Uddin, A. Natural Polymers as a Carrier for the Effective Delivery of Antineoplastic Drugs. Curr. Drug Deliv. 2023, 21, 2. [Google Scholar] [CrossRef]
- Markowiak, T.; Ried, M.; Großer, C.; Hofmann, H.S.; Hillejan, L.; Hecker, E.; Semik, M.; Lesser, T.; Kugler, C.; Seifert, S.; et al. Postoperative outcome after palliative treatment of malignant pleural effusion. Thorac. Cancer 2022, 13, 2158–2163. [Google Scholar] [CrossRef] [PubMed]
- Ampollini, L.F.; Barocelli, E.; Cavazzoni, A.; Bilancia, R.; Mucchino, C.; Cantoni, A.M.; Carbognani, P. Intrapleural polymeric films containing cisplatin for malignant pleural mesothelioma in a rat tumour model: A preliminary study. Eur. J. Cardiothorac. Surg. 2010, 37, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Ampollini, L.; Barocelli, E.; Cavazzoni, A.; Petronini, P.; Mucchino, C.; Cantoni, A.M.; Leonardi, F.; Ventura, L.; Barbieri, S.; Colombo, P.; et al. Polymeric films loaded with cisplatin for malignant pleural mesothelioma: A pharmacokinetic study in an ovine model. J. Thorac. Dis. 2018, 10, S207–S220. [Google Scholar] [CrossRef] [PubMed]
- Banella, S.; Quarta, E.; Colombo, P.; Sonvico, F.; Pagnoni, A.; Bortolotti, F.; Colombo, G. Orphan Designation and Cisplatin/Hyaluronan Complex in an Intracavitary Film for Malignant Mesothelioma. Pharmaceutics 2021, 13, 362. [Google Scholar] [CrossRef]
- Kesharwani, P.; Chadar, R.; Sheikh, A.; Rizg, W.Y.; Safhi, A.Y. CD44-Targeted Nanocarrier for Cancer Therapy. Front. Pharmacol. 2022, 12, 800481. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, Y.; Kato, A.; Hida, Y.; Hamada, J.; Maishi, N.; Hida, K.; Harashima, H. Synergistic Enhancement of Cellular Uptake with CD44-Expressing Malignant Pleural Mesothelioma by Combining Cationic Liposome and Hyaluronic Acid-Lipid Conjugate. J. Pharm. Sci. 2019, 108, 3218–3224. [Google Scholar] [CrossRef] [PubMed]
- Iannelli, F.; Zotti, A.I.; Roca, M.S.; Grumetti, L.; Lombardi, R.; Moccia, T.; Vitagliano, C.; Milone, M.R.; Ciardiello, C.; Bruzzese, F.; et al. Valproic Acid Synergizes with Cisplatin and Cetuximab in vitro and in vivo in Head and Neck Cancer by Targeting the Mechanisms of Resistance. Front. Cell Dev. Biol. 2020, 8, 732. [Google Scholar] [CrossRef] [PubMed]
- Chavez, A.; Quesenberry, C.P., Jr.; Darbinian, J.; Asgari, M.M. Association of Valproic Acid Use, a Potent Histone Deacetylase Inhibitor, and Melanoma Risk. J. Investig. Dermatol. 2020, 140, 2353–2358. [Google Scholar] [CrossRef]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef]
- Misra, S.; Hascall, V.C.; Markwald, R.R.; Ghatak, S. Interactions between Hyaluronan and Its Receptors (CD44, RHAMM) Regulate the Activities of Inflammation and Cancer. Front. Immunol. 2015, 6, 201. [Google Scholar] [CrossRef]
- Cai, S.; Xie, Y.; Bagby, T.R.; Cohen, M.S.; Forrest, M.L. Intralymphatic chemotherapy using a hyaluronan-cisplatin conjugate. J. Surg. Res. 2008, 147, 247–252. [Google Scholar] [CrossRef]
- Cai, S.; Xie, Y.; Davies, N.M.; Cohen, M.S.; Forrest, M.L. Pharmacokinetics and disposition of a localized lymphatic polymeric hyaluronan conjugate of cisplatin in rodents. J. Pharm. Sci. 2010, 99, 2664–2671. [Google Scholar] [CrossRef]
- Penno, M.B.; August, J.T.; Baylin, S.B.; Mabry, M.; Linnoila, R.I.; Lee, V.S.; Croteau, D.; Yang, X.L.; Rosada, C. Expression of CD44 in human lung tumors. Cancer Res. 1994, 54, 1381–1387. [Google Scholar]
- Wei, H.J.; Yin, T.; Zhu, Z.; Shi, P.F.; Tian, Y.; Wang, C.Y. Expression of CD44, CD24 and ESA in pancreatic adenocarcinoma cell lines varies with local microenvironment. Hepatobiliary Pancreat. Dis. Int. 2011, 10, 428–434. [Google Scholar] [CrossRef]
- Fänder, J.; Kielstein, H.; Buttner, M.; Koelblinger, P.; Dummer, R.; Bauer, M.; Handke, D.; Wickenhauser, C.; Seliger, B.; Jasinski-Bergner, S. Characterizing CD44 regulatory microRNAs as putative therapeutic agents in human melanoma. Oncotarget 2019, 10, 6509–6525. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Dai, Y.; Gao, H. Development and application of hyaluronic acid in tumor targeting drug delivery. Acta Pharm. Sin. B 2019, 9, 1099–1112. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.; Ding, X.; Ye, J.; Deng, J.; Cui, S. pH-responsive hyaluronic acid-based nanoparticles for targeted curcumin delivery and enhanced cancer therapy. Colloids Surf. B Biointerfaces 2021, 198, 111455. [Google Scholar] [CrossRef] [PubMed]
- Wawruszak, A.; Luszczki, J.J.; Grabarska, A.; Gumbarewicz, E.; Dmoszynska-Graniczka, M.; Polberg, K.; Stepulak, A. Assessment of Interactions between Cisplatin and Two Histone Deacetylase Inhibitors in MCF7, T47D and MDA-MB-231 Human Breast Cancer Cell Lines—An Isobolographic Analysis. PLoS ONE 2015, 10, e0143013. [Google Scholar] [CrossRef] [PubMed]
- Ishiguro, S.; Cai, S.; Uppalapati, D.; Turner, K.; Zhang, T.; Forrest, W.C.; Forrest, M.L.; Tamura, M. Intratracheal Administration of Hyaluronan-Cisplatin Conjugate Nanoparticles Significantly Attenuates Lung Cancer Growth in Mice. Pharm. Res. 2016, 33, 2517–2529. [Google Scholar] [CrossRef]
- Kim, S.J.; Owen, S.C. Hyaluronic acid binding to CD44S is indiscriminate of molecular weight. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183348. [Google Scholar] [CrossRef]
- Zhang, J.S.; Imai, T.; Suenaga, A.; Otagiri, M. Molecular-weight-dependent pharmacokinetics and cytotoxic properties of cisplatin complexes prepared with chondroitin sulfate A and C. Int. J. Pharm. 2002, 240, 23–31. [Google Scholar] [CrossRef]
- Costa, E.C.; Moreira, A.F.; de Melo-Diogo, D.; Gaspar, V.M.; Carvalho, M.P.; Correia, I.J. 3D tumor spheroids: An overview on the tools and techniques used for their analysis. Biotechnol. Adv. 2016, 34, 1427–1441. [Google Scholar] [CrossRef]
- Sandri, S.; Faião-Flores, F.; Tiago, M.; Pennacchi, P.C.; Massaro, R.R.; Alves-Fernandes, D.K.; Berardinelli, G.N.; Evangelista, A.F.; de Lima Vazquez, V.; Reis, R.M.; et al. Vemurafenib resistance increases melanoma invasiveness and modulates the tumor microenvironment by MMP-2 upregulation. Pharmacol. Res. 2016, 111, 523–533. [Google Scholar] [CrossRef]
- Rathod, D.; Fu, Y.; Patel, K. BRD4 PROTAC as a novel therapeutic approach for the treatment of vemurafenib resistant melanoma: Preformulation studies, formulation development and in vitro evaluation. Eur. J. Pharm. Sci. 2019, 138, 105039. [Google Scholar] [CrossRef]
- Sonvico, F.; Barbieri, S.; Colombo, P.; Mucchino, C.; Barocelli, E.; Cantoni, A.M.; Cavazzoni, A.; Petronini, P.G.; Rusca, M.; Carbognani, P.; et al. Physicochemical and pharmacokinetic properties of polymeric films loaded with cisplatin for the treatment of malignant pleural mesothelioma. J. Thorac. Dis. 2018, 10, S194–S206. [Google Scholar] [CrossRef] [PubMed]
- Crabb, S.J.; Martin, K.; Abab, J.; Ratcliffe, I.; Thornton, R.; Lineton, B.; Ellis, M.; Moody, R.; Stanton, L.; Galanopoulou, A.; et al. COAST (Cisplatin ototoxicity attenuated by aspirin trial): A phase II double-blind, randomised controlled trial to establish if aspirin reduces cisplatin induced hearing-loss. Eur. J. Cancer 2017, 87, 75–83. [Google Scholar] [CrossRef] [PubMed]


| Cell Line | IC50 (µM) | ||
|---|---|---|---|
| cisPt | cisPt/NaHA HMW | cisPt + VA | |
| BxPC3 | 9.63 ± 0.05 | 10.58 ± 0.08 | NA |
| A549 | 9.44 ± 0.10 | 9.14 ± 0.07 | 10.71 ± 0.05 |
| MIA PaCa-2 | 5.04 ± 0.04 | 5.03 ± 0.11 | 5.11 ± 0.05 |
| VEM-responsive A375 | 2.17 ± 0.01 | 2.32 ± 0.04 | 2.39 ± 0.06 |
| Test Solution | cisPt (µM) | NaHA HMW (µM) | VA (µM) |
|---|---|---|---|
| 1 | 0.2–25.0 | 0 | 0 |
| 2 | 0.7–180.5 | 0 | |
| 3 | 0 | 0.4–100.0 |
| Component | % (w/w) |
|---|---|
| Sodium hyaluronate (NaHA) | 40.7 |
| PVA 83,400 | 14.7 |
| PEG 200 | 14.7 |
| PEG 1000s | 14.7 |
| Sorbitol solution (70% w/v) | 14.7 |
| Cisplatin (cisPt) | 0.5 |
| Treatment Group | cisPt (µM) | NaHA (HMW) (µM) | VA (µM) |
|---|---|---|---|
| 1 | 1 or 2.5 | 0 | 0 |
| 2 | 1 or 2.5 | 3.6 or 9.0 | 0 |
| 3 | 0 | 0 | 100 |
| 4 | 1 or 2.5 | 0 | 100 |
| 5 | 1 or 2.5 | 3.6 or 9.0 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banella, S.; Saraswat, A.; Patel, A.; Serajuddin, A.T.M.; Colombo, P.; Patel, K.; Colombo, G. In Vitro Assessment of Cisplatin/Hyaluronan Complex for Loco-Regional Chemotherapy. Int. J. Mol. Sci. 2023, 24, 15725. https://doi.org/10.3390/ijms242115725
Banella S, Saraswat A, Patel A, Serajuddin ATM, Colombo P, Patel K, Colombo G. In Vitro Assessment of Cisplatin/Hyaluronan Complex for Loco-Regional Chemotherapy. International Journal of Molecular Sciences. 2023; 24(21):15725. https://doi.org/10.3390/ijms242115725
Chicago/Turabian StyleBanella, Sabrina, Aishwarya Saraswat, Akanksha Patel, Abu T. M. Serajuddin, Paolo Colombo, Ketan Patel, and Gaia Colombo. 2023. "In Vitro Assessment of Cisplatin/Hyaluronan Complex for Loco-Regional Chemotherapy" International Journal of Molecular Sciences 24, no. 21: 15725. https://doi.org/10.3390/ijms242115725
APA StyleBanella, S., Saraswat, A., Patel, A., Serajuddin, A. T. M., Colombo, P., Patel, K., & Colombo, G. (2023). In Vitro Assessment of Cisplatin/Hyaluronan Complex for Loco-Regional Chemotherapy. International Journal of Molecular Sciences, 24(21), 15725. https://doi.org/10.3390/ijms242115725






